Abstract
In the present study, we synthesized a series of novel 7-methoxy-N-(substituted phenyl)benzofuran-2-carboxamide derivatives in moderate to good yields and evaluated their neuroprotective and antioxidant activities using primary cultured rat cortical neuronal cells and in vitro cell-free bioassays. Based on our primary screening data with eighteen synthesized derivatives, nine compounds (1a, 1c, 1f, 1i, 1j, 1l, 1p, 1q and 1r) exhibiting considerable protection against the NMDA-induced excitotoxic neuronal cell damage at the concentration of 100 μM were selected for further evaluation. Among the selected derivatives, compound 1f (with -CH3 substitution at R2 position) exhibited the most potent and efficacious neuroprotective action against the NMDA-induced excitotoxicity. Its neuroprotective effect was almost comparable to that of memantine, a well-known NMDA antagonist, at 30 μM concentration. In addition to 1f, compound 1j (with -OH substitution at R3 position) also showed marked anti-excitotoxic effects at both 100 and 300 μM concentrations. These findings suggest that -CH3 substitution at R2 position and, to a lesser degree, -OH substitution at R3 position may be important for exhibiting neuroprotective action against excitotoxic damage. Compound 1j was also found to scavenge 1,1-diphenyl-2-picrylhydrazyl radicals and inhibit in vitro lipid peroxidation in rat brain homogenate in moderate and appreciable degrees. Taken together, our structure-activity relationship studies suggest that the compound with -CH3 substitution at R2 and -OH substitution at R3 positions of the benzofuran moiety might serve as the lead exhibiting potent anti-excitotoxic, ROS scavenging, and antioxidant activities. Further synthesis and evaluation will be necessary to confirm this possibility.
Keywords: Neuroprotection, Excitotoxicity, Reactive oxygen species, Antioxidant activity, Benzofuran-2-carboxamide derivatives
INTRODUCTION
Excitotoxicity plays a pivotal role in the onset and development of neurodegeneration, where neuronal cells are subject to damage and extermination (Manev et al., 1989). The process initiates as a consequence of over activation of receptors for the excitatory neurotransmitter glutamate, such as N-methyl-D-aspartic acid (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Accumulating evidence suggests the role of excitotoxicity in traumatic brain injury, stroke and neurodegenerative disorders of the central nervous system (Choi, 1992; Coyle and Puttfarcken, 1993; Lipton and Rosenberg, 1994; Kim et al., 2002). In addition, the involvement of oxidative stress in neuronal cell death has been well documented. An earlier study has reported that during NMDA receptor activation, reactive oxygen species (ROS) is generated by the action of NADPH oxidase in postsynaptic neurons (Girouard et al., 2009). Production of high level of ROS and down regulation of antioxidant mechanisms are known to be among the major causes of neuronal cell death in neurodegenerative diseases (Uttara et al., 2009). Oxidative stress perturbs the structure of protein, lipid and nucleic acid molecules and possibly opens the mitochondrial permeability transition pores which in turn can further stimulates ROS production and triggers the release of proapoptotic factors (Nicholls, 2004; Uttara et al., 2009). Besides, lipid peroxidation (LPO) plays a critical role as a mediator in the pathophysiological processes of many neurological disorders, which are induced by free radicals, predominantly by the major members of ROS (Hall, 1993). All of these findings collectively suggest that the discovery of novel scaffolds with inhibitory effects on excitotoxicity, ROS production and oxidative stress are therapeutically desirable to treat the neurodegenerative disorders.
For the past few years, the heterocyclic benzofuran derivatives have gained a considerable interest due to their diverse biological activities including anti-microbial, anti-inflammatory, anti-tumor, anti-tubercular and anti-plasmodial activities as well as potential pharmacological applications (Dawood, 2013). In addition, accumulating evidence also indicates the neuroprotective activity of benzofurans. For example, a series of benzofuran-based compounds exhibited potent inhibitory effect on β-amyloid aggregation and protected human neuronal SHSY5Y cells from β-amyloid peptide-induced toxic insult (Howlett et al., 1999; Rizzo et al., 2008). In another instance, (−)-1(benzofuran-2-yl)-2-propylaminopentane has been shown to exert neuroprotective effects and has been investigated in the treatment of Alzheimer’s disease, Parkinson’s disease, and clinical depression (Gaszner and Miklya, 2006).
The neuroprotective effects of antioxidants have been well documented which can be mediated via a number of mechanisms including prevention of the formation of free radicals or chemical interference with formed free radicals (Moosmann and Behl, 2002). Substantial evidence indicates the antioxidative properties of benzofuran compounds (Varvaresou et al., 2001; Rindhe et al., 2010; Dawood, 2013) and a recent study has shown the protective effect of a benzofuran derivative against neurotoxin-induced oxidative stress and cell death in a neuronal cell line (Chong et al., 2014). This information prompted us to design and synthesize a series of novel 7-methoxy-N-(substituted phenyl)benzofuran-2-carboxamide derivatives where an amide moiety was linked to the two hydrophobic binding pockets (an aromatic phenyl ring and a benzofuran moiety) in the scaffolds. We then evaluated the anti-excitotoxic, free radical scavenging, and anti-oxidant activities of the synthesized derivatives using primary cultured rat cortical neuronal cells as well as in vitro cell-free bioassays.
MATERIALS AND METHODS
Materials
Minimum essential medium (MEM), fetal bovine serum (FBS), horse serum (HS) and antibiotic-antimycotic agent were purchased from Gibco BRL (Gaithersburg, MD, USA). Cytosine arabinoside, laminin, poly-L-lysine, glucose, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2′, 7′-dichlorofluorescin diacetate (DCFH-DA), 2-thiobarbituric acid and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were from Sigma-Aldrich (St. Louis, MO, USA). NMDA was obtained from Tocris Bioscience (Bristol, UK). All other chemicals were of analytical grade.
General procedure for the synthesis of 7-methoxy-benzofuran-2-carboxylic acid N-(substituted) phenylamides (1a-r)
N,N’-carbonyldiimidazole (1.5 mmol) was added to a solution of 7-methoxy-2-benzofuran-carboxylic acid (1 mmol) in tetrahydrofuran (5 mL). After 1 h, (substituted) aniline (1.5 mmol) was added and stirred for 12–14 h. The mixed solution was then concentrated, acidified with 6 N-HCl and extracted with ethyl acetate. The organic layers were dried with magnesium sulfate, filtered and concentrated in vacuo. The crude material was passed down through a flash chromatography column to yield 7-methoxy-benzofuran-2-carboxylic acid N-(substituted)phenylamides (1a-r). Brief synthetic scheme and the structures of the synthesized compounds are shown in Fig. 1.
Fig. 1.
Scheme for the preparation and chemical structures of 7-methoxy-N-(substituted phenyl)benzofuran-2-carboxamide derivatives. (Upper panel) General synthetic scheme of benzofuran derivatives. (Lower panel) Chemical structures of the synthesized benzofuran derivatives (1a-r).
Analytical data of the synthesized benzofuran derivatives
1) 7-Methoxy-2-benzofurancarboxylic acid N-phenylamide (1a, C16H13NO3): Yield: 42.1%; m.p. 116 ± 1 °C, IR (KBr) 3325 1670 1596 1435 1270 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.74 (dd, 2H, J=7.4, 1.1 Hz, Ar-H), 7.58 (s, 1H, Ar-H), 7.37 (t, 2H, J=7.4 Hz, Ar-H), 7.29 (dd, 1H, J=7.8, 1.2 Hz, Ar-H), 7.25 (t, 1H, J=7.8 Hz, Ar-H), 7.17 (dt, 1H, J=7.4, 1.1 Hz, Ar-H), 7.04 (dd, 1H, J=7.8, 1.2 Hz, Ar-H), 4.04 (s, 3H, OCH3)
2) 7-Methoxy-2-benzofurancarboxylic acid N-(2-methoxyphenyl)amide (1b, C17H15NO4): Yield : 40.0%, m.p. 147 ± 1°C; IR (KBR) 3403, 1606, 1532, 1049 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.50 (d, 1H, J=7.6 Hz, Ar-H), 7.56 (s, 1H, Ar-H), 7.27 (d, 1H, J=7.6 Hz, Ar-H), 7.22 (t, 1H, J=7.6 Hz, Ar-H), 7.10 (t, 1H, J=7.6 Hz, Ar-H), 7.01 (t, 1H, J=7.6 Hz, Ar-H), 6.94 (m, 2H, Ar-H), 4.05 (s, 3H, OCH3), 3.97 (s, 3H, OCH3).
3) 7-Methoxy-2-benzofurancarboxylic acid N-(3-methoxyphenyl) amide(1c, C17H15NO4): Yield : 24.8%; IR (KBR) 3463, 1609, 1577, 1092 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.59 (s, 1H, Ar-H), 7.50 (t, 1H, J=2.0 Hz, Ar-H), 7.30 (m, 2H, Ar-H), 7.25 (t, 1H, J=8.0 Hz, Ar-H), 7.20 (dd, 1H, J=8.0 Hz, 2.0 Hz, Ar-H), 6.95 (dd, 1H, J=7.8 Hz, 1.2 Hz, Ar-H), 6.74 (dd, 1H, J=8.0 Hz, 2.0 Hz, Ar-H), 4.06 (s, 3H, OCH3), 3.85 (s, 3H, OCH3).
4) 7-Methoxy-2-benzofurancarboxylic acid N-(4-methoxyphenyl)amide (1d, C17H15NO4): Yield : 77.8%, m.p. 120 ± 1°C; IR (KBR) 3303, 1666, 1509, 1034 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.63 (dd, 2H, J=6.9 Hz, 1.9 Hz, Ar-H), 7.57 (s, 1H, Ar-H), 7.29 (dd, 1H, J=7.7 Hz, 1.4 Hz, Ar-H), 7.24 (t, 1H, J=7.7, Ar-H), 6.93 (m, 3H, Ar-H), 4.05(s, 3H, -OCH3), 3.82(s, 3H, -OCH3).
5) 7-Methoxy-2-benzofurancarboxylic acid N-(2-methylphenyl)amide (1e, C17H15NO3): Yield : 67 %, m.p. 143 ± 1°C; IR (KBr) 3261 1656 1584 1427 1198 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.95 (d, 1H, J=7.7 Hz, Ar-H), 7.59 (s, 1H, Ar-H), 7.29 (dd, 1H, J=7.7, 1.1 Hz, Ar-H), 7.27 (m, 3H, Ar-H), 7.14 (t, 1H, J=7.7 Hz, Ar-H), 6.94 (dd, 1H, J=7.7, 1.1 Hz, Ar-H), 4.04 (s, 3H, OCH3), 2.41 (s, 3H, Ar-CH3).
6) 7-Methoxy-2-benzofurancarboxylic acid N-(3-methylphenyl)amide (1f, C17H15NO3): Yield : 39.6%, m.p. 122 ± 1°C; IR (KBr) 3262 1656 1584 1428 1266 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.54 (s, 1H, Ar-H), 7.52 (s, 1H, Ar-H), 7.48 (d, 1H, J=8.0 Hz, Ar-H), 7.25 (dd, 1H, J=8.0, 1.2 Hz, Ar-H), 7.21 (t, 1H, J=8.0 Hz, Ar-H), 7.20 (t, 1H, J=8.0 Hz, Ar-H), 7.01 (dd, 1H, J=8.0, 1.2 Hz, Ar-H), 6.95 (d, 1H, J=8.0 Hz, Ar-H), 3.99 (s, 3H, OCH3), 2.31 (s, 3H, Ar-CH3).
7) 7-Methoxy-2-benzofurancarboxylic acid N-(4-methylphenyl)amide (1g, C17H15NO3): Yield : 25.6%, m.p. 138 ± 1°C; IR (KBr) 3296 1668 1598 1405 1240 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.60 (d, 2H, J=8.3 Hz, Ar-H), 7.58 (s, 1H, Ar-H), 7.29 (dd, 1H, J=7.5, 1.2 Hz, Ar-H), 7.24 (t, 1H, J=7.5 Hz, Ar-H), 7.19 (d, 2H, J=8.3 Hz, Ar-H), 6.94 (dd, 1H, J=7.5, 1.2 Hz, Ar-H), 4.05 (s, 3H, OCH3), 2.35 (s, 3H, Ar-CH3).
8) 7-Methoxy-2-benzofuran-carboxylic Acid N-(2-amino phenol)amide (1h, C16H13NO4): Yield : 37.7%, m.p. 241 ± 1°C; IR (KBr) 3380, 1646, 1578, 1454, 1275 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.65 (s, 1H, Ar-H), 7.31 (dd, 1H, J=8.1 Hz, 1.4 Hz, Ar-H), 7.28 (d, 1H, J=7.6 Hz, Ar-H), 7.24 (t, 1H, J=1.4 Hz, Ar-H), 7.19 (td, 1H, J=7.6 Hz, 1.6 Hz, Ar-H), 7.08 (dd, 1H, J=8.1 Hz, 1.4 Hz, Ar-H), 6.97 (dd, 1H, J=7.6 Hz, 1.6 Hz, Ar-H), 6.93 (td, 1H, J =7.6 Hz, 1.6 Hz, Ar-H), 4.05 (s, 3H, OCH3).
9) 7-Methoxy-2-benzofuran-carboxylic Acid N-(3-aminophenol)amide (1i, C16H13NO4): Yield : 42.4%, m.p. 212 ± 1°C; IR (KBr) 3255, 1645, 1583, 1448, 1202 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.78 (t, 1H, J=1.6 Hz, Ar-H), 7.64 (s, 1H, Ar-H), 7.31 (dd, 1H, J=7.7 Hz, 1.2 Hz, Ar-H), 7.25 (m, 2H, Ar-H), 7.00 (dd, 1H, J=7.9 Hz, 1.6 Hz, Ar-H), 6.96 (dd, 1H, J=7.7 Hz, 1.2 Hz, Ar-H), 6.71 (dd, 1H, J =7.9 Hz, 1.6 Hz, Ar-H), 4.05 (s, 3H, OCH3).
10) 7-Methoxy-2-benzofuran-carboxylic Acid N-(4-aminophenol)amide (1j, C16H13NO4): Yield : 35.6%, m.p. 211 ± 1°C; IR (KBr) 3324, 1607, 1437, 1215 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.65 (m, 3H, Ar-H), 7.29 (dd, 1H, J=7.9 Hz, 1.1 Hz, Ar-H), 7.25 (t, 1H, J=7.9 Hz, Ar-H), 6.94 (dd, 1H, J=7.9 Hz, 1.1 Hz, Ar-H), 6.86 (dd, 2H, J =6.7 Hz, 2.1 Hz, Ar-H), 4.05 (s, 3H, OCH3).
11) 7-Methoxy-2-benzofurancarboxylic acid N-(2-chloroaniline)amide (1k, C16H12ClNO3): Yield : 50.6%, m.p. 150 ± 1°C; IR (KBR) 3399, 1680, 1593, 1270, 764 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.52 (dd, 1H, J=7.9 Hz, 1.2 Hz, Ar-H), 7.61 (s, 1H, Ar-H), 7.44 (dd, 1H, J=7.9 Hz, 1.4Hz, Ar-H), 7.34 (td, 1H, J=7.9 Hz, 1.4 Hz, Ar-H), 7.29 (dd, 1H, J=7.8 Hz, 1.2 Hz, Ar-H), 7.24 (t, 1H, J=7.8 Hz, Ar-H), 7.11 (td, 1H, J=7.9 Hz, 1.2 Hz, Ar-H), 6.95 (dd, 1H, J=7.8 Hz, 1.2 Hz, Ar-H), 4.05 (s, 3H, OCH3).
12) 7-Methoxy-2-benzofurancarboxylic acid N-(3-chloroaniline)amide (1l, C16H12ClNO3): Yield : 41.4%, m.p. 135 ± 1°C; IR (KBR) 3233, 1655, 1592, 1270, 675 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.56 (t, 1H, J=2.0 Hz, Ar-H), 7.61 (s, 1H, Ar-H), 7.58 (dd, 1H, J=7.6 Hz, 2.0 Hz, Ar-H), 7.32 (dd, 1H, J=7.9 Hz, 1.4 Hz, Ar-H), 7.25 (m, 2H, Ar-H), 7.15 (dd, 1H, J=7.6 Hz, 2.0 Hz, Ar-H), 6.96 (dd, 1H, J=7.9 Hz, 1.4 Hz, Ar-H), 4.05 (s, 3H, OCH3).
13) 7-Methoxy-2-benzofurancarboxylic acid N-(4-chloroaniline)amide (1m, C16H12ClNO3): Yield : 43.2%, m.p. 181 ± 1°C; IR (KBR) 3169, 1658, 1525, 1270, 719 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.69 (dd, 2H, J=6.8 Hz, 2.1 Hz, Ar-H), 7.60 (s, 1H, Ar-H), 7.35 (dd, 2H, J=6.8 Hz, 2.1 Hz, Ar-H), 7.30 (dd, 1H, J=7.7 Hz, 1.3 Hz, Ar-H), 7.25 (t, 1H, J=7.7 Hz, Ar-H), 6.95 (dd, 1H, J=7.7 Hz, 1.3 Hz, Ar-H), 4.05 (s, 3H, OCH3).
14) 7-Methoxy-2-benzofurancarboxylic acid N-(3-nitroaniline)amide (1n, C16H12N2O5): Yield : 22.7%, m.p. 188 ± 1°C; IR (KBr) 3352 1670 1527 1427 1340 1184 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.61 (t, 1H, J=2.1 Hz, Ar-H), 8.15 (dd, 1H, J=8.2, 2.1 Hz, Ar-H), 8.03 (dd, 1H, J=8.2, 2.1 Hz, Ar-H), 7.65 (s, 1H, Ar-H), 7.57 (t, 1H, J=8.2 Hz, Ar-H), 7.31 (dd, 1H, J=7.6, 1.4 Hz, Ar-H), 7.28 (t, 1H, J=7.6 Hz, Ar-H), 6.98 (dd, 1H, J=7.6, 1.4 Hz, Ar-H), 4.06 (s, 3H, OCH3).
15) 7-Methoxy-2-benzofurancarboxylic acid N-(4-nitroaniline)amide (1o, C16H12N2O5): Yield : 6.4%, m.p. 189 ± 1°C; IR (KBr) 3248 1666 1577 1405 1327 1196 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.29 (dd, 2H, J=7.1, 2.1 Hz, Ar-H), 7.94 (dd, 2H, J=7.1, 2.1 Hz, Ar-H), 7.66 (s, 1H, Ar-H), 7.32 (dd, 1H, J=7.6, 1.4 Hz, Ar-H), 7.28 (t, 1H, J=7.6 Hz, Ar-H), 6.98 (dd, 1H, J=7.6, 1.4 Hz, Ar-H), 4.06 (s, 3H, OCH3).
16) 7-Methoxy-2-benzofurancarboxylic Acid N-(2-trifluoromethylphenyl)amide (1p, C17H12F3NO3): Yield : 21.6%, m.p. 119 ± 1°C; IR (KBr) 3439, 1697, 1592, 1451, 1295, 727 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.39 (d, 1H, J=8.0 Hz, Ar-H), 7.68 (d, 1H, J=8.0 Hz, Ar-H), 7.64 (d, 1H, J=8.0 Hz, Ar-H), 7.61 (s, 1H, Ar-H), 7.3 (m, 2H, Ar-H), 7.23 (d, 1H, J=7.6 Hz, Ar-H), 6.97 (dd, 1H, J=7.6 Hz, 1.2 Hz, Ar-H), 4.06 (s, 3H, OCH3).
17) 7-Methoxy-2-benzofurancarboxylic Acid N-(3-trifluoromethylphenyl)amide (1q, C17H12F3NO3): Yield : 24.9%, m.p. 135 ± 1°C; IR (KBr) 3265, 1662, 1586, 1446, 1266, 1169, 695 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.04 (s, 1H, Ar-H), 7.94 (d, 1H, J=8.0 Hz, Ar-H), 7.62 (s, 1H, Ar-H), 7.50 (t, 1H, J=8.0 Hz, Ar-H), 7.42 (d, 1H, J=8.0 Hz, Ar-H), 7.3 (dd, 1H, J=7.9 Hz, 1.3 Hz, Ar-H), 7.25 (d, 1H, J=7.9 Hz, Ar-H), 6.96 (dd, 1H, J=7.9 Hz, 1.3 Hz, Ar-H), 4.05 (s, 3H, OCH3).
18) 7-Methoxy-2-benzofurancarboxylic Acid N-(4-trifluoromethylphenyl)amide (1r, C17H12F3NO3): Yield : 79.6%, m.p. 121 ± 1°C; IR (KBr) 3609. 1665, 1605, 1578, 1095, 722 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.86 (d, 1H, J=8.6 Hz, Ar-H), 7.64 (d, 1H, J=8.6 Hz, Ar-H), 7.62 (s, 1H, Ar-H), 7.28 (dd, 1H, J=7.8 Hz, 1.2 Hz, Ar-H), 7.24 (d, 1H, J=7.8 Hz, Ar-H), 6.96 (dd, 1H, J=7.8 Hz, 1.2 Hz, Ar-H), 4.05 (s, 3H, OCH3).
Animals
Timed-pregnant Sprague-Dawley (SD) rats were purchased from Daehan Biolink (Chungbuk, Korea). The animals were maintained under controlled temperature (22 ± 2°C) and relative humidity (40–60%) with a 12 h light-dark cycle. They were given access to a standard chow diet and water ad libitum. All experimental procedures including the use, care and handling of the animals were conducted following the international guidelines (Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; National Academy Press: Washington D.C., 1996). Before the study, the rationale, design, and protocols of the experiments were approved by the Institutional Animal Ethical Committee of Dongguk University.
Cell culture
Primary culture of fetal rat brain cortical cells containing neuronal and non-neuronal cells was performed as we reported earlier (Cho et al., 2000; Cho et al., 2001; Kim et al., 2014). Briefly, pregnant SD rats on day 17 of gestation were sacrificed, and their uteri were excised. The embryos were harvested carefully and their cerebral cortices were dissected and mechanically dissociated into single cells by trituration with the help of fire-polished Pasteur pipettes. The isolated cells were seeded on 24-well culture plates (6×105 cells/well) pre-coated with poly-L-lysine and laminin in MEM containing Earle’s salt supplemented with 2 mM glutamine, 25 mM glucose, 5% FBS, 5% HS, and 1% antibiotic-antimycotic agent. The cells were maintained in this condition in an incubator at 37°C with a humidified atmosphere of 95% air/5% CO2. On day 7 of plating, the cultures were exposed to 10 μM cytosine arabinoside to arrest the proliferation of non-neuronal cells. The cultured cells were used in the experimental on days 10–11 of plating.
Treatment of cultured cortical cells
Prior to initiating any treatment, the cultured cortical cells were washed with HEPES-buffered control salt solution (HCSS, 20 mM HEPES, pH 7.4; 120 mM NaCl; 5.4 mM KCl; 1.6 mM MgCl2·6H2O; 2.3 mM CaCl2·2H2O; 15 mM glucose; 10 mM NaOH). To induce excitotoxicity, the HCSS-washed cells were exposed to 300 μM NMDA in Mg2+-free HCSS for 15 min (Cho et al., 2001; Kim et al., 2014). The cells used for control group did not receive any treatment except Mg2+-free HCSS for 15 min. To evaluate the protective effects of benzofuran derivatives on the excitotoxic neuronal damage induced by NMDA, the cultured cells were exposed to various concentrations of these derivatives in combination with 300 μM NMDA for 15 min.
Assessment of cell viability
After the desired treatments, the cells were washed with HCSS and incubated for 22–24 h at 37°C in MEM supplemented with 25 mM glucose. The cell viability was then measured by the MTT reduction assay in accordance to our earlier reports (Cho et al., 2005; Kim et al., 2014). Briefly, MTT was added to the cells at a final concentration of 1.0 mg/ml following which the cells were incubated for 3 h at 37°C. The culture media were then removed carefully and 500 μl DMSO was added following which the cells were incubated under shaking for 15 min to liberate and dissolve the formazan crystal products. Finally, the absorbance was read at 550 nm on a microplate reader (SpectraMax M2e, Molecular Devices, Sunnyvale, CA, USA). The viability of control cells in terms of absorbance was considered 100%.
Determination of intracellular ROS level in the cultured cortical cells
Intracellular ROS level was measured spectrofluorometrically using DCFH-DA fluorogenic dye as a probe as described in our previous report (Kim et al., 2014). Briefly, DCFH-DA was added to the cultured cortical cells at a final concentration of 10 μM. The cells were then incubated for 30 min at 37°C to allow diffusion of the fluorogenic probe into the cells and its subsequent conversion to non-fluorescent polar derivative dichlorofluorescein (DCFH) under the enzymatic action of intracellular esterases. After removing DCFH-DA, the cells were washed with HCSS and then exposed to 300 μM NMDA for 2 h at 37°C in the absence (control) or presence of various concentrations of the benzofuran derivatives. Intracellular ROS production was assessed by the fluorescence detection of 2’,7’-dichlorofluorescein (DCF) as the highly fluorescent oxidized product of DCFH on a microplate reader (SpectraMax M2e, Molecular Devices) with the excitation and emission wavelengths set at 490 nm and 520 nm, respectively. ROS level was quantitated in terms of the fluorescence intensity of DCF and considered 100% in control cells.
Assay of lipid peroxidation in rat brain homogenates
In vitro LPO in the rat forebrain homogenates initiated by Fe2+ (10 μM) and L-ascorbic acid (100 μM) was assessed in accordance to our earlier report (Cho and Lee, 2004). Briefly, the reaction mixture containing an aliquot of the homogenate protein was incubated at 37°C for 1 h in the absence (control) or presence of 10 and 100 μM concentrations of the benzofuran derivatives. The reaction was terminated by adding trichloroacetic acid (28% w/v) followed by the addition of thiobarbituric acid (1% w/v). The mixture was then heated at 100°C for 15 min followed by centrifugation to eliminate the precipitates. The absorbance of the clear supernatant was detected on a microplate reader (SpectraMax M2e, Molecular Devices) at 532 nm and the percent inhibition of the LPO reaction was calculated using the following equation:
Determination of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
DPPH radical scavenging activity was assessed following the method described in our previous report (Cho and Lee, 2004) with some modifications. Briefly, the reaction mixture containing 10 and 100 μM concentrations of the benzofuran derivatives and methanolic solution of DPPH (150 μM) was incubated at 37°C for 30 min following which the absorbance was read on a microplate reader (SpectraMax M2e, Molecular Devices) at 520 nm. The radical scavenging activity of the samples was expressed as % inhibition of absorbance determined in DPPH solution without test sample.
RESULTS
In the present study, we have synthesized a series of novel 7-methoxy-N-(substituted phenyl)benzofuran-2-carboxamide derivatives (Fig. 1). We initiated our synthetic protocol from 7-methoxy-2-benzofuran-carboxylic acid, which upon reaction with various aryl amines and coupling reagent such as 1,1′-carbonyldiimidazole (CDI) in tetrahydrofuran (THF), produced derivatives of novel 7-methoxy-N-(substituted phenyl) benzofuran-2-carboxamides. Following this reaction scheme, a total of eighteen benzofuran derivatives (1a-r) were synthesized (Fig. 1) and their neuroprotective activity against excitotoxic insult, free radical scavenging and anti-oxidant activities were evaluated using cell-free and cell-based assays.
Neuroprotective activity against NMDA-induced excitotoxicity
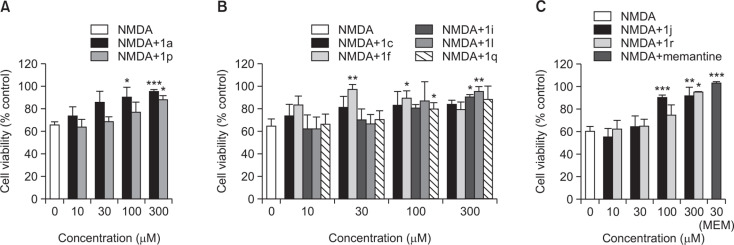
In order to evaluate neuroprotective effects of the synthesized benzofuran derivatives, we treated primary cultured rat cortical cells with NMDA in the absence or presence of the test compounds. NMDA treatment of the cultured neurons induced excitotoxic neuronal cell damage, resulting in reduction of cell viability to 60–70%, as compared to the control cells (Table 1 and Fig. 2). For primary screening, we first determined the effects of test compounds at the concentrations of 10 and 100 μM on the NMDA-induced excitotoxicity. As shown in Table 1, a number of benzofuran derivatives were found to inhibit excitotoxic damage, exhibiting increased viability of the NMDA-treated cells. Among the tested derivatives, nine compounds (1a, 1c, 1f, 1i, 1j, 1l, 1p, 1q and 1r) showing 75% or more cell viability at the concentration of 100 μM were considered to have substantial protective activity against the NMDA-induced excitotoxic damage (Table 1). Further evaluation was carried out with the selected nine compounds (Fig. 2, 3, Table 2). Memantine, an NMDA antagonist currently used to treat Alzheimer’s disease in clinical situations, was also included in this evaluation as a reference drug.
Table 1.
Primary screening of the protective effects of 7-methoxy-N-(substituted phenyl)benzofuran-2-carboxamide derivatives against NMDA-induced excitotoxicity in primary cortical neuronal culture
| Compound | Substituents | Cell viability (% control)1 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| R1 | R2 | R3 | NMDA2 | NMDA + derivatives | ||
|
| ||||||
| 10 μM | 100 μM | |||||
| 1a | H | H | H | 65.86 ± 4.68 | 73.34 ± 8.23 | 89.88 ± 9.41 |
| 1b | OCH3 | H | H | 64.69 ± 6.80 | 67.99 ± 0.23 | |
| 1c | H | OCH3 | H | 73.98 ± 10.06 | 83.14 ± 12.45 | |
| 1d | H | H | OCH3 | 43.70 ± 13.91 | 59.81 ± 1.27 | |
| 1e | CH3 | H | H | 44.21 ± 18.52 | 62.23 ± 4.71 | |
| 1f | H | CH3 | H | 83.57 ± 7.88 | 89.66 ± 6.27 | |
| 1g | H | H | CH3 | 44.60 ± 23.62 | 63.78 ± 0.23 | |
| 1h | OH | H | H | 48.93 ± 28.93 | 63.44 ± 12.04 | |
| 1i | H | OH | H | 62.13 ± 12.48 | 80.79 ± 3.22 | |
| 1j | H | H | OH | 55.13 ± 7.69 | 90.43 ± 2.19 | |
| 1k | Cl | H | H | 68.95 ± 0.40 | 61.36 ± 3.87 | |
| 1l | H | Cl | H | 62.30 ± 10.51 | 87.04 ± 17.18 | |
| 1m | H | H | Cl | 71.16 ± 2.28 | 71.58 ± 2.07 | |
| 1n | H | NO2 | H | 71.56 ± 4.55 | 57.40 ± 4.83 | |
| 1o | H | H | NO2 | 75.06 ± 3.18 | 70.65 ± 9.20 | |
| 1p | CF3 | H | H | 63.45 ± 7.41 | 76.98 ± 8.88 | |
| 1q | H | CF3 | H | 66.76 ± 8.41 | 80.02 ± 5.63 | |
| 1r | H | H | CF3 | 62.38 ± 7.59 | 74.91 ± 8.96 | |
The viability of the cells treated with NMDA or NMDA + derivatives is expressed as % of control.
The values represent the Mean ± SEM of three individual assays.
The value represents the Mean ± SEM of averages of all NMDA data collected from assays of the individual derivatives.
Fig. 2.
Protective effect of the selected 7-methoxy-N-(substituted phenyl)benzofuran-2-carboxamide derivatives with substitutions at R1 (A), R2 (B), and R3 (C) positions against NMDA-induced excitotoxicity in primary cortical neuronal culture. Memantine was tested as a reference. The values are expressed as the Mean ± SEM of three individual assays. Statistical analysis of the data was performed by independent samples t-test. *p<0.05; **p<0.01; ***p<0.001.
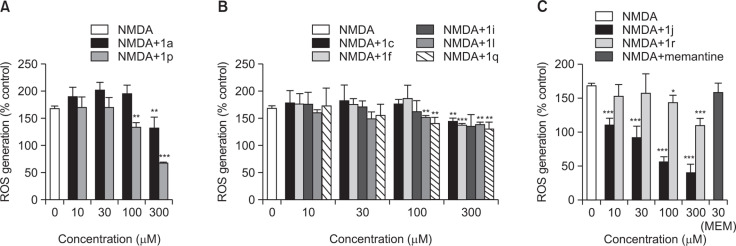
Fig. 3.
Inhibition of NMDA-induced ROS production by the selected 7-methoxy-N-(substituted phenyl)benzofuran-2-carboxamide derivatives with substitutions at R1 (A), R2 (B), and R3 (C) positions in primary cortical neuronal culture. Memantine was tested as a reference. The values are expressed as the Mean ± SEM of three individual assays. Statistical analysis of the data was performed by independent samples t-test to determine the significant inhibition. *p<0.05; **p<0.01; ***p<0.001.
Table 2.
Inhibition of lipid peroxidation and DPPH radical formation by the selected 7-methoxy-N-(substituted phenyl)benzofuran-2-carboxamide derivatives
| Compound | Inhibition of lipid peroxidation (%) | DPPH Scavenging activity (%) | ||
|---|---|---|---|---|
|
|
|
|||
| 10 μM | 100 μM | 10 μM | 100 μM | |
| 1a | −0.2 | 5.9 | −0.3 | −0.8 |
| 1c | −5.5 | 5.5 | −0.9 | −0.4 |
| 1f | −1.7 | 12.3 | 0.0 | 0.9 |
| 1i | 5.9 | 15.1 | 0.2 | −0.6 |
| 1j | 18.9 | 62.0 | 3.5 | 23.5 |
| 1l | 12.6 | 15.6 | 1.0 | 1.6 |
| 1p | 7.1 | 8.9 | 4.6 | 0.9 |
| 1q | 0.9 | 18.6 | 3.3 | 0.1 |
| 1r | 1.7 | 4.5 | −1.2 | −0.3 |
The values are calculated as described in the Materials and methods and expressed as % inhibition of the control.
As illustrated in Fig. 2, the viability of NMDA-treated cells was significantly increased by the co-treatment with all selected compounds except 1c at the concentration ranging from 30 to 300 μM, accounting more than 85% cell survival. Among them, compound 1f exhibited the most potent and efficacious inhibition of NMDA-induced toxicity, showing 97.4% cell survival at the concentration of 30 μM. Notably, the neuroprotective effect of 1f was almost comparable to that of memantine (Fig. 2C). However, the marked protective effect of 1f at 30 μM was not maintained at the higher concentrations, showing 79.6% cell survival at 300 μM (Fig. 2B). In addition to 1f, compound 1j also showed a prominent anti-excitotoxic effect at both 100 and 300 μM concentrations, although its effect was not significant at 10 and 30 μM (Fig. 2C). Compounds 1a, 1i, 1l and 1r also demonstrated significant increases in viability of the NMDA-treated cells to 90% or above at 100 or 300 μM concentration (Fig. 2).
Inhibition of NMDA-induced ROS generation
We next examined the effects of the selected benzofuran derivatives on NMDA-induced ROS generation. NMDA treatment of the cultured neurons increased ROS production to 168.08%, as compared to the control cells (Fig. 3). Co-treatment with the selected benzofuran-2-carboxamide derivatives mostly resulted in significant attenuation of NMDA-induced ROS generation with varied degrees (Fig. 3). Among the tested derivatives, compound 1j exhibited the most potent inhibition of the NMDA-induced ROS generation (Fig. 3C). While compound 1p showed quite marked inhibition of the NMDA-induced ROS at 300 μM, the remaining compounds (1a, 1c, 1f, 1i, 1l, 1q and 1r) tested in this study exhibited mild to moderate inhibition of ROS production at 100–300 μM concentrations. In contrast, memantine did not produce any significant change in NMDA-induced ROS generation at 30 μM concentration (Fig. 3).
Inhibition of LPO and DPPH radical scavenging activity
The antioxidant activities of the selected benzofuran derivatives were evaluated in terms of their ability to inhibit in vitro LPO in rat brain homogenate initiated by Fe2+ and L-ascorbic acid. Additionally, the radical scavenging activity of the selected compounds was also determined using stable free radical DPPH as a probe. Among the tested derivatives, compound 1j exhibited moderate to appreciable antioxidative activities accounting 62% inhibition in LPO and 23.5% inhibition in DPPH radical formation at 100 μM (Table 2). The extents of inhibition of LPO and DPPH radical formation by the other compounds at 10 and 100 μM concentrations were mild or only a negligible to minimal.
DISCUSSION
A total of eighteen benzofuran-2-carboxamide derivatives (1a-r) were synthesized with moderate to good yields (see Fig. 1 for their structures). The chemical reaction process involves substitutions at ortho (R1), meta (R2), or para (R3) position of the benzofuran moiety with electron releasing groups such as -OCH3, -CH3, and -OH or electron withdrawing groups such as -Cl, -NO2, and -CF3. In this study, we evaluated neuroprotective and antioxidative effects of the synthesized benzofuran derivatives using primary cultured rat cortical neuronal cells as well as cell-free bioassays.
As reported previously, primary cultured rat cortical cells are vulnerable to NMDA treatment, resulting in excitotoxic neuronal cell damage (Choi, 1992; Cho et al., 2000; Cho et al., 2005; Kim et al., 2014). We confirmed these findings in our study using the MTT reduction assay, showing that upon exposure to NMDA, the viability of the cultured cortical neurons was reduced to 60–70% as compared to the control cells (Table 1, Fig. 2).
To evaluate the protective effects of the synthesized derivatives on the NMDA-induced excitotoxic damage, the cultured cells were simultaneously treated with the test compounds. We first carried out primary screening using two concentrations (10 and 100 μM) of the test compounds to select derivatives exhibiting substantial neuroprotective effects. Based on our primary screening data (Table 1), nine compounds (1a, 1c, 1f, 1i, 1j, 1l, 1p, 1q and 1r) were selected for further evaluation. The results of our elaborative experiments with these selected derivatives demonstrated that compound 1f (with -CH3 substitution at R2 position) exhibited the most potent and efficacious protective action against the NMDA-induced toxicity (Fig. 2B). The neuroprotective effect of 1f was almost comparable to that of memantine, a well-known NMDA antagonist tested in this study as a reference (Fig. 2C). However, the increased viability of the NMDA-treated cells in the presence of 1f at 30 μM concentration was not maintained when the cells were exposed to 1f at 300 μM, suggesting its potential cytotoxic action at higher concentrations. In addition to 1f, compound 1j (with -OH substitution at R3 position) also showed a potent anti-excitotoxic effect at both 100 and 300 μM concentrations (Fig. 2C). Several other compounds including 1a, 1i, 1l and 1r also demonstrated significant increase in viability of the NMDA-treated cells. These findings are in keeping with a recent report demonstrating the neuroprotectve activity of a benzofuran-based imidazoline compound against NMDA toxicity (Han et al., 2013). In contrast, compound 1c (with -OCH3 substitution at R2 position) did not show any significant protection against the NMDA-induced toxicity (Fig. 2B). These results suggest that -CH3 substitution at R2 position and, to a lesser degree, -OH substitution at R3 position may play important role(s) in contributing protective action against excitotoxic neuronal damage.
As reported previously (Kim et al., 2014) and shown in this study (Fig. 3), treatment of cultured cortical cells with NMDA or L-glutamic acid significantly augmented the intracellular ROS level to 1.7-2-fold over control. The NMDA-induced intracellular ROS generation was inhibited by several derivatives with varied degrees (Fig. 3). Among the tested derivatives, compound 1j exhibited the most potent inhibition of the NMDA-induced ROS generation (Fig. 3C). In contrast, memantine was unable to inhibit NMDA-induced ROS generation at the concentration tested in this study (Fig. 3C).
Previous study reported scavenging of DPPH radicals and inhibition of lipid peroxidation by benzofuran compounds (Grisar et al., 1995). Accordingly, we tested if our benzofuran derivatives have any antioxidant activity. Our study showed that only compound 1j exhibited moderate to appreciable antioxidative activities, inhibiting LPO and scavenging DPPH radicals (Table 2). This is in agreement with our results showing potent inhibition of NMDA-induced ROS generation by compound 1j (Fig. 3C). The presence of electron donating -OH substituent at para (R3) position may play a role in the antioxidant actions of 1j (Rajan et al., 2001). The extents of inhibition of LPO and DPPH radical formation by the other compounds were mild or only a negligible to minimal.
Taken together, our structure-activity relationship studies suggest that both -CH3 substitution at R2 and -OH substitution at R3 positions of the benzofuran moiety may be beneficial to improve neuroprotective effects. Unlike memantine, this hypothetical compound may have better pharmacological profile with considerable antioxidant activity. Therefore, the compound with -CH3 at R2 and -OH at R3 positions may serve as the lead for future synthesis of benzofuran derivatives with potent neuroprotective activities. Additional substitution may be necessary to minimize the potential toxicity shown in compound 1f at higher concentrations.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2009381) and the GRRC program of Gyeonggi province (GRRC-DONGGUK 2014-B01).
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no competing interests to disclose.
REFERENCES
- Cho J, Joo NE, Kong JY, Jeong DY, Lee KD, Kang BS. Inhibition of excitotoxic neuronal death by methanol extract of Acori graminei rhizoma in cultured rat cortical neurons. J Ethnopharmacol. 2000;73:31–37. doi: 10.1016/S0378-8741(00)00262-2. [DOI] [PubMed] [Google Scholar]
- Cho J, Kong JY, Jeong DY, Lee KD, Lee DU, Kang BS. NMDA recepter-mediated neuroprotection by essential oils from the rhizomes of Acorus gramineus. Life Sci. 2001;68:1567–1573. doi: 10.1016/S0024-3205(01)00944-4. [DOI] [PubMed] [Google Scholar]
- Cho J, Lee HK. Wogonin inhibits excitotoxic and oxidative neuronal damage in primary cultured rat cortical cells. Eur J Pharmacol. 2004;485:105–110. doi: 10.1016/j.ejphar.2003.11.064. [DOI] [PubMed] [Google Scholar]
- Cho J, Kim HM, Ryu JH, Jeong YS, Lee YS, Jin C. Neuroprotective and antioxidant effects of the ethyl acetate fraction prepared from Tussilago farfara L. Biol Pharm Bull. 2005;28:455–460. doi: 10.1248/bpb.28.455. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Chong CM, Shen M, Zhou ZY, Pan P, Hoi PM, Li S, Liang W, Ai N, Zhang LQ, Li CW, Yu H, Hou T, Lee SM. Discovery of a benzofuran derivative (MBPTA) as a novel ROCK inhibitor that protects against MPP+-induced oxidative stress and cell death in SH-SY5Y cells. Free Radic Biol Med. 2014;74:283–293. doi: 10.1016/j.freeradbiomed.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dawood KM. Benzofuran derivatives: a patent review. Expert OpinTher Pat. 2013;23:1133–1156. doi: 10.1517/13543776.2013.801455. [DOI] [PubMed] [Google Scholar]
- Gaszner P, Miklya I. Major depression and the synthetic enhancer substances, (-)-deprenyl and R-(-)-1-(benzofuran-2-yl)-2-propylaminopentane. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:5–14. doi: 10.1016/j.pnpbp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, Iadecola C. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci. 2009;29:2545–2552. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisar JM, Bolkenius FN, Petty MA, Verne J. 2,3-Di-hydro-1-benzofuran-5-ols as analogues of alpha-tocopherol that inhibit in vitro and ex vivo lipid autoxidation and protect mice against central nervous system trauma. J Med Chem. 1995;38:453–458. doi: 10.1021/jm00003a008. [DOI] [PubMed] [Google Scholar]
- Hall ED. Role of oxygen radicals in central nervous system trauma. In: Tarr M, Samson F, editors. In oxygen free radicals in tissue damage. Birkhäuser; Boston: 1993. pp. 153–173. [Google Scholar]
- Han Z, Yang JL, Jiang SX, Hou ST, Zheng RY. Fast, non-competitive and reversible inhibition of NMDA-activated currents by 2-BFI confers neuroprotection. PLoS One. 2013;8:e64894. doi: 10.1371/journal.pone.0064894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett DR, Perry AE, Godfrey F, Swatton JE, Jennings KH, Spitzfaden C, Wadsworth H, Wood SJ, Markwell RE. Inhibition of fibril formation in beta-amyloid peptide by a novel series of benzofurans. Biochem J. 1999;340:283–289. doi: 10.1042/0264-6021:3400283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Kerchner GA, Choi DW. Blocking excitotoxicity. In: Marcoux FW, Choi DW, editors. In handbook of experimental pharmacology. Springer; New York: 2002. pp. 3–36. [DOI] [Google Scholar]
- Kim S, Lee Y, Cho J. Korean red ginseng extract exhibits neuroprotective effects through inhibition of apoptotic cell death. Biol Pharm Bull. 2014;37:938–946. doi: 10.1248/bpb.b13-00880. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989;36:106–112. [PubMed] [Google Scholar]
- Moosmann B, Behl C. Antioxidants as treatment for neurodegenerative disorders. Expert Opin Investig Drugs. 2002;11:1407–1435. doi: 10.1517/13543784.11.10.1407. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med. 2004;4:149–177. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- Rajan P, Vedernikova I, Cos P, Berghe DV, Augustyns K, Haemers A. Synthesis and evaluation of caffeic acid amides as antioxidants. Bioorg Med Chem Lett. 2001;11:215–217. doi: 10.1016/S0960-894X(00)00630-2. [DOI] [PubMed] [Google Scholar]
- Rindhe SS, Rode MA, Karale BK. New benzofuran derivatives as an antioxidant agent. Indian J Pharm Sci. 2010;72:231–235. doi: 10.4103/0250-474X.65022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo S, Rivière C, Piazzi L, Bisi A, Gobbi S, Bartolini M, Andrisano V, Morroni F, Tarozzi A, Monti JP, Rampa A. Benzofuran-based hybrid compounds for the inhibition of cholinesterase activity, beta amyloid aggregation, and abeta neurotoxicity. J Med Chem. 2008;51:2883–2886. doi: 10.1021/jm8002747. [DOI] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvaresou A, Iakovou K, Filippatos E, Souli C, Calogeropoulou T, Ioannidou I, Kourounakis AP, Pannecouque C, Witvrouw M, Padalko E, Neyts J, De Clercq E, Tsotinis A. Synthesis, antiretroviral and antioxidant evaluation of a series of new benzo[b]furan derivatives. Arzneimittelforschung. 2001;51:156–162. doi: 10.1055/s-0031-1300018. [DOI] [PubMed] [Google Scholar]