Abstract
Large-sized calvarial defects in pediatric patients pose a reconstructive challenge because of children's unique physiology, developing anatomy, and dynamic growth. We review the current literature and outcomes with autologous and alloplastic cranioplasty in the pediatric population.
Keywords: pediatric, cranioplasty, calvarial defect, skull reconstruction
Large-sized cranial defects can arise from many etiologies, including open skull trauma, infection, tumor, and craniofacial reconstruction complications. With advances in neurotrauma care and early surgical decompression, trauma outcomes have improved over time; survivors are left with cranial defects. While large retrospective studies and meta-analyses of adult cranioplasty have been conducted, less is known about outcomes in the pediatric population.
Reimplantation of the autologous bone flaps in children is thought to be advantageous. The osseous material can become reincorporated over the process of a child's maturation and growth. However, bone resorption is seen in up to 50% in the pediatric population1 2 compared with up to 6.5% in adults,3 necessitating further reconstructive surgery either with further autologous materials (split-thickness cranial bone graft, particulate bone graft with or without resorbable mesh), or other alloplastic materials (methyl methacrylate, hydroxyapatite cement, demineralized bone, and titanium mesh).
We review the literature on special considerations, materials, and outcomes of large-sized cranioplasty in children such as after decompressive craniectomy (Table 1).
Table 1. General characteristics of pediatric cranioplasty type.
| Cranioplasty type | Precision | Durability | Osseointegration | Growth potential | Donor site morbidity | Cost |
|---|---|---|---|---|---|---|
| Autologous | Difficult to contour/shape | Potential to resorb with nonvascularized bone | Excellent | Excellent | Significant with large pediatric cranioplasty defects | Minimal |
| Alloplastic | Good precision with potential to customize | Durable; however, relatively nonresistant to infection | Poor | Poor | None | High |
Autologous Reconstruction
Autologous cranioplasty after decompressive craniectomy is considered the gold standard in pediatric care because of the ability of the bone graft to reincorporate into the skull (osseointegration), lower risk of material rejection, and ability to allow growth of the skull. There are several approaches to this form of reconstruction (Table 2). The three main ways to perform an autologous cranioplasty use bone stored in the body, cryopreserved bone, or bone flaps harvested from a donor site. With pediatric patients, additional considerations must account for the high incidence of bone resorption (Fig. 1a–c), the immature osseous skeleton, and future growth.
Table 2. Autologous cranioplasty options.
| Adult | Pediatric (< 5 y) |
|---|---|
| Nonvascularized | Nonvascularized |
| Split-calvarial bone graft | Spilt-calvarial bone graft (impractical due to poorly formed/absent diploic space) |
| Rib | Rib (may result in chest wall deformity) |
| Iliac crest | Iliac crest (may cause growth disturbance) |
| Tibia | Particulate bone graft (± resorbable mesh |
| Vascularized | Vascularized |
| Rib | Requires highly expert microsurgical skills and resources |
| Scapula | |
| Iliac crest |
Fig. 1.
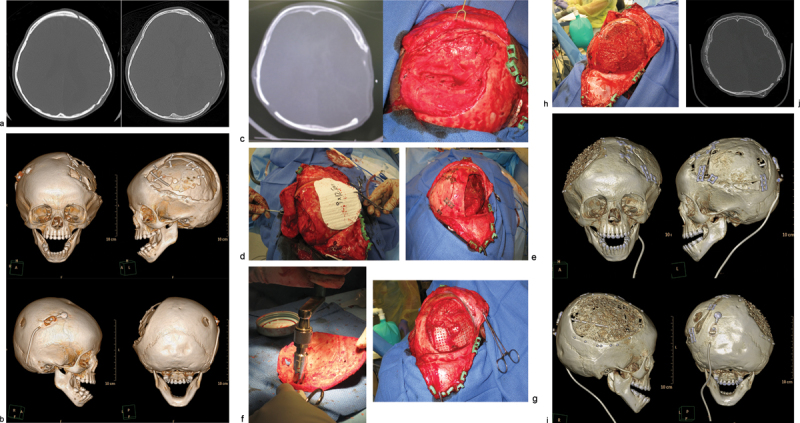
Case 1: An 18-month-old child with history of decompressive craniectomy for acute traumatic subdural hematoma, with at right frontal ventriculoperitoneal shunt in place for posttraumatic hydrocephalus. The autologous bone flap was retrieved from bone freezer storage and replaced 3 months after the initial trauma to repair the large left frontoparietotemporal skull defect. The autologous cranioplasty failed with bone flap resorption noted 6 weeks later, which was near complete by 4 months after autologous cranioplasty. An exchange cranioplasty was done, with re-siting of the ventriculoperitoneal shunt to the left occipital approach to avoid the bilateral cranioplasty areas. (a) Axial head computed tomographic (CT) scan immediately after autologous cranioplasty (left); axial CT scan of the same patient with bone flap resorption 4 months later (right). (b) Three-dimensional (3D) CT reconstructions showing bone flap resorption and right frontal ventriculoperitoneal shunt in place. (c) Axial CT scan (left) with corresponding intraoperative skull defect (right) at time of second reconstruction (exchange cranioplasty) after bone flap resorption. (d) Exchange cranioplasty. Measuring the donor site for the exchange and templating the cranial defect. (e) Exchange cranioplasty in process after placement of the freshly harvested contralateral bone graft. (f) Harvesting autologous particulates for bone grafting from the inner surface of a full-thickness calvarial flap. (g) Bilaminate mesh cranioplasty: inner layer of mesh in the right frontoparietotemporal calvarial defect, forming the epidural inner cortex. (h) Exchange cranioplasty completed with the recreated “diploic space” in between mesh layers filled autologous particulate bone mixed with demineralized bone matrix and autologous blood, then covered with the outer layer of mesh. (i) 3D CT scan 3 months after exchange cranioplasty showing partial consolidation of particulate graft on the left, incorporation of the full-thickness calvarial graft on the right with good skull contour. The shunt was re-sited to avoid the cranioplasty areas. (j) Axial CT scan 3 months after exchange cranioplasty.
Bone Flap Storage Methods
Methods of bone preservation each have advantages and drawbacks. Cryopreservation of the bone flap obviates a second surgical site and is favored by most of the U.S. centers. However, cryopreservation requires facilities for bone storage; in cases of geographic transfer of patient care, logistics of transport pose additional difficulty.4 In addition, the freezing process can devitalize the bone and result in an increased rate of complications including infection, resorption, and cosmetic deformity.1 4 5 The risk of resorption is known to be high, in up to 50% of pediatric cases.1 2 Different methods of bone sterilization and cryopreservation have been explored, including autoclaving, altering temperatures of freezer storage, irradiation, and other sterilization methods. Autoclaving may increase devitalization of bone and thereby increase complication rates after reimplantation, although there is no consensus on this finding among studies.1 3 6 7
Subcutaneous storage is utilized, including storing the bone flap in an abdominal pocket, anterolateral thigh, or under the scalp.6 Subcutaneous storage is thought to potentially be capable of nourishing osteocytes in the skull bone flap, of reducing devitalization of the bone,8 and of potentially aiding in clearing infective agents.9 These are especially important to consider when planning to minimize the risk of bone flap resorption. For practical purposes, subcutaneous pocket storage also ensures that the bone is transported with the patient. However, small children may not anatomically have adequate subcutaneous storage area. Patients have reported discomfort with the bone flap stored in the abdomen, especially in thin patients8 with risk of bone flap extrusion or wound dehiscence. There is also the added morbidity of a second surgical site. While it is proposed that subcutaneous pockets may provide a favorable vascularized environment,9 some authors show that bone flaps are more susceptible to bone resorption and infection while subcutaneously stored.10 11
There are conflicting data when comparing these two storage methods. Inamasu et al looked at a group of 70 patients of all ages and found no significant statistical difference between outcomes for subcutaneous bone storage and cryopreservation, except in cases of traumatic brain injury, where cryopreservation had a much higher rate of surgical site infection.9 In the pediatric population, Grant et al reported on 40 cases1 and Bowers et al examined 54 cases2 both studies found high rates of bone resorption, which occurred in 50% of their patients after cranioplasty with cryopreserved bone.
In studies including adults, Yadla et al, in a systematic review of 2,254 patients found no statistical difference between outcomes with the two storage methods.3 In addition, Huang et al also found no significant relationship between storage method and infection, though follow-up time for the 153 patients was short 2 weeks12 without attempt to detect infections beyond that perioperative time period. Pediatric studies are more limited in number. Further study is needed to confirm a relationship between storage method and outcome, although the higher resorption rate in children with cryopreserved bone1 2 13 might suggest that devitalization of bone with cryopreservation may be higher in the pediatric population.
Timing of Reconstruction
Another major question when dealing with cranial reconstruction is timing. The time between craniotomy and cranioplasty is largely determined by the condition of the individual patient. There are conflicting results when considering optimal cranioplasty timing. Schuss et al examined 280 adult patients and showed that cranioplasties performed greater than 2 months after craniectomy had lower rates of complication than earlier ones; however, only 19% of patients had cranioplasties done earlier than 2 months.14 Piedra et al retrospectively examined 61 pediatric cases and found that earlier autologous cranioplasties (< 6 weeks) result in significantly less bone resorption (14.3 vs. 42.4% in the early vs. late cranioplasty group respectively, p = 0.016) and no difference in other complications.13 Other pediatric and adult studies report that cranioplasty timing with autologous bone flaps had no significant influence on reconstruction outcomes.1 2 3 Experiments on bone preservation support this latter conclusion as most of the total osteocyte damage occurred quickly during or after craniotomy and stayed relatively stable throughout storage.5
On the occasion, traumatic defects of the scalp result in a composite defect requiring little or negligible bony reconstruction. If only soft tissue coverage is needed for the cranial defect, locoregional flaps, or free tissue transfer in the form of the latissimus dorsi, anterolateral thigh, radial forearm and rectus abdominus donor tissue have been found to be effective, depending on the size of the patient and size of the defect.15 16 17 18 Unlike the latissimus dorsi, free radial forearm flaps, anterolateral thigh flaps, and rectus abdominus flaps all have the advantage of being harvested without need for repositioning the patient, allowing for simultaneous operations and shorter operative time.15 19 In children, size is more of a limitation than in adult patients. For this reason, most pediatric free flap reconstructions have been performed with latissimus dorsi, anterolateral thigh flaps and, on occasion, rectus abdominus flaps. Though technically more difficult because of the small size of both the donor and recipient vessels, outcomes are favorable.16 18 20 21 Because of the small size of the pediatric patients and the cosmetic issues at the harvest site, free radial forearm flap reconstructions are often not feasible for the pediatric population. Though still uncommon procedures, Jia-Ao et al report a favorable outcome in 94% of patients in their series of 37 cases of pediatric anterolateral free flap reconstruction for coverage of large cranial defects.16
When bone is needed to provide cranial coverage, there are several options if the removed bone is no longer available because of trauma, loss, or infection. These options from the cranium include full-thickness grafts, split-thickness grafts, and particulate bone grafts. These three reconstructive approaches all involve using bone from the healthy parts of the skull to repair the defect and have been shown to have a lower rate of resorption than grafts from elsewhere on the body.22 Full-thickness calvarial grafts are often used when there is concern about healing, such as, in the cases of the previous failed reconstruction. This approach provides good coverage of the cranial defect but creates a donor site to be repaired. This type of defect can be done using an exchange cranioplasty (Fig. 1d, e ), in which the existing defect is repaired with the full-thickness calvarial graft, and the donor site is repaired with particulate bone graft harvested from the full-thickness graft. Rogers et al reviewed 20 cases, and found that 15 of 20 had complete healing, and the size of the defects decreased by an average of 96%. This exchange cranioplasty method, although it involves an additional operative site, has been found to be highly effective even for large cranial defects as children have high-osteogenic potential.23
Particulate grafts can also be used to repair defects directly. In young children, the supply of autologous bone is limited; particulate grafts can be advantageous as they can be harvested with minimal morbidity ( Fig. 1f).24 Bilaminate constructs with particulate grafts can be made by placing bioresorbable mesh endocranially on the dura, laying in bone particulates mixed with blood, demineralized bone matrix paste and other bone growth enhancing agents, and buttressing with an outer layer of bioresorbable mesh. (Fig. 1g, h) This method has been used successfully to augment small and large defects. Chao et al report that all the 11 patients in their initial experience achieved clinically stable reconstructions.25 This method has the benefit that the donor site returns to full thickness over time. It is best used when the reconstructive site has good healing potential and unscarred dura. Alternatively, an exchange cranioplasty or other reconstructive method may be indicated.23 26 (Fig. 1i, j).
A split-thickness cranial bone graft is also an option for cranial reconstructions that call for bony coverage. These involve harvesting a bone flap from the skull and separating the outer and inner table of the flap through its diploic space. One table is then used to reconstruct the donor site and the other is used to repair the defect.27 This method has the advantage of using autologous bone in reconstruction, but adds morbidity of a donor site and results in two reconstructions both with reduced thickness and stability.28 The bone flaps over the donor and recipient sites do not heal to full thickness. A well-developed diploic space is required so this method is less feasible in children younger than the age of 5 years.22 The ability to harvest split cranial bone graft in the young child is a controversial topic. Inoue et al advocate this method for patients over 7 years of age with cranial defects exposed to infection.29 Recently, Vercler et al reported experience with safely and predictably splitting the cranial bone between the inner and outer cortex in 418 patients younger than 3 years with an average age of 9 months in their series.30 This report suggests a valuable expanded option for rigid bone in this younger population. However, it should be noted that the mean patient age in this study was 328 days (10 months), an age where dural regeneration of bone is still possible. Furthermore, the indication of splitting bone in this setting (cranial vault reconstruction for craniosynostosis), where the surgeon is encountering a primary surgical field with a finite defect size is in stark contrast to the massive, critical-sized defects pertinent to this review where splitting bone may not be a practical exercise. To this point, it is interesting that the authors of this article do not disclose data on their primary defect size. Nonetheless, the authors' results strongly point toward a debunking of the surgical myth that cranial bone cannot be split in infancy and so such a source of autologous cranioplasty may be an option in finite defects.
When a composite defect of soft tissue and bone is encountered, a vascularized cranioplasty may be in order (Fig. 2a–d). Three main donor sites typically used are as follows: rib and latissimus dorsi, scapula, and iliac crest. These composite tissue free flaps can be considered when a cranial defect has significant wound healing challenges such as radiation, infection, and/or scar tissue seen in multiple operated cases. The theory behind this approach is that the vascular nature of the flap will improve osseointegration and wound healing. The donor site is determined by defect size and surgeon's preference. In our experience, a variety of autologous vascularized cranioplasties have been performed with good outcomes in adults providing structural support and cranial coverage in the face of scarred, irradiated, or infected cranial environment.31 These types of complex reconstructions are infrequently performed in children. Given the rarity of these procedures, there exists little in the way of large studies of these types of reconstructions and virtually none in terms of pediatric patients. However, as medical and oncologic treatments improve, these will likely become considerations in the pediatric cases with hostile healing environments.
Fig. 2.
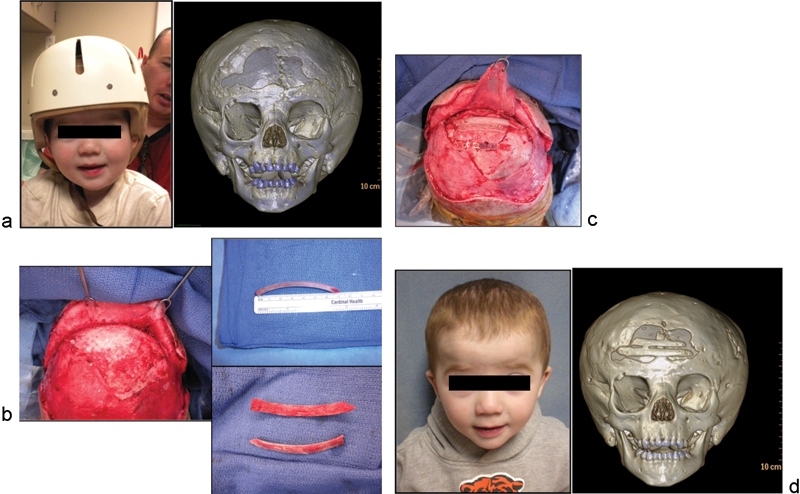
Case 2: A 2-year-old boy with history of metopic craniosynostosis and fronto-orbital advancement with frontal cranial defect because of infected bifrontal craniotomy piece and subsequent bone resorption. (a) Child with protective helmet preoperatively, with three-dimensional (3D) computed tomographic (CT) scan demonstrating frontal cranial defect. (b) Intraoperative vertex view demonstrating frontal defect; harvesting and splitting of nonvascularized autologous rib graft for interpositional cranioplasty. (c) Graft in place with resorbable plates to repair defect for good reconstruction of the forehead contour. (d) 6 months after reconstruction: frontal view photograph and 3D CT demonstrating partial reconstitution of defect due to secondary partial resorption of split rib grafts.
Another emerging avenue of autologous cranial reconstruction is that of adjuvants to improve or augment osteogenesis. These modalities include bone morphogenetic protein 2 (BMP2) and other growth factors such as vascular endothelial growth factor. These factors can improve osteogenesis and wound healing. Animal experiments with these agents have been encouraging and show potential for improvement in bone healing.32 33 34 However, its use in humans has been restricted. To this end, BMP2 has been shown to be proinflammatory, and in one case, its off-label use was aborted when cranioplasty in an infant with metopic craniosynostosis led to severe facial and scalp edema, necessitating urgent removal of the recombinant BMP2.35 More safety testing is required before its full use in cranioplasty reconstruction.
Alloplastic Reconstruction
The human skull is approximately 50% of its adult size at birth and a critical period between 6 and 12 months of age characterizes a time when the majority of active brain growth occurs.36 Risks for the use of synthetic implants in pediatric cranioplasty include inadequate cosmesis, potential need for replacement of the implant as the skull grows, restriction of normal growth, long-term compromise of overlying tissues (culminating in implant extrusion or migration), and risk of infection.37 However, given the high rates of complications of cranioplasty using autologous stored bone flaps in pediatric patients, alloplastic alternatives for cranial reconstruction are an important consideration.
There is little consensus among surgeons regarding the ideal way to reconstruct a skull defect. The patient's own bone may not an option because of extensive damage, osteolysis, or infection. It is reported in the neurosurgical literature that rates of autologous bone flap resorption in children may be as high as 50%; some posit that alloplastic reconstruction may be warranted as an initial option given the high rate of autologous bone flap resorption. There are a variety of alloplastic choices based on patient factors, cost, and surgeon preference. These materials are not able to remodel with a growing child, and also have a chance of extrusion. When autologous bone is not available or not advisable, alloplastic materials should be considered.
Acrylics
Polymethyl methacrylate (PMMA) is an acrylic widely used in nonautologous adult cranioplasties. Some of its noteworthy properties include biocompatibility, hardness characteristics similar to that of natural bone, ease of prefabrication, and ability for intraoperative molding.38 With significant advances in computer-aided design and computer-aided modeling (CAD/CAM) a prefabricated PMMA prosthesis may be generated before the surgery, reducing time needed for intraoperative molding. PMMA shows fewer problems than some other materials and can be offered at lower cost than hydroxyapatite-based ceramics.39 40 41 The use of PMMA is not without risks. It has been associated with an increased risk of infection6 and the exothermic reaction during the setting process may damage tissues.40 42 Prefabrication of PMMA may lessen some of the danger of heat exposure.38 Retrospective studies have noted significantly higher rates of complications with PMMA in children.38 Acrylics do not integrate well with growing pediatric calvarium and are generally avoided in younger children younger than 5 years.43
Ceramics
A variety of ceramics have offered potential options for calvarial repair. Hydroxyapatite bone cements (HBCs) are nonresorbable, available in unlimited supply, and obviate donor site morbidity.44 They bear a chemical composition similar to natural bone.45 One such material is carbonated calcium phosphate paste (CCPP) which has shown greater compressive and tensile strength than other HBCs, with its crystalline structure similar to that of the mineral phase of human bone.44 Of special interest to the pediatric population, Kirschner et al demonstrated that this CCPP could be used in animal models to repair calvarial defects in immature animals (3-week old Yorkshire piglets). The use of CCPP did not restrict craniofacial growth and the material was remodeled into living bone.46 The group concluded that CCPP's ability to remodel into living bone may be a function of the host's remodeling capacity.47 Their research showed that although there was significant remodeling in the 3-week old Yorkshire piglet immature craniofacial skeleton, there was only modest remodeling in the 16-week-old more mature skeleton. These results suggest that there may be a distinct age period representing a period of maximal dural osteogenic potential when this particular type of graft is indicated. Further investigation will be necessary to isolate this time period.
A noteworthy characteristic of hydroxyapatite (HA) compounds is the reported potential for postfracture self-repair. This was described in a retrospective analysis of HA complications.45 The authors postulate in case-report-level evidence that osteointegration can reach up to 60 to 80% of the total mass.
A primary problem of the calcium phosphate cements stems from the pulsations of the underlying intradural contents interfering with the crystallization of these cements. These pulsations lead to microfragmentation of the material. This problem may be mitigated by using resorbable mesh between the dura and the cement.48 Early fragility has also been noted to be a problem leading to alloplast fracture.45 One long-term study of HA cements also suggested a delayed immune-mediated inflammatory reaction.40 In addition, bone flap removal in the setting of secondary infection may be challenging with CCPP as it binds tightly to the skull.
There have been conflicting results from large studies concerning the potential risk/benefit profile of HA compounds. Moreira-Gonzalez et al found HA to be associated with a high rate of complications (32.8%), while Stefini et al reviewed 1,500 patients and found this rate closer to 5% (4.78% in first-line treatments and 5.02% in second-line treatments).45 In the study of Moreira-Gonzalez et al, HA compounds were used more often in complicated cases, which may have affected the rate of complications.
Although there are few studies of HA focused in the pediatric population, one of the largest (n = 78) by Singh et al resulted in a total complication rate of 8.9%. The authors attribute this low complication rate adhering closely to specific practices such as avoiding the use of HA in proximity to paranasal sinuses, as well as using pericranial flaps to cover the HA area whenever possible. They also report meticulous apposition to avoid microfragmentation and burring of the skull bone edge to improve contour.49 In contrast, Wong et al reported on 17 cases with a focus on the complication rate of HA use in secondary pediatric craniofacial repair. They cited high rates of infection (59%) when using HA in the pediatric population.50
Ultimately, HA is a viable synthetic cranioplasty material in the pediatric population. As in the adult population, the risks, benefits, and alternatives must be weighed carefully. Caution is needed in proximity to sinus or nasal mucosa and for full-thickness calvarial reconstructions larger than 25 cm2.51 Complications such as infection and fragmentation have been described even months to years after implantation.51
Titanium
Titanium mesh has a long history of use in cranioplasty; the introduction of CAD and CAM of this material has allowed a new level of cosmetic results to be achieved (Fig. 3). In a large Australian study with long-term follow-up, not a single patient noted a poor cosmetic result52; multicenter experiences in Europe and the United States also report favorable results.53 These encouraging outcomes, combined with mechanical strength, low infection rates, and biocompatibility make titanium an attractive option.54
Fig. 3.
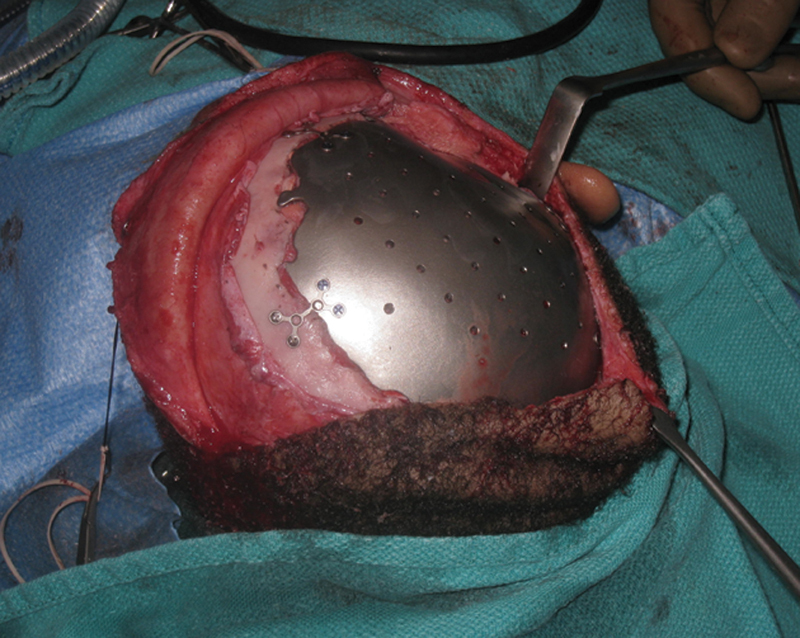
Reconstruction using computer-modeled titanium cranial prosthetic.
Titanium is sometimes described as a material of last resort in the adult population. A significant portion of patients who have had complications arising from previous cranioplasties receive titanium in the second operation.54 Titanium mesh is also commonly used in combination with HA as a primary repair. Either as a primary or secondary option, titanium offers a good choice for adults. As the implant cannot grow with a young child, some authors suggest reserving this material for children over 10 years of age.43
Plastics
Types of plastics commonly used in cranioplasty include polyether ether ketone (PEEK) (Shenzhen Yataixing Industrial Co., Ltd., Guangdong, China) (Fig. 4) and porous polyethylene (Medpor®, Stryker, Kalamazoo, MI) (Fig. 5a–d).
Fig. 4.
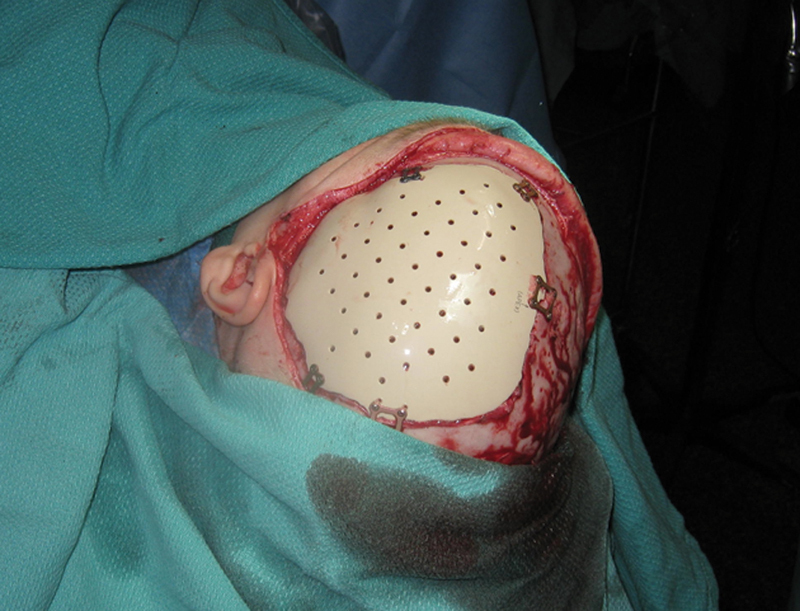
Reconstruction using computer-modeled polyether ether ketone cranial prosthetic.
Fig. 5.
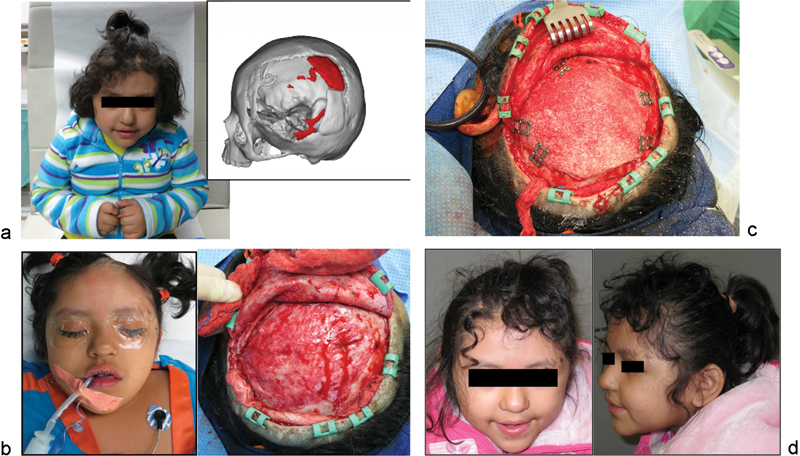
Case 3: A 5-year-old girl with large left frontotemporoparietal defect after emergent decompressive craniectomy for acute subdural hematoma, subsequent loss of bone from resorption, and multiple attempts at autologous cranioplasty during her “immature” skeletal years. She eventually underwent computer-modeled alloplastic reconstruction as her definitive treatment after she turned 5 years old. (a) Frontal view and three-dimensional (3D) computed tomographic (CT) image used for planning and customized implant fabrication.(b) On-table and intraoperative views demonstrating large defect. (c) Fixation of customized porous polyethylene implant. (d) Six-month postoperative views with good contour reconstruction.
PEEK is not osteoinductive. In a direct comparison between PEEK and titanium, it was shown that PEEK's elasticity and energy-absorbing properties were closer to that of bone.55 It does not osseointegrate, so its removal (if necessary) is relatively more straightforward than for osseointegrated materials.
Porous polyethylene has been used as a substitute for autologous bone and has a large number of pores which allow for bony ingrowth.56 The pores have diameters ranging from 100 to 250 µm.57 While early animal model studies of porous polyethylene suggest that the porosity of the material allowed bone ingrowth (osteoconduction),58 more recent in vivo studies have demonstrated that while there is soft tissue ingrowth, there is no bony ingrowth as previously believed.59 Clinical studies have given positive early results for the use of Medpor in children. For instance, in children with large-scale defects averaging 152 cm2, acceptable contour results were reported with no major complications.37 In 23 patients retrospectively reviewed from 1999 through 2011, Medpor was found to have satisfactory results in all but 2 patients.60 Additional studies consisting of larger samples and greater follow-up duration will be necessary to understand outcomes.
Site of Implant
Recently, Gordon et al61 reported a pericranial-onlay technique, where the traditional epidural dissection of the scalp flap is eschewed in favor of dissection in the loose areolar plane overlying the pericranium, leaving a vascularized pericranial layer on the dura. The bone replacement (autologous or alloplastic) is placed on top of the pericranium in the skull defect and secured to the bone edges. While only two patients (9 and 17 years old) in the 50-patient series were in the pediatric age group, this technique is presented to reduce perioperative complications by encasing the bone flap or cranial implant with vascularized tissue and minimizing risk of dural injury. There may be challenges imposed by a younger patient's scalp and by a technical learning curve, but this pericranial-onlay technique may be considered for its advantages. Further studies are needed to explore this technique in pediatric patients.
On the Horizon
An interesting investigation is the use silver (Ag) in nanoparticulate form (AgNP). Ag+ ions have displayed the ability to hinder bacterial replication. AgNP delivery systems in PMMA prostheses could theoretically reduce rates of infection. Although development is still hindered by mechanical weakness generated by the addition of the delivery system, an antimicrobial acrylic resin shows significant promise and has potential for lowering postsurgical implant-centered infections.62
Another development is the use of BMP-7 in cranioplasty for a growing infant. BMP-7 is a protein that has been shown to play a role in bone development. Early work shows promise in its ability to aid in the development of the growing bone in infants more effectively than particulate bone transplants.63 Along these lines, further characterization of other osteogenic BMPs in murine models have revealed that BMP-9 is the most potent osteogenic BMP64; however, this protein is not yet available in recombinant form.
Our Experience
Each child with a large-sized calvarial defect poses unique challenges. We share example cases with various techniques. Our existing bias is to “replace like with like” and so whenever practical, autologous bone, and more specifically cranial bone, is our preferred cranioplasty material. When defect sizes are massive (> 50 cm2), particulate cranial bone is our technique of choice. In such cases, exchange particulate cranioplasty (i.e., placing a full-thickness craniotomy bone piece in the scarred, traumatic defect and particulate bone graft from the inner table of the craniotomized bone into the fresh craniotomy defect) is our technique of choice.23 Within this group, we have even found supplementation of the particulate bone with demineralized bone matrix to aid in osseous regeneration.26 Extracranial sites are also an option; however, they are of limited supply in the young child and should be used sparingly.
However, the stakes differ when one encounters a serially infected, scarred, or irradiated defect. In this scenario, nonvascularized autologous cranioplasty alone may indeed fail, not only because of the dependency of the bone graft for vascularity from the recipient site, but also because of the lack of an adequate soft tissue envelope, which may lead to skin necrosis, wound dehiscence, and graft/hardware exposure. Therefore, our experience has led to use of vascularized bone/soft tissue in the form of a chimeric free flap to combat the caustic environment of what we have termed the “hostile cranium.”31 However, one should note that this experience is mainly derived from our experience in the adult population as there is a paucity of data on the success of vascularized cranioplasty in the pediatric population.
Finally, in the case of a pediatric patient who has reached skeletal, cranial maturity (age 5 years or older) with no wound comorbidities such as radiation or infection, an alloplast would be safe to use for a massive defect. In those defects of limited size, split calvarial bone would be the gold standard.
Conclusion
There is a wide range of developments in calvarial reconstruction including autologous grafts and alloplastic materials. While outcomes are encouraging, little of the research is focused specifically on the pediatric population. Current decisions are often made by extrapolation from research on adults. More research into cranioplasty for children throughout stages of growth and development will be required before definitive conclusions can be drawn.
References
- 1.Grant G A Jolley M Ellenbogen R G Roberts T S Gruss J R Loeser J D Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents J Neurosurg 2004100(2, Suppl Pediatrics ):163–168. [DOI] [PubMed] [Google Scholar]
- 2.Bowers C A, Riva-Cambrin J, Hertzler D A II, Walker M L. Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J Neurosurg Pediatr. 2013;11(5):526–532. doi: 10.3171/2013.1.PEDS12483. [DOI] [PubMed] [Google Scholar]
- 3.Yadla S Campbell P G Chitale R Maltenfort M G Jabbour P Sharan A D Effect of early surgery, material, and method of flap preservation on cranioplasty infections: a systematic review Neurosurgery 20116841124–1129., discussion 1130 [DOI] [PubMed] [Google Scholar]
- 4.Baldo S, Tacconi L. Effectiveness and safety of subcutaneous abdominal preservation of autologous bone flap after decompressive craniectomy: a prospective pilot study. World Neurosurg. 2010;73(5):552–556. doi: 10.1016/j.wneu.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Oppenheimer A J, Mesa J, Buchman S R. Current and emerging basic science concepts in bone biology: implications in craniofacial surgery. J Craniofac Surg. 2012;23(1):30–36. doi: 10.1097/SCS.0b013e318240c6d9. [DOI] [PubMed] [Google Scholar]
- 6.Matsuno A Tanaka H Iwamuro H et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty Acta Neurochir (Wien) 20061485535–540., discussion 540 [DOI] [PubMed] [Google Scholar]
- 7.Osawa M, Hara H, Ichinose Y, Koyama T, Kobayashi S, Sugita Y. Cranioplasty with a frozen and autoclaved bone flap. Acta Neurochir (Wien) 1990;102(1-2):38–41. doi: 10.1007/BF01402184. [DOI] [PubMed] [Google Scholar]
- 8.Morina A, Kelmendi F, Morina Q. et al. Cranioplasty with subcutaneously preserved autologous bone grafts in abdominal wall-Experience with 75 cases in a post-war country Kosova. Surg Neurol Int. 2011;2:72. doi: 10.4103/2152-7806.81735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inamasu J Kuramae T Nakatsukasa M Does difference in the storage method of bone flaps after decompressive craniectomy affect the incidence of surgical site infection after cranioplasty? Comparison between subcutaneous pocket and cryopreservation J Trauma 2010681183–187., discussion 187 [DOI] [PubMed] [Google Scholar]
- 10.Yap C, Macarthur D C, Hope D T. 'Mind the gap': resorption of a bone flap stored subcutaneously for 6 months. Br J Neurosurg. 2002;16(5):523–524. doi: 10.1080/0268869021000030951. [DOI] [PubMed] [Google Scholar]
- 11.Bhaskar I P, Inglis T J, Lee G Y. Clinical, radiological, and microbiological profile of patients with autogenous cranioplasty infections. World Neurosurg. 2014;82((3–4)):e531–e534. doi: 10.1016/j.wneu.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y H, Yang T M, Lee T C, Chen W F, Yang K Y. Acute autologous bone flap infection after cranioplasty for postinjury decompressive craniectomy. Injury. 2013;44(1):44–47. doi: 10.1016/j.injury.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Piedra M P, Thompson E M, Selden N R, Ragel B T, Guillaume D J. Optimal timing of autologous cranioplasty after decompressive craniectomy in children. J Neurosurg Pediatr. 2012;10(4):268–272. doi: 10.3171/2012.6.PEDS1268. [DOI] [PubMed] [Google Scholar]
- 14.Schuss P, Vatter H, Marquardt G. et al. Cranioplasty after decompressive craniectomy: the effect of timing on postoperative complications. J Neurotrauma. 2012;29(6):1090–1095. doi: 10.1089/neu.2011.2176. [DOI] [PubMed] [Google Scholar]
- 15.Sweeny L, Eby B, Magnuson J S, Carroll W R, Rosenthal E L. Reconstruction of scalp defects with the radial forearm free flap. Head Neck Oncol. 2012;4:21. doi: 10.1186/1758-3284-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia-Ao Y, Hong-Jing L, Zheng-Hua J, Kai S, Zhen-Hai N. Reconstruction of a large pediatric scalp defect with skull exposure by a free anterolateral thigh flap. Plast Reconstr Surg. 2012;129(1):178e–180e. doi: 10.1097/PRS.0b013e3182365d8a. [DOI] [PubMed] [Google Scholar]
- 17.Serletti J M, Schingo V A Jr, Deuber M A, Carras A J, Herrera H R, Reale V F. Free tissue transfer in pediatric patients. Ann Plast Surg. 1996;36(6):561–568. doi: 10.1097/00000637-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Uusitalo M, Ibarra M, Fulton L. et al. Reconstruction with rectus abdominis myocutaneous free flap after orbital exenteration in children. Arch Ophthalmol. 2001;119(11):1705–1709. doi: 10.1001/archopht.119.11.1705. [DOI] [PubMed] [Google Scholar]
- 19.Ozkan O, Coskunfirat O K, Ozgentas H E, Derin A. Rationale for reconstruction of large scalp defects using the anterolateral thigh flap: structural and aesthetic outcomes. J Reconstr Microsurg. 2005;21(8):539–545. doi: 10.1055/s-2005-922433. [DOI] [PubMed] [Google Scholar]
- 20.Iida T, Mihara M, Yoshimatsu H. et al. Reconstruction of an extensive anterior skull base defect using a muscle-sparing rectus abdominis myocutaneous flap in a 1-year-old infant. Microsurgery. 2012;32(8):622–626. doi: 10.1002/micr.22025. [DOI] [PubMed] [Google Scholar]
- 21.Yu J A, Lin H J, Jin Z H, Shi K, Niu Z H, Zhao J C. Free anterolateral thigh flap for coverage of scalp large defects in pediatric burn population. J Burn Care Res. 2012;33(4):e180–e185. doi: 10.1097/BCR.0b013e318239f80b. [DOI] [PubMed] [Google Scholar]
- 22.Rogers G F, Greene A K. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg. 2012;23(1):323–327. doi: 10.1097/SCS.0b013e318241dcba. [DOI] [PubMed] [Google Scholar]
- 23.Rogers G F, Greene A K, Mulliken J B, Proctor M R, Ridgway E B. Exchange cranioplasty using autologous calvarial particulate bone graft effectively repairs large cranial defects. Plast Reconstr Surg. 2011;127(4):1631–1642. doi: 10.1097/PRS.0b013e31821084f0. [DOI] [PubMed] [Google Scholar]
- 24.Greene A K, Mulliken J B, Proctor M R, Rogers G F. Pediatric cranioplasty using particulate calvarial bone graft. Plast Reconstr Surg. 2008;122(2):563–571. doi: 10.1097/PRS.0b013e31817d61c1. [DOI] [PubMed] [Google Scholar]
- 25.Chao M T, Jiang S, Smith D. et al. Demineralized bone matrix and resorbable mesh bilaminate cranioplasty: a novel method for reconstruction of large-scale defects in the pediatric calvaria. Plast Reconstr Surg. 2009;123(3):976–982. doi: 10.1097/PRS.0b013e31819ba46f. [DOI] [PubMed] [Google Scholar]
- 26.Beederman M, Alkureishi L W, Lam S, Warnke P, Reid R R. Exchange hybrid cranioplasty using particulate bone graft and demineralized bone matrix: the best of both worlds. J Craniofac Surg. 2014;25(2):451–454. doi: 10.1097/SCS.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 27.Jaskolka M S, Olavarria G. Reconstruction of skull defects. Atlas Oral Maxillofac Surg Clin North Am. 2010;18(2):139–149. doi: 10.1016/j.cxom.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Agrawal A, Garg L N. Split calvarial bone graft for the reconstruction of skull defects. J Surg Tech Case Rep. 2011;3(1):13–16. doi: 10.4103/2006-8808.78465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue A, Satoh S, Sekiguchi K. et al. Cranioplasty with split-thickness calvarial bone. Neurol Med Chir (Tokyo) 1995;35(11):804–807. doi: 10.2176/nmc.35.804. [DOI] [PubMed] [Google Scholar]
- 30.Vercler C J, Sugg K B, Buchman S R. Split cranial bone grafting in children younger than 3 years old: debunking a surgical myth. Plast Reconstr Surg. 2014;133(6):822e–827e. doi: 10.1097/PRS.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J C, Kleiber G M, Pelletier A T, Reid R R, Gottlieb L J. Autologous immediate cranioplasty with vascularized bone in high-risk composite cranial defects. Plast Reconstr Surg. 2013;132(4):967–975. doi: 10.1097/PRS.0b013e31829f4b59. [DOI] [PubMed] [Google Scholar]
- 32.Smith D M, Afifi A M, Cooper G M, Mooney M P, Marra K G, Losee J E. BMP-2-based repair of large-scale calvarial defects in an experimental model: regenerative surgery in cranioplasty. J Craniofac Surg. 2008;19(5):1315–1322. doi: 10.1097/SCS.0b013e3181843369. [DOI] [PubMed] [Google Scholar]
- 33.Smith D M, Cooper G M, Afifi A M. et al. Regenerative surgery in cranioplasty revisited: the role of adipose-derived stem cells and BMP-2. Plast Reconstr Surg. 2011;128(5):1053–1060. doi: 10.1097/PRS.0b013e31822b65e4. [DOI] [PubMed] [Google Scholar]
- 34.Yonamine Y, Matsuyama T, Sonomura T. et al. Effectable application of vascular endothelial growth factor to critical sized rat calvaria defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(2):225–231. doi: 10.1016/j.tripleo.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Shah M M, Smyth M D, Woo A S. Adverse facial edema associated with off-label use of recombinant human bone morphogenetic protein-2 in cranial reconstruction for craniosynostosis. Case report. J Neurosurg Pediatr. 2008;1(3):255–257. doi: 10.3171/PED/2008/1/3/255. [DOI] [PubMed] [Google Scholar]
- 36.Vignes J R, Jeelani Nu, Dautheribes M, San-Galli F, Liguoro D. Cranioplasty for repair of a large bone defect in a growing skull fracture in children. J Craniomaxillofac Surg. 2007;35(3):185–188. doi: 10.1016/j.jcms.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Lin A Y, Kinsella C R Jr, Rottgers S A. et al. Custom porous polyethylene implants for large-scale pediatric skull reconstruction: early outcomes. J Craniofac Surg. 2012;23(1):67–70. doi: 10.1097/SCS.0b013e318240c876. [DOI] [PubMed] [Google Scholar]
- 38.Lee S C, Wu C T, Lee S T, Chen P J. Cranioplasty using polymethyl methacrylate prostheses. J Clin Neurosci. 2009;16(1):56–63. doi: 10.1016/j.jocn.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Marchac D Greensmith A Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery J Plast Reconstr Aesthet Surg 2008617744–752., discussion 753 [DOI] [PubMed] [Google Scholar]
- 40.Moreira-Gonzalez A, Jackson I T, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg. 2003;14(2):144–153. doi: 10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Jaberi J, Gambrell K, Tiwana P, Madden C, Finn R. Long-term clinical outcome analysis of poly-methyl-methacrylate cranioplasty for large skull defects. J Oral Maxillofac Surg. 2013;71(2):e81–e88. doi: 10.1016/j.joms.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Golz T, Graham C R, Busch L C, Wulf J, Winder R J. Temperature elevation during simulated polymethylmethacrylate (PMMA) cranioplasty in a cadaver model. J Clin Neurosci. 2010;17(5):617–622. doi: 10.1016/j.jocn.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Josan V A, Sgouros S, Walsh A R, Dover M S, Nishikawa H, Hockley A D. Cranioplasty in children. Childs Nerv Syst. 2005;21(3):200–204. doi: 10.1007/s00381-004-1068-2. [DOI] [PubMed] [Google Scholar]
- 44.Baker S B, Weinzweig J, Kirschner R E, Bartlett S P. Applications of a new carbonated calcium phosphate bone cement: early experience in pediatric and adult craniofacial reconstruction. Plast Reconstr Surg. 2002;109(6):1789–1796. doi: 10.1097/00006534-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Stefini R, Esposito G, Zanotti B, Iaccarino C, Fontanella M M, Servadei F. Use of “custom made” porous hydroxyapatite implants for cranioplasty: postoperative analysis of complications in 1549 patients. Surg Neurol Int. 2013;4:12. doi: 10.4103/2152-7806.106290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirschner R E Karmacharya J Ong G et al. Repair of the immature craniofacial skeleton with a calcium phosphate cement: quantitative assessment of craniofacial growth Ann Plast Surg 200249133–38., discussion 38 [DOI] [PubMed] [Google Scholar]
- 47.Smartt J M Jr, Karmacharya J, Gannon F H. et al. Repair of the immature and mature craniofacial skeleton with a carbonated calcium phosphate cement: assessment of biocompatibility, osteoconductivity, and remodeling capacity. Plast Reconstr Surg. 2005;115(6):1642–1650. doi: 10.1097/01.prs.0000161466.74294.1e. [DOI] [PubMed] [Google Scholar]
- 48.Losee J E, Karmacharya J, Gannon F H. et al. Reconstruction of the immature craniofacial skeleton with a carbonated calcium phosphate bone cement: interaction with bioresorbable mesh. J Craniofac Surg. 2003;14(1):117–124. doi: 10.1097/00001665-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 49.Singh K A, Burstein F D, Williams J K. Use of hydroxyapatite cement in pediatric craniofacial reconstructive surgery: strategies for avoiding complications. J Craniofac Surg. 2010;21(4):1130–1135. doi: 10.1097/SCS.0b013e3181e482c6. [DOI] [PubMed] [Google Scholar]
- 50.Wong R K, Gandolfi B M, St-Hilaire H, Wise M W, Moses M. Complications of hydroxyapatite bone cement in secondary pediatric craniofacial reconstruction. J Craniofac Surg. 2011;22(1):247–251. doi: 10.1097/SCS.0b013e3181f7b7db. [DOI] [PubMed] [Google Scholar]
- 51.Gilardino M S, Cabiling D S, Bartlett S P. Long-term follow-up experience with carbonated calcium phosphate cement (Norian) for cranioplasty in children and adults. Plast Reconstr Surg. 2009;123(3):983–994. doi: 10.1097/PRS.0b013e318199f6ad. [DOI] [PubMed] [Google Scholar]
- 52.Wiggins A Austerberry R Morrison D Ho K M Honeybul S Cranioplasty with custom-made titanium plates—14 years experience Neurosurgery 2013722248–256., discussion 256 [DOI] [PubMed] [Google Scholar]
- 53.Eufinger H. et al. Management of cranial and craniofacial bone defects with prefabricated individual titanium implants: follow-up and evaluation of 166 patients with 169 titanium implants from 1994–2000. Int J CARS. 2006;1(4):197–203. [Google Scholar]
- 54.Cabraja M, Klein M, Lehmann T N. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009;26(6):E10. doi: 10.3171/2009.3.FOCUS091. [DOI] [PubMed] [Google Scholar]
- 55.Lethaus B, Safi Y, ter Laak-Poort M. et al. Cranioplasty with customized titanium and PEEK implants in a mechanical stress model. J Neurotrauma. 2012;29(6):1077–1083. doi: 10.1089/neu.2011.1794. [DOI] [PubMed] [Google Scholar]
- 56.Maas C S, Merwin G E, Wilson J, Frey M D, Maves M D. Comparison of biomaterials for facial bone augmentation. Arch Otolaryngol Head Neck Surg. 1990;116(5):551–556. doi: 10.1001/archotol.1990.01870050051005. [DOI] [PubMed] [Google Scholar]
- 57.Carboni A. et al. Long-term follow-up of 105 porous polyethylene implants used to correct facial deformity. Eur J Plast Surg. 2002;25(6):310–314. [Google Scholar]
- 58.Cestero H J Jr, Salyer K E, Toranto I R. Bone growth into porous carbon, polyethylene, and polypropylene prostheses. J Biomed Mater Res. 1975;9(4):1–7. doi: 10.1002/jbm.820090403. [DOI] [PubMed] [Google Scholar]
- 59.Tark W H, Yoon I S, Rah D K, Park B Y, Kim Y O. Osteoconductivity of porous polyethylene in human skull. J Craniofac Surg. 2012;23(1):78–80. doi: 10.1097/SCS.0b013e318240c85d. [DOI] [PubMed] [Google Scholar]
- 60.Wang J C, Wei L, Xu J, Liu J F, Gui L. Clinical outcome of cranioplasty with high-density porous polyethylene. J Craniofac Surg. 2012;23(5):1404–1406. doi: 10.1097/SCS.0b013e31825e3aeb. [DOI] [PubMed] [Google Scholar]
- 61.Gordon C R Fisher M Liauw J et al. Multidisciplinary approach for improved outcomes in secondary cranial reconstruction: introducing the pericranial-onlay cranioplasty technique Neurosurgery 20141002179–189., discussion 189–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oei J D, Zhao W W, Chu L. et al. Antimicrobial acrylic materials with in situ generated silver nanoparticles. J Biomed Mater Res B Appl Biomater. 2012;100(2):409–415. doi: 10.1002/jbm.b.31963. [DOI] [PubMed] [Google Scholar]
- 63.Springer I N, Açil Y, Kuchenbecker S. et al. Bone graft versus BMP-7 in a critical size defect—cranioplasty in a growing infant model. Bone. 2005;37(4):563–569. doi: 10.1016/j.bone.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Cheng H, Jiang W, Phillips F M. et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]


