Abstract
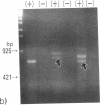
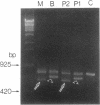
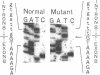
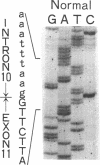
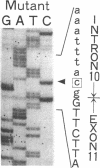
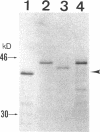
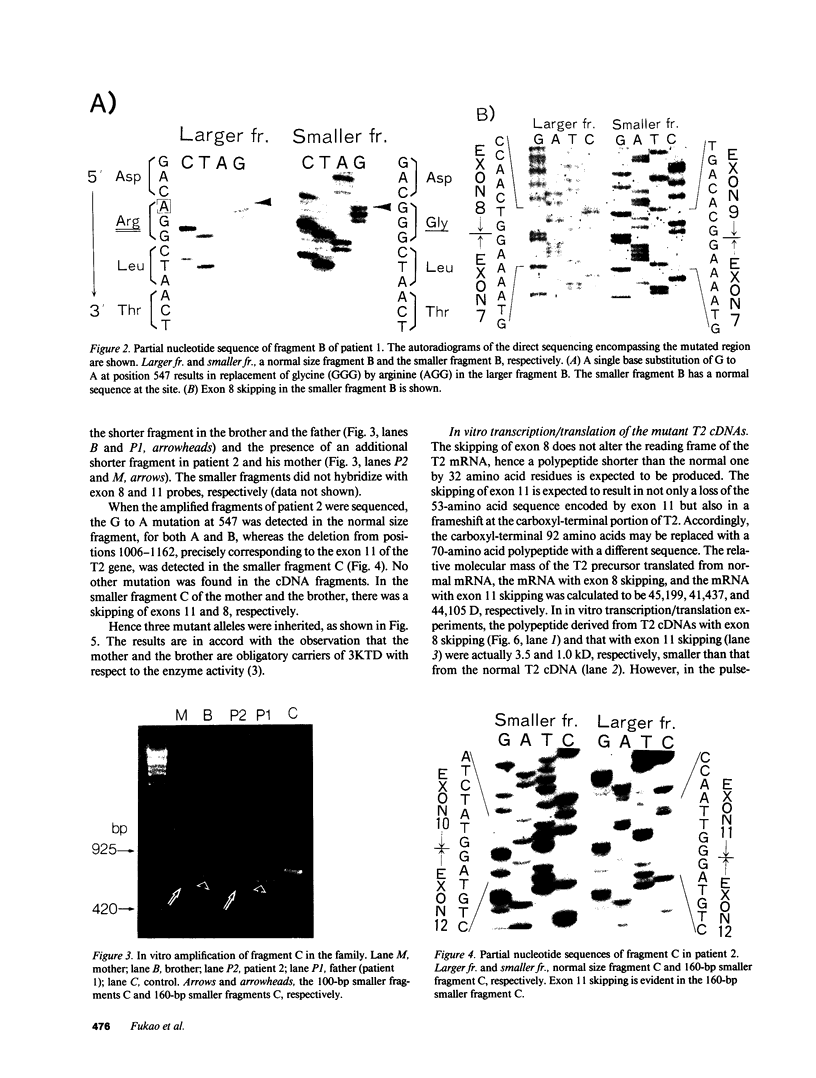
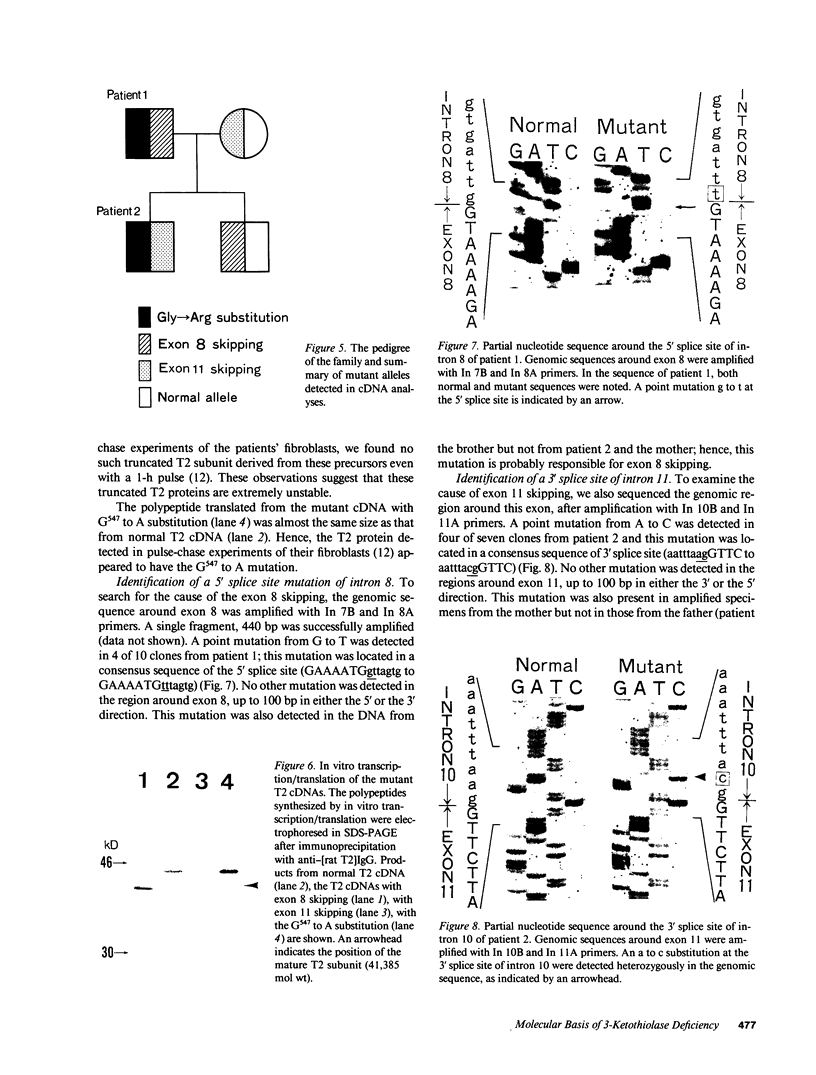
3-Ketothiolase deficiency (3KTD) stems from a deficiency of mitochondrial acetoacetyl-coenzyme A thiolase (T2). We analyzed the molecular basis of 3KTD in two generations of a family. A boy (patient 2, GK04), his father (patient 1, GK05), his mother, and his brother were studied; three mutant alleles of T2 gene were identified. Patient 1 is a compound heterozygote: one allele has a point mutation of G to A at position 547 on his T2 cDNA, causing Gly150 to Arg substitution of the mature T2 subunit, and the other allele has GT to TT transition at the 5' splice site of intron 8, causing exon 8's skipping of the T2 cDNA. Patient 2 is also a compound heterozygote: one allele inherited from his mother has AG to CG transition at the 3' splice site of intron 10, causing exon 11's skipping of the T2 cDNA, and the other allele derived from patient 1 has the G to A mutation (Gly to Arg). The brother of patient 2 is an obligatory carrier with the mutant allele causing the exon 8 skipping. This report seems to be the first complete molecular definition of 3KTD at the gene level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akli S., Chelly J., Mezard C., Gandy S., Kahn A., Poenaru L. A "G" to "A" mutation at position -1 of a 5' splice site in a late infantile form of Tay-Sachs disease. J Biol Chem. 1990 May 5;265(13):7324–7330. [PubMed] [Google Scholar]
- Antonarakis S. E., Irkin S. H., Cheng T. C., Scott A. F., Sexton J. P., Trusko S. P., Charache S., Kazazian H. H., Jr beta-Thalassemia in American Blacks: novel mutations in the "TATA" box and an acceptor splice site. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1154–1158. doi: 10.1073/pnas.81.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H., Takiguchi M., Amaya Y., Nagata S., Hayashi H., Mori M. cDNA-derived amino acid sequence of rat mitochondrial 3-oxoacyl-CoA thiolase with no transient presequence: structural relationship with peroxisomal isozyme. EMBO J. 1987 May;6(5):1361–1366. doi: 10.1002/j.1460-2075.1987.tb02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Chiodo A. A., Lamande S. R., Janeczko R., Ramirez F., Dahl H. H., Chan D., Bateman J. F. A base substitution at a splice site in the COL3A1 gene causes exon skipping and generates abnormal type III procollagen in a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1990 Oct 5;265(28):17070–17077. [PubMed] [Google Scholar]
- Daum R. S., Lamm P. H., Mamer O. A., Scriver C. R. A "new" disorder of isoleucine catabolism. Lancet. 1971 Dec 11;2(7737):1289–1290. doi: 10.1016/s0140-6736(71)90605-2. [DOI] [PubMed] [Google Scholar]
- Daum R. S., Scriver C. R., Mamer O. A., Delvin E., Lamm P., Goldman H. An inherited disorder of isoleucine catabolism causing accumulation of alpha-methylacetoacetate and alpha-methyl-beta -hydroxybutyrate, and intermittent metabolic acidosis. Pediatr Res. 1973 Mar;7(3):149–160. doi: 10.1203/00006450-197303000-00007. [DOI] [PubMed] [Google Scholar]
- Dequin S., Gloeckler R., Herbert C. J., Boutelet F. Cloning, sequencing and analysis of the yeast S. uvarum ERG10 gene encoding acetoacetyl CoA thiolase. Curr Genet. 1988 Jun;13(6):471–478. doi: 10.1007/BF02427752. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Lidsky A. S., Güttler F., Woo S. L. Tight linkage between a splicing mutation and a specific DNA haplotype in phenylketonuria. 1986 Aug 28-Sep 3Nature. 322(6082):799–803. doi: 10.1038/322799a0. [DOI] [PubMed] [Google Scholar]
- Fairbairn L. J., Tanner M. J. Complete cDNA sequence of human foetal liver peroxisomal 3-oxoacyl-CoA thiolase. Nucleic Acids Res. 1989 May 11;17(9):3588–3588. doi: 10.1093/nar/17.9.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T., Kamijo K., Osumi T., Fujiki Y., Yamaguchi S., Orii T., Hashimoto T. Molecular cloning and nucleotide sequence of cDNA encoding the entire precursor of rat mitochondrial acetoacetyl-CoA thiolase. J Biochem. 1989 Aug;106(2):197–204. doi: 10.1093/oxfordjournals.jbchem.a122832. [DOI] [PubMed] [Google Scholar]
- Fukao T., Yamaguchi S., Kano M., Orii T., Fujiki Y., Osumi T., Hashimoto T. Molecular cloning and sequence of the complementary DNA encoding human mitochondrial acetoacetyl-coenzyme A thiolase and study of the variant enzymes in cultured fibroblasts from patients with 3-ketothiolase deficiency. J Clin Invest. 1990 Dec;86(6):2086–2092. doi: 10.1172/JCI114946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Emi M., Luc G., Basdevant A., Gambert P., Iverius P. H., Lalouel J. M. Compound heterozygote for lipoprotein lipase deficiency: Ser----Thr244 and transition in 3' splice site of intron 2 (AG----AA) in the lipoprotein lipase gene. Am J Hum Genet. 1990 Oct;47(4):721–726. [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Ishii N., Kagamiyama H., Osumi T., Hashimoto T. Structural analysis of cDNA for rat peroxisomal 3-ketoacyl-CoA thiolase. J Biol Chem. 1987 Jun 15;262(17):8151–8158. [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Fukao T., Yamaguchi S., Orii T., Osumi T., Hashimoto T. Structure and expression of the human mitochondrial acetoacetyl-CoA thiolase-encoding gene. Gene. 1991 Dec 30;109(2):285–290. doi: 10.1016/0378-1119(91)90623-j. [DOI] [PubMed] [Google Scholar]
- Middleton B., Bartlett K. The synthesis and characterisation of 2-methylacetoacetyl coenzyme A and its use in the identification of the site of the defect in 2-methylacetoacetic and 2-methyl-3-hydroxybutyric aciduria. Clin Chim Acta. 1983 Mar 14;128(2-3):291–305. doi: 10.1016/0009-8981(83)90329-7. [DOI] [PubMed] [Google Scholar]
- Middleton B. The oxoacyl-coenzyme A thiolases of animal tissues. Biochem J. 1973 Apr;132(4):717–730. doi: 10.1042/bj1320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Urlaub G., Chasin L. Spontaneous splicing mutations at the dihydrofolate reductase locus in Chinese hamster ovary cells. Mol Cell Biol. 1986 Jun;6(6):1926–1935. doi: 10.1128/mcb.6.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Furuta S., Osumi T., Hashimoto T., Ui N. Properties of peroxisomal 3-ketoacyl-coA thiolase from rat liver. J Biochem. 1981 Aug;90(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a133499. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T. The presence of a new 3-oxoacyl-CoA thiolase in rat liver peroxisomes. Eur J Biochem. 1980 Feb;103(3):589–596. doi: 10.1111/j.1432-1033.1980.tb05984.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Yamaguchi S., Orii T., Schutgens R. B., Sweetman L., Hashimoto T. Heterogeneity of defects in mitochondrial acetoacetyl-CoA thiolase biosynthesis in fibroblasts from four patients with 3-ketothiolase deficiency. Pediatr Res. 1989 Aug;26(2):145–149. doi: 10.1203/00006450-198908000-00016. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Kono N., Yamasaki T., Hotta K., Kawachi M., Kuwajima M., Noguchi T., Tanaka T., Tarui S. Genetic defect in muscle phosphofructokinase deficiency. Abnormal splicing of the muscle phosphofructokinase gene due to a point mutation at the 5'-splice site. J Biol Chem. 1990 Jun 5;265(16):9392–9395. [PubMed] [Google Scholar]
- Orkin S. H., Sexton J. P., Goff S. C., Kazazian H. H., Jr Inactivation of an acceptor RNA splice site by a short deletion in beta-thalassemia. J Biol Chem. 1983 Jun 25;258(12):7249–7251. [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Peoples O. P., Masamune S., Walsh C. T., Sinskey A. J. Biosynthetic thiolase from Zoogloea ramigera. III. Isolation and characterization of the structural gene. J Biol Chem. 1987 Jan 5;262(1):97–102. [PubMed] [Google Scholar]
- Peoples O. P., Sinskey A. J. Poly-beta-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding beta-ketothiolase and acetoacetyl-CoA reductase. J Biol Chem. 1989 Sep 15;264(26):15293–15297. [PubMed] [Google Scholar]
- Robinson B. H., Sherwood W. G., Taylor J., Balfe J. W., Mamer O. A. Acetoacetyl CoA thiolase deficiency: a cause of severe ketoacidosis in infancy simulating salicylism. J Pediatr. 1979 Aug;95(2):228–233. doi: 10.1016/s0022-3476(79)80656-3. [DOI] [PubMed] [Google Scholar]
- Schutgens R. B., Middleton B., vd Blij J. F., Oorthuys J. W., Veder H. A., Vulsma T., Tegelaers W. H. Beta-ketothiolase deficiency in a family confirmed by in vitro enzymatic assays in fibroblasts. Eur J Pediatr. 1982 Sep;139(1):39–42. doi: 10.1007/BF00442077. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T. S., Lin L. H. Analysis of a splice acceptor site mutation which produces multiple splicing abnormalities in the human argininosuccinate synthetase locus. J Biol Chem. 1990 Nov 15;265(32):19716–19720. [PubMed] [Google Scholar]
- Weil D., Bernard M., Combates N., Wirtz M. K., Hollister D. W., Steinmann B., Ramirez F. Identification of a mutation that causes exon skipping during collagen pre-mRNA splicing in an Ehlers-Danlos syndrome variant. J Biol Chem. 1988 Jun 25;263(18):8561–8564. [PubMed] [Google Scholar]
- Yamaguchi S., Orii T., Sakura N., Miyazawa S., Hashimoto T. Defect in biosynthesis of mitochondrial acetoacetyl-coenzyme A thiolase in cultured fibroblasts from a boy with 3-ketothiolase deficiency. J Clin Invest. 1988 Mar;81(3):813–817. doi: 10.1172/JCI113388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Y., Yang X. Y., Healy-Louie G., Schulz H., Elzinga M. Nucleotide sequence of the fadA gene. Primary structure of 3-ketoacyl-coenzyme A thiolase from Escherichia coli and the structural organization of the fadAB operon. J Biol Chem. 1990 Jun 25;265(18):10424–10429. [PubMed] [Google Scholar]