Abstract
Methodologies for generating functional neuronal cells directly from human fibroblasts [induced neuronal (iN) cells] have been recently developed, but the research so far has only focused on technical refinements or recapitulation of known pathological phenotypes. A critical question is whether this novel technology will contribute to elucidation of novel disease mechanisms or evaluation of therapeutic strategies. Here we have addressed this question by studying Tay-Sachs disease, a representative lysosomal storage disease, and Dravet syndrome, a form of severe myoclonic epilepsy in infancy, using human iN cells with feature of immature postmitotic glutamatergic neuronal cells. In Tay-Sachs disease, we have successfully characterized canonical neuronal pathology, massive accumulation of GM2 ganglioside, and demonstrated the suitability of this novel cell culture for future drug screening. In Dravet syndrome, we have identified a novel functional phenotype that was not suggested by studies of classical mouse models and human autopsied brains. Taken together, the present study demonstrates that human iN cells are useful for translational neuroscience research to explore novel disease mechanisms and evaluate therapeutic compounds. In the future, research using human iN cells with well-characterized genomic landscape can be integrated into multidisciplinary patient-oriented research on neuropsychiatric disorders to address novel disease mechanisms and evaluate therapeutic strategies.
Keywords: Direct conversion, Induced neuronal cells, iN cells, Lysosomal storage diseases, Channelopathy, Polyglutamine diseases
Introduction
Modern medicine has developed through direct examination of patients' tissues and cells displaying major pathologies. In particular, cancer research has clearly benefitted from this strategy [1]. The same strategy has not been easily applied to brain disorders because of the difficulty in accessing brain tissues and cells from living patients. Therefore, many investigators have focused on techniques to generate “surrogate” brain cells, which reflect some, although not all, features of brain neurons and glia and can be used as a probe in exploring disease mechanisms [2-5]. A novel methodology that directly converts peripheral fibroblasts into neuronal cells, induced neuronal (iN) cells, has potential to accelerate this direction in translational research [6]. Nonetheless, it is currently unclear whether this technological advance leads to elucidation of novel mechanisms of brain diseases or evaluation of therapeutic strategies. Here we demonstrate the utility of human iN cells for both elucidation of novel mechanisms of disease and evaluation of therapeutic strategies in translational research on brain disorders relevant for neuropsychiatric disorders.
Materials and Methods
Fibroblasts
Primary human fibroblasts were collected from several sources. Normal control skin fibroblasts were collected from healthy volunteers at the Johns Hopkins Hospital or obtained from the Coriell Institute (Camden, NJ) (Supplementary Material, Table S1). Fibroblasts for Tay-Sachs disease, Sandhoff disease, Gaucher disease, GM1 gangliosidosis, and metachromatic leukodystrophy were obtained from Drs. D. Batista and E. Wohler (Cytogenetics Laboratory at the Kennedy Krieger Institute). Fibroblasts from patients with Machado-Joseph Disease (GM06153), dentatorubral-pallidoluysian atrophy (GM06917), Huntington's disease (GM09197), and Tay-Sachs disease (GM00502, GM01110) were obtained from the Coriell Institute. Dravet syndrome fibroblasts (D1, D2, D3) were collected from patients at Fukuoka University. Fibroblasts were maintained in minimal essential media (MEM) supplemented with 10% fetal bovine serum (FBS), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and 1% Pen Strep (all from Invitrogen). Human primary astrocytes were purchased from ScienCell and maintained in astrocyte medium provided by the manufacturer. 293 FT cells were purchased from Invitrogen and maintained in DMEM supplemented with 10% FBS/1 mM sodium pyruvate/0.1 mM nonessential amino acids.
Plasmid construction and lentiviral preparation
Human ASCL1, POU3F2, and MYT1L cDNA were purchased from GeneCopoeia or Open Biosystems, and cloned into pLenti6.3-TO/V5 lentiviral vectors by using Gateway shuttle vector system (Invitrogen). Human synapsin (hSyn) promoter-GFP lentivirus vector was prepared by inserting hSyn-EGFP region from pAAV-hSyn1-EGFP vector (a gift from Dr. S. Kugler, Germany) into pGIPZ lentiviral vector (Open Biosystems). Human wild-type SCN1A full-length cDNA (a gift from Dr. A. George, Vanderbilt University) was cloned into a CSII-EF-MCS-IRES-Venus lentiviral vector (a gift from Dr. H. Miyoshi, Japan) to generate CSII-EF-SCN1A-IRES-Venus vector. A CaMKII promoter-driven GFP reporter construct (FCKGW) was a gift from Dr. P. Osten (Cold Spring Harbor Laboratory). 293FT cells were transfected with each pLenti6.3 or GFP lentiviral vector and a set of virus packaging vectors by using Lipofectamine 2000 (Invitrogen). Lentiviral supernatants were collected 72 h later, and concentrated with either ultracentrifugation or PEG 8000 precipitation. Virus titers (infectious units: IFU) were determined using a Lenti-X p24 Rapid-Titer kit (Clontech). Aliquots of viruses were kept frozen at -80°C until use.
Generation of induced neuronal (iN) cells from fibroblasts
Fibroblasts were seeded on 6-well culture dishes, and spin-infected at 1,800 rpm for 60 min with lentivirus (MOI of 9) in the presence of polybrene (8 μg/mL). Infected cells were maintained in fibroblast medium, described above, for 3 to 4 days, then cells were gently detached by TrypLE (Invitrogen) and passaged onto poly-D-lysine or Matrigel-coated (ECM gel, Sigma-Aldrich) coverslips. For some experiments, infected cells were maintained on the original plate. Infected cells were subsequently maintained in a 1:1 mixture of human astrocyte-conditioned medium and Neurobasal medium supplemented with N2 and B27 supplements and Glutamax (all from Invitrogen). Half the medium was removed every 3-4 days and replaced with fresh 1:1 mixture. To prepare astrocyte-conditioned medium, human astrocytes (from ScienCell) were cultured with Neurobasal medium supplemented with B27 and L-glutamine (Invitrogen) for 4 days, and then the culture supernatants were collected and filtered to remove any cell debris; this conditioned medium was stored at -80°C until use. Chromosomal comparisons between the fibroblasts and iN cells would be a powerful assay to exclude the possibility of unexpected chromosomal changes caused by iN cell generation that may result in non-disease related phenotypes. In our current work, the disease phenotypes are rescued by pharmacological or genetic manipulation. Thus, we believe that our observed phenotypes are not caused by iN cell generation but by diseases.
Immunofluorescence staining
Cells were fixed in 4% paraformaldehyde (PFA) for 15 min at room temperature, and then incubated with 0.1-0.3% Triton X-100 in PBS for 15 min at room temperature. Cells were then blocked in PBS with 10% normal goat serum and 1% bovine serum albumin for 1 h at room temperature or overnight at 4°C, followed by incubation with primary antibodies for 1-2 h at room temperature or overnight at 4°C. Secondary antibodies conjugated with Alexa-488, Alexa-568, Alexa-647 (all from Invitrogen), or Cy5 (Jackson ImmunoResearch Lab) were applied for 1-2 h at room temperature. Finally, coverslips were briefly placed in 4′,6-diamidino-2-phenylindole (DAPI) solution (Invitrogen) for nuclei staining, and mounted on glass slides. The following primary antibodies were used: rabbit anti-βIII tubulin (1:2,000, Covance); mouse anti-βIII tubulin (Tuj1) (1:500, Sigma); rabbit anti-MAP2 (1:1,000, Millipore); mouse anti-MAP2 (1:1,000, Sigma); chicken-anti-MAP2 (1:20,000, Abcam); rabbit anti-VGLUT1 (1:200, Abcam); rabbit anti-GM2 (1:1,000-5,000, Matreya); rabbit anti-Nav1.1 (1:100, Alomone labs); mouse anti-tyrosine hydroxylase (1:200, Millipore); mouse anti-glutamic acid decarboxylase 67 (1:500, Millipore); and chicken anti-GFP (1:2,000, Abcam). Cells were visualized with Zeiss epifluorescence or confocal microscopy.
Quantitative analysis of microscopy images
To calculate the percentage of MAP2-positive cells per total cells in iN cell culture, we took pictures of 5-6 randomly selected 20x visual fields with an epifluorescence microscope and counted the number of cells with MAP2-positive staining and neuronal morphology. We then divided the number by the total number of DAPI-positive cells to estimate the enrichment of MAP2-positive neuronal cells in culture. To evaluate the extent of GM2 ganglioside accumulation, we counted the number of cells with GM2 accumulation (bright dots around nuclei detected by anti-GM2 antibodies). Pictures of randomly selected 20x visual fields were used to calculate the ratio of MAP2-positive neuronal cells with GM2 accumulation against total MAP2-positive neuronal cells.
Electrophysiology
Coverslips containing neuronal cells were loaded into an upright microscope (Olympus BX-50-WI) fitted with a Warner RC-27 submerged recording chamber. Patch pipettes (7–10 MΩ) were filled with 0.125% Neurobiotin solution containing 115 mM potassium gluconate, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2 mM MgCl2, 20 mM KCl, 2 mM MgATP, 2 mM Na2-ATP, and 0.3 mM GTP (pH 7.3, 280 ± 5 mOsm). Recordings were made at 32-35°C in oxygenated artificial cerebrospinal fluid (aCSF) (pH 7.4, osmolarity 290 ± 5 mOsm) perfused at 2 mL/min containing125 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 3.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, and 1 mM MgCl2. Neuronal cells were identified under visual guidance with infrared differential interference contrast optics (Olympus BX-50-WI) with a 40× water-immersion objective. The image was captured with an infrared-sensitive CCD camera (Dage-MTI) and displayed on a monitor. In addition, the image was sent to a computer with a USB connector and captured (KWorld). Whole-cell current clamp recordings were made with a headstage (CV-7B) connected to a computer-controlled amplifier (MultiClamp 700B, Axon Instruments), and acquired at a sampling rate of 10 kHz. All current steps to set membrane potential and elicit action potentials were delivered through the recording pipette and controlled by Axoscope 9.0 (Axon). Liquid junction potential was not corrected, and electrode potentials were adjusted to zero before recording. Cells were held at approximately -70 mV with negative current delivered through the recording electrode. In some experiments, tetrodotoxin (TTX) (1 μM, Tocris) was used to confirm that action potentials were sodium-dependent.
Pharmacological and genetic rescue experiments
For rescue experiments in Tay-Sachs disease, Tay-Sachs iN cells were treated with NB-DNJ (10 or 50 μM, EMD bioscience or Toronto Research Chemicals) or vehicle control (DMSO) three times every 3-4 days from day 10 to 20. For rescue experiments in Dravet syndrome, Dravet iN cells were transfected with CSII-EF-SCN1A-IRES-Venus vector by using HilyMax transfection reagents (Dojindo) at days 15-25 and electrophysiological assessment was performed 4-7 days after transfection.
Statistical analysis
Student's t-test was used to determine the difference between two groups. For the comparison among three or more groups, one-way ANOVA followed by Tukey's correction was used. A p-value <0.05 was regarded as statistically significant. Data are shown as mean ± S.E.M. or mean ± S.D. as indicated in the figure legends.
Results
We established a protocol to directly generate induced neuronal (iN) cells, immature postmitotic neuronal cells, from human fibroblasts by lentiviral transduction of human ASCL1 (also known as MASH1), POU3F2 (also known as BRN2), and MYT1L cDNA into early-passage human fibroblasts, a method similar to that was used in previous studies on iN cells [6-8] (Supplementary Material, Text; Table S1; Figs. S1A-D). These iN cells are able to fire action potentials and mostly glutamatergic in subtype (Supplementary Material, Figs. S1E-H). In this study, using these human iN cells, we studied two specific types of disorders, lysosomal storage diseases and channelopathy, in order to explore their clinical utility in neuropsychiatric research.
We first studied lysosomal storage diseases in which unavailability of human neuronal cell models has hampered development of translational studies. Tay-Sachs disease, a representative neurological lysosomal storage disease, is caused by β-hexosaminidase deficiency, due to mutations in the α-subunit of β-hexosaminidase (HEXA) gene, that results in the accumulation of its substrate GM2 gangliosides in neurons and a progressive neurodegenerative disease [9, 10]. The incidence of Tay-Sachs disease is approximately 1 per 220,000 in the general population, but is higher in the selected populations such as Ashkenazi Jewish and French Canadian, in which recent implementation of several screening programs of genetic carriers have reduced the incidence [11, 12]. Sandhoff disease is also caused by β-hexosaminidase deficiency, but the mutations occur in the β-subunit of β-hexosamindase (HEXB) gene. The different rate of the progressive accumulation of GM2 gangliosides leads to a clinical phenotype ranging from a very severe, rapidly progressive infantile form to a milder, chronic adult-onset form with mostly peripheral nerve and psychiatric symptoms [13]. Investigation of the molecular pathogenesis and development of mechanism-based therapeutic strategies have been limited because endogenous GM2 accumulation is not observed in peripheral cells from patients [14]. Although mouse models for GM2 gangliosidosis are available, due to the presence of surrogate degradation pathway for GM2 gagnliosides in mice (known as sialidase pathway), they fail to recapitulate GM2 gangliosidosis [15]. A spontaneous feline model of GM2 gangliosidosis [16] was reported to be more similar to human conditions than rodent ones, but it is still different from human GM2 gangliosidosis [17]. Therefore, human iN cells have a great potential to fulfill the needs in the current research on GM2 gangliosidosis.
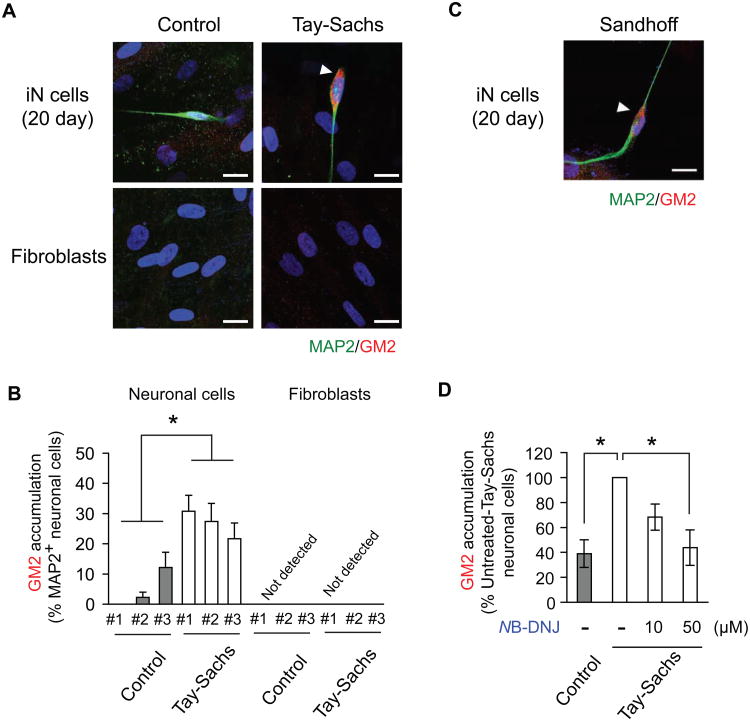
We generated iN cells directly from fibroblasts of Tay-Sachs patients. We observed robust accumulation of GM2 gangliosides in the patient-derived iN cells, but not in those from normal controls (Figs. 1A and B; Supplementary Material, Fig. S2A). No accumulation of GM2 gangliosides was detected in patient and control fibroblasts (Figs 1A and B; Supplementary Material, Fig. S2A). iN cells from Sandhoff disease, another GM2 gangliosidosis caused by β-hexosaminidase B deficiency [9], also showed robust GM2 accumulation (Fig. 1C). N-Butyldeoxynojirimycin (NB-DNJ), or miglustat (Zavesca®), inhibits glycosphingolipid biosynthesis and is known to reduce lysosomal storage in patients with Gaucher disease and Hexa-deficient mice, a model of Tay-Sachs disease [18]. We found that NB-DNJ decreased GM2 accumulation in Tay-Sachs iN cells without affecting the number of total iN cells (Fig. 1D; Supplementary Material, Fig. S2B). These data are pharmacological evidence for the inability of Tay-Sachs iN cells to remove GM2 gangliosides and also demonstrate the utility of human iN cell culture for testing potential therapeutic compounds. Thus, our study on lysosomal storage diseases establishes that patient fibroblast-derived iN cells have the potential to greatly aid the investigation of lysosomal storage disease phenotypes and screening of potential therapeutic compounds.
Figure 1. GM2 ganglioside accumulation in iN cells from patients with Tay-Sachs disease.
(A) Representative picture of GM2 ganglioside accumulation in iN cells derived from Tay-Sachs disease fibroblasts at day 20 after neuronal induction with lentivirus infection. Arrowhead indicates GM2 accumulation. (B) Quantification of MAP2-positive iN cells with GM2 accumulation per total MAP2-positive neuronal cells. Data are presented as mean ± S.E.M. of at least three independent experiments for each Tay-Sachs patient and control subject (14-23 neuronal cells per experiment). *p<0.05. (C) Representative pictures of GM2 ganglioside accumulation in MAP2-positive iN cells from a patient with Sandhoff disease. Arrowhead indicates GM2 accumulation. (D) Dose-dependent reduction of GM2 ganglioside accumulation in Tay-Sachs iN cells treated with N-Butyldeoxynojirimycin (NB-DNJ). Data are presented as mean ± S.E.M. of four independent experiments (16-24 neuronal cells per experiment). *p<0.05. Scale bars, 20 μm (A, C).
Previous studies with either iN cells or induced pluripotent stem cell (iPSC)-derived neuronal cells have successfully recapitulated brain pathology described in patient peripheral tissues, autopsied brains, and animal models [19-21]. We questioned whether human iN cells could be used as a tool to identify novel mechanisms that have not been uncovered by other research methodologies. Dravet syndrome is a form of severe myoclonic epilepsy in infancy, caused by de novo mutations in the SCN1A gene encoding the α-subunit of the NaV1.1 (SCN1A) channel [22, 23]. The incidence of Dravet syndrome is estimated at 1/20,000 to 1/40,000 birth in the general population [24]. SCN1A is expressed in glutamatergic pyramidal neurons in the human brain[25], whereas expression in mice is confined to GABAergic interneurons [26]. Thus, mouse genetic models such as SCN1A knockout mice currently indicated that the mutation mainly affects GABAergic interneurons, and less attention was paid to any pathological changes in glutamatergic neurons [26, 27]. Consistent with the expression pattern in the human brain [25], we found that SCN1A is expressed in human iN cells, which has a feature of glutamatergic neuronal cells (Fig. 2A). In our experiments, most of the MAP2-positive iN cells (defined as MAP2-positive cells with neuronal morphology) were CaMKII-GFP-positive glutamatergic cells, and there was no difference in the percentage of MAP2-positive cells per CaMKII-GFP positive cells between control and Dravet iN cell cultures (Supplementary Material, Fig. S1D).
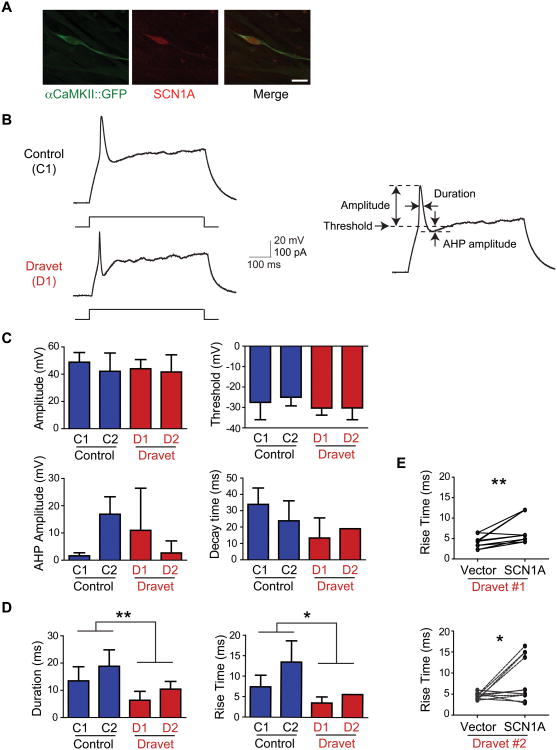
Figure 2. Altered patterns of action potential firings in iN cells from Dravet syndrome patients.
(A) SCN1A staining in Dravet glutamatergic iN cells. (B) Representative traces of membrane potential changes induced by current injection in control and Dravet iN cells. Parameters for electrophysiological data analysis are shown to the right. Threshold was measured as the point of inflection leading to the rising phase of the action potential following the current stimulus. The difference in membrane potential between the threshold and the peak of the action potential was considered the amplitude. Duration was measured as the distance between the rising and the falling phases of the action potential at half the total amplitude. When present, afterhyperpolarization (AHP) amplitude was calculated as the difference between the threshold and the lowest point of the undershoot in the falling phase. Duration was measured as the width at half-amplitude. Rise time and decay time of action potentials were measured as the width from the threshold to half-amplitude and from the half-amplitude to the threshold, respectively. (C) No differences in action potential amplitude, threshold, afterhyperpolarization (AHP) amplitude, and decay time in Dravet iN cells compared to control iN cells. (D) Decrease in action potential durations and rise time in Dravet iN cells compared to control iN cells. **p<0.01, *p<0.05. (E) Effects of wild-type SCN1A overexpression on action potential rise time in Dravet iN cells. **p<0.01, *p<0.05. Scale bars, 20 μm (A). For C and D, data are presented as mean ± S.D. of at least four experiments per subject.
Unexpectedly, excitatory glutamatergic iN cells from Dravet patients showed action potentials with significantly shorter duration relative to neuronal cells derived from healthy control subjects (n=10 experiments per subject); other action potential characteristics such as threshold, amplitude, and afterhyperpolarization amplitude did not differ between the two groups (Figs. 2B-D; Supplementary Material, Figs. S3 and S4; Tables S2 and S3). Further analysis of the action potential waveform revealed a reduction in the rise time with no significant change in decay time (Figs. 2C and D). The duration of the action potential is determined by both sodium and potassium channel kinetics. The depolarized resting membrane potential, high input resistance, and small afterhyperpolarization of the iN cells suggest that potassium channels may be absent or expressed at low levels. Because sodium but not potassium channels contribute to the rising phase of the action potential, this measure accurately reflects the altered sodium channel kinetics in Dravet syndrome-derived iN cells. These data suggest that excitatory glutamatergic iN neurons from Dravet patients may have altered sodium channel activity.
We next attempted to rescue the Dravet-associated abnormal action potentials by transfecting the iN cells with a vector encoding full-length human SCN1A cDNA. We compared matched pairs of neuronal cells from the same cultures, one expressing SCN1A cDNA and the other a mock vector. In both of the two patients (D1 and D2), iN cells expressing SCN1A cDNA showed significantly rescued phenotypes (i.e., normalized rise time) (Fig. 2E): on the contrary, iN cells expressing a mock vector did not show any rescued phenotype.
Dravet syndrome can result from a number of different mutations. Thus, we analyzed another patient (D3) with a mutation resulting in a much shorter truncated protein than the other two patients (D1 and D2), and found almost no changes on the cellular electrophysiology of glutamatergic iN cells in this patient (data not shown). The mutation in this patient (D3) completely removes the channel pore while the mutations in the other two patients preserve it. We speculate that an endogenous compensatory mechanism may be at work in iN cells with Nav1.1 channels lacking the pore (in case of D3), but not in those with malfunctioning Nav1.1 (in case of D1 and D2). The malfunctioning Nav1.1 in patients D1 and D2 were likely replaced by fully functioning Nav1.1 in our rescue experiments.
Our data show a cell-autonomous alteration of glutamatergic neuronal cells caused by genetic mutations associated with Dravet syndrome. This alteration may shape the excitability of cells in these specific patients, and widely open new field of further research on how such cellular susceptibility underlies circuitry and phenotypic abnormalities in this disease. Of note, recent studies using iPSC-derived neuronal cells from Dravet patients characterized electrophysiological properties in the mixed culture in which GABAergic and glutamatergic cells co-existed and showed a cell-intrinsic hyperexcitability in glutamatergic neuronal cells [28, 29].
We have thus far generated iN cells from patients over a wide range of ages with various neurological disorders including polyglutamine diseases [dentatorubral-pallidoluysian atrophy (DRPLA), Huntington's disease, and Machado-Joseph disease], lysosomal storage diseases (Tay-Sachs disease, Sandhoff disease, Gaucher disease, GM1 gangliosidosis, and metachromatic leukodystrophy), and channelopathy (Dravet syndrome) (Figs. 1 and 2; Supplementary Material, Fig. S5). We did not observe any remarkable difference in the efficiency or toxicity of neuronal conversion from various disease and control fibroblasts. Thus, iN cells can be useful for research in any brain disorders.
Discussion
Here we report the utility of postmitotic glutamatergic iN cells directly converted from fibroblasts. We show disease-relevant molecular (GM2 ganglioside accumulation in Tay-Sachs disease) and functional (altered sodium channel kinetics in Dravet syndrome) changes, indicating that these immature neuronal cells are sufficiently useful in studying key neuronal phenotypes of brain disorders. We also acknowledge the limitation of these cells due to insufficient synapse formation [30], which is crucial in different classes of brain disorders. We believe that direct neuronal conversion to generate iN cells is an important, higher throughput, and complementary methodology to the technology of iPSCs for studying brain disorders.
Ethical and technical issues have so far limited access to living brain tissues and have hampered understanding of the mechanisms underlying neuropsychiatric disorders. iPSCs provide an innovative method to generate neuronal cells, but there are several limitations to their translational application including time-consuming and costly experimental procedures. Here we show that iN cells directly converted from patient fibroblasts are useful for identifying novel disease phenotypes and for testing the efficacy of therapeutic compounds against key disease phenotypes in the brain. Although iN cells have some limitations because of their postmitotic features, including the inability to expand the cell culture and difficulties in freezing cells for long-term storage, these neuronal cells can be utilized as complementary resource to iPSC-derived neuronal cells in translational research for neuropsychiatric disorders. Because the use of Sendai virus has enabled integration-free generation of iPSC lines and is believed to be beneficial for modeling of complex genetic disorders and regenerative medicine, the use of Sendai virus, instead of lentivirus, in iN cell generation may prove to be beneficial in the future. As in research using iPSCs, effects of direct conversion on genomic landscape may need to be characterized in research using iN cells.
Conclusion
Our study demonstrates that induced neuronal (iN) cells directly converted from patient fibroblasts are useful for identifying novel disease phenotypes and for testing the efficacy of therapeutic compounds against key disease phenotypes in the brain. Research using iN cells can be integrated into multidisciplinary patient-oriented research on neuropsychiatric disorders to address novel disease mechanisms and evaluate therapeutic strategies.
Supplementary Material
Figure S1: Generation of neuronal cells directly from human fibroblasts by using human ASCL1, POU3F2, and MYT1L
Figure S2: GM2 ganglioside accumulation in neuronal cells from Tay-Sachs disease and Sandhoff disease
Figure S3: Representative action potential traces in neuronal cells from control (C1 and C2) and Dravet syndrome subjects (D1 and D2)
Figure S4: Electrophysiological characterization of neuronal cells from Dravet patients and controls
Figure S5: iN cell generation from various brain disorders.
Table S1: List of fibroblasts used for the generation of iN cells
Table S2: Summary of electrophysiological properties of iN cells
Table S3: Variability in the Dravet's electrophysiology data (action potential duration) within versus between groups
Acknowledgments
We thank Drs. P. Talalay, Y. Nakagawa, and M. Li for critical reading of the manuscript and discussion; Drs. A. George, H. Miyoshi, P. Osten, and S. Kugler for reagents; Ms. Y. Lema for help in organizing the manuscript. This work was supported by the National Institute of Health [MH069853, MH084018, MH085226, and MH088753 to A.S., MH085226 to P.O., NS071535 and MH098689 to G.M., and MH093458 to S.K.]; the Maryland Stem Cell Research Fund to M.K., P.O., and A.S.]; the National Tay-Sachs and Allied Diseases Association to G.M.; the Brain and Behavioral Foundation to A.S.; Stanley Foundation to A.S.; the S-R foundation to A.S.; the Hammerschlag Family to S.K.; Grant-in-Aids for Scientific Research in Japan [#21249062, #23659529 to S.H. and #22791011 to N.H.]; Research grant for Central Research Institute for the Molecular Pathomechanisms of Epilepsy of Fukuoka University to S.H. and N.H.
Abbreviations
- iN
induced neuronal
- iPSC
induced pluripotent stem cell
- NB-DNJ
N-Butyldeoxynojirimycin
Footnotes
Conflict of Interest: Patricio O'Donnell is now employed by Pfizer, Inc.
References
- 1.Contran RS, Kumar V, Robbins S. Robbins Pathologic Basis of Disease. 4th. Philadelphia, USA: Saunders; 1989. [Google Scholar]
- 2.Dolmetsch R, Geschwind DH. The Human Brain in a Dish: The Promise of iPSC-Derived Neurons. Cell. 2011;145(6):831–4. doi: 10.1016/j.cell.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meissner A, Jaenisch R. Mammalian nuclear transfer. Dev Dyn. 2006;235(9):2460–9. doi: 10.1002/dvdy.20915. [DOI] [PubMed] [Google Scholar]
- 4.Wilson A, Sawa A. Human cell models for schizophrenia. In: Silverstein SM, Moghaddam B, Wykes T, editors. Schizophrenia: Evolution and Synthesis Strungmann Forum Reports. Cambridge, USA: MIT Press; 2013. [PubMed] [Google Scholar]
- 5.Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A, et al. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry. 2013;18(7):740–2. doi: 10.1038/mp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476(7359):220–3. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476(7359):224–7. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 9.Gravel RA, Clarke JTR, Kaback MM, Mahuran D, Sandhoff K, Suzu-ki K. The GM2 gangliosidoses. In: Scriver CR, B AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7th. New York, USA: McGraw-Hill; 1995. pp. 2839–79. [Google Scholar]
- 10.Svennerholm L. Identification of the accumulated ganglioside. Adv Genet. 2001;44:33–41. doi: 10.1016/s0065-2660(01)44068-5. [DOI] [PubMed] [Google Scholar]
- 11.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249–54. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 12.Kaback MM. Population-based genetic screening for reproductive counseling: the Tay-Sachs disease model. Eur J Pediatr. 2000;159(Suppl 3):S192–5. doi: 10.1007/pl00014401. [DOI] [PubMed] [Google Scholar]
- 13.Maegawa GH, Stockley T, Tropak M, Banwell B, Blaser S, Kok F, et al. The natural history of juvenile or subacute GM2 gangliosidosis: 21 new cases and literature review of 134 previously reported. Pediatrics. 2006;118(5):e1550–62. doi: 10.1542/peds.2006-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyakumar M, Butters TD, Dwek RA, Platt FM. Glycosphingolipid lysosomal storage diseases: therapy and pathogenesis. Neuropathol Appl Neurobiol. 2002;28(5):343–57. doi: 10.1046/j.1365-2990.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 15.Zigdon H, Meshcheriakova A, Futerman AH. From sheep to mice to cells: Tools for the study of the sphingolipidoses. Biochim Biophys Acta. 2014;1841(8):1189–99. doi: 10.1016/j.bbalip.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Cork LC, Munnell JF, Lorenz MD, Murphy JV, Baker HJ, Rattazzi MC. GM2 ganglioside lysosomal storage disease in cats with beta-hexosaminidase deficiency. Science. 1977;196(4293):1014–7. doi: 10.1126/science.404709. [DOI] [PubMed] [Google Scholar]
- 17.Baek RC, Martin DR, Cox NR, Seyfried TN. Comparative analysis of brain lipids in mice, cats, and humans with Sandhoff disease. Lipids. 2009;44(3):197–205. doi: 10.1007/s11745-008-3268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platt FM, Neises GR, Reinkensmeier G, Townsend MJ, Perry VH, Proia RL, et al. Prevention of lysosomal storage in Tay-Sachs mice treated with N-butyldeoxynojirimycin. Science. 1997;276(5311):428–31. doi: 10.1126/science.276.5311.428. [DOI] [PubMed] [Google Scholar]
- 19.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363(15):1397–409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 20.Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482(7384):216–20. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imaizumi Y, Okada Y, Akamatsu W, Koike M, Kuzumaki N, Hayakawa H, et al. Mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain. 2012;5(1):35. doi: 10.1186/1756-6606-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meisler MH, O'Brien JE, Sharkey LM. Sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. J Physiol. 2010;588(Pt 11):1841–8. doi: 10.1113/jphysiol.2010.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol. 2010;588(Pt 11):1849–59. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl 2):3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- 25.Whitaker WR, Faull RL, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Brain Res Mol Brain Res. 2001;88(1-2):37–53. doi: 10.1016/s0169-328x(00)00289-8. [DOI] [PubMed] [Google Scholar]
- 26.Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, et al. Na(v)1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27(22):5903–14. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9(9):1142–9. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Lopez-Santiago LF, Yuan Y, Jones JM, Zhang H, O'Malley HA, et al. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Annals of Neurology. 2013 doi: 10.1002/ana.23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higurashi N, Uchida T, Lossin C, Misumi Y, Okada Y, Akamatsu W, et al. A human Dravet syndrome model from patient induced pluripotent stem cells. Mol Brain. 2013;6:19. doi: 10.1186/1756-6606-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78(5):785–98. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Generation of neuronal cells directly from human fibroblasts by using human ASCL1, POU3F2, and MYT1L
Figure S2: GM2 ganglioside accumulation in neuronal cells from Tay-Sachs disease and Sandhoff disease
Figure S3: Representative action potential traces in neuronal cells from control (C1 and C2) and Dravet syndrome subjects (D1 and D2)
Figure S4: Electrophysiological characterization of neuronal cells from Dravet patients and controls
Figure S5: iN cell generation from various brain disorders.
Table S1: List of fibroblasts used for the generation of iN cells
Table S2: Summary of electrophysiological properties of iN cells
Table S3: Variability in the Dravet's electrophysiology data (action potential duration) within versus between groups