Abstract
Objective
To determine if serum urate concentration is associated with development of hypertension in young adults.
Methods
Retrospective cohort analysis from 4752 participants with available serum urate and without hypertension at baseline from the Coronary Artery Risk Development in Young Adults (CARDIA) Study; a mixed race (African-American and White) cohort established in 1985 with 20 years of follow-up data for this analysis. Associations between baseline serum urate concentration and incident hypertension (defined as a blood pressure greater or equal to 140/90 or being on antihypertensive drugs) were investigated in sex-stratified bivariate and multivariable Cox-proportional analyses.
Results
Mean age (standard deviation) at baseline was 24.8 (3.6) years for men and 24.9 (3.7) years for women. Compared with the referent category, we found a greater hazard of developing hypertension starting at 345 μmol/L (5.8 mg/ dL) of serum urate for men and 214 μmol/L (3.6 mg/dL) for women. There was a 25% increase in the hazard of developing hypertension in men (HR1.25 [95% CI 1.15-1.36]) per each mg/dL increase in serum urate but no significant increase in women (HR 1.06 [95%CI 0.97-1.16]).
Conclusions
We found a significant independent association between higher serum urate concentrations and the subsequent hazard of incident hypertension, even at concentrations below the conventional hyperuricemia threshold of 404 μmol/L (6.8 mg/dL).
Keywords: health services research, hypertension, epidemiology
INTRODUCTION
An association between elevated serum urate and hypertension has been well described in adults above the fifth decade of life. 1-11 Animal models suggest that decreasing urate levels can reverse urate-induced hypertension, but only if this occurs early in life. 12-14 A small number of epidemiological studies also support the concept that with advancing age, the association between serum urate and incident hypertension is attenuated.15-17 It is possible that young persons are more sensitive to the mechanisms by which serum urate contributes to incident hypertension such as pre-glomerular vascular disease.18 With the exception of a small randomized clinical trial in adolescents,19 most studies have predominately focused on adults over 40 years of age who may already have arterial stiffness and in whom there is less potential role for an early intervention to prevent hypertension.
The concentration at which serum urate is associated with the development of incident hypertension has been evaluated predominantly with regards to thresholds related to the current definitions for hyperuricemia (404 micromoles per liter [μmol/L] or 6.8 milligrams/deciliter [mg/dL]).20 These definitions are based on the solubility point of serum urate at normal body temperature and normal pH that is relevant to the pathophysiology and management of gout. It is uncertain if this established threshold for hyperuricemia is as relevant for other putative pathogenic associations of serum urate, such as those with hypertension and other cardiovascular outcomes.
The Coronary Artery Risk Development in Young Adults (CARDIA) cohort, established to investigate the development of cardiovascular disease in White and African-American young adults, is uniquely suited to examine prospective associations of higher serum urate levels with conditions such as hypertension starting at a younger age. Using CARDIA, we prospectively evaluated the association between serum urate concentrations and the hazard for incident hypertension, along with the serum urate concentrations at which the risk for incident hypertension increases in young adults.
METHODS
Study population
The CARDIA cohort was established in 1985-86 and is still following participants with the primary objective of investigating factors that contribute to the development of coronary heart disease in young adults 21. Study design, recruitment of participants, and the Institutional Review Board approvals with individual informed consent processes have been described in detail 22. Institutional Review Boards at all the participating institutions: the University of Alabama at Birmingham (Birmingham, coordinating center, IRB registration 00000726), the University of Minnesota (Minneapolis), Northwestern University (Chicago), and Kaiser Permanente (Oakland) gave approval for the CARDIA study that is sponsored by the United States National Heart, Lung, and Blood Institute. All participants provided a written informed consent prior to enrollment. The sample of 5115 subjects 18-30 years of age was stratified to achieve nearly equal numbers of African-Americans and Whites, men and women, persons older and younger than 25 years of age, and persons with high school graduation or less and more than high school graduation. A majority of participants were recruited by telephone and after a brief screening interview they were invited for an initial enrolling examination. Over the past 25 years, eight examinations of this cohort have been completed, with a majority of those enrolled participating in these follow-up examinations (ranging from 90% at year 2 to 72% at year 20) 23. Participants without serum urate information at baseline, with no follow-up time after enrollment, or that met the study hypertension definition at enrollment were excluded from the analyses.
Study design and variables
With the purpose of prospectively studying the association between serum urate concentrations and incident hypertension, we performed two main analyses in which serum urate was the independent variable. In the first, serum urate concentration at baseline, divided in 5 categories, was the independent variable. We based these categories on sex-specific quintiles because lower serum urate levels are induced by the uricosuric effect of estrogen in pre-menopausal women.24,25 In the second analysis we used serum urate collected at years 0, 10, 15, and 20 as a linear time-dependent variable with replacement (e.g., at year 10 of follow-up serum urate at year 10 replaced serum urate from year 0 as the independent variable). Participants were asked to fast at least 12 hours and to avoid smoking and heavy physical activity before each examination. For each participant, an overnight-fasting blood sample was collected between 7am and 10am, and divided into two EDTA-containing vacuum sealed tubes. Serum and plasma samples were prepared separately, shipped (in dry ice) to each research locations and stored at −70°C until analysis (within a maximum four months after collection). At baseline, serum urate measurements were assessed using the uricase method.2 In years 10, 15, and 20, CARDIA used a modification of this method in which urate was oxidized by uricase to produce peroxide (measured at year 20 as part of the Young Adult Longitudinal Trends in Antioxidants (YALTA) ancillary study) 26. To allow full comparability of urate measures from these time points with baseline urate levels and to conform to National Institute of Standards Standard Reference Materials, serum urate levels were recalibrated based on a re-run of frozen samples. Development of hypertension, defined as a resting seated blood pressure greater or equal than a systolic of 140 millimeters of mercury or diastolic of 90 millimeters of mercury or being on medication for hypertension (including diuretics) by self-report, was the dependent variable. The values for systolic blood pressure of 140 and diastolic of 90 millimeters of mercury were based on the accepted definition for stage I hypertension .27 Participants were seated and rested for five minutes before their resting systolic and diastolic blood pressure were measured three times at 1-minute intervals using a Hawksley random zero sphygmomanometer on the right arm. The first phase (systolic) and fifth phase (diastolic) Korotkoff sounds were recorded. The average of the second and the third measurements was used in the analyses. Medications considered anti-hypertensives (besides diuretics) included methyldopa, beta-blockers, alpha-blockers, calcium channel blockers, inhibitors of the angiotensin-converting enzyme, and angiotensin receptor-blockers.
Covariates obtained at the baseline exam were included based on their potential associations with a blood-pressure and serum urate level. Age at enrollment based on self-report and race as defined by the participant (CARDIA only enrolled participants that identified themselves as Whites or African-Americans, race was considered because of its association with the outcome and the exposure variable) were considered relevant. Body mass index (BMI in kilograms per squared meter) was calculated during clinic visits in which participants wore light clothing and no shoes when measured for height (in meters, to the nearest 0.5 centimeter) using a vertical ruler and weight (kilograms, to the nearest 0.2 kilogram) using a balance beam scale. Glomerular filtration rates (in milliliters per minute per 1.73 squared meters) were estimated using the modification of diet in renal disease (MDRD) equation (based on creatinine measurements done by the nephelometry method and calibrated to national standards as described in previous CARDIA publications)28,29. Total alcohol intake in milliliters per day was estimated from the recorded average frequency of wine, beer, and liquor intake. Current smoking status (yes/no) was assessed by direct interview. Serum triglyceride levels, serum high density (HDL) and low density (LDL) lipoprotein levels were performed in plasma through enzymatic and dextran-sulfate/magnesium chloride precipitation methods and reported in milligrams per deciliter. Serum insulin levels were measured by radioimmunoassay in micro units per milliliter. Physical activity scores were estimated from questionnaires in validated exercise units30. Education level (less than high school, high school completed, college and graduate) was directly assessed through sociodemographic questionnaires. Center of subject recruitment was also included as a covariate.
Statistical analysis
Survival analyses were performed in order to examine the bivariate association between baseline serum urate categories and the development of incident hypertension at years 2, 5, 7, 10, 15, and 20 of follow-up. This was followed by a Cox-proportional hazard analysis in which the hazard of developing incident hypertension was estimated per quintile of serum urate compared with the referent quintile. In order to estimate the risk associated with higher values of serum urate as a continuous value, we repeated this analysis with baseline serum urate and time-dependent serum urate (with replacement with most current serum urate value as the independent variable, covariate values were not replaced). For the purpose of this analysis hypertension onset was assigned at the time it was first known to CARDIA investigators. Participants developing hypertension were excluded from further contribution to the longitudinal analysis. Given the small proportion of participants with missing values for any covariate (< 2%), imputation techniques were not considered and instead individuals with missing data were excluded from the longitudinal analysis. Statistical model's goodness of fit was tested through graphical methods using log-logistic and log-normal models. Proportional hazard assumptions were tested by evaluating the significance of time-dependent covariates, and if violations were identified, models were stratified on those variables and compared with the original model. Differences in risk were considered significant at an alpha level of 0.05. SAS 9.2 (SAS Inc., Cary, North Carolina) was used for all statistical analyses.
RESULTS
Patient population
Of the 5115 CARDIA participants at baseline, 66 were excluded from analyses because they did not have serum urate levels measured at baseline, 181 were excluded because they did not contribute any follow-up after their initial enrollment visit, and 116 because they met the study definition of hypertension at the baseline (Figure 1). This rendered an initial study sample of 4752 individuals (2135 men and 2617 women). The mean follow-up time per participant (until the development of hypertension or lost to follow-up) was 15.3 years for men and 15.9 years for women with 2788 participants contributing 20 years of follow up. Characteristics of the CARDIA population at the baseline examination are presented in Tables 1 (men) and 2 (women). In both men and women there were no significant age differences across serum urate quintiles. Both sexes had higher proportions of African-Americans in the lower serum urate quintiles. Participants in the higher serum urate quintiles had higher average BMI, lower average glomerular filtration rates, higher average LDL cholesterol and triglycerides, and lower average HDL cholesterol. Higher proportions of smokers were present among women in the higher serum urate quintiles, with an opposite trend among men. Physical activity scores did not show significant variation across quintiles and were was not included in subsequent multivariable analysis. There were a greater proportion of men with fewer years of completed education in the higher serum urate quintiles. A total of 70 participants did not contribute any information to the multivariable analysis because of incomplete covariate data (14 African-American men, 22 White men, 18 African-American women, and 16 White women).
Figure 1.
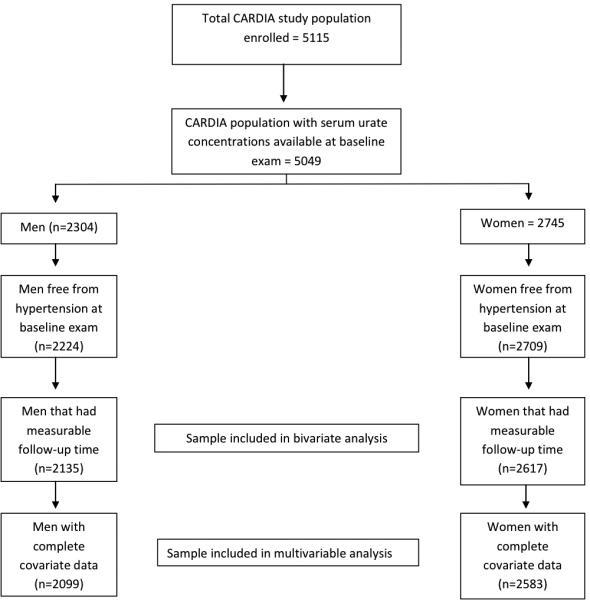
CARDIA study population and exclusion criteria.
Table 1.
Distribution of variables by quintiles of serum urate level (μmol/L*) at study baseline in men
| Variable† | Quintile 1 < 309 n=391 | Quintile 2 309-344 n=409 | Quintile 3 345-380 n=451 | Quintile 4 381-415 n=428 | Quintile 5 ≥416 n=456 | p‡ |
|---|---|---|---|---|---|---|
| Age | 24.8 (3.7) | 24.7 (3.6) | 24.8 (3.6) | 24.8 (3.7) | 24.9 (3.5) | 0.93 |
| Race§ | 62.4 | 51.8 | 41.7 | 41.1 | 45.8 | <0.0001 |
| Body mass index∥ | 22.8 (2.8) | 23.6 (3.2) | 24.0 (3.4) | 24.6 (3.6) | 26.4 (4.8) | <0.0001 |
| Glomerular filtration rate¶ | 100.4 (17.9) | 94.6 (15.4) | 91.8 (18.0) | 89.9 (15.3) | 88.0 (16.5) | <0.0001 |
| Alcohol intake** | 14.6 (22.8) | 14.6 (23.3) | 17.2 (26.8) | 20.0 (29.0) | 22.8 (31.8) | <0.0001 |
| Physical activity score†† | 510 (320) | 512 (302) | 516 (318) | 551 (339) | 506 (314) | 0.22 |
| Insulin level‡‡ | 60 (37) | 62 (41) | 67 (42) | 69 (42) | 95 (74) | <0.0001 |
| Low density lipoprotein§§ | 2.67 (0.78) | 2.81 (0.82) | 2.80 (0.78) | 2.90 (0.79) | 2.96 (0.89) | <0.0001 |
| High density lipoprotein§§ | 1.38 (0.33) | 1.32 (0.29) | 1.31 (0.33) | 1.25 (0.30) | 1.22 (0.35) | <0.0001 |
| Triglycerides§§ ∥∥ | 0.64 (0.47-0.84) | 0.68 (0.51-0.89) | 0.73 (0.55-0.99) | 0.79 (0.56-1.05) | 0.94 (0.63-1.35) | <0.0001 |
| Current smoking | 42.5 | 34.2 | 32.4 | 31.5 | 34.3 | 0.009 |
| Education completed | <0.0001 | |||||
| • Less than high school | 15.6 | 13.0 | 11.3 | 8.6 | 10.1 | |
| • High school complete | 36.1 | 30.6 | 25.5 | 28.3 | 27.4 | |
| • College or higher | 48.3 | 56.5 | 63.2 | 63.1 | 62.5 |
To convert micromoles per liter to milligram per deciliter divide by 59.48
Mean (standard deviation) for continuous variables unless otherwise specified. Categorical variables reported as (%)
Differences tested by analysis of variance for continuous variables and chi-square test for categorical variables
Percentage of African-Americans
In kilograms per squared meter (m2)
In mililiters/minute/1.73 m2 calculated using the MDRD equation
In milliliters per day
In exercise units as validated in reference (30)
In picomoles per liter
In milimoles per liter
Median and interquartile range reported given non-normal distribution
Table 2.
Distribution of variables by quintiles of serum urate level (μmol/L) at study baseline in women
| Variable† | Quintile 1 < 214 n=434 | Quintile 2 214-249 n=610 | Quintile 3 250-273 n=476 | Quintile 4 274-314 n=568 | Quintile 5 ≥315 n=529 | p‡ |
|---|---|---|---|---|---|---|
| Age | 24.8 (3.7) | 24.9 (3.7) | 24.8 (3.8) | 25.1 (3.6) | 24.9 (3.7) | 0.67 |
| Race§ | 60.8 | 54.6 | 48.5 | 47.9 | 47.5 | <0.0001 |
| Body mass index∥ | 22.7 (4.0) | 23.2 (4.4) | 23.6 (4.9) | 25.4 (6.1) | 27.1 (6.9) | <0.0001 |
| Glomerular filtration rate¶ | 96.9 (21.9) | 92.4 (18.9) | 89.6 (18.7) | 88.8 (18.1) | 85.0 (17.7) | <0.0001 |
| Alcohol intake** | 4.8 (8.7) | 5.2 (9.9) | 6.5 (11.6) | 8.3 (16.5) | 9.6 (19.4) | <0.0001 |
| Physical activity score†† | 331 (261) | 324 (237) | 343 (265) | 352 (240) | 337 (258) | 0.38 |
| Insulin level‡‡ | 72 (51) | 75 (48) | 72 (42) | 85 (57) | 103 (85) | <0.0001 |
| Low density lipoprotein§§ | 2.78 (0.77) | 2.73 (0.82) | 2.76 (0.75) | 2.82 (0.79) | 2.90 (0.82) | <0.0001 |
| High density lipoprotein§§ | 1.51 (0.31) | 1.48 (0.33) | 1.44 (0.32) | 1.41 (0.33) | 1.37 (0.35) | <0.0001 |
| Triglycerides§§ ∥∥ | 0.59 (0.45-0.78) | 0.60 (0.46-0.81) | 0.67 (0.50-0.85) | 0.69 (0.51-0.91) | 0.76 (0.58-1.08) | <0.0001 |
| Current smoking | 28.9 | 25.9 | 30.3 | 29.2 | 37.4 | 0.008 |
| Education completed | 0.59 | |||||
| • Less than high school | 6.7 | 8.2 | 9.7 | 7.4 | 7.9 | |
| • High school complete | 30.0 | 27.9 | 28.8 | 26.9 | 31.4 | |
| • College or higher | 63.4 | 63.9 | 61.6 | 65.7 | 60.7 |
* To convert micromoles per liter to milligram per deciliter divide by 59.48
Mean (standard deviation) for continuous variables unless otherwise specified. Categorical variables reported as (%)
Differences tested by analysis of variance for continuous variables and chi-square test for categorical variables
Percentage of African-Americans
In kilograms per squared meter (m2)
In mililiters/minute/1.73 m2 calculated using the MDRD equation
In milliliters per day
In exercise units as validated in reference (30)
In picomoles per liter
In milimoles per liter
Median and interquartile range reported given non-normal distribution
Bivariate analyses
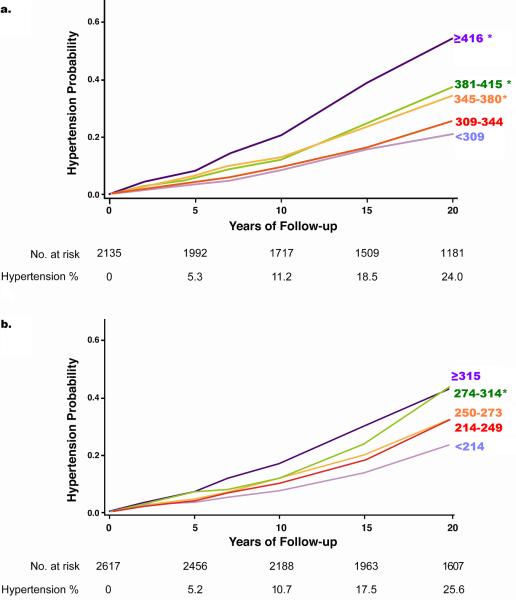
Results from bivariate analyses are shown in Figure 2. The cumulative incidence of hypertension after 20 years of follow-up was 24.0% for men and 25.6% for women. The proportions of men and women classified as hypertensive based solely on medication use increased with follow-up time on both men and women. For men this proportion increased from 29% at year 5, to 30% at year 10, and 69% at year 20. For women this proportion was of 56% at year 5, 50% at year 10, and 67% at year 20.
Figure 2.
Negative logarithmic survival plots on the bivariate association between serum urate quintiles (in micromoles per liter) at baseline and risk of developing hypertension in a) men and b) women. Hypertension % is the cumulative incidence of hypertension. * Significantly different from the referent category (p<0.05) by log-rank test adjusted for multiple comparisons
For both men and women there were significant associations between the baseline serum urate quintile and the risk of developing hypertension. Significant differences in hypertension incidence started at the third quintile in men (≥ 345 μmol/L or 5.80 mg/dL) and fourth quintile in women (≥ 274 μmol/L or 4.60 mg/dL) compared with the referent value. Log-logistic and log-normal model analyses for both men and women indicated that the hazards trended to increase with time.
Multivariable analyses
Both men and women had a significantly greater hazard of developing hypertension with higher concentrations of serum urate compared with the lowest quintile of serum urate at baseline, as shown in Table 3. For men, we noted significant differences starting at the third quintile (≥ 345 μmol/L or 5.80 mg/dL), without an inflection point at 404 μmol/L (6.80 mg/dL.) For women, significant differences were noted starting at the second quintile (≥ 214 μmol/L or 3.60 mg/dL). However, the associations were weaker than for men.
Table 3.
Multivariable Cox-proportional hazard analysis of the association of baseline serum urate quintiles (Q) in men (n=2099) and women (n=2583) with incident hypertension over 20 years of follow-up*†
| Men | Women | ||||
|---|---|---|---|---|---|
| Serum urate quintile (μmol/L) | Hypertension incidence (events/1000 person-years) | Hazard ratio (95% CI) | Serum urate quintile (μmol/L) | Hypertension incidence (events/1000 person-years) | Hazard ratio (95% CI) |
| Q1: < 309 (n=386) | 9.8 | 1.00 (Referent) | Q1: < 214 (n=426) | 11.0 | 1.00 (Referent) |
| Q2: 309-344 (n=405) | 11.7 | 1.27 (0.90-1.80) | Q2: 214-249 (n=602) | 14.5 | 1.33 (1.00-1.76) |
| Q3: 345-380 (n=439) | 16.7 | 1.86 (1.34-2.57) | Q3: 250-273 (n=469) | 14.9 | 1.38 (1.03-1.85) |
| Q4: 381-415 (n=422) | 15.4 | 1.67 (1.19-2.34) | Q4: 274-314 (n=562) | 19.2 | 1.54 (1.17-2.04) |
| Q5: ≥416 (n=447) | 24.3 | 2.05 (1.47-2.84) | Q5: ≥315 (n=524) | 20.1 | 1.37 (1.02-1.83) |
CI= confidence interval, mg/dL = micromoles per liter, to convert micromoles per liter to milligram per deciliter divide by 59.48
Linear hazard ratio across serum urate quintiles for men is 1.18 (95% CI 1.10-1.26) with a p for trend of <0.0001. For women, the linear hazard ratio across serum urate quintiles is 1.07 (95% CI 1.01-1.14), and a p for trend of 0.03.
Adjusted for baseline age, race, body-mass index, glomerular filtration rate, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum triglyceride level, serum insulin level, daily alcohol intake, smoking status, education achieved, and center of recruitment.
The analysis of the linear trend with continuous serum urate at baseline as the independent variable showed a significant 25% increase in the hazard of developing hypertension in men (HR 1.25 [95% CI 1.15-1.36]) but without a significant increase in women (HR 1.06 [95%CI 0.97-1.16]). In men, the time-dependent serum urate association (with replacement with the most current serum urate value) with the hazard of developing hypertension showed that each mg/dL greater serum urate concentration during follow-up was associated with a 12% greater hazard (HR 1.12 [95% CI 1.02-1.20]) of incident hypertension. In women, the associated hazard was 7% (HR 1.07 [95% CI 1.01-1.14]). Models utilizing serum creatinine instead of GFR estimates (because of risk of overadjusting for age, sex, and race/ethnicity) did not show meaningful differences from the ones presented (data not shown). In addition, models omitting covariates that showed evidence of collinearity did not show differences in the final estimates (data not shown). The proportional hazards assumption was satisfied in the men's analysis and required stratification of two variables (race/ethnicity and center of recruitment) in women. Stratification by race/ethnicity in women showed minimal variability when compared to the main analysis while stratification by center of recruitment in women showed significant variability between sites (Appendix tables 1a and 1b).
DISCUSSION
We found a significant association between higher serum urate concentrations and an increased hazard of incident hypertension in a prospective cohort of young adults. This hazard increase was more pronounced for men than for women, possibly a reflection of higher absolute urate levels in men. While our results are supportive of prior reports describing a positive association between urate levels and incident hypertension in older adults, 1,5,6,8,11,17,31-33 there are several important novel aspects to our study. In our study of younger adults of both Caucasian and African American race, several of the categories we constructed based on serum urate quintiles were below the accepted threshold for hyperuricemia (404 μmol/L or 6.8 mg/dL). Even in these lower quintiles we found significant associations between most serum urate levels and a greater hazard for incident hypertension. The association with hypertension was linear for men and did not demonstrate an inflection point at the currently accepted hyperuricemia thresholds. In women, we found a non-linear association, as indicated by the non-significant test for trend.
There is evidence that chronic hypertension causes anatomical changes leading to an increase in the wall/lumen ratio of the arteries 34,35, a phenomenon expected to be more common in older adults and less associated with serum urate levels. As a consequence, we expected that the association between serum urate levels and incident hypertension would be stronger in a population of young adults in which chronic vascular remodeling induced by hypertension is less likely to have occurred. It is difficult to compare the magnitude of the hazard increase in our study to those in previous studies in older adults because of the variations in hyperuricemia definitions, differences in methodology, and overall different ranges of hyperuricemia values studied. However, several studies report linear hazard ratios that are comparable to ours. For example, the MRFIT and Beaver Dam studies approach our study design by being based on large populations in the US, recruitment at similar dates, having a long follow-up period, studying the association between urate levels and incident hypertension with a similar methodology, and having comparable widths in the serum urate categories constructed. In the MRFIT study population (mean age 45 years at enrollment and all men), the HR for hypertension associated with each milligram per deciliter increased among men (time-dependent covariates) was 1.02 (95% CI 1.01-1.04). 5 In the Beaver Dam study population (57-60 years of age) the pooled reported linear hazard ratio per each higher mg/dL at baseline was 1.31 (95% CI 1.14-1.51).32 Our reported hazards are larger than those in the MRFIT study but smaller than in the Beaver Dam study. The analysis based on the MRFIT cohort did not include individuals with metabolic syndrome, which could have contributed to smaller estimates since subjects with metabolic syndrome have higher serum urate levels. The Beaver Dam study included a highly uniform population of Caucasians, limiting the generalizability of the findings. Although we consider the hypothesis that hypertension in adolescents and young adults has a stronger association with serum urate than hypertension at older ages to be compelling, 36 we cannot support that concept from our data alone.
We found that serum urate was associated with increases in the risk for hypertension at values below the traditional definition of hyperuricemia in both men and women. Our data supports the hypothesis that the hyperuricemia threshold of 6.8 mg/dl, which is well-accepted for the development of gout ,20 may not be so for putative cardiovascular associations which may begin at lower serum urate levels. Other studies in adults have also described an increase in the risk of developing cardiovascular events associated with increases in serum urate even at concentrations below the solubility threshold. 37,38 This finding relates to animal models describing the induction of hypertension by urate through endothelial dysfunction and oxidative stress, instead of arterial deposition of monosodium urate (the latter occurring in humans at serum concentrations exceeding 404 μmol/L or 6.8 mg/dl).39
Our study was conducted in a unique, well-established prospective cohort that was recruited at a young age, with a good retention rate, and a long follow-up of 20 years. There were comparable proportions of African-Americans and White men and women. Serum urate and creatinine levels were recalibrated across time points in order to adjust to national reference standards and blood pressure measurements were carefully obtained according to recognized standards. The careful collection of information about medication use allowed for a more inclusive definition of the outcome event (hypertension defined as blood pressure or being on hypertension medications) to minimize missed hypertension events or those in which the blood pressure was rendered normal during the evaluation by the use of an anti-hypertensive.. Although the use of medications as a surrogate for incident hypertension (when used for non-hypertension indications) could lead to misclassification, given that these medications could be used for indications other than hypertension, we believe that any resulting misclassification would likely be non-differential and bias our results towards the null. The assessment of covariates was thorough and included elements of the metabolic syndrome.
Despite these strengths, limitations of our study include the inability to construct categories of serum urate with significant numbers of women above the typical solubility threshold of hyperuricemia. This was inevitable given the young age of this population at the time of recruitment, even with our relatively large sample size. The low values in serum urate categories also could have led to less consistent findings of an association between serum urate levels and incident hypertension among women. The CARDIA cohort did not specifically address the issue of secondary hypertension and it was not possible to separate persons with alternate hypertension etiologies from the primary (essential) hypertension cases that were the main objective of this parent study. Secondary hypertension cases should be a minority of all hypertension events and, given that they usually present before age 30 and our results show no differences by that age (Figure 2), a small number of secondary hypertension cases is unlikely to have affected our main outcome. In addition, it is possible that despite the careful study methodology, incident hypertension episodes could have been missed based on the discrete nature of the CARDIA interim evaluations. These missed hypertension episodes are expected to be equally distributed among all serum urate categories and not affect the final estimates. Finally, and despite careful covariate adjustment, we cannot completely rule out residual confounding as a complete or partial explanation for the associations we detected.
In conclusion, we describe significant positive associations between increasing concentrations of serum urate in men and women and the development of incident hypertension in young adults. Our findings are in concordance with the growing body of data that supports a causal role for elevated serum urate in the development of hypertension. We consider these results to be especially relevant since young adults are an understudied population with respect to the association of serum urate and hypertension, and should a causal relationship be established, these findings could lead to interventions that could lower serum urate at younger ages to prevent later hypertension. In addition, if a true causal association between serum urate and hypertension starts below the traditional threshold for hyperuricemia, this could lead to a shift in the way serum urate levels and urate-lowering therapy are used in clinical practice. Confirmation of our compelling observational data with interventional studies is necessary before advocating pharmacological serum urate lowering as a novel therapeutic approach for hypertension in younger adults. Non-pharmacological approaches to lower or prevent further increases in serum urate, such as maintenance of a healthy weight or weight loss and moderation in the intake of meat, seafood, and beer40,41 could be advocated in young adults found to be hyperuricemic and at risk for incident hypertension.
Supplementary Material
ACKNOWLEDGEMENTS
FUNDING
Work on this manuscript was supported (or partially supported) by contracts from the University of Alabama at Birmingham, Coordinating Center, N01-HC-95095; University of Alabama at Birmingham, Field Center, N01-HC-48047; University of Minnesota, Field Center and Diet Reading Center (Year 20 Exam), N01-HC-48048; Northwestern University, Field Center, N01-HC-48049; Kaiser Foundation Research Institute, N01-HC-48050; N01-HC-05187; Wake Forest University (Year 20 Exam), N01-HC-45205; New England Medical Center (Year 20 Exam), N01-HC-45204 from the National Heart, Lung and Blood Institute.
Footnotes
Authorship contributions:
Angelo L Gaffo: Conception and design, analysis and interpretation of data, drafting of the manuscript, statistical analysis
David R Jacobs: Conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis
Femke Sijtsma: Conception and design, analysis and interpretation of data, drafting of the manuscript
Cora E Lewis: Acquisition of data, analysis and interpretation of data, drafting of the manuscript
Ted R Mikuls: Analysis and interpretation of data, drafting of the manuscript
Kenneth G Saag: Conception and design, analysis and interpretation of data, drafting of the manuscript
COMPETING INTEREST
Angelo L Gaffo, David R Jacobs, Femke Sijstma, Cora Elizabeth Lewis, and Ted R Mikuls report no conflict of interest. Kenneth G Saag discloses having received research funding from Takeda Pharmaceuticals and being a consultant for Ardea Pharmaceuticals, URL Pharma, and Novartis.
REFERENCES
- 1.Grayson PC, Kim SY, Lavalley M, Choi HK. Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63(1):102–10. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyer AR, Liu K, Walsh M, Kiefe C, Jacobs DR, Jr., Bild DE. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13(1):13–21. doi: 10.1038/sj.jhh.1000740. [DOI] [PubMed] [Google Scholar]
- 3.Hunt SC, Stephenson SH, Hopkins PN, Williams RR. Predictors of an increased risk of future hypertension in Utah. A screening analysis. Hypertension. 1991;17(6 Pt 2):969–76. doi: 10.1161/01.hyp.17.6.969. [DOI] [PubMed] [Google Scholar]
- 4.Jossa F, Farinaro E, Panico S, Krogh V, Celentano E, Galasso R, et al. Serum uric acid and hypertension: the Olivetti heart study. J Hum Hypertens. 1994;8(9):677–81. [PubMed] [Google Scholar]
- 5.Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49(2):298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 6.Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension. 2006;48(6):1037–42. doi: 10.1161/01.HYP.0000249768.26560.66. [DOI] [PubMed] [Google Scholar]
- 7.Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. 2004;27(11):835–41. doi: 10.1291/hypres.27.835. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18(6):523–30. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 9.Panoulas VF, Douglas KM, Milionis HJ, Nightingale P, Kita MD, Klocke R, et al. Serum uric acid is independently associated with hypertension in patients with rheumatoid arthritis. J Hum Hypertens. 2008;22(3):177–82. doi: 10.1038/sj.jhh.1002298. [DOI] [PubMed] [Google Scholar]
- 10.Perlstein TS, Gumieniak O, Williams GH, Sparrow D, Vokonas PS, Gaziano M, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031–6. doi: 10.1161/01.HYP.0000248752.08807.4c. [DOI] [PubMed] [Google Scholar]
- 11.Selby JV, Friedman GD, Quesenberry CP., Jr. Precursors of essential hypertension: pulmonary function, heart rate, uric acid, serum cholesterol, and other serum chemistries. Am J Epidemiol. 1990;131(6):1017–27. doi: 10.1093/oxfordjournals.aje.a115593. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Lozada LG, Tapia E, Bautista-Garcia P, Soto V, Avila-Casado C, Vega-Campos IP, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2008;294(4):F710–8. doi: 10.1152/ajprenal.00454.2007. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Lozada LG, Tapia E, Soto V, Avila-Casado C, Franco M, Wessale JL, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 2008;108(4):p69–78. doi: 10.1159/000127837. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Lozada LG, Tapia E, Soto V, Avila-Casado C, Franco M, Zhao L, et al. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrol Dial Transplant. 2008;23(4):1179–85. doi: 10.1093/ndt/gfm783. [DOI] [PubMed] [Google Scholar]
- 15.Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol. 2007;18(1):287–92. doi: 10.1681/ASN.2006080865. [DOI] [PubMed] [Google Scholar]
- 16.Lu Z, Dong B, Wu H, Chen T, Zhang Y, Wu J, et al. Serum uric acid level in primary hypertension among Chinese nonagenarians/centenarians. J Hum Hypertens. 2009;23(2):113–21. doi: 10.1038/jhh.2008.104. [DOI] [PubMed] [Google Scholar]
- 17.Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45(1):28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 18.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282(6):F991–7. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 19.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972;15(2):189–92. doi: 10.1002/art.1780150209. [DOI] [PubMed] [Google Scholar]
- 21.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr., et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 22.Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, Hughes GH, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12(1 Suppl):1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 23.Stamp L, Ha L, Searle M, O'Donnell J, Frampton C, Chapman P. Gout in renal transplant recipients. Nephrology (Carlton) 2006;11(4):367–71. doi: 10.1111/j.1440-1797.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 24.Adamopoulos D, Vlassopoulos C, Seitanides B, Contoyiannis P, Vassilopoulos P. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol (Copenh) 1977;85(1):198–208. doi: 10.1530/acta.0.0850198. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. 1973;1(5851):449–51. doi: 10.1136/bmj.1.5851.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hozawa A, Jacobs DR, Jr., Steffes MW, Gross MD, Steffen LM, Lee DH. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem. 2007;53(3):447–55. doi: 10.1373/clinchem.2006.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs DR, Jr., Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155(12):1114–9. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Sidney S, Jacobs DR, Jr., Haskell WL, Armstrong MA, Dimicco A, Oberman A, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133(12):1231–45. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 31.Imazu M, Yamamoto H, Toyofuku M, Sumii K, Okubo M, Egusa G, et al. Hyperinsulinemia for the development of hypertension: data from the Hawaii-Los Angeles-Hiroshima Study. Hypertens Res. 2001;24(5):531–6. doi: 10.1291/hypres.24.531. [DOI] [PubMed] [Google Scholar]
- 32.Shankar A, Klein R, Klein BE, Nieto FJ. The association between serum uric acid level and long-term incidence of hypertension: Population-based cohort study. J Hum Hypertens. 2006;20(12):937–45. doi: 10.1038/sj.jhh.1002095. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi Y, Hayashi T, Tsumura K, Endo G, Fujii S, Okada K. Serum uric acid and the risk for hypertension and Type 2 diabetes in Japanese men: The Osaka Health Survey. J Hypertens. 2001;19(7):1209–15. doi: 10.1097/00004872-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Izzard AS, Rizzoni D, Agabiti-Rosei E, Heagerty AM. Small artery structure and hypertension: adaptive changes and target organ damage. J Hypertens. 2005;23(2):247–50. doi: 10.1097/00004872-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Prewitt RL, Rice DC, Dobrian AD. Adaptation of resistance arteries to increases in pressure. Microcirculation. 2002;9(4):295–304. doi: 10.1038/sj.mn.7800143. [DOI] [PubMed] [Google Scholar]
- 36.Kosugi T, Nakagawa T, Kamath D, Johnson RJ. Uric acid and hypertension: an age-related relationship? J Hum Hypertens. 2009;23(2):75–6. doi: 10.1038/jhh.2008.110. [DOI] [PubMed] [Google Scholar]
- 37.Strasak A, Ruttmann E, Brant L, Kelleher C, Klenk J, Concin H, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83,683 Austrian men. Clin Chem. 2008;54(2):273–84. doi: 10.1373/clinchem.2007.094425. [DOI] [PubMed] [Google Scholar]
- 38.Strasak AM, Kelleher CC, Brant LJ, Rapp K, Ruttmann E, Concin H, et al. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. Int J Cardiol. 2008;125(2):232–9. doi: 10.1016/j.ijcard.2007.11.094. [DOI] [PubMed] [Google Scholar]
- 39.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67(5):1739–42. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 40.Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52(1):283–9. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
- 41.Gaffo AL, Roseman JM, Jacobs DR, Jr., Lewis CE, Shikany JM, Mikuls TR, et al. Serum urate and its relationship with alcoholic beverage intake in men and women: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. Ann Rheum Dis. 2010;69(11):1965–70. doi: 10.1136/ard.2010.129429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.