Abstract
Objective
Combination antiretroviral therapy (ART) is now the global standard for HIV-infected pregnant and breastfeeding women at all CD4 cell counts. We compared the efficacy and safety of an efavirenz versus lopinavir/ritonavir regimen for HIV-infected pregnant women initiating ART in rural Uganda.
Design
Randomized clinical trial.
Methods
We performed a planned secondary analysis comparing viral load suppression (HIV-1 RNA ≤400 copies/ml), safety, and HIV transmission to infants in a trial designed to test the hypothesis that lopinavir/ritonavir- versus efavirenz-based ART would reduce placental malaria (PROMOTE, ClinicalTrials.gov, NCT00993031). HIV-infected, ART-naïve pregnant women at 12–28 weeks gestation and any CD4 cell count were randomized. ART was provided and participants were counseled to breastfeed for one year postpartum.
Results
The median age of the 389 study participants was 29 years; median CD4 cell count was 370 cells/mm3. At delivery, virologic suppression was 97.6% in the efavirenz arm and 86.0% in the lopinavir/ritonavir arm, p <0.001. At 48 weeks postpartum, 91.0% of women on efavirenz and 88.4% on lopinavir/ritonavir had viral suppression, p = 0.49. Grade 1 or 2 gastrointestinal adverse events were higher among women on lopinavir/ritonavir versus efavirenz. Only two infants acquired HIV (both in the lopinavir/ritonavir arm) and HIV-free infant survival was similar between study arms: 92.9% (lopinavir/ritonavir) versus 97.2% (efavirenz), p = 0.10.
Conclusions
Virologic suppression at delivery was higher with an efavirenz- versus lopinavir/ritonavir-based regimen. However, women in both arms achieved high levels of virologic suppression through one year postpartum and the risk of transmission to infants was low.
Keywords: Prevention of mother-to-child transmission of HIV, pregnancy, breastfeeding, efavirenz, lopinavir/ritonavir
BACKGROUND
Combination antiretroviral therapy (ART) is now the global standard for all HIV-infected pregnant and breastfeeding women per the 2013 World Health Organization (WHO) Consolidated Guidelines on antiretroviral therapy. [1] Benefits of this new policy include preservation or restoration of a woman’s health, prevention of HIV transmission during gestation and breastfeeding, and reduction of sexual transmission. [2–5] However, there are few randomized studies to guide optimal ART regimens for pregnant and breastfeeding women. [3, 5–7]
Challenges to optimizing ART for HIV-infected pregnant and breastfeeding women may include the need to modify antiretroviral (ARV) drug dosing due to alterations in drug metabolism during pregnancy, as in the case of lopinavir/ritonavir. [8] Pregnant women may also be less able to tolerate ART and may face barriers to adherence that differ from those in non-pregnant adults, increasing the risk of virologic failure and drug resistance. [9] Finally, several prior studies have raised concerns that certain ARVs may pose risks to infants, such as teratogenicity, preterm delivery, hematologic abnormalities, and adrenal insufficiency. [10–13]
The 2013 WHO guidelines recommend a single-pill, fixed-dose, efavirenz-based combination regimen for all HIV-infected pregnant and breastfeeding women, including those in the first trimester of pregnancy, regardless of CD4 cell count. [1] These guidelines harmonize first-line ART recommendations with those for non-pregnant adults. Protease inhibitors are considered second-line by the WHO and remain a preferred option for pregnant women in higher income countries such as the United States. [14] We report here on the first randomized comparison of lopinavir/ritonavir- versus efavirenz-based ART in HIV-infected pregnant and breastfeeding women.
METHODS
Study Population and Design
This was a planned secondary analysis of the efficacy and safety of lopinavir/ritonavir- versus efavirenz-based ART in pregnant and breastfeeding women in the PROMOTE-Pregnant Women and Infants (PIs) study (ClinicalTrials.gov, NCT00993031). This open-label, single-site, randomized study was designed to test the hypothesis that lopinavir/ritonavir would reduce placental malaria. It was conducted from December 2009 to March 2013 in Tororo, a municipality in rural eastern Uganda. The primary study endpoint, placental malaria, did not differ between the groups, as previously reported. [15]
Women were recruited from the Tororo District Hospital antenatal clinic and HIV testing service, the AIDS Support Organization (TASO, an HIV clinic in Tororo), and other health centers in the area. Inclusion criteria were age ≥16 years, confirmed HIV-1 infection, residence within 30 kilometers of the study site, and pregnancy at 12–28 weeks gestation by last menstrual period with ultrasound confirmation. Women were eligible for enrollment at any CD4 cell count. Exclusion criteria included any prior use of ART or exposure to single-dose nevirapine or abbreviated ARV monotherapy or dual therapy within 24 months before enrollment, dose-limited toxicity to trimethoprim-sulfamethoxazole (TS) within 14 days, active tuberculosis or other WHO Stage 4 disease, cardiac disease, or abnormal screening laboratory values (ClinicalTrials.gov, NCT00993031).
All participants provided written informed consent in their preferred language. The study protocol was approved by the Makerere University School of Medicine Research and Ethics Committee, the Uganda National Council of Science and Technology, and the University of California, San Francisco Committee on Human Research.
Study Procedures
Participants were randomized in a 1:1 ratio to efavirenz- or lopinavir/ritonavir-based ART after stratification by gravidity (gravida 1 versus gravida ≥2) and gestational age at enrollment (<24 weeks versus ≥24 weeks). Randomization was performed in permuted blocks of 2 or 4. The dosing of study drugs was as follows: efavirenz 600 mg once daily; lopinavir/ritonavir 200 mg/50 mg, two tablets twice daily, increased to three tablets twice daily from 30 weeks gestation until delivery, then reduced to two tablets twice daily. Women in both arms received lamivudine/zidovudine 150 mg/300 mg twice daily. Tenofovir was used in cases of zidovudine intolerance. AbbVie Pharmaceuticals (North Chicago, Illinois) provided lopinavir/ritonavir (Aluvia) but had no other role in study design, data accrual and analysis, or manuscript preparation. All women received daily TS prophylaxis and a long-lasting insecticide-treated bednet.
Women received antenatal care per Uganda Ministry of Health (MOH) guidelines. In addition, women returned to the study clinic every 4 weeks for scheduled visits and medications, as well as for any health conditions requiring evaluation. CD4 cell counts were measured at screening, 12 and 24 weeks after enrollment, delivery, and 24 and 48 weeks postpartum. HIV-1 RNA PCR was tested at screening, 8 weeks after ART initiation, delivery, and 8, 24, and 48 weeks postpartum. HIV-1 RNA PCR testing was performed via COBAS AMPLICOR Version 1.5 (Roche Molecular Diagnostics, Pleasanton, CA) until September 2012 and thereafter with the m2000 RealTime HIV-1 assay (Abbott Laboratories, Abbott Park, IL). Small hair samples were collected from a subset of women at 30 weeks gestation, delivery, and 12 weeks postpartum to analyze ARV concentrations in hair via liquid chromatography/tandem mass spectrometry. [16, 17]
Women were counseled to breastfeed until one year postpartum, with exclusive breastfeeding for the first 6 months. All infants received HIV prophylaxis after delivery with zidovudine for 7 days until November 2010, when infants began receiving nevirapine for 6 weeks due to a change in MOH guidelines. Infants received daily TS from 6 weeks of life through 6 weeks after weaning. HIV-1 DNA PCR testing (COBAS AMPLICOR Version 1.5, Roche Molecular Diagnostics, Pleasanton, CA) was performed at birth, 24 weeks, and 6 weeks after weaning; infection was confirmed with a second positive PCR test. At study completion, coordinators facilitated linkage of women to HIV care at local clinics for continuation of ART.
Outcomes
Virologic suppression was defined as plasma HIV-1 RNA ≤400 copies/ml based on the lower limit of detection of the available test. Virologic failure was defined as two consecutive non-suppressed HIV-1 RNA values after achieving virologic suppression. Immunologic efficacy was assessed by mean change in CD4 cell count. ART adherence was determined by self-reported recall in the three days prior to each study visit. Adverse events were classified according to the Division of AIDS grading table. [18] Clinical progression of HIV was categorized according to 2007 WHO criteria. [19]
Statistical Analysis
The sample size of the study was driven by the primary malaria endpoint. [15] We performed a planned non-inferiority analysis of virologic suppression at delivery and 24 weeks after ART initiation. We used a two-sample comparison of difference in proportions with a non-inferiority margin of 11% with an 80% or greater power and 5% type-I error rate. Using Fisher’s exact test, we also compared the proportion of women in each arm who achieved virologic suppression at individual time points, although the study was not specifically powered for these comparisons. Generalized estimating equations with correction for repeated measures were used for between-group comparison of the odds of virologic suppression from delivery to study end. Predictors of virologic suppression at delivery, including ART regimen and treatment duration, baseline HIV-1 RNA, weight gain, BMI, and ARV hair concentrations, were evaluated using logistic regression. With the exception of delivery, a 4-week measurement window was used for assessing virologic and immunologic outcomes. Virologic failure was assessed using Cox proportional hazards. Kaplan-Meier survival estimates were used to calculate HIV-free infant survival (absence of confirmed HIV infection or death) among live-born infants with censoring at 58 weeks or last study visit. The log-rank test was used to compare differences in survival between treatment arms. Between-group comparisons of baseline characteristics and adverse events were tested using Pearson’s chi-squared test, Fisher’s exact test, or the Wilcoxon two-sample test. All statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Study Participants
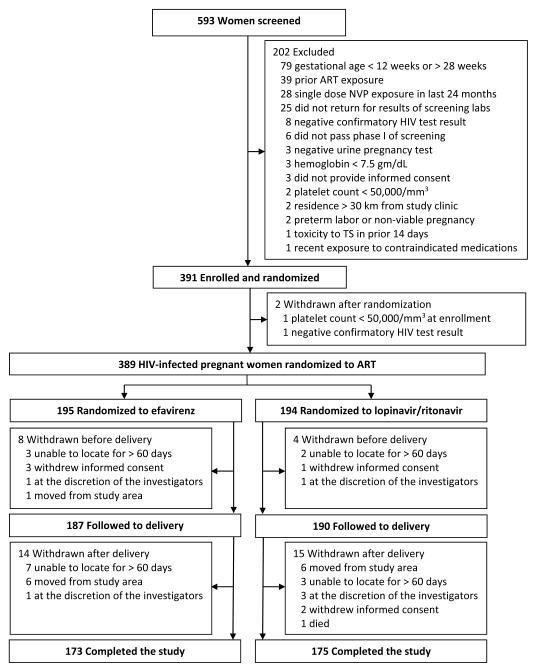
Of 593 women screened for the study, a total of 391 patients were enrolled (Figure 1). The majority of exclusions resulted from women presenting outside the criteria for gestational age and having a history of ARV exposure. Prior to delivery, women received ART for a mean duration of 17.5 weeks (SD 4.9) in the efavirenz arm and 17.1 weeks (SD 5.1) in the lopinavir/ritonavir arm. 374 live-born infants were delivered. Women were followed for up to 52 weeks postpartum or until study completion in March 2013. After delivery, 14 women in the efavirenz arm and 15 women in the lopinavir/ritonavir arm were withdrawn from the study. A total of 173 women in the efavirenz arm and 175 women in the lopinavir/ritonavir arm completed the study, of whom 124 women in the efavirenz arm and 122 women in the lopinavir/ritonavir arm were followed for at least 1 year after delivery. Forty-nine women in the efavirenz arm and 53 women in the lopinavir/ritonavir arm were followed for less than 1 year postpartum because the study was completed in March 2013. Demographic and clinical characteristics were similar between the arms, including baseline median CD4 cell count, median log10 plasma HIV-1 RNA, and WHO stage (Table 1).
Figure 1.
Screening, randomization, and follow-up of study patients
ART, antiretroviral therapy; NVP, nevirapine; TS, trimethoprim-sulfamethoxazole.
Table 1.
Characteristics of pregnant women at enrollment
| Characteristic | Efavirenz (N = 195) | Lopinavir/ritonavir (N = 194) |
|---|---|---|
| Maternal age, years, mean (SD) | 29.5 (5.4) | 29.0 (5.4) |
| Gestational age, weeks, median (IQR) | 21.3 (17.9, 24.4) | 21.2 (17.6, 25.0) |
| Education level, n (%) | ||
| None | 24 (12.3) | 24 (12.4) |
| Primary | 125 (64.1) | 135 (70.0) |
| More than primary | 46 (23.6) | 34 (17.6) |
| Number of previous pregnancies, n (%) | ||
| None | 16 (8.2) | 8 (4.1) |
| 1–2 | 50 (25.6) | 53 (27.3) |
| 3 or more | 129 (66.2) | 133 (68.6) |
| Number of living children, n (%) | ||
| None | 22 (11.3) | 16 (8.3) |
| 1–2 | 65 (33.3) | 67 (34.5) |
| 3 or more | 108 (55.4) | 111 (57.2) |
| HIV diagnosed in current pregnancy, n (%) | 87 (44.6) | 73 (37.6) |
| Body mass index, kg/m2, mean (SD) | 21.8 (3.1) | 22.1 (2.9) |
| Hemoglobin, g/dl, mean (SD) | 10.9 (1.3) | 11.0 (1.2) |
| CD4 cell count, cells/mm3, median (IQR) | 374 (270, 485) | 368 (282, 506) |
| HIV-1 RNA, log10 copies/ml, median (IQR) | 4.3 (3.5, 4.8) | 4.1 (3.3, 4.7) |
| WHO stage, n (%) | ||
| 1 | 181 (92.8) | 189 (97.4) |
| 2 | 13 (6.7) | 5 (2.6) |
| 3 | 1 (0.5) | 0 (0.0) |
| On TS prophylaxis prior to enrollment, n (%) | 125 (64.1) | 124 (63.9) |
NOTE. TS, trimethoprim-sulfamethoxazole
Virologic, Immunologic, and Clinical Outcomes
In a planned non-inferiority analysis, the difference in virologic suppression at delivery was 11.7% with a 95% confidence interval of 5.8% to 17.3%, which did not exclude the pre-specified non-inferiority margin of 11%. Viral suppression at delivery was significantly higher in the efavirenz arm than in the lopinavir/ritonavir arm, with a difference in magnitude of at least 5.8%. At 24 weeks, the treatment difference between the efavirenz and lopinavir/ritonavir arms was 2.8% (95% CI −3.9% to 9.4%), with lopinavir/ritonavir meeting the non-inferiority criterion.
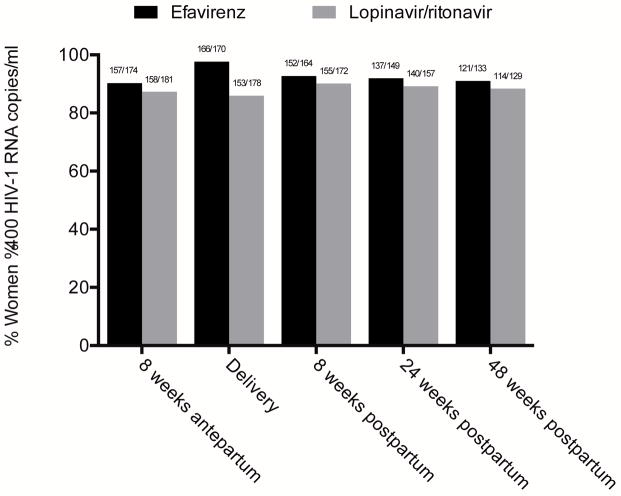
In further comparisons of virologic suppression at individual time points, at 8 weeks following ART initiation, 166 of 186 women (89.3%) in the efavirenz arm and 162 of 186 women (87.1%) in the lopinavir/ritonavir arm achieved virologic suppression, p = 0.52 (Table 2). At delivery, 166 of 170 women (97.6%) in the efavirenz arm and 153 of 178 women (86.0%) in the lopinavir/ritonavir arm demonstrated virologic suppression, p <0.001 (Figure 2). At 48 weeks postpartum, 121 of 133 women (91.0%) on efavirenz and 114 of 129 women (88.4%) on lopinavir/ritonavir had achieved virologic suppression, p = 0.49. Women in the efavirenz arm had higher odds of viral suppression from delivery to study end (odds ratio [OR] 1.98, 95% confidence interval [CI] 1.21–3.22, p = 0.006), adjusting for repeated measures. Including all 195 women in the efavirenz arm and 194 women in the lopinavir/ritonavir arm who enrolled in the study and assigning virologic non-suppression to women who withdrew or did not complete the study, more women on efavirenz were suppressed at delivery (166 [85.1%] versus 153 [78.9%] on lopinavir/ritonavir), and there remained no difference in virologic suppression at 24 and 48 weeks postpartum.
Table 2.
Proportion of women with virologic suppression (HIV-1 RNA ≤400 copies/ml)
| Efavirenz n/N (%) | Lopinavir/ritonavir n/N (%) | p | |
|---|---|---|---|
| 8 weeks on ART | 166/186 (89.3) | 162/186 (87.1) | 0.52 |
| Delivery | 166/170 (97.7) | 153/178 (86.0) | <0.001 |
| 24 weeks postpartum | 137/149 (92.0) | 140/157 (89.2) | 0.41 |
| 48 weeks postpartum | 121/133 (91.0) | 114/129 (88.4) | 0.49 |
Figure 2.
Proportion of women with virologic suppression (HIV-1 RNA ≤400 copies/ml) by pregnancy status
* p <0.001 by Fisher’s exact test
Predictors of virologic suppression at delivery included ART regimen of efavirenz versus lopinavir/ritonavir (OR 6.78, 95% CI 2.31–19.93, p <0.001) and log10 baseline HIV-1 RNA (OR 0.50, 95% CI 0.31–0.81, p = 0.005). Among a subset of women with hair samples collected for analysis, in a model adjusting for baseline HIV-1 RNA, the odds of achieving virologic suppression at delivery was 2.25 (95% CI 1.53–3.30, p <0.001) for every doubling in hair ARV concentration of efavirenz, lopinavir, or ritonavir.
Fifteen women taking lopinavir/ritonavir and 6 women taking efavirenz experienced virologic failure (hazard ratio 2.58, 95% CI 1.002–6.66, p <0.05). Among women with ≥6 subsequent months of follow-up and two subsequent HIV-1 RNA measurements, 6 of 8 in the lopinavir/ritonavir arm achieved virologic re-suppression with adherence counseling. None of the women experiencing virologic failure in the efavirenz arm had enough follow-up time to evaluate re-suppression.
Women on lopinavir/ritonavir experienced a greater mean CD4 cell count recovery than women on efavirenz at delivery (+57 versus −7 cells/mm3, p = 0.002) and at 24 weeks post-ART initiation (+178 versus +109 cells/mm3, p = 0.01). One woman in the efavirenz arm was diagnosed with Kaposi’s sarcoma and progressed from WHO stage 2 to stage 4 HIV infection. One woman on lopinavir/ritonavir was diagnosed with pulmonary tuberculosis, requiring a switch to efavirenz to minimize drug interactions with TB medications.
Adverse Events
Among pregnant and postpartum women, there were no differences between study arms in grade 3 or 4 adverse events (AEs), including anemia and neutropenia (Table 3). Significantly more women on lopinavir/ritonavir than efavirenz experienced grade 1 or 2 diarrhea and grade 1 or 2 nausea/vomiting. Three women in each arm experienced central nervous system AEs. There was no difference between arms in non-obstetrical hospitalizations. One maternal death occurred: a woman in the lopinavir/ritonavir arm died in a motor vehicle accident at 30 weeks postpartum. Among infants, there were no statistically significant differences between study arms in adverse events or hospitalizations (Table 3). There was a trend toward a higher rate of grade 3 or 4 anemia and infant death in the lopinavir/ritonavir arm. There were no differences between study arms in the numbers of stillbirths, preterm deliveries, low birth weight infants, or neonatal deaths. [15, 20]
Table 3.
Adverse events among women and live-born infants
| Efavirenz n (%) | Lopinavir/ritonavir n (%) | p | |
|---|---|---|---|
| Pregnant women | N = 195 | N = 194 | |
| Any grade 1 or 2 adverse event | 177 (90.8) | 182 (93.8) | 0.26 |
| Diarrhea | 18 (9.2) | 43 (22.2) | <0.001 |
| Nausea/vomiting | 29 (14.9) | 53 (27.3) | 0.003 |
| Any grade 3 or 4 adverse event | 12 (6.2) | 8 (4.1) | 0.36 |
| Anemia | 5 (2.6) | 3 (1.6) | 0.72 |
| Neutropenia | 3 (1.5) | 2 (1.0) | 1.00 |
| Non-obstetrical hospitalization | 7 (3.6) | 8 (4.1) | 0.78 |
| Death | 0 | 0 | N/A |
| Postpartum women | N = 187 | N = 190 | |
| Any grade 1 or 2 adverse event | 179 (95.7) | 187 (98.4) | 0.12 |
| Diarrhea | 54 (28.9) | 74 (39.0) | 0.04 |
| Nausea/vomiting | 20 (10.7) | 34 (17.9) | <0.05 |
| Any grade 3 or 4 adverse event | 32 (17.1) | 36 (19.0) | 0.64 |
| Anemia | 4 (2.1) | 6 (3.2) | 0.75 |
| Neutropenia | 24 (12.8) | 27 (14.2) | 0.70 |
| Non-obstetrical hospitalization | 5 (2.7) | 1 (0.5) | 0.12 |
| Death | 0 | 1 | 1.00 |
| Live-born infants | N = 183 | N = 191 | |
| Any grade 1 or 2 adverse event | 176 (96.2) | 179 (93.7) | 0.28 |
| Any grade 3 or 4 adverse event | 40 (21.9) | 51 (26.7) | 0.28 |
| Anemia | 19 (10.4) | 32 (16.8) | 0.07 |
| Neutropenia | 17 (9.3) | 14 (7.3) | 0.49 |
| Hospitalization | 5 (2.7) | 6 (3.1) | 0.81 |
| Death | 5 (2.7) | 12 (6.3) | 0.10 |
Breastfeeding and ART Adherence
Breastfeeding was reported until 6 months postpartum by 96.7% of women on lopinavir/ritonavir and 98.7% of women on efavirenz; 59.6% (lopinavir/ritonavir) and 60% (efavirenz) did so exclusively; 72.3% (lopinavir/ritonavir) and 73.5% (efavirenz) continued partial breastfeeding for 12 months postpartum.
Self-reported ART adherence was 99% in the lopinavir/ritonavir arm and 97.6% in the efavirenz arm during pregnancy and 99% (lopinavir/ritonavir) and 99.2% (efavirenz) during breastfeeding.
Perinatal Transmission and HIV-Free Infant Survival
Two infants were infected with HIV; both were born to mothers in the lopinavir/ritonavir arm. One infant was infected in utero. The mother’s HIV-1 RNA was 191,000 copies/ml at screening (26 weeks gestation) and ≤400 copies/ml 8 weeks after ART initiation and at delivery. The infant’s HIV-1 DNA PCR was positive at delivery and confirmed at 27 weeks. The second infant was infected during breastfeeding. The mother’s HIV-1 RNA measurements were: 253,000 copies/ml at screening (23 weeks gestation); ≤400 copies/ml at 8 weeks after ART initiation; 30,800 copies/ml at delivery; 4,270 copies/ml at 8 weeks postpartum; and ≤400 copies/ml at 24 and 48 weeks postpartum. The infant received extended nevirapine prophylaxis beyond 6 weeks and was exclusively breastfed for 28 weeks and partially breastfed until one year of age. Infant HIV-1 DNA PCR testing was negative at birth and 24 weeks of life but was positive at 58 and 61 weeks. Overall HIV-free infant survival at 58 weeks was 92.9% in the lopinavir/ritonavir arm and 97.2% in the efavirenz arm, p = 0.10.
DISCUSSION
In this randomized study conducted in rural Uganda, HIV-infected women achieved high rates of virologic suppression in both study arms through one year postpartum. Virologic suppression was higher among women treated with efavirenz than lopinavir/ritonavir at delivery and women on efavirenz had higher odds of suppression from delivery to study end. Both regimens were relatively well tolerated, although there were more grade 1 or 2 gastrointestinal adverse events among women on lopinavir/ritonavir. There were only two maternal AIDS events and one maternal death, and only two infants acquired HIV during the study period.
These results are among the first randomized data on ART virologic efficacy and safety among HIV-infected pregnant and breastfeeding women with high CD4 cell counts in resource-limited settings, a population that did not qualify for lifelong ART until the 2013 WHO guidelines. [1] Over half of the women enrolled in this study had CD4 cell counts above 350 cells/mm3. Previously, these women were routinely offered punctuated antiretroviral regimens to reduce the risk of perinatal HIV transmission. This study shows that pregnant and breastfeeding women with high CD4 cell counts can adhere to ART, tolerate it well, and can achieve high rates of virologic suppression, with women on efavirenz-based ART demonstrating higher rates of virologic suppression at delivery.
To our knowledge, only two other published trials have evaluated virologic outcomes among HIV-infected pregnant women randomized to receive different ART regimens or triple ARV prophylaxis for reduction of perinatal transmission risk. [3, 5] Both studies evaluated regimens including lopinavir/ritonavir but neither included efavirenz, a mainstay of first-line ART regimens by current guidelines. [1] A trial conducted in Botswana which randomized pregnant women at 26–34 weeks gestation to ART with a nucleoside backbone plus abacavir (triple NRTI) versus lopinavir/ritonavir showed excellent rates of virologic suppression to <400 copies/ml at delivery (96% [triple NRTI] and 93% [lopinavir/ritonavir]) and during breastfeeding up to 6 months postpartum (92% [triple NRTI] and 93% [lopinavir/ritonavir]) with transmission of HIV to 1.1% of infants. [5] Another trial conducted in Burkina Faso, Kenya, and South Africa found higher rates of virologic suppression at delivery among women randomized at 28–36 weeks gestation to lopinavir/ritonavir with a nucleoside backbone versus zidovudine plus single-dose nevirapine. [3]
Among non-pregnant adults, several randomized trials have compared the efficacy of efavirenz- to lopinavir/ritonavir-based ART. [21–23] Our results are consistent with the largest randomized trial in non-pregnant adults comparing lopinavir/ritonavir to efavirenz. [23] In that trial, at 96 weeks after ART initiation, efavirenz recipients had higher rates of virologic suppression to <200 copies/ml than lopinavir/ritonavir recipients (93% versus 86%, p = 0.04) and to <50 copies/ml (89% versus 77%, p = .003), but had a smaller median increase in CD4 cell counts (230 versus 287 cells/mm3, p = .01).
Virologic suppression may have been higher for efavirenz than lopinavir/ritonavir at delivery because of differences in adherence or drug exposure. Similar to other trials, we found more grade 1 or 2 gastrointestinal adverse events in the lopinavir/ritonavir arm, which may have adversely affected adherence. [23] To compensate for alterations in lopinavir/ritonavir metabolism during pregnancy, we used an adjusted dosing regimen; however, despite this, drug exposure may not have been optimal during the third trimester. PROMOTE enrolled many food-insecure women, for whom ARV drug exposure was reduced compared to normally nourished women. [24] Although women in both arms reported high rates of adherence, pharmacokinetic analyses from this study and others suggest that lopinavir/ritonavir exposure may be inadequate antepartum. [8, 24, 25] Similar to other studies, hair ARV concentrations were strong independent predictors of virologic suppression. [26–28]
The rate of perinatal HIV transmission (2 per 374 live-born infants) that occurred in this study is one of the lowest reported in breastfeeding women in Africa. [3, 5–7, 29, 30] These results are encouraging, showing that in rural areas, prevention of perinatal transmission can be achieved for women over a wide spectrum of CD4 cell counts. Nevertheless, concerns may persist about adverse effects among infants due to ART exposure from maternal use during gestation and breastfeeding. Recent studies in humans have not demonstrated an increased risk of birth defects with efavirenz, [31, 32] a concern that arose from neural tube defects observed among efavirenz-exposed animals and retrospective case reports in humans. Women were enrolled in PROMOTE no earlier than 12 weeks gestation, following organogenesis; thus, we cannot comment on the impact of efavirenz exposure on birth defects in this cohort. However, the low rate of grade 3 or 4 infant adverse events in both groups is reassuring.
Limitations of this study include its unblinded nature, which could have led to bias in some reporting of adverse events. Standardized evaluation schedules and toxicity scales were used to mitigate this possible effect. In terms of study generalizability, a backbone of zidovudine and lamivudine was used in the ART regimens. This combination is now being replaced with tenofovir and emtricitabine or lamivudine in many countries, per 2013 WHO guidelines. Furthermore, HIV-1 RNA monitoring, used in this study, is not yet available in many resource-limited settings and may have influenced adherence. Women in both arms of the study were monitored with HIV-1 RNA testing and received the same nucleoside backbone, thus reducing the possibility that these factors impacted comparisons between study arms. Due to subject withdrawals and study termination in March 2013, we were unable to follow all women in the study to at least 1 year postpartum. As a result, the relatively small size of this study may have limited our ability to detect differences between the treatment arms at time points other than delivery. Because of the lower limit of detection of the available HIV-1 RNA test, we are unable to comment on the presence of low-level viremia below 400 copies/ml.
In conclusion, this study of ART-naïve pregnant and breastfeeding women that included a population with high CD4 cell counts provides evidence to support current WHO guidelines that recommend efavirenz as a first-line regimen and lopinavir/ritonavir as an alternative. Both regimens were highly effective at preventing perinatal HIV transmission among women in this study who initiated efavirenz- or lopinavir/ritonavir-based ART at 12 to 28 weeks gestation. These results demonstrate that women can successfully initiate ART when they present to antenatal clinic and maintain therapy thereafter.
Acknowledgments
The authors thank the participants in the PROMOTE-Pregnant Women and Infants trial, the dedicated PROMOTE study staff, and the practitioners at Tororo District Hospital. We thank Peter Bacchetti, Ph.D. for statistical analyses involving the hair concentration data.
Footnotes
Authorship: D.C., D.V.H., E.D.C. and M.R.K. designed the study. P.N, F.L., J.M, V.A., B.N., and T.D.C. contributed significantly to the acquisition of data. D.C., P.N., C.A.K., A.P., E.D.C., M.G., M.R.K., and D.V.H. analyzed and interpreted the data. D.V.H., C.A.K., and E.D.C. authored the manuscript with input and important revisions from all authors, including D.C., P.N., A.P., F.L., J.M., V.A., M.G., T.D.C., J.A., T.R., B.N., and M.R.K.
Conflicts of Interest and Source of Funding: This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01 HD059454, D.V.H. and K23 HD60459, T.R.) and the National Institute of Allergy and Infectious Diseases (NIAID, T32 AI060530, D.V.H./C.A.K.) at the National Institutes of Health. A grant from NIAID supported the hair analyses (R01 AI098472, M.G.). AbbVie Pharmaceuticals donated lopinavir/ritonavir (Aluvia) for the parent trial. Gilead donates medications for participants in a separate NIH-funded study led by Dr. Havlir but provides no financial support.
References
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, Switzerland: 2013. [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kesho Bora Study Group. de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 4.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson DJ, Chasela CS, Hudgens MG, King CC, Kourtis AP, Kayira D, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012;379:2449–2458. doi: 10.1016/S0140-6736(12)60321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best BM, Stek AM, Mirochnick M, Hu C, Li H, Burchett SK, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54:381–388. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26:2039–2052. doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206:1695–1705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzi P, Spicher VM, Laubereau B, Hirschel B, Kind C, Rudin C, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS. 1998;12:F241–247. doi: 10.1097/00002030-199818000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Newell ML, Bunders MJ. Safety of antiretroviral drugs in pregnancy and breastfeeding for mother and child. Curr Opin HIV AIDS. 2013;8:503–509. doi: 10.1097/COH.0b013e3283632b88. [DOI] [PubMed] [Google Scholar]
- 13.Simon A, Warszawski J, Kariyawasam D, Le Chenadec J, Benhammou V, Czernichow P, et al. Association of prenatal and postnatal exposure to lopinavir-ritonavir and adrenal dysfunction among uninfected infants of HIV-infected mothers. JAMA. 2011;306:70–78. doi: 10.1001/jama.2011.915. [DOI] [PubMed] [Google Scholar]
- 14.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States, 2014. 2014 [Google Scholar]
- 15.Natureeba P, Ades V, Luwedde F, Mwesigwa J, Plenty A, Okong P, et al. Lopinavir/Ritonavir-Based Antiretroviral Treatment (ART) Versus Efavirenz-Based ART for the Prevention of Malaria Among HIV-Infected Pregnant Women. J Infect Dis. 2014 doi: 10.1093/infdis/jiu346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi M, Mwesigwa J, Aweeka F, Plenty A, Charlebois E, Ruel TD, et al. Hair and plasma data show that lopinavir, ritonavir, and efavirenz all transfer from mother to infant in utero, but only efavirenz transfers via breastfeeding. J Acquir Immune Defic Syndr. 2013;63:578–584. doi: 10.1097/QAI.0b013e31829c48ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3401–3409. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Table for Grading the Severity of Adult and Pediatric Adverse Events Version 1.0 - December 2004 (Clarification dated August 2009).
- 19.World Health Organization. WHO case definitions for HIV surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children, 2007. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 20.Koss CA, Natureeba P, Plenty A, Luwedde F, Mwesigwa J, Ades V, et al. Risk Factors for Preterm Birth Among HIV-Infected Pregnant Ugandan Women Randomized to Lopinavir/Ritonavir- or Efavirenz-Based Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2014;67:128–135. doi: 10.1097/QAI.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron DW, da Silva BA, Arribas JR, Myers RA, Bellos NC, Gilmore N, et al. A 96-week comparison of lopinavir-ritonavir combination therapy followed by lopinavir-ritonavir monotherapy versus efavirenz combination therapy. J Infect Dis. 2008;198:234–240. doi: 10.1086/589622. [DOI] [PubMed] [Google Scholar]
- 22.The Phidisa II Writing Team for Project Phidisa. Ratsela A, Polis M, Dhlomo S, Emery S, Grandits G, et al. A randomized factorial trial comparing 4 treatment regimens in treatment-naive HIV-infected persons with AIDS and/or a CD4 cell count <200 cells/uL in South Africa. J Infect Dis. 2010;202:1529–1537. doi: 10.1086/656718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, Klingman KL, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartelink IH, Savic RM, Mwesigwa J, Achan J, Clark T, Plenty A, et al. Pharmacokinetics of lopinavir/ritonavir and efavirenz in food insecure HIV-infected pregnant and breastfeeding women in Tororo, Uganda. J Clin Pharmacol. 2013 doi: 10.1002/jcph.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52:1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhi M, Ameli N, Bacchetti P, Gange SJ, Anastos K, Levine A, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009;23:471–478. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, Decloedt E, et al. Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011;56:333–339. doi: 10.1097/QAI.0b013e31820dc0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 30.Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, Misore A, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding--the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford N, Calmy A, Mofenson L. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS. 2011;25:2301–2304. doi: 10.1097/QAD.0b013e32834cdb71. [DOI] [PubMed] [Google Scholar]
- 32.Ford N, Mofenson L, Kranzer K, Medu L, Frigati L, Mills EJ, et al. Safety of efavirenz in first-trimester of pregnancy: a systematic review and meta-analysis of outcomes from observational cohorts. AIDS. 2010;24:1461–1470. doi: 10.1097/QAD.0b013e32833a2a14. [DOI] [PubMed] [Google Scholar]