Abstract
During angiogenesis, endothelial cells (ECs1) initiate new blood vessel growth and invade into the extracellular matrix (ECM). Membrane type-1 matrix metalloproteinase (MT1-MMP) facilitates this process and translocates to the plasma membrane following activation to promote ECM cleavage. The N-terminal pro-domain within MT1-MMP must be processed for complete activity of the proteinase. This study investigated whether MT1-MMP activation was altered by sphingosine 1-phosphate (S1P) and wall shear stress (WSS), which combine to stimulate EC invasion in three dimensional (3D) collagen matrices. MT1-MMP was activated rapidly and completely by WSS but not S1P. Proprotein convertases (PCs) promoted MT1-MMP processing, prompting us to test whether WSS or S1P treatments increased PC activity. Like MT1-MMP, PC activity increased with WSS, while S1P had no effect. A pharmacological PC inhibitor completely blocked S1P- and WSS-induced EC invasion and MT1-MMP translocation to the plasma membrane. Further, a recombinant PC inhibitor reduced MT1-MMP activation and decreased lumen formation in invading ECs, a process known to be controlled by MT1-MMP. Thus, we conclude that PC and MT1-MMP activation are mechanosensitive events that are required for EC invasion into 3D collagen matrices.
Keywords: angiogenesis, mechanotransduction, sprouting, furin
Introduction
Angiogenesis is the formation of new capillaries from pre-existing vessels and is important during physiological and pathological events such as wound healing, embryonic development, the female reproductive cycle, and tumor vascularization [1]. During angiogenesis, endothelial cells (ECs) respond to biochemical factors such as vascular endothelial growth factor (VEGF) [2], basic fibroblast growth factor (bFGF) [3], placental growth factor [4], and sphingosine 1-phosphate (S1P) [5], as well as mechanical shear forces created by blood flow [6]. Wall shear stress (WSS) rates have been estimated at 1–8 dyn/cm2 for the microcirculation, the site of angiogenic initiation [7]. Although multiple signaling pathways are activated by growth factors, lipids, and mechanical forces [4,6,8,9], the precise underlying signals and intracellular events that control EC sprouting in response to shear forces remain incompletely understood.
Membrane type-1 matrix metalloproteinase (MT1-MMP) is a member of the matrix metalloproteinase family of enzymes and is vital during angiogenesis. Mice deficient in MT1-MMP demonstrate defective vascular infiltration of cartilage [10] and corneal angiogenesis [10]. Also, MT1-MMP exclusively promoted endothelial-dependent vessel formation in vitro and in vivo [11], indicating a clear requirement for MT1-MMP in initiating new blood vessel growth. In addition, MT1-MMP is required for EC tubulogenesis and lumen formation [12,13,14,15]. Together, these studies establish a critical role for MT1-MMP in angiogenic responses.
To be functional, the propeptide sequence of MT1-MMP must be removed by one of several intracellular proprotein convertases (PCs), which include furin, PC1/3, PC2, PACE4, PC4, PC5/6, and PC7 [16,17]. Of these, furin, PC5/6, PC7, and PACE4 cleave the first 111 amino acids of MT1-MMP at a defined site (Arg-Arg-Lys-Arg111) to unmask the catalytic domain and promote surface localization of MT1-MMP [18,19]. Furin, PC1, PC6, and PC7 mRNA have been detected in ECs [20], and furin colocalized with MT1-MMP in the trans-Golgi network [21]. Although it is well-recognized that MT1-MMP promotes vessel outgrowth and lumen formation [11,13] and proprotein convertases activate MT1-MMP [18,19], it is not known whether WSS affects MT1-MMP activation. We observed here that WSS activated proprotein convertases, and proprotein convertases activated MT1-MMP to facilitate EC invasion of 3D collagen matrices.
Materials and Methods
Endothelial cell invasion stimulated by shear stress
Human umbilical vein endothelial cells (HUVECs) (Lonza BioProducts) were used at passage 4–6. Collagen type I was isolated as previously described [22]. Experiments applying 1μM S1P and 5.3 dyn/cm2 WSS were conducted as previously described [23,24].
Quantification of endothelial sprouting responses stimulated by shear stress
Invading cell cultures were fixed in 3% glutaraldehyde in PBS overnight and stained (15min) with 0.1% toluidine blue in 30% methanol. Invasion density was quantified as the average number of structures invading beneath the monolayer per standardized 1mm2 field (n>3 fields). The percentage of cells forming lumens and the lumen diameter were quantified from images taken of a side view of invasion. For each treatment group, n>100 cells were measured.
Proprotein convertase inhibition
ECs were pre-incubated with 25μM of proprotein convertase inhibitor, decanoyl-RVKR-chloromethylketone (ALX-260-022, Enzo) or vehicle control (DMSO) during attachment to collagen matrices (1hr) and for the duration of WSS application (24hr).
Proprotein convertase activity
Cells were seeded on polymerized collagen matrices containing S1P, exposed to 5.3 dyn/cm2 WSS, and allowed to invade for 3hr before homogenization in lysis buffer [100mM HEPES (pH 7.5), 0.5% TX-100, 1mM CaCl2, 1mM 2-mercaptoethanol, Complete Protease Inhibitor Cocktail (Roche), and Halt Phosphatase Inhibitor Cocktail (Pierce)] at 4°C for 10min. Samples were vortexed every 5min for 20min and centrifuged at 13,000×g at 4°C for 10min. Supernatants were collected and stored at −80°C until use. Assay buffer, fluorogenic substrate peptides (Boc-RVRR-AMC, ALX-260-040, Enzo), and reactants were prepared according to manufacturer’s instructions and measured for fluorescence intensity at excitation/emission wavelengths of 380(±20nm)/520(±20nm) using a Victor X3 plate reader (PerkinElmer Life Sciences) in triplicate wells.
Immunoblotting
Immunoblotting was conducted as previously described [23,24]. Band intensities were measured using ImageJ. Antisera used in this study were raised against GAPDH (ab8245, Abcam), MT1-MMP (SC30074, Santa Cruz; MAB3328, Millipore), Zyxin (cs3553, Cell Signaling), and antitrypsin (ab9400, Abcam). In all figures, densitometric analyses of band intensities were compiled from 3 independent experiments. Pro and active MT1-MMP levels were normalized to GAPDH. Data presented are mean values ±S.D.
Cell transfection and immunofluorescence analyses
Transient transfections of MT1-MMP-GFP expression plasmids were performed and quantified as previously described [24,25]. Transfection efficiency was approximately 20%. ECs were serum-starved for 1hr and then untreated or treated with the proprotein convertase inhibitor in the presence of 1μM S1P and WSS for 2hr. Cells were fixed in 4% paraformaldehyde before quantifying MT1-MMP-GFP localization to the cell periphery as previously described [24,25]. Data shown were averaged from 3 experiments (n=39 cells total/group).
Proprotein convertase inhibitor cloning and lentiviral transduction
Full-length α1-antitrypsin (α1-AT), α1-antitrypsin variant Pittsburgh (α1-PIT), and α1-antitrypsin variant Portland (α1-PDX) constructs were kind gifts from Gary Thomas (Oregon Health Sciences University) [26]. The inserts were subcloned into the pIEx-5 vector (Novagen) using the Acc65I and HindIII sites, generating a C-terminal S-tag. Positive clones confirmed by sequence analysis were subcloned into the pENTR4 vector (Invitrogen) using the Acc65I and XhoI sites and recombined into the pLenti6/V5 DEST vector (Invitrogen) using the GATEWAY system. Lentiviruses were generated as previously described [24,25]. ECs were transduced for 3d and selected with blasticidin (1μg/ml) for 8d. Blasticidin was removed for 24hr and invasion assays were conducted.
Statistical analyses
All data are presented as the mean ± standard deviation (S.D.) or standard error of the mean (SEM) for each group. Individual statistical analyses were performed using SAS software. Lowercase letters denote groupings from one-way ANOVA followed by post hoc pairwise comparison testing using Tukey’s method (p<0.05).
Results
MT1-MMP activation increases with time during EC invasion in 3D collagen matrices
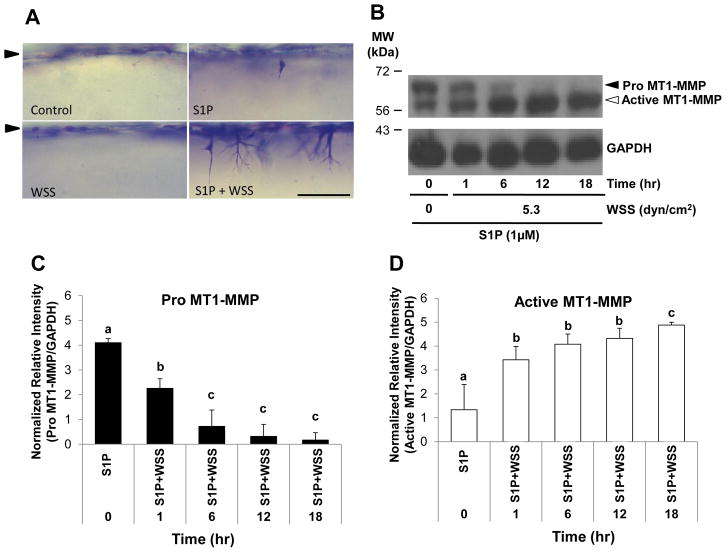
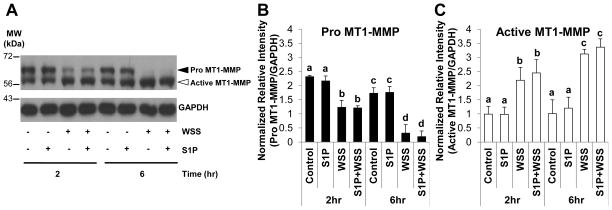
We have shown that surface translocation of MT1-MMP facilitates EC invasion in multiple assay systems [24,25]. However, whether WSS directly affects MT1-MMP activation has not been investigated. We used an established model [23,24], where 5.3 dyn/cm2 WSS combined with 1μM S1P promoted EC sprouting. Robust sprouting required S1P and WSS to promote EC invasion of 3D collagen matrices (Fig. 1A). Extracts were collected from invading cultures at 0, 1, 6, 12, and 18hr following stimulation with S1P and WSS. MT1-MMP was converted from the pro (63kDa) to an active, lower molecular weight form (60kDa) almost completely by 12hr (Fig. 1B). Quantification of band intensities from multiple experiments revealed a significant decrease in pro MT1-MMP with time in S1P- and WSS-treated ECs (Fig. 1C), which corresponded with an increase in active MT1-MMP over time (Fig. 1D). To test the effects of S1P and WSS separately, ECs were treated with or without WSS in the presence or absence of S1P. We observed increased active MT1-MMP in all groups exposed to WSS, while S1P appeared to have no effect on MT1-MMP activation (Fig. 2A). Quantification of both pro and active forms of MT1-MMP revealed that pro MT1-MMP levels were decreased significantly at 2hr compared to control treatment (Fig. 2B). At 6hr WSS treatment, pro MT1-MMP was nearly undetectable (Fig. 2B). In accordance with decreased levels of pro MT1-MMP, active MT1-MMP levels increased significantly with 2hr and 6hr WSS treatment compared to controls (Fig. 2C). No differences in levels of pro (Fig. 2B) or active (Fig. 2C) MT1-MMP were observed between Control and S1P treatment groups, indicating that S1P did not enhance MT1-MMP activation. Altogether, these data indicate that treatment of EC monolayers on 3D collagen matrices with 5.3 dyn/cm2 WSS enhanced conversion of pro MT1-MMP to the active form, while treatment with S1P had no effect on MT1-MMP activation.
Figure 1. MT1-MMP activation occurred during S1P- and WSS-stimulated EC invasion in 3D collagen matrices.
(A) Representative photographs of invasion responses. Scale bar,100μm. (B) Western blots are shown from ECs treated with or without 5.3 dyn/cm2 WSS and allowed to invade for 0, 1, 6, 12, and 18hr in the presence of 1μM S1P. Cell extracts were probed with antibodies directed to MT1-MMP (top blot) or GAPDH (bottom blot). Black and white arrowheads indicate pro and active MT1-MMP, respectively in all figures. Densitometric analyses of band intensities for (C) Pro MT1-MMP and (D) Active MT1-MMP levels normalized to GAPDH using ImageJ software from 3 independent experiments (means ±S.D.). In all figures, lowercase letters denote groupings from one-way ANOVA followed by post hoc pairwise comparison testing using Tukey’s method (p<0.05).
Figure 2. WSS, but not S1P, stimulated conversion of pro MT1-MMP to the active form in ECs seeded on 3D collagen matrices.
(A) Representative western blots are shown from ECs treated with or without 5.3 dyn/cm2 WSS for 2 or 6hr in the presence or absence of 1μM S1P. Cell extracts were probed with antibodies directed to MT1-MMP (top blot) or GAPDH (bottom blot). Densitometric analyses of band intensities for (B) Pro MT1-MMP and (C) Active MT1-MMP levels normalized to GAPDH.
WSS increases proprotein convertase (PC) activity
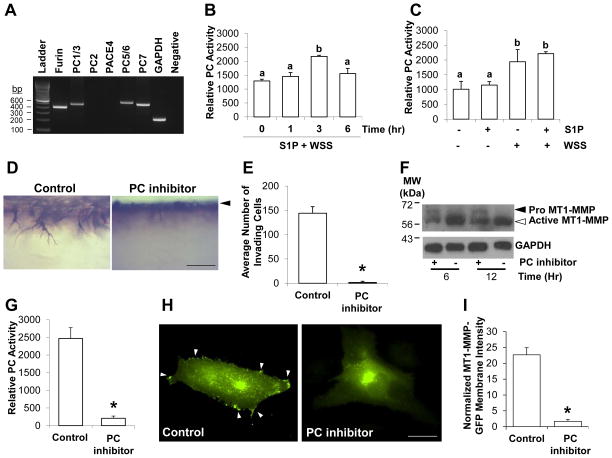
Because proprotein convertases (PCs) convert pro MT1-MMP to the active form by cleaving the propeptide sequence of MT1-MMP [18,19,27], we first tested whether proprotein convertases were expressed in ECs. Using validated primer sets [28], we observed ECs express furin, PC1/3, PC5/6, and PC7 (Fig. 3A), which agrees with a previous report [20]. We next determined whether PC activity increased with time during S1P- and WSS-induced EC invasion using a fluorogenic substrate. ECs were treated with WSS and S1P and allowed to invade 3D collagen matrices for 0, 1, 3, and 6hrs. PC activity initially increased from 0 to 3hr, reaching a maximum value at 3hr, and then decreased slightly at 6hrs (Fig. 3B). To test whether S1P or WSS activated PCs, ECs cultured on 3D collagen matrices containing or lacking S1P were treated with or without WSS for 3hr. PC activity increased significantly with WSS stimulation but was not affected by S1P treatment (Fig. 3C), which was consistent with the trend observed for conversion of pro MT1-MMP to the active form (cf. Fig. 2A).
Figure 3. WSS increased proprotein convertase activity, which was required for EC invasion.
(A) Representative RT-PCR using primers validated to amplify proprotein convertases [28]. ECs derived from 3 independent donors were tested with identical results. A representative example is shown. (B) ECs were treated with 5.3 dyn/cm2 WSS for 0, 1, 3, and 6hrs in the presence of 1μM S1P and lysed to measure proprotein convertase activity. (C) ECs were treated with or without 5.3 dyn/cm2 for 3hr in the presence or absence of 1μM S1P and lysed to measure proprotein convertase activity. (D) Photographs showing a side view of invading ECs stimulated with S1P and 5.3 dyn/cm2 WSS in the presence of vehicle control or 25μM PC inhibitor for 24hr. Scale bar, 100μm. Arrowhead indicates original monolayer. (E) Quantification of invasion density from 4 individual experiments (n=20 total wells). Data shown are average number of invading cells +/−S.D. (F) Cell extracts were collected at 6 and 12hr and probed with antibodies directed to MT1-MMP (top blot) or GAPDH (bottom blot). (G) Quantification of PC activity from cell lysates collected at 3hr. (H) ECs were transiently transfected with a vector expressing MT1-MMP-GFP and treated with 5.3 dyn/cm2 WSS and 1μM S1P for 2hr. Photographs of ECs expressing MT1-MMP-GFP are shown treated with or without 25μM PC inhibitor. White arrowheads indicate MT1-MMP-GFP at the cell periphery. Scale bar, 10μm. (I) Quantification of MT1-MMP-GFP localization to the cell periphery as described in “Materials and Methods” from 3 independent experiments. * indicates significant difference from control (Student’s t-test; n=39 cells, p<0.01).
Proprotein convertase activity is required for S1P- and WSS-induced EC invasion and MT1-MMP translocation to the plasma membrane
The results above indicate that WSS enhanced PC activity and the conversion of pro MT1-MMP to the active form. We next determined whether PC activity was necessary for EC invasion in 3D collagen matrices. ECs were stimulated with S1P and WSS in the presence of vehicle control or PC inhibitor. Photographs capturing a side view of invading structures are shown in Figure 3D. No decrease in EC viability was observed in the presence of the PC inhibitor (not shown), which significantly blocked EC invasion responses (Fig. 3E). Compared to controls, PC inhibition reduced the conversion of pro MT1-MMP to the active form at 6 and 12hr (Fig. 3F), supporting that proprotein convertases regulated MT1-MMP activation in invading ECs.
Béliveau and colleagues observed that S1P stimulated MT1-MMP translocation to the plasma membrane [29], and we have previously shown that increased MT1-MMP-GFP plasma membrane translocation correlated with increased EC sprouting responses [24,25]. We next determined whether proprotein convertase activity was required for MT1-MMP-GFP translocation to the membrane in response to stimulation by S1P and WSS. As expected, the PC inhibitor almost completely abolished PC activity (Fig. 3G) and blocked localization of MT1-MMP-GFP to the plasma membrane (Fig. 3H) in response to stimulation by S1P and WSS. Quantitative analysis of these images indicated that PC inhibition significantly reduced the ability of S1P and WSS to stimulate MT1-MMP-GFP membrane localization (Fig. 3I). Altogether, these data reinforce that PC activity is required for successful plasma membrane translocation of MT1-MMP [18], which is vital for ECM proteolysis during endothelial sprouting responses.
Exogenous expression of a PC inhibitor attenuated lumen formation in invading ECs
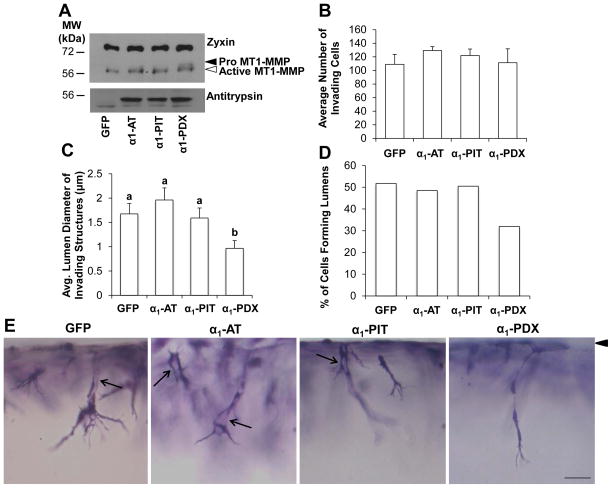
To reinforce the findings from pharmacological PC inhibition, we investigated whether expression of a protein-based PC inhibitor altered EC invasion in response to WSS and S1P. ECs were transduced to express GFP or various antitrypsin constructs. Human α1-antitrypsin (α1-AT) is a natural inhibitor of elastase and trypsin [30,31], while the α1-AT Pittsburgh (α1-PIT) mutant (Arg358) acts as specific inhibitor of thrombin [26]. The α1-AT Portland (α1-PDX) mutant contains a bait sequence (Arg355-X-X-Arg358) that binds to furin and PC5/6 to function as a competitive inhibitor [26,32,33]. Transduced ECs were stimulated with S1P and WSS for 2 or 24hrs on 3D collagen matrices. At 2hrs, expression of all antitrypsin constructs was detected (Fig. 4A), and GFP expression was confirmed visually (not shown). As expected, α1-PDX expression resulted in increased levels of pro-MT1-MMP, indicative of reduced PC-dependent processing of pro MT1-MMP to the active form (Fig. 4A). No difference in invasion density was observed with expression of α1-AT, α1-PIT, or α1-PDX (Fig. 4B). MT1-MMP is required for EC lumen formation [12,13] and associates with several signaling complexes known to be crucial for lumen formation and endothelial sprouting [25,34,35]. Fitting with these findings, expression of α1-PDX significantly reduced average lumen diameter (Fig. 4C) and the percentage of cells forming lumens (Fig. 4D). Representative photographs are shown in Figure 4E. Together, these findings support that WSS promotes PC-dependent activation of MT1-MMP.
Figure 4. Exogenous expression of proprotein convertase inhibitor decreased lumen formation.
ECs were transduced to express GFP, α1-AT, α1-PIT, or α1-PDX and allowed to invade 2 or 24hr in the presence of 5.3 dyn/cm2 WSS and 1μM S1P. (A) Cell extracts (2hr) were immunoblotted with antibodies against Zyxin to normalize for total protein (top blot), MT1-MMP (middle blot), and Antitrypsin (bottom blot) to demonstrate overexpression of antitrypsin-derived constructs. (B) Quantification of invasion density (24hr invasion). (C) Quantification of the average lumen diameter of invading cells (24hr invasion; n>100 cells, means ±SEM). (D) Percentage of invading structures which contained a lumen (n>100 cells). (E) Photographs illustrating a side view of EC invasion after 24hr. Arrowhead indicates monolayer; arrows indicate lumens. Scale bar, 50μm.
Discussion
We report that WSS induced PC-dependent activation of MT1-MMP. Our data show a corresponding increase in PC activity and MT1-MMP processing that was stimulated by 5.3 dyn/cm2 WSS, while S1P had no effect on PC activity or MT1-MMP processing. These results support that PC-dependent activation of MT1-MMP is a mechanosensitive event that facilitates proper plasma membrane localization of MT1-MMP to allow successful endothelial sprouting in 3D collagen matrices.
The data presented here suggest subsets of PCs may be responsible for WSS-dependent processing of MT1-MMP in ECs. Furin, PC1/3, PC5/6, and PC7 mRNA were expressed (Fig. 3A), confirming a previous report [20]. Although the PC responsible for MT1-MMP processing and activation has not been definitively determined, furin, PC5/6, PC7, and PACE4 cleave MT1-MMP [19], and α1-PDX specifically inhibits furin and PC5/6 [32,33], suggesting a role for furin and/or PC5/6 in WSS-induced MT1-MMP activation. Notably, our data agree with previous reports that α1-PDX partially blocked MT1-MMP activation [18,19]. Although we cannot rule out a role for PC7 or furin-independent MT1-MMP activation [18,36], the data presented here support that furin and/or PC5/6 are, at least partially, responsible for WSS-dependent MT1-MMP processing needed for EC invasion of collagen matrices.
While the present study investigated alterations in PC activity in response to WSS, others have reported that alterations in flow affect furin expression. Expression of furin and its associated substrate, transforming growth factor-β, increased in the endothelium of the carotid vein proximal to implantation of a carotid arteriovenous shunt in vivo [37]. In addition, 15 dyn/cm2 WSS increased furin mRNA expression in bovine aortic endothelial cells in vitro [37]. In separate studies, both increasing and decreasing flow levels within carotid and femoral arteries upregulated furin and MT1-MMP mRNA expression in arterial extracts containing endothelial and smooth muscle cells [38]. These data indicate that furin and MT1-MMP mRNA expression levels were regulated by changes in flow and correlated with arterial remodeling, but isolated effects on the endothelium were not determined. Our study has not examined whether WSS affected the expression of PC mRNA in invading ECs, and thus we cannot rule out a contribution of elevated PC expression contributing to the increased PC activity observed here. However, because the assay system used here consists exclusively of ECs, we can confirm a definitive EC-specific effect of WSS in upregulating PC activity.
WSS has been estimated in post-capillary venules to range from 1–8 dyn/cm2 [7]. We found that 5.3 dyn/cm2 WSS was effective at converting pro MT1-MMP to the active form. To promote matrix degradation, MT1-MMP must be activated and present at the plasma membrane [11,13]. Although we find here that S1P did not change PC activity (Fig. 3B), S1P has been reported to promote MT1-MMP plasma membrane localization in ECs [24,25,39], and treatment with both S1P and 5.3 dyn/cm2 WSS resulted in sustained localization of MT1-MMP to the plasma membrane and robust sprouting [24] that was blocked by PC inhibition. When considering key events required for successful EC invasion, WSS enhanced MT1-MMP activation (shown here), while S1P combined with WSS was needed for membrane retention [24,25]. Altogether, these data illuminate intracellular signaling events downstream of physiological stimulation with WSS and S1P, which combine to promote robust EC sprouting.
Highlights.
Physiological levels of WSS seen at post-capillary venules robustly convert pro MT1-MMP to the active form
Proprotein convertase activity is required for WSS-mediated conversion of pro to active MT1-MMP and EC invasion responses
Proprotein convertase activity and subsequent MT1-MMP activation are mechanosensitive and enable EC invasion
Acknowledgments
We thank Dr. Gary Thomas for providing the α1-AT, α1-PIT, and α1-PDX expression constructs. This work was supported by NIH Public Health Service Grant HL095786 to KJB.
Footnotes
Abbreviations used: ECs, endothelial cells; MT1-MMP, Membrane type-1 matrix metalloproteinase; ECM, extracellular matrix; WSS, wall shear stress; S1P, sphingosine 1-phosphate; PC, pro protein convertase; 3D, threedimensional; HUVEC, human umbilical vein endothelial cell; GFP, green fluorescent protein; GAPDH, glyceraldehyde phosphate dehydrogenase; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 3.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 4.Gigante B, Morlino G, Gentile MT, Persico MG, De Falco S. Plgf−/−eNos−/− mice show defective angiogenesis associated with increased oxidative stress in response to tissue ischemia. FASEB J. 2006;20:970–972. doi: 10.1096/fj.05-4481fje. [DOI] [PubMed] [Google Scholar]
- 5.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta. 2002;1582:72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 6.Kaunas R, Kang H, Bayless KJ. Synergistic Regulation of Angiogenic Sprouting by Biochemical Factors and Wall Shear Stress. Cell Mol Bioeng. 2011;4:547–559. doi: 10.1007/s12195-011-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim MB, Sarelius IH. Distributions of wall shear stress in venular convergences of mouse cremaster muscle. Microcirculation. 2003;10:167–178. doi: 10.1038/sj.mn.7800182. [DOI] [PubMed] [Google Scholar]
- 8.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aird WC. Endothelial Biomedicine. Cambridge University Press; New York, NY: 2007. [Google Scholar]
- 10.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009;114:237–247. doi: 10.1182/blood-2008-12-196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards DR. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs) J Cell Sci. 2002;115:3427–3438. doi: 10.1242/jcs.115.17.3427. [DOI] [PubMed] [Google Scholar]
- 15.Nisato RE, Hosseini G, Sirrenberg C, Butler GS, Crabbe T, Docherty AJ, Wiesner M, Murphy G, Overall CM, Goodman SL, Pepper MS. Dissecting the role of matrix metalloproteinases (MMP) and integrin alpha(v)beta3 in angiogenesis in vitro: absence of hemopexin C domain bioactivity, but membrane-Type 1-MMP and alpha(v)beta3 are critical. Cancer Res. 2005;65:9377–9387. doi: 10.1158/0008-5472.CAN-05-1512. [DOI] [PubMed] [Google Scholar]
- 16.Fugere M, Day R. Cutting back on pro-protein convertases: the latest approaches to pharmacological inhibition. Trends Pharmacol Sci. 2005;26:294–301. doi: 10.1016/j.tips.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidah NG, Chretien M. Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr Opin Biotechnol. 1997;8:602–607. doi: 10.1016/s0958-1669(97)80036-5. [DOI] [PubMed] [Google Scholar]
- 18.Yana I, Weiss SJ. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remacle AG, Rozanov DV, Fugere M, Day R, Strongin AY. Furin regulates the intracellular activation and the uptake rate of cell surface-associated MT1-MMP. Oncogene. 2006;25:5648–5655. doi: 10.1038/sj.onc.1209572. [DOI] [PubMed] [Google Scholar]
- 20.Campan M, Yoshizumi M, Seidah NG, Lee ME, Bianchi C, Haber E. Increased proteolytic processing of protein tyrosine phosphatase mu in confluent vascular endothelial cells: the role of PC5, a member of the subtilisin family. Biochemistry. 1996;35:3797–3802. doi: 10.1021/bi952552d. [DOI] [PubMed] [Google Scholar]
- 21.Roghi C, Jones L, Gratian M, English WR, Murphy G. Golgi reassembly stacking protein 55 interacts with membrane-type (MT) 1-matrix metalloprotease (MMP) and furin and plays a role in the activation of the MT1-MMP zymogen. FEBS J. 2010;277:3158–3175. doi: 10.1111/j.1742-4658.2010.07723.x. [DOI] [PubMed] [Google Scholar]
- 22.Bayless K, Kwak HI, Su SC. Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nature protocols. 2009;4:1888–1898. doi: 10.1038/nprot.2009.221. [DOI] [PubMed] [Google Scholar]
- 23.Kang H, Bayless KJ, Kaunas R. Fluid shear stress modulates endothelial cell invasion into three-dimensional collagen matrices. Am J Physiol Heart Circ Physiol. 2008;295:H2087–2097. doi: 10.1152/ajpheart.00281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang H, Kwak HI, Kaunas R, Bayless KJ. Fluid shear stress and sphingosine 1-phosphate activate calpain to promote membrane type 1 matrix metalloproteinase (MT1-MMP) membrane translocation and endothelial invasion into three-dimensional collagen matrices. J Biol Chem. 2011;286:42017–42026. doi: 10.1074/jbc.M111.290841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak HI, Kang H, Dave JM, Mendoza EA, Su SC, Maxwell SA, Bayless KJ. Calpain-mediated vimentin cleavage occurs upstream of MT1-MMP membrane translocation to facilitate endothelial sprout initiation. Angiogenesis. 2012;15:287–303. doi: 10.1007/s10456-012-9262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson ED, Thomas L, Hayflick JS, Thomas G. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed alpha 1-antitrypsin variant. J Biol Chem. 1993;268:24887–24891. [PubMed] [Google Scholar]
- 27.Pei D, Weiss SJ. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J Biol Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- 28.Cheng M, Watson PH, Paterson JA, Seidah N, Chretien M, Shiu RP. Pro-protein convertase gene expression in human breast cancer. Int J Cancer. 1997;71:966–971. doi: 10.1002/(sici)1097-0215(19970611)71:6<966::aid-ijc10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Langlois S, Gingras D, Beliveau R. Membrane type 1-matrix metalloproteinase (MT1-MMP) cooperates with sphingosine 1-phosphate to induce endothelial cell migration and morphogenic differentiation. Blood. 2004;103:3020–3028. doi: 10.1182/blood-2003-08-2968. [DOI] [PubMed] [Google Scholar]
- 30.Perlmutter DH, Pierce JA. The alpha 1-antitrypsin gene and emphysema. Am J Physiol. 1989;257:L147–162. doi: 10.1152/ajplung.1989.257.4.L147. [DOI] [PubMed] [Google Scholar]
- 31.Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 32.Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J. 1998;17:4735–4743. doi: 10.1093/emboj/17.16.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason AJ, Thomas G. alpha1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci U S A. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacharidou A, Koh W, Stratman AN, Mayo AM, Fisher KE, Davis GE. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood. 2010;115:5259–5269. doi: 10.1182/blood-2009-11-252692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh W, Sachidanandam K, Stratman AN, Sacharidou A, Mayo AM, Murphy EA, Cheresh DA, Davis GE. Formation of endothelial lumens requires a coordinated PKCepsilon-, Src-, Pak- and Raf-kinase-dependent signaling cascade downstream of Cdc42 activation. J Cell Sci. 2009;122:1812–1822. doi: 10.1242/jcs.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato T, Kondo T, Fujisawa T, Seiki M, Ito A. Furin-independent pathway of membrane type 1-matrix metalloproteinase activation in rabbit dermal fibroblasts. J Biol Chem. 1999;274:37280–37284. doi: 10.1074/jbc.274.52.37280. [DOI] [PubMed] [Google Scholar]
- 37.Negishi M, Lu D, Zhang YQ, Sawada Y, Sasaki T, Kayo T, Ando J, Izumi T, Kurabayashi M, Kojima I, Masuda H, Takeuchi T. Upregulatory Expression of Furin and Transforming Growth Factor- by Fluid Shear Stress in Vascular Endothelial Cells. Arterioscler Thromb Vasc Biol. 2001;21:785–790. doi: 10.1161/01.atv.21.5.785. [DOI] [PubMed] [Google Scholar]
- 38.de Kleijn DP, Sluijter JP, Smit J, Velema E, Richard W, Schoneveld AH, Pasterkamp G, Borst C. Furin and membrane type-1 metalloproteinase mRNA levels and activation of metalloproteinase-2 are associated with arterial remodeling. FEBS Lett. 2001;501:37–41. doi: 10.1016/s0014-5793(01)02622-9. [DOI] [PubMed] [Google Scholar]
- 39.Nyalendo C, Michaud M, Beaulieu E, Roghi C, Murphy G, Gingras D, Beliveau R. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration. J Biol Chem. 2007;282:15690–15699. doi: 10.1074/jbc.M608045200. [DOI] [PubMed] [Google Scholar]