Abstract
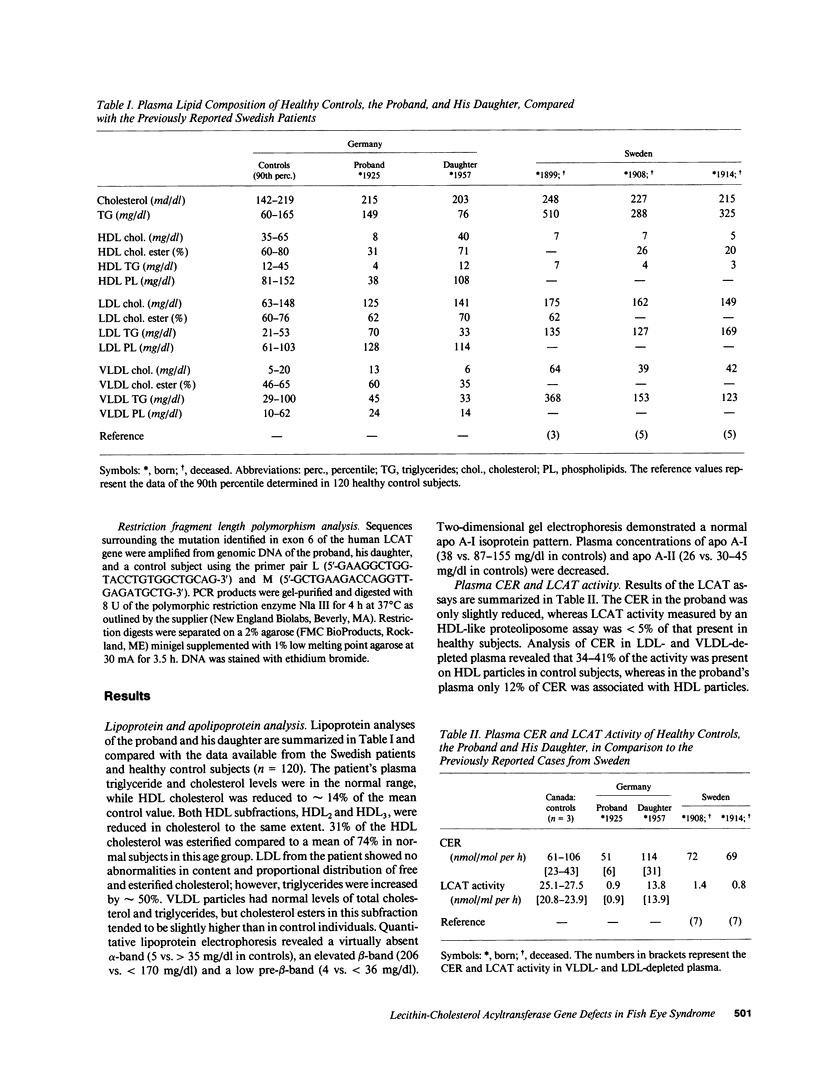
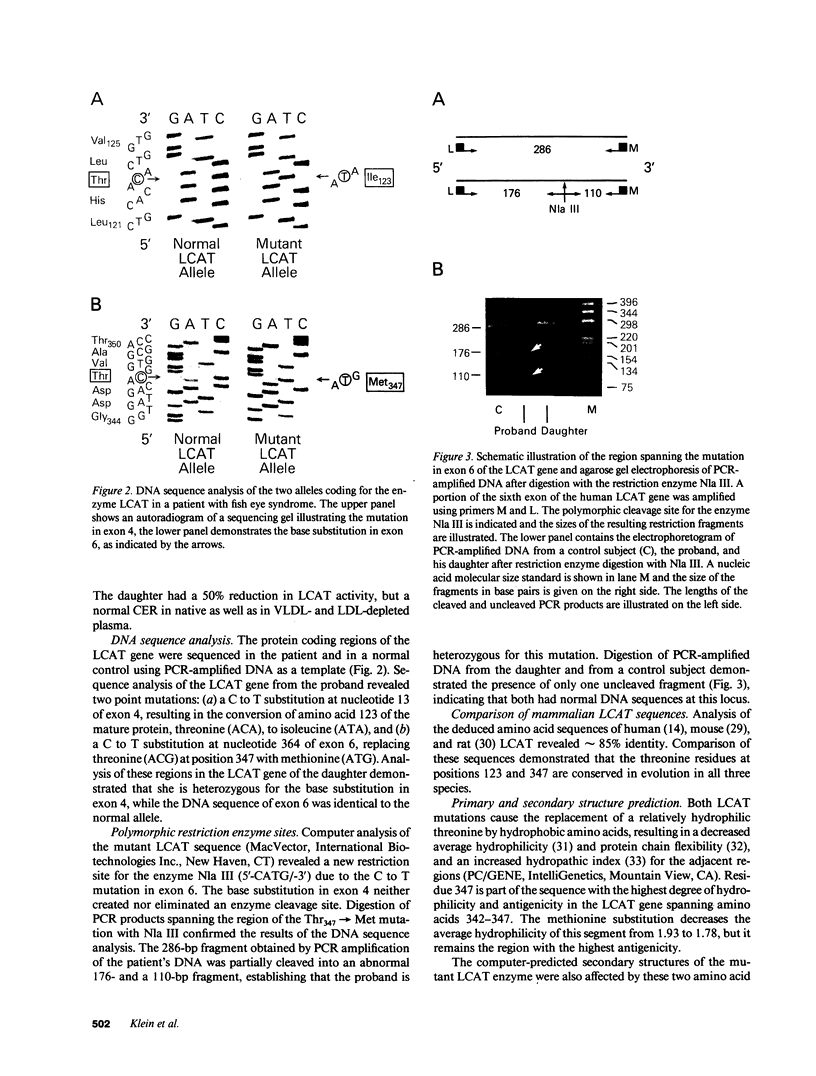
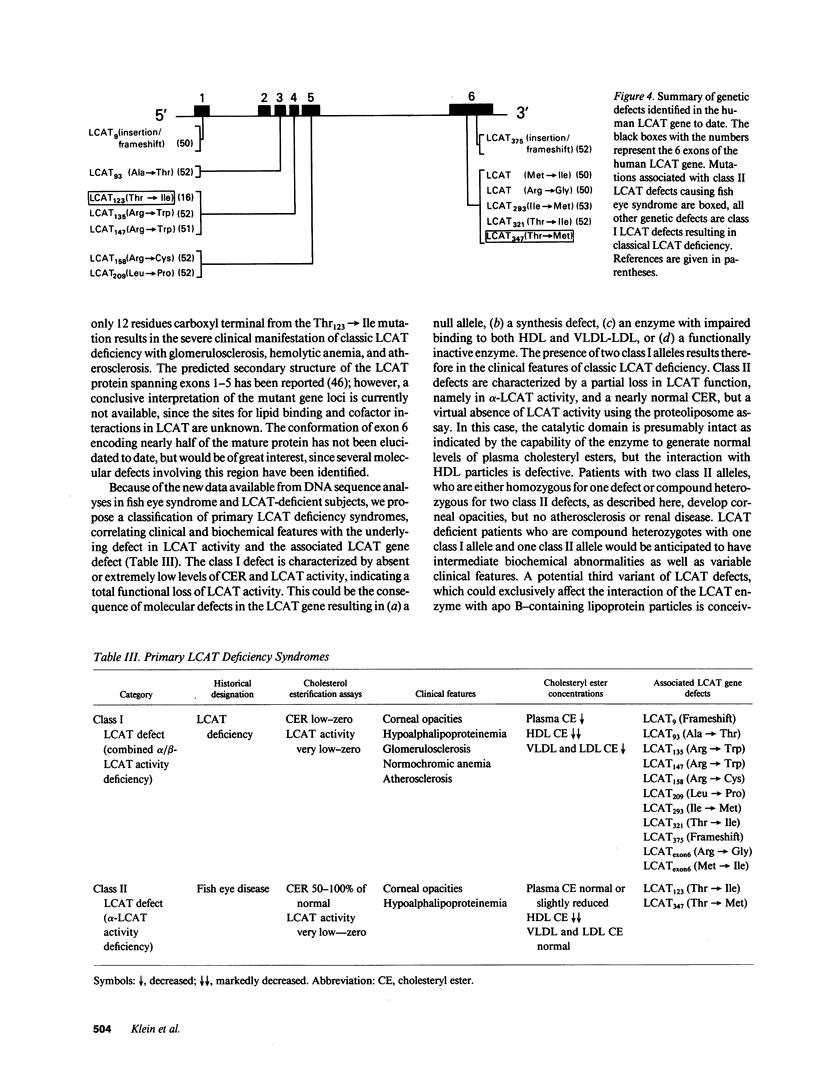
We have elucidated the genetic defect in a 66-yr-old patient with fish eye syndrome (FES) presenting with severe corneal opacities and hypoalphalipoproteinemia. The patient's plasma concentration of high density lipoprotein (HDL) cholesterol was reduced at 7.7 mg/dl (35.1-65.3 mg/dl in controls) and the HDL cholesteryl ester content was 31% (60-80% in controls); however, total plasma cholesteryl esters were similar to normal (60% of total cholesterol vs. a mean of 66% in controls). The patient's plasma cholesterol esterification rate was slightly reduced at 51 nmol/ml per h (control subjects: 61-106 nmol/ml per h), whereas lecithin-cholesterol acyltransferase (LCAT) activity, assayed using a HDL-like exogenous proteoliposome substrate, was virtually absent (0.9 nmol/ml per h vs. 25.1-27.9 nmol/ml per h in control subjects). DNA sequence analysis of the proband's LCAT gene revealed two separate C to T transitions resulting in the substitution of Thr123 with Ile and Thr347 with Met. The mutation at codon 347 created a new restriction site for the enzyme Nla III. Analysis of the patient's polymerase chain reaction-amplified DNA containing the region of the Thr347 mutation by digestion with Nla III confirmed that the proband is a compound heterozygote for both defects. The patient's daughter, who is asymptomatic despite a 50% reduction of LCAT activity, is heterozygous for the Thr123----Ile mutation. Our data indicate that the regions adjacent to Thr123 and Thr347 of LCAT may play an important role in HDL cholesterol esterification, suggesting that these regions may contain a portion of the LCAT binding domain(s) for HDL.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Gjone E., Adolphson J. L., Chen C. H., Teisberg P., Torsvik H. Familial lecithin-cholesterol acyltransferase deficiency in four Norwegian Families. Evidence for low levels of a functionally defective enzyme. Acta Med Scand. 1981;210(6):455–459. doi: 10.1111/j.0954-6820.1981.tb09849.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Calvert G. D., Carlson L. A. Plasma lipid transfer in fish-eye disease. Acta Med Scand. 1983;213(4):253–254. doi: 10.1111/j.0954-6820.1983.tb03729.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A. A further case of fish-eye disease. Lancet. 1979 Dec 22;2(8156-8157):1376–1377. doi: 10.1016/s0140-6736(79)92867-8. [DOI] [PubMed] [Google Scholar]
- Carlson L. A. Fish eye disease: a new familial condition with massive corneal opacities and dyslipoproteinaemia. Eur J Clin Invest. 1982 Feb;12(1):41–53. doi: 10.1111/j.1365-2362.1982.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Evidence for deficiency of high density lipoprotein lecithin: cholesterol acyltransferase activity (alpha-LCAT) in fish eye disease. Acta Med Scand. 1985;218(2):189–196. doi: 10.1111/j.0954-6820.1985.tb08846.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Evidence for the presence in human plasma of lecithin: cholesterol acyltransferase activity (beta-LCAT) specifically esterifying free cholesterol of combined pre-beta- and beta-lipoproteins. Studies of fish eye disease patients and control subjects. Acta Med Scand. 1985;218(2):197–205. doi: 10.1111/j.0954-6820.1985.tb08847.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Paradoxical esterification of plasma cholesterol in fish eye disease. Acta Med Scand. 1985;217(5):491–499. doi: 10.1111/j.0954-6820.1985.tb03252.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Studies on high density lipoproteins in fish eye disease. Acta Med Scand. 1983;213(3):177–182. doi: 10.1111/j.0954-6820.1983.tb03713.x. [DOI] [PubMed] [Google Scholar]
- Dobiasova M., Stribrna J., Sparks D. L., Pritchard P. H., Frohlich J. J. Cholesterol esterification rates in very low density lipoprotein- and low density lipoprotein-depleted plasma. Relation to high density lipoprotein subspecies, sex, hyperlipidemia, and coronary artery disease. Arterioscler Thromb. 1991 Jan-Feb;11(1):64–70. doi: 10.1161/01.atv.11.1.64. [DOI] [PubMed] [Google Scholar]
- Francone O. L., Fielding C. J. Effects of site-directed mutagenesis at residues cysteine-31 and cysteine-184 on lecithin-cholesterol acyltransferase activity. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1716–1720. doi: 10.1073/pnas.88.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich J., Hoag G., McLeod R., Hayden M., Godin D. V., Wadsworth L. D., Critchley J. D., Pritchard P. H. Hypoalphalipoproteinemia resembling fish eye disease. Acta Med Scand. 1987;221(3):291–298. doi: 10.1111/j.0954-6820.1987.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Frohlich J., McLeod R., Pritchard P. H., Fesmire J., McConathy W. Plasma lipoprotein abnormalities in heterozygotes for familial lecithin:cholesterol acyltransferase deficiency. Metabolism. 1988 Jan;37(1):3–8. doi: 10.1016/0026-0495(88)90021-2. [DOI] [PubMed] [Google Scholar]
- Funke H., von Eckardstein A., Pritchard P. H., Albers J. J., Kastelein J. J., Droste C., Assmann G. A molecular defect causing fish eye disease: an amino acid exchange in lecithin-cholesterol acyltransferase (LCAT) leads to the selective loss of alpha-LCAT activity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4855–4859. doi: 10.1073/pnas.88.11.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke H., von Eckardstein A., Pritchard P. H., Karas M., Albers J. J., Assmann G. A frameshift mutation in the human apolipoprotein A-I gene causes high density lipoprotein deficiency, partial lecithin: cholesterol-acyltransferase deficiency, and corneal opacities. J Clin Invest. 1991 Jan;87(1):371–376. doi: 10.1172/JCI114997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gascuel O., Golmard J. L. A simple method for predicting the secondary structure of globular proteins: implications and accuracy. Comput Appl Biosci. 1988 Aug;4(3):357–365. doi: 10.1093/bioinformatics/4.3.357. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist L., Carlson L. A. In vitro normalization of cholesteryl ester content and particle size of fish eye disease high density lipoproteins. Acta Med Scand. 1987;221(3):283–289. doi: 10.1111/j.0954-6820.1987.tb00895.x. [DOI] [PubMed] [Google Scholar]
- Holmquist L., Carlson L. A. Net lipid transfer between lipoproteins in fish-eye disease plasma supplemented with normal high density lipoproteins. Lipids. 1987 May;22(5):305–311. doi: 10.1007/BF02533997. [DOI] [PubMed] [Google Scholar]
- Holmquist L., Carlson L. A. Normalization of high density lipoprotein in fish eye disease plasma by purified normal human lecithin: cholesterol acyltransferase. Lipids. 1988 Mar;23(3):225–229. doi: 10.1007/BF02535462. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhiainen M., Stevenson K. J., Dolphin P. J. Human plasma lecithin-cholesterol acyltransferase. The vicinal nature of cysteine 31 and cysteine 184 in the catalytic site. J Biol Chem. 1988 May 15;263(14):6525–6533. [PubMed] [Google Scholar]
- Jauhiainen M., Yuan W., Gelb M. H., Dolphin P. J. Human plasma lecithin-cholesterol acyltransferase. Inhibition of the phospholipase A2-like activity by sn-2-difluoroketone phosphatidylcholine analogues. J Biol Chem. 1989 Feb 5;264(4):1963–1967. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LEES R. S., HATCH F. T. Sharper separation of lipoprotein species by paper electrophoresis in albumin-containing buffer. J Lab Clin Med. 1963 Mar;61:518–528. [PubMed] [Google Scholar]
- Maeda E., Naka Y., Matozaki T., Sakuma M., Akanuma Y., Yoshino G., Kasuga M. Lecithin-cholesterol acyltransferase (LCAT) deficiency with a missense mutation in exon 6 of the LCAT gene. Biochem Biophys Res Commun. 1991 Jul 31;178(2):460–466. doi: 10.1016/0006-291x(91)90129-u. [DOI] [PubMed] [Google Scholar]
- McIntyre N. Familial LCAT deficiency and fish-eye disease. J Inherit Metab Dis. 1988;11 (Suppl 1):45–56. doi: 10.1007/BF01800570. [DOI] [PubMed] [Google Scholar]
- McLean J., Wion K., Drayna D., Fielding C., Lawn R. Human lecithin-cholesterol acyltransferase gene: complete gene sequence and sites of expression. Nucleic Acids Res. 1986 Dec 9;14(23):9397–9406. doi: 10.1093/nar/14.23.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni G., Malgaretti N., Magnaghi P., Taramelli R. Nucleotide sequence of the cDNA for lecithin-cholesterol acyl transferase (LCAT) from the rat. Nucleic Acids Res. 1990 Sep 11;18(17):5308–5308. doi: 10.1093/nar/18.17.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Norum R. A., Lakier J. B., Goldstein S., Angel A., Goldberg R. B., Block W. D., Noffze D. K., Dolphin P. J., Edelglass J., Bogorad D. D. Familial deficiency of apolipoproteins A-I and C-III and precocious coronary-artery disease. N Engl J Med. 1982 Jun 24;306(25):1513–1519. doi: 10.1056/NEJM198206243062503. [DOI] [PubMed] [Google Scholar]
- Novotný J., Auffray C. A program for prediction of protein secondary structure from nucleotide sequence data: application to histocompatibility antigens. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):243–255. doi: 10.1093/nar/12.1part1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogne S., Skretting G., Larsen F., Myklebost O., Mevåg B., Carlson L. A., Holmquist L., Gjone E., Prydz H. The isolation and characterisation of a cDNA clone for human lecithin:cholesterol acyl transferase and its use to analyse the genes in patients with LCAT deficiency and fish eye disease. Biochem Biophys Res Commun. 1987 Oct 14;148(1):161–169. doi: 10.1016/0006-291x(87)91090-4. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer E. J., Ordovas J. M., Law S. W., Ghiselli G., Kashyap M. L., Srivastava L. S., Heaton W. H., Albers J. J., Connor W. E., Lindgren F. T. Familial apolipoprotein A-I and C-III deficiency, variant II. J Lipid Res. 1985 Sep;26(9):1089–1101. [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Sprecher D. L., Taam L., Brewer H. B., Jr Two-dimensional electrophoresis of human plasma apolipoproteins. Clin Chem. 1984 Dec;30(12 Pt 1):2084–2092. [PubMed] [Google Scholar]
- Stein E. A., DiPersio L., Pesce A. J., Kashyap M., Kao J. T., Srivastava L., McNerney C. Enzyme-linked immunoabsorbant assay of apolipoprotein AII in plasma, with use of a monoclonal antibody. Clin Chem. 1986 Jun;32(6):967–971. [PubMed] [Google Scholar]
- Taramelli R., Pontoglio M., Candiani G., Ottolenghi S., Dieplinger H., Catapano A., Albers J., Vergani C., McLean J. Lecithin cholesterol acyl transferase deficiency: molecular analysis of a mutated allele. Hum Genet. 1990 Jul;85(2):195–199. doi: 10.1007/BF00193195. [DOI] [PubMed] [Google Scholar]
- Warden C. H., Langner C. A., Gordon J. I., Taylor B. A., McLean J. W., Lusis A. J. Tissue-specific expression, developmental regulation, and chromosomal mapping of the lecithin: cholesterol acyltransferase gene. Evidence for expression in brain and testes as well as liver. J Biol Chem. 1989 Dec 25;264(36):21573–21581. [PubMed] [Google Scholar]
- Yamazaki S., Mitsunaga T., Furukawa Y., Nishida T. Interaction of lecithin-cholesterol acyltransferase with human plasma lipoproteins and with lecithin-cholesterol vesicles. J Biol Chem. 1983 May 10;258(9):5847–5853. [PubMed] [Google Scholar]
- Yang C. Y., Manoogian D., Pao Q., Lee F. S., Knapp R. D., Gotto A. M., Jr, Pownall H. J. Lecithin:cholesterol acyltransferase. Functional regions and a structural model of the enzyme. J Biol Chem. 1987 Mar 5;262(7):3086–3091. [PubMed] [Google Scholar]