Abstract
Bacopa monnieri has been used in Ayurvedic medicine to improve memory and cognition. The active constituent responsible for its pharmacological effects is bacoside A, a mixture of dammarane-type triterpenoid saponins containing sugar chains linked to a steroid aglycone skeleton. Triterpenoid saponins have been reported to be transformed in vivo to metabolites that give better biological activity and pharmacokinetic characteristics. Thus, the activities of the parent compounds (bacosides), aglycones (jujubogenin and pseudojujubogenin) and their derivatives (ebelin lactone and bacogenin A1) were compared using a combination of in silico and in vitro screening methods. The compounds were docked into 5-HT1A, 5-HT2A, D1, D2, M1 receptors and acetylcholinesterase (AChE) using AutoDock and their central nervous system (CNS) drug-like properties were determined using Discovery Studio molecular properties and ADMET descriptors. The compounds were screened in vitro using radioligand receptor binding and AChE inhibition assays. In silico studies showed that the parent bacosides were not able to dock into the chosen CNS targets and had poor molecular properties as a CNS drug. In contrast, the aglycones and their derivatives showed better binding affinity and good CNS drug-like properties, were well absorbed through the intestines and had good blood brain barrier (BBB) penetration. Among the compounds tested in vitro, ebelin lactone showed binding affinity towards M1 (Ki = 0.45 μM) and 5-HT2A (4.21 μM) receptors. Bacoside A and bacopaside X (9.06 μM) showed binding affinity towards the D1 receptor. None of the compounds showed any inhibitory activity against AChE. Since the stimulation of M1 and 5-HT2A receptors has been implicated in memory and cognition and ebelin lactone was shown to have the strongest binding energy, highest BBB penetration and binding affinity towards M1 and 5-HT2A receptors, we suggest that B. monnieri constituents may be transformed in vivo to the active form before exerting their pharmacological activity.
Introduction
Bacopa monnieri (Linn.) Pennell (Scrophulariaceae), also known as Brahmi, is a reputed Ayurvedic herb noted to improve memory and cognition [1]. These traditional claims have been supported by extensive in vitro, in vivo and clinical studies conducted over the last two decades using the plant extract and its constituents [2]. A meta-analysis of randomized controlled trials on the cognitive effects of B. monnieri extract also suggests that B. monnieri has the potential to improve cognition [3]. Other important pharmacological activities shown by B. monnieri include antiepileptic, anxiolytic, antidepressant, sedative, antioxidant and anti-inflammatory activities [4]. Various mechanisms of action for its cognitive effects have been proposed including acetylcholinesterase (AChE) inhibition, β-amyloid reduction, antioxidant neuroprotection, neurotransmitter modulation (acetylcholine [ACh], 5-hydroxytryptamine [5-HT], dopamine [DA]), choline acetyltransferase activation and increased cerebral blood flow [5].
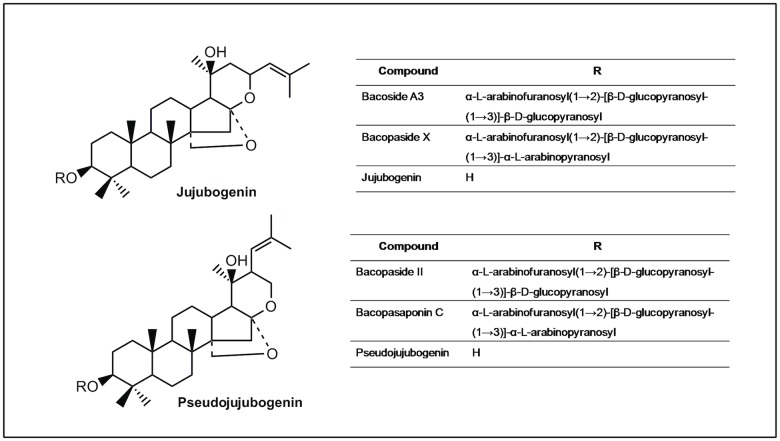
Characteristic saponins called ‘bacosides’, especially bacoside A, have been considered to be the main bioactive constituents responsible for the cognitive effects of B. monnieri [6–8]. Bacoside A is a mixture of four triglycosidic saponins, namely bacoside A3, bacopaside II, bacopasaponin C and the jujubogenin isomer of bacosaponin C (bacopaside X) [9]. These bacosides are dammarane types of triterpenoid saponins with jujubogenin or pseudojujubogenin moieties as the aglycone units (Fig 1) [10].
Fig 1. Structures of bacoside A saponin glycosides and aglycones.
Bacoside A is a mixture of bacoside A3, bacopaside II, bacopaside X and bacopasaponin C. These bacosides are dammarane-type triterpenoid saponins that have three sugar chains linked to a nonpolar triterpene aglycone skeleton.
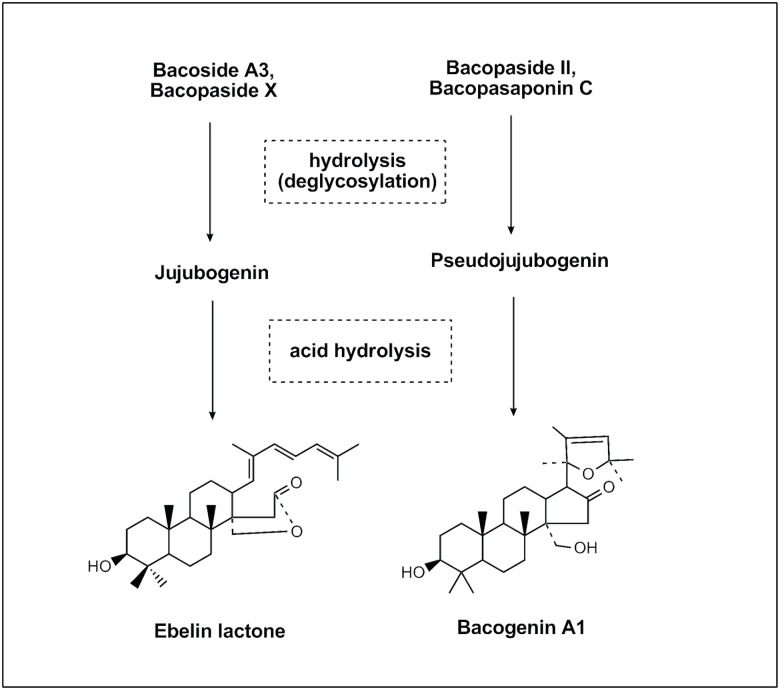
Saponins are susceptible to glycosidic cleavage at various sites to form secondary metabolites and finally the aglycone [11]. Triterpenoid saponins from other neuropharmacologically active plants such as ginsenoside [12] and jujuboside [13, 14] have shown that instead of the parent saponins, the metabolites transformed in vivo give better biological activity and pharmacokinetic characteristics. A recent study by Le et al. [15] showed that a B. monnieri extract had a negligible effect on the in vitro activity of AChE of brain tissues, whereas its daily systemic administration reduced the ex vivo activity of AChE in brain tissues. The study proposed that chemical constituent(s) of B. monnieri may be converted to their active form(s) in vivo with the ability to inhibit the activity of AChE in the brain. A recent report shows B. monnieri extracts inhibit some human cytochrome P450 (CYP) drug metabolizing enzymes [16]. It can also alter the expression of rat hepatic and intestinal CYP drug metabolizing enzymes and intestinal P-glycoprotein [17]. Thus, it is conceivable that the bacoside constituents present in B. monnieri extracts may be metabolized in vivo to active forms before exerting their pharmacological activities. Through sequential deglycosylation, bacoside A3, bacopaside II, bacopaside X and bacopasaponin C can be transformed to their aglycones jujubogenin or pseudojujubogenin. Jujubogenin and pseudojujubogenin can be further acid hydrolyzed to form ebelin lactone and bacogenin A1, respectively (Fig 2) [18].
Fig 2. Formation of ebelin lactone and bacogenin A1.
Bacoside A components form aglycone jujubogenin and pseudojujubogenin through deglycosylation and further acid hydrolysis yields ebelin lactone and bacogenin A1.
Therefore, in this study, we aim to compare the activity of the parent compounds (bacosides) with the aglycones (jujubogenin and pseudojujubogenin) and their derivatives (ebelin lactone and bacogenin A1) by studying the central nervous system (CNS) receptor (muscarinic, serotonin and dopamine) binding and AChE inhibition activities using a combination of in silico and in vitro screening methods. These assays were chosen because most CNS-related disorders such as schizophrenia, Alzheimer’s disease, epilepsy, and Parkinson's disease are related to neurotransmitters such as acetylcholine, dopamine, 5-hydroxytryptamine (5-HT) and their receptors [19]. In addition, physicochemical and ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) properties of the compounds were calculated using web-based applications and software to predict whether the compounds are orally active and have CNS drug properties. To the best of our knowledge, this is the first study on bacoside A aglycone and its derivatives.
Materials and Methods
Chemicals and reagents
Recombinant human membrane preparations for M1 (expressed in CHO cells; Lot no. 1706299), 5-HT1A (HEK293 cells; no. 1812363), 5-HT2A (CHO cells; no. 1813092), D1 (L cells; no. 1840059), and D2S (CHO cells; no. 1820984) receptors and MicroScint-O scintillation cocktail were procured from Perkin Elmer (Waltham, MA, USA). [3H] N-methylscopolamine (NMS), [3H] 8-OH-DPAT, [3H] ketanserin, [3H] SCH 23390 and [3H] methylspiperone were purchased from American Radiolabeled Chemicals (St. Louis, MO, USA). Atropine, 5-carboxamidotryptamine (5-CT), ketanserin, SCH 23390 hydrochloride, haloperidol, serotonin hydrochloride, mianserin, human recombinant acetylcholinesterase expressed in HEK 293 cells, acetylthiocholine iodide (ATChI), 5, 5’-dithiobis [2-nitrobenzoic acid] (DTNB) and donepezil hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO, USA). Bacoside A, bacoside A3, bacopaside II, bacopaside X and bacopasaponin C were purchased from Chromadex Inc. (Irvine, CA, USA). Jujubogenin and ebelin lactone were purchased from Shanghai IS Chemical Technology Ltd. (Jinshan, Shanghai, China). Unless stated otherwise, all other reagents of analytical grade were obtained through standard commercial sources.
In silico studies
Molecular docking
The two-dimensional (2-D) structures of the ligands (compounds) were built using ChemBioDraw Ultra 11.0 (Perkin Elmer) and converted to 3-dimensional (3-D) structures using Chem3D Pro 12.0 (Perkin Elmer). The resulting structures were subjected to energy minimization by MM2 force field and saved as MOL files. Finally the pdbqt formats (the input format of the docking software) of the ligands were prepared with AutoDockTools 1.5.6 [20] using default parameters.
For AChE, crystal structures of human AChE (hAChE) in complex with donepezil (2.35 Å) (PDB ID: 4EY7) and hAChE in complex with fasciculin II (2.76 Å) (PDB ID: 1B41) were extracted from the protein data bank (PDB) as a pdb file. The heteroatoms and water molecules were removed using Discovery Studio Visualizer 3.1 (Accelrys, San Diego, CA, USA). Hydrogens were added and double coordinates were corrected using HyperChem Pro 6.0 (Hypercube Inc., Gainesville, FL, USA). Then, hydrogens were added again, non-polar hydrogens were merged and the missing atoms were repaired using AutoDockTools 1.5.6. Finally, Kollman charges were added and AutoDock 4 type atoms were assigned to the protein. For the CNS receptors [serotonin: 5-HT1A, 5-HT2A; dopamine: D1, D2 and muscarinic acetylcholine (mACh): M1], validated homology models built from the previous work of our group were used [21, 22].
Docking studies were performed with AutoDock 4.2 [20], using a Lamarckian genetic algorithm [23] with a flexible ligand and a rigid receptor, a population size of 300, a maximum of 250,000 generations and 2,500,000 evaluations for 100 GA runs. The root mean square deviation (RMSD) tolerance was set to 2.0 Å for the clustering of docked results. Docking grids and the grid box was set to cover the transmembrane (TM) domain (for CNS receptors) and entire protein (for AChE enzyme). Ligand-receptor interactions were viewed using Discovery Studio Visualizer 3.1. Maestro 9.2 and PyMOL 1.3 (Schrödinger, LLC, New York, USA) were used to produce 2-D and 3-D figures.
Drug-like properties
Discovery Studio 4.0 molecular properties and ADMET descriptors were used to determine the CNS drug-like properties of the compounds. The ADMET descriptor estimates a range of properties for the compounds using QSAR models. Since these compounds are taken orally and were screened for CNS activity, intestinal absorption properties and blood brain barrier (BBB) penetration were evaluated. The molecular properties include molecular weight, polar surface area (PSA), log P (octanol-water partition coefficient), H-bond donors, H-bond acceptors and number of rotatable bonds.
In vitro radioligand receptor binding assay
The assay was performed according to the methods published previously [24, 25]. Bacoside A, bacopasaponin C, bacopaside X and bacoside A3 were assayed up to 100 μM whereas bacopaside II, jujubogenin and ebelin lactone were only assayed up to 30 μM due to poor solubility. Pseudojujubogenin and bacogenin A1 were not available for purchase at the time when the work was carried out. All compounds were dissolved in dimethylsulfoxide (DMSO) and the solvent was kept below 1% (v/v) in the final reaction mixture. Briefly, in each well, 100 μl of the respective membrane preparations (μg/well), 50 μl of the respective [3H]-ligand and 50 μl of the test compounds were added and the total assay reaction volume was made up to 200 μl by adding assay buffer. In place of the test compounds, 50 μl of 4x concentration of atropine (M1), serotonin (5-HT1A), mianserin (5-HT2A) and haloperidol (D1, D2) was added to respective wells to determine the non-specific binding (NSB) (or 100% inhibition) whereas 50 μl of assay buffer was added to determine the total binding (TB) of radioligand (or 0% inhibition). The reaction mixture was incubated for 60 or 120 min at room temperature or 37°C for the respective membranes. The reaction was stopped by rapid filtration onto GF/C filter plates (presoaked in 0.5% polyethyleneimine) using FilterMate (Perkin Elmer) cell harvester and washed with wash buffer (200 μl for 5 times) to remove unbound ligands. The filter plates were dried at 60°C for 15 minutes before the application of Bottom Seal and addition of 50 μl MicroScint-O scintillation cocktail. The top of the plate was then sealed with TopSeal A. Radioactivity (CPM) was counted using TopCount NXT microplate scintillation counter (Perkin-Elmer). A summary of the reaction components and the assay conditions are listed in Table 1. The data were analyzed by non-linear regression using Prism Version 5.0 (GraphPad Inc., San Diego CA, USA). The percentage of specific binding of radioligand in the presence of test compounds was calculated using the standard data reduction algorithm: ([B-NSB] / [TB-NSB]) × 100) where B is the binding in the presence of test compounds, NSB is the non-specific binding in the presence of excess reference ligand and TB is the total binding. K i values were calculated from the IC50 values using the Cheng-Prusoff equation [26].
Table 1. Summary of radioligand receptor binding assay components and reactions according to each receptor.
| Assay Components | Muscarinic (M1) | Serotonin (5-HT1A) | Serotonin (5-HT2A) | Dopamine (D1) | Dopamine (D2s) |
|---|---|---|---|---|---|
| Assay Buffer | PBS pH 7.4 | 50 mM Tris-HCl pH 7.4, 5 mM MgSO4 | 50 mM Tris-HCl pH 7.4, 4 mM CaCl2, 0.1% Ascorbic acid | 50 mM Tris-HCl pH 7.4, 1.5 mM CaCl2, 5 mM MgCl2, 5 mM EDTA, 5 mM KCl | 50 mM Tris-HCl pH 7.4, 120 mM NaCl, 5 mM KCl, 5 mM MgCl2, 1 mM EDTA |
| [ 3 H] Ligand, nM | 0.1 nM ([3H]-NMS) | 2 nM ([3H]-8-OH-DPAT) | 1 nM ([3H]-Ketanserin) | 0.2 nM ([3H]-SCH 23390) | 0.2 nM ([3H]-Methylspiperone) |
| NSB Ligand | Atropine (10 μM) | Serotonin (10 μM) | Mianserin (20 μM) | Haloperidol (20 μM) | Haloperidol (10 μM) |
| Human Recombinantmembranes | 17.5 μg/well | 16 μg/well | 5 μg/well | 2 μg/well | 3 μg/well |
| Incubation | 120 min @ 27°C | 120 min @ 37°C | 60 min @ 27°C | 60 min @ 27°C | 120 min @ 27°C |
| Wash Buffer | 50 mM Tris-HCl pH 7.4, 154 mM NaCl | 50 mM Tris-HCl pH 7.4 | 50 mM Tris-HCl pH 7.4 | 50 mM Tris-HCl pH 7.4 | 50 mM Tris-HCl pH 7.4, 154 mM NaCl |
| Washes (200 μl/well) | 5 | 5 | 5 | 5 | 5 |
In vitro AChE inhibition assay
The inhibitory activity of the compounds toward AChE was determined by following the method of Ellman et al. [27] with minor modifications using human recombinant AChE and acetylthiocholine as a substrate. Fifty-microliters (50 μl) of AChE enzyme (0.1 ng/well) in assay buffer [0.1 M sodium phosphate, 0.05% (w/v) Brij35], pH 7.5 and 25 μl of 4× concentrations of the test compounds were mixed in a microplate and left to incubate at room temperature for 30 minutes. Subsequently, 25 μl of a 4× ATChI / DTNB mixture (final concentration 200 μM / 100 μM) was added to the respective wells. This substrate mixture was prepared 5 min prior to being added to the plate in equal volumes of ATChI and DTNB. The hydrolysis of acetylthiocholine was monitored by measuring the absorbance due to yellow 5-thio-2-nitrobenzoate anion in a kinetic mode at a wavelength of 405 nm for 10 min. The enzyme activity was measured in the presence (Asample) and in the absence (Acontrol) of the test compounds. All the tests were carried out in triplicate and the enzyme inhibition was calculated as: % Inhibition = 100 –[(Asample) / (Acontrol) x 100].
Results
The aglycones show better docking than the parent bacosides
Docking results were analyzed and the best-docked conformation was chosen based on the number of conformations in a cluster and the estimated free energy of binding. Higher numbers of conformations and the lowest binding energy indicate better affinity of the compound to the CNS receptor and AChE enzyme. As shown in Table 2, the docking of the parent compounds, bacopasaponin C, bacopaside X, bacopaside II and bacoside A3 in the CNS receptors and AChE gave a very low number of conformations in a cluster and were not able to fit into the orthosteric site of the CNS receptors and AChE. In contrast, the aglycones (jujubogenin, pseudojujubogenin, ebelin lactone and bacogenin A1) with higher number of conformations in a cluster, docked better than the parent compounds in the CNS receptors and AChE. These results indicate that the binding pockets of the CNS receptors and AChE were not able to accommodate the large glycoside groups on the bacosides. Among these compounds, ebelin lactone showed the strongest binding towards all the CNS receptors, with the lowest estimated free energy of binding.
Table 2. Total number of conformations in a cluster and binding energy of compounds for 5-HT1A, 5-HT2A, D1, D2 and M1 receptors and AChE (in complex with fasciculin or donepezil) enzyme.
| Compound | Total number of conformations in a cluster (estimated binding energy kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|
| 5-HT1A | 5-HT2A | D1 | D2 | M1 | AChE (fasciculin) | AChE (donepezil) | |
| Bacopa-saponin C | 13 (-9.99) | 6 (-12.25) | 1 (-11.12) | 4 (-13.33) | 4 (-12.94) | 5 (-8.63) | 5 (-10.90) |
| Bacopaside X | 7 (-6.97) | 6 (-12.25) | 2 (-10.50) | 1 (-10.64) | 1 (-11.68) | 3 (-9.61) | 5 (-8.35) |
| Bacopaside II | 8 (-6.43) | 1 (-11.18) | 7 (-10.51) | 2 (-10.44) | 5 (-12.06) | 3 (-8.02) | 3 (-7.88) |
| Bacoside A3 | 3 (-6.03) | 1 (-11.38) | 1 (-9.74) | 1 (-9.75) | 3 (-12.23) | 6 (-8.21) | 4 (-7.30) |
| Jujubogenin | 41 (-9.31) | 25 (-8.54) | 63 (-8.93) | 50 (-8.90) | 70 (-10.68) | 99 (-11.65) | 99 (-11.63) |
| Psuedo-jujubogenin | 79 (-9.42) | 24 (-8.28) | 16 (-8.99) | 63 (-9.26) | 100 (-11.33) | 90 (-11.41) | 96 (-11.40) |
| Ebelin Lactone | 97 (-9.58) | 38 (-10.79) | 28 (-9.27) | 78 (-11.06) | 79 (-11.71) | 83 (-11.59) | 83 (-11.59) |
| Bacogenin A1 | 49 (-9.07) | 24 (-9.19) | 38 (-8.56) | 28 (-9.52) | 68 (-10.71) | 74 (-9.26) | 65 (-11.59) |
The aglycones have better CNS drug-like properties than the parent bacosides
Since B. monnieri is taken orally and has neuropharmacological activities, the active constituents that give the pharmacological activity are necessarily orally and CNS active, i.e., the compound must be absorbed through the intestine and penetrate the BBB. Through various studies, the accepted criteria for CNS drug properties have been found to include molecular weight < 450, polar surface area (PSA) < 60–70 Å2 (upper limit is 90 Å2), Log P < 5, H-bond donor < 3, H-bond acceptor < 7 and number of rotatable bonds < 8 [28]. From Table 3, the parent bacosides (bacopasaponin C, bacopaside X, bacopaside II, bacoside A3) fail to meet all but one (Log P < 5) criteria of oral CNS drug candidates. In particular, they fail to meet the criteria for molecular weight (> 899 Da), hydrogen-bonding capacity (hydrogen bond acceptors, 17 to 18; hydrogen bond donors 9 to 10) and molecular flexibility (number of rotatable bonds, 9 to 10). These unfavorable physiochemical traits of the parent bacosides most likely result in poor membrane permeability through the intestine and BBB. However, the aglycones (jujubogenin, pseudojujubogenin, ebelin lactone and bacogenin A1) showed better CNS drug-like properties by meeting four of the required criteria. The removal of the sugar moieties decreases the molecular weight, hydrogen-bonding capacity and molecular flexibility, and increases the lipophilicity of the aglycones (Log P, 5.46 to 7.22) compared to the corresponding parent bacosides (Log P, 3.30 to 3.72). Although the molecular weight for the aglycones are slightly more than 450, according to Hansch et al. [29] small molecules may undergo significant passive lipid-mediated transport through the BBB when the molecular mass is kept in or below the 400–600 Da range. Furthermore, increasing lipophilicity of aglycones also tends to increase their brain permeation [28].
Table 3. The physicochemical properties of bacopasaponin C, bacopaside X, bacopaside II, bacoside A3, jujubogenin, pseudojujubogenin, ebelin lactone and bacogenin A1.
| Compounds | Molecular Weight (< 450) | PSA (< 60–70 Å2) | Log P (< 5) | H-bond donor (< 3) | H-bond acceptor (< 7) | No. of rotatable bonds (< 8) |
|---|---|---|---|---|---|---|
| Bacopasaponin C | 899.07 | 256 | 3.72 | 9 | 17 | 9 |
| Bacopaside X | 899.07 | 256 | 3.54 | 9 | 17 | 9 |
| Bacopaside II | 929.10 | 279 | 3.48 | 10 | 18 | 10 |
| Bacoside A3 | 929.10 | 279 | 3.30 | 10 | 18 | 10 |
| Jujubogenin | 472.70 | 59 | 7.22 | 2 | 4 | 1 |
| Psuedojujubogenin | 472.70 | 59 | 6.89 | 2 | 4 | 1 |
| Ebelin Lactone | 454.68 | 47 | 6.77 | 1 | 3 | 3 |
| Bacogenin A1 | 472.70 | 67 | 5.46 | 2 | 4 | 2 |
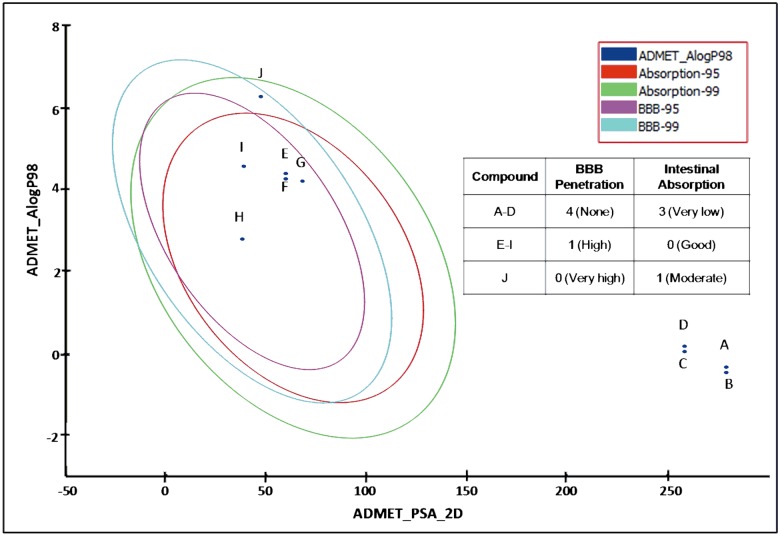
Compounds that are taken orally for CNS activity should be able to be absorbed from the intestines and penetrate the BBB. The ADMET human intestinal absorption (HIA) and BBB penetration model are defined by 95% and 99% confidence ellipses in the ADMET_PSA_2D, ADMET_AlogP98 plane (Fig 3) [30, 31]. These ellipses describe the regions where compounds that are both well-absorbed and able to penetrate the BBB are expected to be found. Compounds outside the 95% and 99% confidence ellipsoids are considered to have very low intestinal absorption and BBB penetration. The ADMET descriptor gives four prediction levels within the 95% and 99% confidence ellipsoids for HIA and BBB. The four levels for HIA are 0 (good), 1 (moderate), 2 (poor), 3 (very poor); whereas for BBB, 0 (very high penetrant), 1 (high), 2 (medium), 3 (low) and 4 (undefined). As shown in Fig 3, parent compounds bacopasaponin C, bacopaside X, bacopaside II and bacoside A3 had no BBB penetration and showed very poor intestinal absorption. In contrast, the aglycones jujubogenin, pseudojujubogenin, ebelin lactone and bacogenin A1 were well absorbed through the intestine and had high BBB penetration. Among the compounds ebelin lactone had the highest BBB penetration and predicted to be moderately absorbed through the intestine.
Fig 3. BBB penetration and intestinal absorption properties by ADMET descriptors.
(A) bacoside A3, (B) bacopaside II, (C) bacopasaponin C, (D) bacopaside X, (E) jujubogenin, (F) pseudojujubogenin, (G) bacogenin A1, (H) tacrine, (I) donepezil and (J) ebelin lactone. Tacrine and donepezil were used as standard orally active CNS drugs. ADMET prediction level for Human intestinal absorption (HIA)- 0 (good), 1 (moderate), 2 (poor), 3 (very poor); Blood brain barrier (BBB)- 0 (very high penetrant), 1 (high), 2 (medium), 3 (low) and 4 (undefined). The aglycones and its acid hydrolysis derivatives showed better intestinal absorption and BBB penetration compared to the parent bacosides.
Overall, the in silico studies demonstrated that the parent bacosides with glycones attached were not able to dock into CNS receptors and AChE, and had poor molecular and ADMET properties as CNS drugs. On the other hand, the aglycones and their acid hydrolysis derivatives showed better binding affinity towards the CNS receptors and AChE enzyme and conform to the required criteria for intestinal absorption and penetration of the BBB.
Ebelin lactone interacts with M1 and 5-HT2A receptors
At the time of this study, pseudojujubogenin and bacogenin A1 were not available for purchase. Hence, bacopasaponin C, bacopaside X, bacopaside II, bacoside A3, bacoside A (mixture of the four bacosides), jujubogenin and ebelin lactone were further analyzed by in vitro assays. The compounds were assayed in vitro, for their ability to displace [3H] NMS, [3H] 8-OH-DPAT, [3H] ketanserin, [3H] SCH 23390 and [3H] methylspiperone from M1, 5-HT1A, 5-HT2A, D1, and D2 receptors, respectively, and to inhibit AChE activity. The results of the receptor binding assays are shown in Table 4.
Table 4. CNS receptor binding affinities of bacosides and aglycones.
| Compound a | IC50 (μM) | ||||
|---|---|---|---|---|---|
| M1 | 5-HT1A | 5-HT2A | D1 | D2 | |
| Bacoside A | > 100 | > 100 | > 100 | 24.65 ± 3.76 (K i, 12.14 ± 1.68 μM) | > 100 |
| Bacopa-saponin C | > 100 | > 100 | > 100 | > 100 | > 100 |
| Bacopaside X | > 100 | > 100 | > 100 | 19.49 ± 3.07 (K i, 9.06 ± 1.36 μM) | > 100 |
| Bacoside A3 | > 100 | > 100 | > 100 | > 100 | > 100 |
| Bacopaside II | > 30 | > 30 | > 30 | > 30 | > 30 |
| Jujubogenin | > 30 | > 30 | > 30 | > 30 | > 30 |
| Ebelin Lactone | 0.80 ± 0.19 (K i, 0.45 ± 0.11 μM) | > 30 | 14.48 ± 4.98 (K i, 4.21 ± 1.45 μM) | > 30 | > 30 |
Values are expressed as the mean ± S.D. of three determinations with two independent experiments.
aBacoside A, bacopasaponin C, bacopaside X and bacoside A3 were assayed up to 100 μM whereas bacopaside II, jujubogenin and ebelin lactone were assayed up to 30 μM due to poor solubility.
Most of the parent bacoside compounds did not show binding affinity towards M1, 5-HT1A, 5-HT2A, D1 and D2 receptors except bacoside A and bacopaside X, which showed some affinity towards D1 receptor. Since bacopaside X is part of the mixture in bacoside A (K i = 12.14 μM), the binding might be due to bacopaside X (K i = 9.06 μM). Contrary to the in silico result, the aglycone jujubogenin did not show binding affinity towards all the receptors tested here. However, its derivative ebelin lactone showed affinity towards M1 (K i = 0.45 μM) and 5-HT2A (K i = 4.21 μM) receptors, which are implicated in memory and learning processes [32, 33]. The K i values of ebelin lactone for M1 and 5-HT2A are comparable to that of M1 agonists acetylcholine and pilocarpine (K i = 59 and 2.7 μM, respectively) [25] and 5-HT agonist serotonin (K i = 23.9 μM) [34]. However, none of the compounds showed any inhibitory activity against AChE.
Ebelin lactone may act as an allosteric modulator of M1 and 5-HT2A receptors via non-polar interactions
The investigation of the complexes obtained from the docking of ebelin lactone into M1 muscarinic acetylcholine receptor (mAChR) and 5-HT2A models revealed that it does not fit into their primary/orthosteric binding sites (Fig 4). In the case of the M1 mAChR, ebelin lactone bound to a cavity directly above the orthosteric site and established interactions with a set of residues that formed the binding pocket, mainly through non-polar interactions (Figs 4 and 5). Among these interacting residues, L183 (from the extracellular loop 2), Y82 and L86 are postulated to be responsible for the muscarinic subtype selectivity. These residues are non-conserved residues among the subtypes, located above the orthosteric site that was identified by site-directed mutagenesis experiments [35–40]. It is also obvious that there is an overlapping of the ebelin lactone binding pocket with the orthosteric binding pocket, as Y106, T192, Y381, and Y404 from the orthosteric site are found within 4 Å from the bound ebelin lactone. The superposition of the recent crystal structure of M2 mAChR in complex with an allosteric modulator LY2119620 (PDB code: 4MQS) [41] and the docked ebelin lactone in M1 mAChR showed that both ligands share part of the binding cavity, suggesting that ebelin lactone could be an allosteric modulator, with a good selectivity profile (Fig 6) [42]. However, further studies are required to definitively determine the selectivity of ebelin lactone on other muscarinic subtypes.
Fig 4. Docking of the ebelin lactone to (A) the M1 mAChR and (B) the 5-HT2A models.
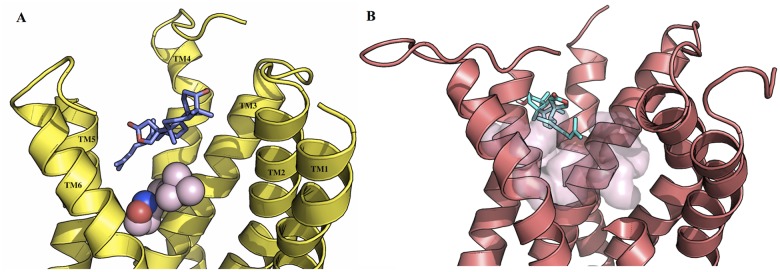
Iperoxo (sphere) from the crystal structure of M2 mAChR (PDB code: 4MQS) was used to show the orthosteric site in the M1 mAChR, and the transparent surface represents the orthosteric site of the 5-HT2A receptor. Ebelin lactone bound to a cavity directly above the orthosteric site suggesting it could be an allosteric modulator. For the purpose of clarity, some of the loops and transmembrane helix are not shown.
Fig 5. 2-D interaction map of ebelin lactone in complex with the M1 mAChR model.
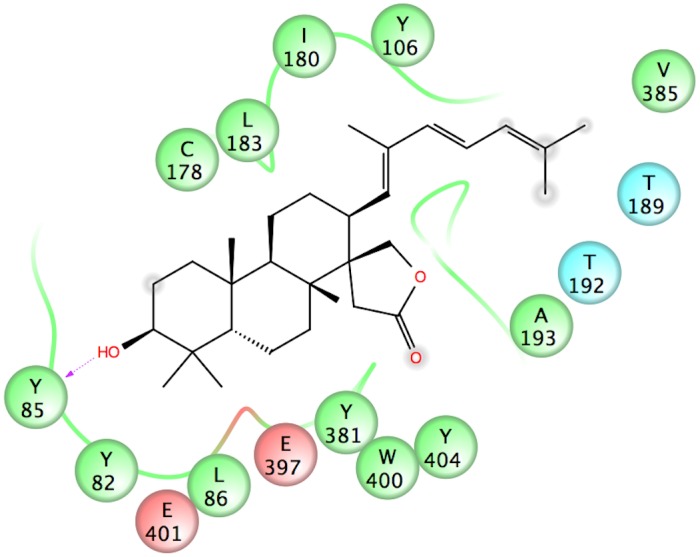
Negatively-charged, polar and hydrophobic residues are depicted with red, light blue and green circles, respectively. The hydrogen bond between the OH group at position-3 and Y85 residue is indicated by a purple dashed arrow. Ebelin lactone established non-polar interactions with L183, Y82 and L86 (non-conserved residues) which are postulated to be responsible for the allosteric subtype selectivity in muscarinic receptors.
Fig 6. Superposition of ebelin lactone (blue) in complex with the M1 mAChR model and LY2119620 (green) in complex with the M2 mAChR.
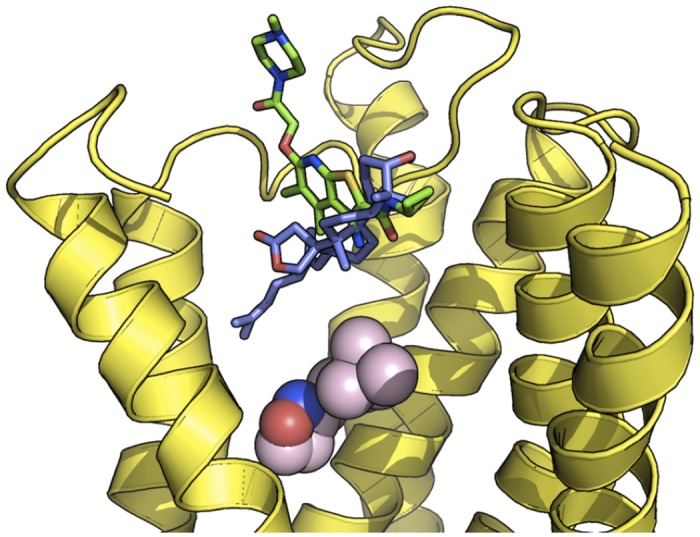
Superposition of the crystal structure of the allosteric modulator LY2119620 in complex with M2 mAChR (PDB code: 4MQS) with the docked ebelin lactone in M1 mAChR shows the overlapping binding positions of these ligands, suggesting ebelin lactone could be a M1 allosteric modulator. Iperoxo is shown in spheres to depict the orthosteric site of the receptor.
Ebelin lactone docked to the 5-HT2A receptor in a very distinct way. It did not bind to the cavity on top of the orthosteric site but, instead, almost half of the structure (the tricyclic terpenoid moiety) is found fitted in the cavity in between TM4 and TM5. The pocket seems to be an almost horizontal extension of the orthosteric binding pocket in 5-HT2A. Ebelin lactone is coordinated by a set of residues only through non-polar interactions, including those that are found in the orthosteric site, such as, D155, S159, S242, W336, and F339 (Fig 7) [43–47]. This unique binding of ebelin lactone is possible as the recent crystal structure of free-fatty acid receptor 1 [48] in complex with an allosteric modulator, TAK-875 (PDB code: 4PHU), revealed that the ligand binds to a non-canonical binding pocket, between TM3 and TM4 (Fig 8). Overall, binding interactions of ebelin lactone with the M1 and 5-HT2A receptor models suggest that ebelin lactone is most likely an allosteric modulator that interacts with the residues mainly through non-polar interactions.
Fig 7. 2-D interaction map of ebelin lactone in complex with the 5-HT2A receptor model.
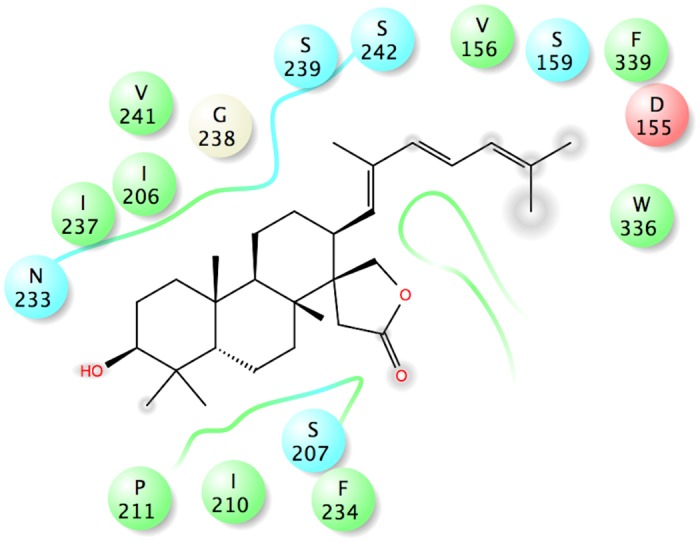
Negatively-charged, polar and hydrophobic residues are depicted with red, light blue and green circles, respectively. Ebelin lactone is coordinated by a set of residues only through non-polar interactions.
Fig 8. Superposition of ebelin lactone (cyan) in complex with the 5-HT2A receptor and TAK-875 (green) in complex with free-fatty acid receptor 1.
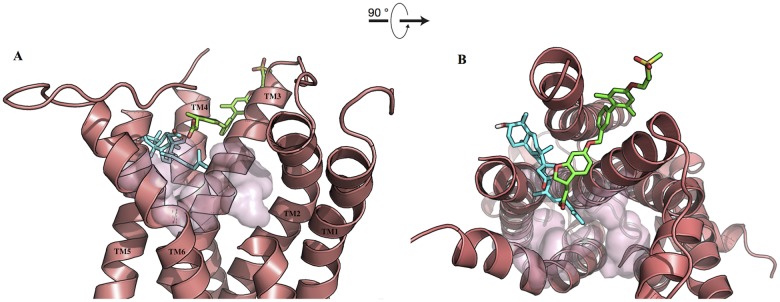
A. Front view and B. Top view (from the extracellular surface). Superposition of ebelin lactone in complex with the 5-HT2A receptor from the docking studies and the allosteric modulator TAK-875 in complex with the free-fatty acid receptor 1 from the crystal structure (PDB code: 4PHU) shows both ligands bound to cavities in between the transmembrane helices suggesting ebelin lactone could be a 5-HT2A allosteric modulator. The transparent surface represents the orthosteric site of the 5-HT2A receptor. For the purpose of clarity, some of the loops and transmembrane helix are not shown.
Discussion
The memory enhancing and cognitive effects of B. monnieri are believed to be mediated by bacoside A, a mixture of bacoside A3, bacopacide II, bacopasaponin C and bacopaside X. However, evidence regarding the bacoside components responsible for the activity and the mechanisms of action are still unclear. This study shows that bacoside A is unlikely to be absorbed through the intestine or to penetrate the BBB, using in silico models. Therefore, the bacosides are likely to undergo transformation in vivo to remove the sugar units as well as other biotransformations, that result in metabolites that may mediate the memory enhancing and cognitive activities. This is consistent with other neuropharmacologically active plants such as ginseng [12], Ginkgo biloba [49] and jujube (red date) [13, 14], where their respective active constituents are formed via the metabolism of the parent compounds in vivo.
Unlike bacosides, the aglycones (jujubogenin and pseudo-jujubogenin) and their acid hydrolyzed derivatives (ebelin lactone and bacogenin A1) produced higher predicted binding affinity towards all the CNS receptors and stronger docking to AChE in silico. They also had CNS drug-like properties which suggested that they would show better oral absorption and penetration through the BBB. Among the ligands, ebelin lactone had the strongest binding of all the CNS receptors and the highest expected BBB penetration.
Although until now there are no pharmacokinetics studies on bacosides, similar studies on other saponin glycosides such as ginsenoside [50] and flavonoid glycosides such as quercetin glucoside [51] have been reported. In these studies, the parent glycosides were not found in the plasma after oral administration, while their metabolites were detected. This poor intestinal absorption of the glycosides is most likely due to their low membrane permeability. Therefore, prior to intestinal absorption into the systemic circulation, these glycosides undergo deglycosylation in the intestinal tract. In the case of ginsenoside RB1, deglycosylation of the glycosides is by gastric acid, which remove the sugar units [52]. Another proposed mechanism is the hydrolysis of glycosides by lactase phloridzin hydrolase (LPH) and cytosolic β-glucosidase (CBG). LPH, a β-glucosidase found on the outside of the brush border membrane of the small intestine, hydrolyzes the glycosides and the liberated aglycones can then be absorbed into the systemic circulation from the small intestine through passive diffusion [53]. CBG on the other hand is located intracellularly in the enterocytes and so requires active transport of the hydrophilic glycosides into the cells via the sugar transporter sodium-dependent glucose co-transporter 1 (SGLT-1). CBG is capable of hydrolyzing a broad range of glycosides including glucosides, galactosides, xylosides, arabinosides, and fucosides [54]. Besides this, glycosides that are not substrates for LPH, CBG and SGLT-1, will be transported towards the colon where they may be hydrolyzed by colonic bacteria to release the aglycones, which can then be absorbed into the systemic circulation via passive uptake [12, 14, 55].
The absorbed aglycones in the systemic circulation can then cross the BBB into the brain. This mechanism is supported by the findings that 18β-glycyrrhetinic acid, a metabolite of glycyrrhizin [56], and kaempferol and isorhamnetin [49] are detected in the brain after oral administration of the parent glycosides. In other instances, the absorbed aglycone from the intestine may go through conjugation (methylation, sulphatation and glucuronidation) and exist in the plasma in the conjugated forms as with flavonoid aglycones [57]. Flavonoids in the form of aglycones and conjugated forms are able cross the BBB. During passage of the BBB, the conjugates may be metabolized back to the parent aglycone, which then enters the central nervous system [58]. Therefore, similar to glycosides from other CNS active plants and our in silico results here a similar pharmacokinetics behavior is expected for the bacosides.
The findings from the in vitro radioligand receptor binding assays confirmed the favorable affinity of the aglycone derivative, ebelin lactone towards M1 (K i = 0.45 μM) and 5-HT2A (4.21 μM) receptors, where the binding activities are similar to other known M1 and 5-HT agonists. In contrast, the aglycone jujubogenin did not give significant binding affinity towards the receptors. This difference could be explained by the presence of the carbonyl oxygen of the lactone ring in ebelin lactone, which is lacking in jujubogenin. The carbonyl oxygen of a lactone ring has previously been reported to be essential for the activity of pilocarpine at M1 receptors [59]. In the current work, the in silico studies were unable to identify the precise role of the carbonyl oxygen in the binding of ebelin lactone, perhaps due to limitations in the docking and scoring functions used, such as not allowing full conformational flexibility in the receptor. However, they did suggest that ebelin lactone could act as an allosteric modulator via non-polar interactions. Such allosteric binding of the aglycone derivatives to the M1 and 5-HT2A receptors, distinct from orthosteric interaction, may offer greater selectivity and reduced side effects, and may conceivably contribute to the cognitive function and safety of B. monnieri [60–63].
M1 mAChR and serotonin 5-HT2A receptors are expressed abundantly in brain regions essential for cognitive functions such as the prefrontal cortex and hippocampus. The stimulation of these receptors by their respective agonists has been shown to improve cognition and to enhance learning in humans and animal models [33, 64]. M1 receptors are associated with cholinergic transmission whereas 5-HT2A receptors are associated with both cholinergic and glutamatergic transmission, and are implicated in cognition by regulating the release of these and other neurotransmitters [32, 33]. Cognitive functions are said to be dependent on the ability of neurons to change their function i.e. neural plasticity [65]. The pyramidal neurons (pyramidal cells) are the primary excitation units in the mammalian cortical structures which play important roles in cognition through their neural plasticity (synaptic plasticity) function and are also expressed abundantly in the prefrontal cortex and hippocampus [66]. At cellular levels the M1 receptors are located on the dendrites of cortical pyramidal cells [67, 68] whereas the 5-HT2A receptors are located on both the dendrites of cortical pyramidal cells and the interneurons [69]. Activation of M1 and 5-HT2A metabotrobic receptors in pyramidal cells activates phospholipase C (PLC), which subsequently promotes the release of diacylglycerol (DAG) and inositol triphosphate (IP3), stimulate protein kinase C (PKC) activity and Ca2+ release, leading to activations of signal transduction pathways that result in increased neural plasticity [70, 71]. In addition, the location of 5-HT2A receptors in the cortex and hippocampus on cholinergic [72] and glutamatergic [73] axon terminals serves to regulate the release of these transmitters where the increased release of acetylcholine and glutamate are expected to enhance learning [33]. Pyramidal cells use glutamate as their excitatory neurotransmitter, and GABA as their inhibitory neurotransmitter [74].
There is evidence that the mechanisms of action of B. monnieri could be attributed to a combination of cholinergic modulation especially through the muscarinic cholinergic receptor. B. monnieri extract has been reported to alleviate the amnesic effects of scopolamine, a muscarinic receptor antagonist, suggesting a crucial role of muscarinic receptors in the action of B. monnieri [15, 75]. Furthermore, the administration of B. monnieri for two weeks reversed the depletion of acetylcholine, reduced choline acetylase activity and decreased muscarinic cholinergic receptor binding in the frontal cortex and hippocampus of rats with AD, induced by the neurotoxin colchicine [76]. In addition to this, B. monnieri extract was found to induce neurite and neuronal dendritic growth [77, 78], and studies have shown that muscarinic receptor activation plays a key role in neurite outgrowth [79, 80]. Previous work has demonstrated that treatment with B. monnieri extract caused an increase in 5-HT levels in the hippocampus, hypothalamus and cerebral cortex of rats [8, 81]. Charles et al. [82] also found that B. monnieri extract caused a significant up-regulation in the synthesis of 5-HT and altered the ACh level, and proposed that the elevated 5-HT level may activate their receptor to facilitate the release of ACh and thus enhance learning ability and memory.
In vitro studies suggest B. monnieri extract did not inhibit AChE directly [15, 83]. However, brain homogenate obtained from rats fed with B. monnieri extract showed anti-AChE activity [15, 84]. Treatment with bacosides on aged rats for a 3 months period appeared to enhance the synthesis and availability of acetylcholine rather than affecting the activity of AChE [85]. Our in silico findings suggest that the aglycones dock on the catalytic site of AChE better than the parent bacosides. However, the same pattern is not reflected in the in vitro AChE inhibition study. The reason for the discrepancy has not been determined. It is conceivable that the crystal structure of AChE for this study optimized for fasciculin and donepezil is not suitable for bacosides and their aglycones as their structures are quite different. It is also worth while noting that pseudojujubogenin and bacogenin A1, which have not been tested, may bind to AChE and cause inhibition.
Our findings are based on bacoside A (bacoside A3, bacopaside X, bacopaside II, bacopasaponin C), its aglycones (jujubogenenin and pseudojujubogenin) and its derivatives (ebelin lactone and bacogenin A1). Although both pseudojujubogenin and bacogenin A1 were evaluated by in silico studies, they were not evaluated with in vitro receptor binding assays and the AChE inhibition assay. It is conceivable that they may interact with the CNS receptors and bind to the catalytic site of AChE to contribute to the cognitive effect of B. monnieri.
This study used validated in silico receptor and AChE models from our previous work to predict the activities of bacosides, aglycones and their derivatives. However, the adopted in silico models were built and validated using ligands structurally different from that of the bacosides tested. Therefore, the models may not be fully reliable for bacosides. Nevertheless, this combination of in silico and in vitro studies gives an overall picture of absorption (pharmacokinetics) and pharmacodynamics of bacosides.
Conclusions
In this study, we have demonstrated through a combination of in silico and in vitro experiments that the bacoside aglycone derivative, ebelin lactone, has better CNS drug-like and receptor-binding properties compared to the parent compound. Hence, we suggest B. monnieri constituents may be transformed and metabolized to the active form in vivo before exerting their pharmacological activity. Additional studies are required to determine the actual metabolites of B. monnieri in order to further elucidate the memory enhancing and cognitive actions of this plant. Ebelin lactone may also be an interesting CNS drug candidate that is worthy of further investigation. The results from such studies will give more of an indication of the potential of B. monnieri for the treatment of AD.
Acknowledgments
The authors would like to express their utmost gratitude to the late Dr. Khalit Mohamad for his support during the research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors wish to acknowledge the grants RG032/10BIO provided by the University of Malaya, and High Impact Research Grants HIR-MOHE (UM.C/625/1/HIR/MOHE/MED/17 and UM.C/625/1/HIR/MOHE/MED/33) provided by the Ministry of Higher Education, Malaysia, for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Indian Drug Manufacturers Association (IDMA). Indian Herbal Pharmacopeia, Rev. new ed Mumbai: The Association; 2002. 76 p. [Google Scholar]
- 2. Rajani M. Bacopa monnieri, a nootropic drug In: Ramawat KG, Merillon JM, editors. Bioactive molecules and medicinal plants. Berlin: Springer; 2008. p. 175–195. [Google Scholar]
- 3. Kongkeaw C, Dilokthornsakul P, Thanarangsarit P, Limpeanchob N, Norman Scholfield C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J Ethnopharmacol. 2014;151: 528–535. 10.1016/j.jep.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 4. Russo A, Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine. 2005;12: 305–317. [DOI] [PubMed] [Google Scholar]
- 5. Aguiar S, Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri . Rejuvenation Res. 2013;16: 313–326. 10.1089/rej.2013.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh HK, Rastogi RP, Srimal RC, Dhawan BN. Effect of bacoside A and B on the avoidance response in rats. Phytother Res. 1988;2: 70–75. [Google Scholar]
- 7. Dhawan BN, Singh HK. Pharmacological studies on Bacopa monnieri, an Ayurvedic nootropic agent. Eur Neuropsychopharmacol. 1996;6: 144. [Google Scholar]
- 8. Singh HK, Dhawan BN. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi). Indian J Pharmacol. 1997;29: S359–S365. [Google Scholar]
- 9. Deepak M, Sangli GK, Arun PC, Amit A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem Anal. 2005;16: 24–29. [DOI] [PubMed] [Google Scholar]
- 10. Sivaramakrishna C, Rao CV, Trimurtulu G, Vanisree M, Subbaraju GV. Triterpenoid glycosides from Bacopa monnieri . Phytochemistry. 2005;66: 2719–2728. [DOI] [PubMed] [Google Scholar]
- 11. Kren V, Martínková L. Glycosides in medicine: "The role of glycosidic residue in biological activity". Curr Med Chem. 2001;8: 1303–1328. [DOI] [PubMed] [Google Scholar]
- 12. Leung KW, Wong AS. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5: 20 10.1186/1749-8546-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen CYC. Chemoinformatics and pharmacoinformatics approach for exploring the GABA-A agonist from Chinese herb suanzaoren. J Taiwan Inst Chem Eng. 2009;40: 36–47. [Google Scholar]
- 14. Zhang Y, Xie J, Zhang Y, Zhang M. Degradation kinetics of jujuboside A by rat intestinal flora and identification of the metabolites by HPLC-MS/MS. Int J Food Prop. 2014;17: 1841–1849. [Google Scholar]
- 15. Le XT, Pham HT, Do PT, Fujiwara H, Tanaka K, Li F, et al. Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice: possible involvement of glutamatergic and cholinergic systems. Neurochem Res. 2013;38: 2201–2215. 10.1007/s11064-013-1129-6 [DOI] [PubMed] [Google Scholar]
- 16. Ramasamy S, Kiew LV, Chung LY. Inhibition of human cytochrome P450 enzymes by Bacopa monnieri standardized extract and constituents. Molecules. 2014;19: 2588–2601. 10.3390/molecules19022588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh R, Panduri J, Kumar D, Kumar D, Chandsana H, Ramakrishna R, et al. Evaluation of memory enhancing clinically available standardized extract of Bacopa monniera on P-glycoprotein and cytochrome P450 3A in Sprague-Dawley rats. Plos One. 2013;8: e72517 10.1371/journal.pone.0072517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kulshreshtha DK, Rastogi RP. Identification of ebelin lactone from Bacoside A and the nature of genuine saponin. Phytochemistry. 1973;12: 2074–2076. [Google Scholar]
- 19. Lefkowitz RJ, Hoffman BB, Taylor P. Neurohumoral transmission: the autonomic and somatic motor nervous systems In: Gilman AG, Rall TW, Nites AS, Taylor P, editors. The Pharmacological Basis of Therapeutics, ed 8 New York: Pergamon Press; 1990. p. 84–121. [Google Scholar]
- 20. Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30: 2785–2791. 10.1002/jcc.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yap BK, Buckle MJ, Doughty SW. Homology modeling of the human 5-HT1A, 5-HT2A, D1, and D2 receptors: model refinement with molecular dynamics simulations and docking evaluation. J Mol Model. 2012;18: 3639–3655. 10.1007/s00894-012-1368-5 [DOI] [PubMed] [Google Scholar]
- 22. Chin SP, Buckle MJ, Chalmers DK, Yuriev E, Doughty SW. Toward activated homology models of the human M1 muscarinic acetylcholine receptor. J Mol Graph Model. 2014;49: 91–98. 10.1016/j.jmgm.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 23. Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19: 1639–1662. [Google Scholar]
- 24. Chung LY, Goh SH, Imiyabir Z. Central nervous system receptor activities of some Malaysian plant species. Pharm Biol. 2005;43: 280–288. [Google Scholar]
- 25. Swaminathan M, Chee CF, Chin SP, Buckle MJ, Rahman NA, Doughty SW, et al. Flavonoids with M1 muscarinic acetylcholine receptor binding activity. Molecules. 2014;19: 8933–8948. 10.3390/molecules19078933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22: 3099–3108. [DOI] [PubMed] [Google Scholar]
- 27. Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7: 88–95. [DOI] [PubMed] [Google Scholar]
- 28. Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. Neuro Rx. 2005;2: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansch C, Björkroth JP, Leo A. Hydrophobicity and central nervous system agents: on the principle of minimal hydrophobicity in drug design. J Pharm Sci. 1987;76: 663–687. [DOI] [PubMed] [Google Scholar]
- 30. Egan WJ, Merz KM Jr, Baldwin JJ. Prediction of drug absorption using multivariate statistics. J Med Chem. 2000;43: 3867–3877. [DOI] [PubMed] [Google Scholar]
- 31. Egan WJ, Lauri G. Prediction of intestinal permeability. Adv Drug Deliv Rev. 2002;54: 273–289. [DOI] [PubMed] [Google Scholar]
- 32. Fisher A. Cholinergic treatments with emphasis on M1 muscarinic agonists as potential disease-modifying agents for Alzheimer's disease. Neurotherapeutics. 2008;5: 433–442. 10.1016/j.nurt.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harvey JA. Role of the serotonin 5-HT(2A) receptor in learning. Learn Mem. 2003;10: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chung LY, Lo MW, Mustafa MR, Goh SH, Imiyabir Z. 5-Hydroxytryptamine2A receptor binding activity of compounds from Litsea sessilis . Phytother Res. 2009;23: 330–334. 10.1002/ptr.2627 [DOI] [PubMed] [Google Scholar]
- 35. Allman K, Page KM, Curtis CA, Hulme EC. Scanning mutagenesis identifies amino acid side chains in transmembrane domain 5 of the M(1) muscarinic receptor that participate in binding the acetyl methyl group of acetylcholine. Mol Pharmacol. 2000;58: 175–184. [DOI] [PubMed] [Google Scholar]
- 36. Blüml K, Mutschler E, Wess J. Functional role in ligand binding and receptor activation of an asparagine residue present in the sixth transmembrane domain of all muscarinic acetylcholine receptors. J Biol Chem. 1994;269: 18870–18876. [PubMed] [Google Scholar]
- 37. Huang XP, Nagy PI, Williams FE, Peseckis SM, Messer WS Jr. Roles of threonine 192 and asparagine 382 in agonist and antagonist interactions with M1 muscarinic receptors. Br J Pharmacol. 1999;126: 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu ZL, Hulme EC. The functional topography of transmembrane domain 3 of the M1 muscarinic acetylcholine receptor, revealed by scanning mutagenesis. J Biol Chem. 1999;274: 7309–7315. [DOI] [PubMed] [Google Scholar]
- 39. Lu ZL, Saldanha JW, Hulme EC. Transmembrane domains 4 and 7 of the M(1) muscarinic acetylcholine receptor are critical for ligand binding and the receptor activation switch. J Biol Chem. 2001;276: 34098–34104. [DOI] [PubMed] [Google Scholar]
- 40. Ward SD, Curtis CA, Hulme EC. Alanine-scanning mutagenesis of transmembrane domain 6 of the M(1) muscarinic acetylcholine receptor suggests that Tyr381 plays key roles in receptor function. Mol Pharmacol. 1999;56: 1031–1041. [DOI] [PubMed] [Google Scholar]
- 41. Kruse AC, Ring AM, Manglik A, Hu J, Hu K, Eitel K, et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504: 101–106. 10.1038/nature12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kruse AC, Kobilka BK, Gautam D, Sexton PM, Christopoulos A, Wess J. Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov. 2014;13: 549–560. 10.1038/nrd4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang CD, Gallaher TK, Shih JC. Site-directed mutagenesis of the serotonin 5-hydroxytrypamine2 receptor: identification of amino acids necessary for ligand binding and receptor activation. Mol Pharmacol. 1993;43: 931–940. [PubMed] [Google Scholar]
- 44. Kristiansen K, Kroeze WK, Willins DL, Gelber EI, Savage JE, Glennon RA, et al. A highly conserved aspartic acid (Asp-155) anchors the terminal amine moiety of tryptamines and is involved in membrane targeting of the 5-HT(2A) serotonin receptor but does not participate in activation via a "salt-bridge disruption" mechanism. J Pharmacol Exp Ther. 2000;293: 735–746. [PubMed] [Google Scholar]
- 45. Almaula N, Ebersole BJ, Zhang D, Weinstein H, Sealfon SC. Mapping the binding site pocket of the serotonin 5-hydroxytryptamine2A receptor. Ser3.36(159) provides a second interaction site for the protonated amine of serotonin but not of lysergic acid diethylamide or bufotenin. J Biol Chem. 1996;271: 14672–14675. [DOI] [PubMed] [Google Scholar]
- 46. Braden MR, Nichols DE. Assessment of the roles of serines 5.43(239) and 5.46(242) for binding and potency of agonist ligands at the human serotonin 5-HT2A receptor. Mol Pharmacol. 2007;72: 1200–1209. [DOI] [PubMed] [Google Scholar]
- 47. Roth BL, Shoham M, Choudhary MS, Khan N. Identification of conserved aromatic residues essential for agonist binding and second messenger production at 5-hydroxytryptamine2A receptors. Mol Pharmacol. 1997;52: 259–266. [DOI] [PubMed] [Google Scholar]
- 48. Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, et al. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014;513: 124–127. 10.1038/nature13494 [DOI] [PubMed] [Google Scholar]
- 49. Rangel-Ordóñez L, Nöldner M, Schubert-Zsilavecz M, Wurglics M. Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba extract EGb 761. Planta Med. 2010;76: 1683–1690. 10.1055/s-0030-1249962 [DOI] [PubMed] [Google Scholar]
- 50. Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 51. Hollman PCH. Absorption, bioavailability, and metabolism of flavonoids. Pharm Biol. 2004;42: 74–83. [Google Scholar]
- 52. Shen H, Leung WI, Ruan JQ, Li SL, Lei JP, Wang YT, et al. Biotransformation of ginsenoside Rb1 via the gypenoside pathway by human gut bacteria. Chin Med. 2013;8: 22 10.1186/1749-8546-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Day AJ, Canada FJ, Diaz JC, Kroon PA, Mclauchlan R, Faulds CB, et al. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468: 166–170. [DOI] [PubMed] [Google Scholar]
- 54. Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, et al. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr. 2003;42: 29–42. [DOI] [PubMed] [Google Scholar]
- 55. Ruan JQ, Leong WI, Yan R, Wang YT. Characterization of metabolism and in vitro permeability study of notoginsenoside R1 from Radix notoginseng. J Agric Food Chem. 2010;58: 5770–5776. 10.1021/jf1005885 [DOI] [PubMed] [Google Scholar]
- 56. Tabuchi M, Imamura S, Kawakami Z, Ikarashi Y, Kase Y. The bloodbrain barrier permeability of 18b-glycyrrhetinic acid, a major metabolite of glycyrrhizin in glycyrrhiza root, a constituent of the traditional Japanese medicine yokukansan. Cell Mol Neurobiol. 2012;32: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79: 727–747. [DOI] [PubMed] [Google Scholar]
- 58. Jäger AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16: 1471–1485. 10.3390/molecules16021471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shapiro G, Floersheim P, Boelsterli J, Amstutz R, Bolliger G, Gammenthaler H, et al. Muscarinic activity of the thiolactone, lactam, lactol, and thiolactol analogues of pilocarpine and a hypothetical model for the binding of agonists to the ml receptor. J Med Chem. 1992;35: 15–27. [DOI] [PubMed] [Google Scholar]
- 60. Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8: 41–54. 10.1038/nrd2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Keov P, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology. 2011;60: 24–35. 10.1016/j.neuropharm.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 62. Pravina K, Ravindra KR, Goudar KS, Vinod DR, Joshua AJ, Wasim P, et al. Safety evaluation of BacoMind in healthy volunteers: a phase I study. Phytomedicine. 2007;14: 301–308. [DOI] [PubMed] [Google Scholar]
- 63. Barbhaiya HC, Desai RP, Saxena VS, Pravina K, Wasim P, Geetharani P, et al. Efficacy and tolerability of BacoMind on memory improvement in elderly participants—a double blind placebo controlled study. J Pharmacol Toxicol. 2008;3: 425–434. [Google Scholar]
- 64. Clader JW, Wang Y. Muscarinic receptor agonists and antagonists in the treatment of Alzheimer’s disease. Curr Pharm Des. 2005;11: 3353–3361. [DOI] [PubMed] [Google Scholar]
- 65. Mercado E 3rd. Neural and cognitive plasticity: from maps to minds. Psychol Bull. 2008;134: 109–137. 10.1037/0033-2909.134.1.109 [DOI] [PubMed] [Google Scholar]
- 66. Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9: 206–221. 10.1038/nrn2286 [DOI] [PubMed] [Google Scholar]
- 67. Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1–m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci. 1995;15: 4077–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yamasaki M, Matsui M, Watanabe M. Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci. 2010;30: 4408–4418. 10.1523/JNEUROSCI.5719-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci. 1998;95: 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Van der Zee EA, Kronforst-Collins MA, Maizels ET, Hunzicker-Dunn M, Disterhoft JF. gamma Isoform-selective changes in PKC immunoreactivity after trace eyeblink conditioning in the rabbit hippocampus. Hippocampus. 1997;7: 271–285. [DOI] [PubMed] [Google Scholar]
- 71. Weiss C, Preston AR, Oh MM, Schwarz RD, Welty D, Disterhoft JF. The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons J Neurosci. 2000;20: 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Quirion R, Richard J, Dam TV. Evidence for the existence of serotonin type-2 receptors on cholinergic terminals in rat cortex. Brain Res. 1985;333: 345–349. [DOI] [PubMed] [Google Scholar]
- 73. Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: Emerging role of glutamate mechanisms. Brain Res Rev. 2000;31: 302–312. [DOI] [PubMed] [Google Scholar]
- 74. Megías M, Emri Z, Freund TF, Gulyás AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102: 527–540. 11226691 [Google Scholar]
- 75. Saraf MK, Prabhakar S, Khanduja KL, Anand A. Bacopa monniera attenuates scopolamine-induced impairment of spatial memory in mice. Evid Based Complement Alternat Med. 2011;2011: 236186 10.1093/ecam/neq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bhattacharya SK, Kumar A, Ghosal S. Effect of Bacopa monniera on animal models of Alzheimer's disease and perturbed central cholinergic markers of cognition in rats. Res Commun Pharmacol Toxicol. 1999;4: II.1-II.12. [Google Scholar]
- 77. Vollala VR, Upadhya S, Nayak S. Enhancement of basolateral amygdaloid neuronal dendritic arborization following Bacopa monniera extract treatment in adult rats. Clinics. 2011;66: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sharma RJ, Chaphalkar SR, Dikshit M. Screening of plant derived secondary metabolites that help the axonal regrowth in neurons after the spinal cord injury. Neurosci Res Lett. 2012;3: 35–39. [Google Scholar]
- 79. De Jaco A, Augusti-Tocco G, Biagioni S. Muscarinic acetylcholine receptors induce neurite outgrowth and activate the synapsin I gene promoter in neuroblastoma clones. Neuroscience. 2002;113: 331–338. [DOI] [PubMed] [Google Scholar]
- 80. Anelli T, Mannello F, Salani M, Tonti GA, Poiana G, Biagioni S. Acetylcholine induces neurite outgrowth and modulates matrix metalloproteinase 2 and 9. Biochem Biophys Res Commun. 2007;362: 269–274. [DOI] [PubMed] [Google Scholar]
- 81. Sheikh N, Ahmad A, Siripurapu KB, Kuchibhotla VK, Singh S, Palit G. Effect of Bacopa monniera on stress induced changes in plasma corticosterone and brain monoamines in rats. J Ethnopharmacol. 2007;111: 671–676. [DOI] [PubMed] [Google Scholar]
- 82. Charles PD, Ambigapathy G, Geraldine P, Akbarsha MA, Rajan KE. Bacopa monniera leaf extract up-regulates tryptophan hydroxylase (TPH2) and serotonin transporter (SERT) expression: implications in memory formation. J Ethnopharmacol. 2011;134: 55–61. 10.1016/j.jep.2010.11.045 [DOI] [PubMed] [Google Scholar]
- 83. Das A, Shanker G, Nath C, Pal R, Singh S, Singh H. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: anticholinesterase and cognitive enhancing activities. Pharmacol Biochem Behav. 2002;73: 893–900. [DOI] [PubMed] [Google Scholar]
- 84. Ahirwar S, Tembhre M, Gour S, Namdeo A. Anticholinesterase efficacy of Bacopa monnieri against the brain regions of rat—a novel approach to therapy for Alzheimer’s disease. Asian J Exp Sci. 2012;26: 65–70. [Google Scholar]
- 85. Rastogi M, Ojha RP, Prabu PC, Devi BP, Agrawal A, Dubey GP. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontology. 2012;13: 183–195. 10.1007/s10522-011-9367-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.