Abstract
The physiological control of steroid hormone secretion from the adrenal cortex depends on the function of potassium channels. The “two-pore domain K+ channels” (K2P) TWIK-related acid sensitive K+ channel 1 (TASK1), TASK3, and TWIK-related K+ channel 1 (TREK1) are strongly expressed in adrenocortical cells. They confer a background K+ conductance to these cells which is important for the K+ sensitivity as well as for angiotensin II and adrenocorticotropic hormone-dependent stimulation of aldosterone and cortisol synthesis. Mice with single deletions of the Task1 or Task3 gene as well as Task1/Task3 double knockout mice display partially autonomous aldosterone synthesis. It appears that TASK1 and TASK3 serve different functions: TASK1 affects cell differentiation and prevents expression of aldosterone synthase in the zona fasciculata, while TASK3 controls aldosterone secretion in glomerulosa cells. TREK1 is involved in the regulation of cortisol secretion in fasciculata cells. These data suggest that a disturbed function of K2P channels could contribute to adrenocortical pathologies in humans.
Keywords: KCNK2, KCNK3, KCNK9, TASK, TREK, Aldosterone
Introduction
The distinct zones of the adrenal cortex produce different steroid hormones, which regulate several important physiological functions. The mineralocorticoid aldosterone is synthesized by the outermost cell layer (zona glomerulosa) beneath the capsule of the adrenal gland. Glucocorticoids are produced in the zona fasciculata that consists of column-like organized cells below zona glomerulosa. In humans, but not in rodents, the innermost zona reticularis cells produce androgenic steroid hormones. In cells of all three zones of the adrenal cortex, the function of K+ channels is an important determinant for controlling hormone secretion, cell differentiation, proliferation, and possibly apoptosis. This review aims to discuss the physiology and pathophysiology of “two-pore domain K+ channels” (K2P) in the adrenal cortex, especially in aldosterone-producing glomerulosa cells.
Aldosterone controls the extracellular fluid and salt balance by stimulation of sodium reabsorption and potassium secretion in the distal nephron of the kidney, in the distal colon, and in sweat glands. By controlling water and salt balance and by direct effects on the cardiovascular system, aldosterone has a major impact on blood pressure control. So-called primary aldosteronism is characterized by inappropriately high plasma aldosterone levels due to autonomous aldosterone synthesis. Inadequately high aldosterone secretion is believed to be causal for about 3 % of the cases of arterial hypertension [97]. Additionally, aldosterone contributes to cardiac fibrosis, cardiovascular dysfunction, and progressive kidney disease [59, 111]. The relevance of aldosterone as clinical risk factor has been stressed by clinical trials (Aldosterone Evaluation Study (RALES); EPlerenone HEart failure and SUrvival Study (EPHESUS)) [16, 148]. Therefore, understanding the physiology and pathophysiology of aldosterone synthesis is of great relevance for the diagnosis and treatment of arterial hypertension and cardiovascular disease.
It is known for a long time that the regulation of aldosterone synthesis strongly depends on the modulation of the membrane potential of glomerulosa cells. Also cortisol synthesis appears to be stimulated by depolarization of the plasma membrane. The membrane potential of resting adrenocortical cells is mainly determined by the function of K+ channels. Accordingly, the disturbed function of adrenal K+ channels has pathological consequences for the regulation of steroid hormone production, and it may lead to excessive proliferation of adrenocortical cells.
Adrenocortical K+ channels
The resting membrane potential of human glomerulosa cells is set by a number of K+ channels, particularly of the K2P family, which are highly expressed among species (Table 1). In rodents, two members of the TWIK-related acid sensitive K+ (Task) family (Task1 and Task3) were shown to play an important role in the regulation of aldosterone secretion and adrenocortical cell differentiation [7, 28, 34, 51, 55, 103]. TWIK-related K+ channel 1 (Trek1) is important for the normal function of the bovine adrenal cortex [38, 41]. The role of K2P channels in the human adrenal gland is still under investigation. Several studies indicate that K2P channels contribute to the physiological control of aldosterone synthesis in human adrenocortical cells. TASK1 is strongly expressed in the human adrenal cortex [22] and in the human adrenocortical NCI-H295R cell line [96]. Silencing of TASK1 expression stimulates aldosterone secretion in NCI-H295R cells [96]. TREK1 and TASK3 K+ channels are also expressed in NCI-H295R cells, albeit on a much lower level than TASK1 [96]. Inactivation of TREK1 and TASK3 depolarizes the membrane potential of NCI-H295R cells. However, the amount of this depolarization is likely small, because aldosterone production is not significantly increased [14]. Decreased expression of TASK2 was found in adrenal adenomas [5, 72], and suppression of TASK2 activity in NCI-H295R cells increased aldosterone synthesis [72]. TREK1 was shown to dominate the K+ conductance of human fasciculata cells [39]. Most of the knowledge about the functional role of Task K+ channels has been obtained by phenotyping different knockout mouse models. The following paragraphs aim at providing a comprehensive overview of the specific role and relevance of K2P channels for the regulation of steroid hormone synthesis and zonal differentiation of the adrenal gland.
Table 1.
Adrenal K+ channels
| Channel | Expression | Function | Pathology | Reference |
|---|---|---|---|---|
| TASK1 (KCNK3) | Mouse: ZG>ZF>inner adrenal cortex | Maintenance and regulation of membrane potential of adrenocortical and medullary cells; Inhibition by Ang-II and endothelin-1; prevention of Cyp11b2 expression in ZF of ♀ mice | Task1 −/− mouse: sex-dependent hyperaldosteronism due to ectopic Cyp11b2 expression in ZF | [22, 27, 28, 55, 62, 63, 86, 96, 121] |
| Rat: ZG, medulla | Human: pulmonary hypertension | |||
| Guinea pig: medulla | ||||
| Human: ZG>ZF>ZR (unpublished data), aldosterone-producing adenoma, adrenocortical cell line (NCI-H295R cells) | ||||
| TASK3 (KCNK9) | Mouse: ♂ ZG, ZF; ♀ ZG | Maintenance and regulation of membrane potential of adrenal cortex; inhibition by Ang-II; probably heterodimers with Task1 | Mild hyperaldosteronism in adult Task3 −/− mice; severe hyperaldosteronism in neonatal Task3 −/− mice | [6, 9, 14, 22, 25, 26, 28, 51, 83, 103] |
| Rat: ZG | ||||
| Human: low adrenal expression compared to TASK1 and TASK2; adrenocortical cell line (NCI-H295R cells) | Human: hypotension and mental retardation | |||
| TASK2 (KCNK5) | Mouse: inner adrenal cortex (unpublished data) | Probably maintenance and regulation of membrane potential of adrenal cortex; expression of a dominant negative TASK2 mutant in NCI-H295R cells stimulated aldosterone synthesis | Decreased expression in aldosterone-producing adenomas | [5, 22, 26, 72] |
| Human: adrenal cortex | ||||
| TREK1 (KCNK2) | Bovine: ZG, ZF | Maintenance and regulation of membrane potential of adrenal cortex; inhibition by Ang-II, ACTH and vasopressin; expression induced by ACTH and cAMP | [22, 36–39, 41, 80–82] | |
| Human: ZF | ||||
| Mouse: unknown | ||||
| KCNJ5 (Kir3.4/GIRK4) | Human: ZG>ZF, aldosterone-producing adenoma, adrenocortical cell line (NCI-H295R cells) | Function in ZG still unknown; Gßγ activated; Ang-II reduced KCNJ5 expression | Somatic mutations in 30–40 % of aldosterone-producing adenomas; germline mutations in patients with familial hyperaldosteronism type III | [5, 22, 69, 98] |
| Pig: ZG (unpublished data) | ||||
| KCNQ1/KCNE1 | Mouse: adrenal cortex | Repolarization of membrane potential; KCNE1 as regulatory subunit; voltage activated | Kcnq1 −/− mouse: hypoaldosteronism | [4, 22, 116, 138] |
| Human: adrenal cortex (K+ channel with the highest level of expression), adrenocortical cell line (NCI-H295R cells) | Kcne1−/− mouse: hyperaldosteronism under hyperkalemia | |||
| Maxi K (KCNMA1/KCNMB1) | Mouse: ZG and medulla | Repolarization of membrane potential; Ca2+ and voltage activated; channel activation by ANP inhibits aldosterone production; KCNMB1 as regulatory subunit | Kcnma1 −/− mouse: hyperaldosteronism | [22, 46, 48, 118, 143, 146, 147] |
| Human: adrenal cortex | Kcnmb1 −/− mouse: hyperaldosteronism |
ZG zona glomerulosa, ZF zona fasciculata
Stimulation of aldosterone secretion
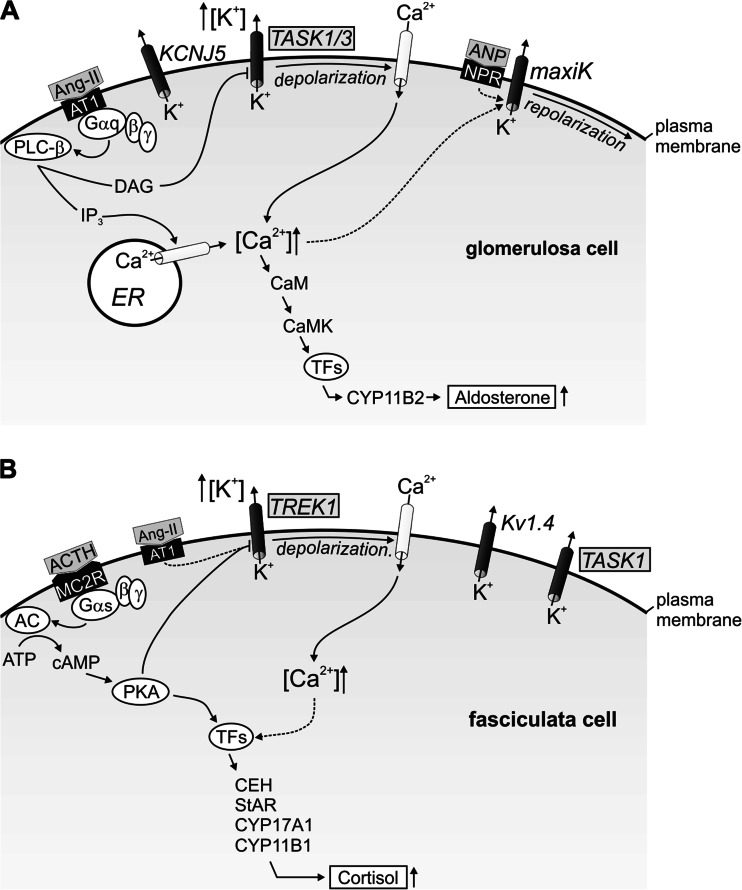
Aldosterone synthesis in adrenal zona glomerulosa cells is mainly stimulated by angiotensin II (Ang-II), by high plasma K+ concentrations, and, to a minor extent, by the adrenocorticotropic hormone (ACTH). For the stimulation of aldosterone synthesis by Ang-II or hyperkalemia, modulation of the membrane potential is an early and critical early event in the cellular signaling cascade (Fig. 1). Therefore, precise control of the membrane voltage is very important. A large proportion of the K+ channels that determine the resting membrane voltage of glomerulosa cells are constitutively open, e.g., “background” or “leak” K+ channels of the K2P family. Due to the high K+ conductance, the resting membrane potential of glomerulosa cells is hyperpolarized (−80 mV), close to the K+ equilibrium potential. An increase of the extracellular K+ concentration, according to Nernst’s equation, leads to a positive shift of the K+ equilibrium potential and to a depolarization. By this mechanism, glomerulosa cells are able to sense changes of plasma K+ concentration, reminiscent of K+-selective electrodes. Upon depolarization of the membrane, voltage-dependent T-type and L-type Ca2+ channels are activated, thereby translating the membrane depolarization into a rise of the intracellular Ca2+ activity. High intracellular Ca2+ activity, via binding to calmodulin and activation of calmodulin-dependent kinases, induces transcription of particular enzymes needed for aldosterone synthesis, e.g., aldosterone synthase (CYP11B2), and steroidogenic acute regulatory protein (StAR) [23]. Aldosterone synthase catalyzes the final three-step reaction from 11-deoxycorticosterone to aldosterone, and it is considered to be the rate-limiting enzyme of aldosterone synthesis. StAR is a transport protein facilitating the shuttling of cholesterol from the outer to the inner mitochondrial membrane where cholesterol is converted to pregnenolone, a precursor of steroid hormones.
Fig. 1.
Simplified models for the regulation of aldosterone synthesis in zona glomerulosa cells (a) and of cortisol synthesis in zona fasciculata cells (b). a Stimulatory action of Ang-II and increased plasma K+ concentration on aldosterone synthesis depends on membrane voltage depolarization and on increased cytosolic Ca2+. G-Protein-dependent activation of phospholipase-C (PLC-ß) via binding of Ang-II to angiotensin receptor 1 (AT1) leads to generation of inositol-triphosphate (IP3) and diacylglycerol (DAG). IP3 stimulates Ca2+ store release from the endoplasmatic reticulum (ER). DAG-dependent inhibition of TASK1 and TASK3 K+ channels or a high K+-induced shift of the Nernst potential depolarize the membrane. The depolarization activates voltage-dependent Ca2+ channels. Ca2+-calmodulin activates CaM-Kinases, and this leads to activation of transcription factors (TFs) and increased transcription of CYP11B2 (aldosterone synthase). MaxiK K+ channels are activated by the atrial natriuretic peptide (ANP), which binds to the natriuretic peptide receptor (NPR), or by increases of cytosolic Ca2+. MaxiK channels repolarize glomerulosa cells and decrease aldosterone synthesis. KCNJ5 K+ channels are highly expressed in human glomerulosa cells, but seem to be inactive under control conditions. b The stimulatory effect of ACTH on cortisol synthesis depends on cAMP-dependent signaling, but also involves membrane depolarization and increased cytosolic Ca2+. ACTH binds to the melanocortic-2-receptor (MC2R) and leads to activation of a Gαs-protein that stimulates adenylate cyclase (AC). cAMP-activated protein kinase A (PKA) activates transcription factors (TFs) inducing transcription of steroidogenic enzymes. These enzymes are required for cortisol synthesis (e.g., CHE: cholesterolester hydrolase, StAR: steroidogenic acute regulated protein, CYP17A1, CYP11B1). PKA also inhibits TREK1 K+ channels, depolarizes the membrane and promotes Ca2+ influx and consecutive activation of transcription factors. TREK1 is also inhibited by Ang-II. Additionally, TASK1 and Kv1.4 K+ channels are expressed in fasciculata cells
The mechanism by which Ang-II depolarizes the membrane is different from the one of high extracellular K+. Ang-II depolarizes the plasma membrane by inhibiting background K2P K+ channels. The molecular mechanism of the Ang-II-mediated K+ channel inhibition was a matter of debate for a long time [19, 79, 87, 121] but was solved only recently. Binding of Ang-II to the AT1 receptor activates phospholipase-C via Gαq-proteins. By cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2), phospholipase-C generates diacylglycerol (DAG) and inositol-triphosphate (IP3). Interestingly, it appears that DAG acts as a K2P channel-inhibiting factor leading to a strong decrease of the fractional K+ conductance and depolarization of the membrane [145]. Similar to the high K+-induced depolarization, the Ang-II-induced depolarization activates voltage-gated Ca2+ channels and leads to Ca2+ influx [1, 58, 137]. In addition, Ang-II facilitates the opening of Ca2+ channels by lowering the voltage threshold for activation [84], and it induces a release of Ca2+ from IP3-sensitive intracellular Ca2+ stores [129]. The cytosolic rise of Ca2+ is further amplified by store-operated Ca2+ entry [95, 106, 112, 139].
Besides its effects on gene transcription of steroidogenic enzymes, intracellular Ca2+ induces a very fast non-transcriptional stimulation of aldosterone secretion. In mice, a strong peak of aldosterone secretion is observed within 10 min after injection of Ang-II [130], although aldosterone is not believed to be stored within the cells. Probably, a variety of non-genomic effects of Ca2+ underlies this fast response, e.g., an increase of intracellular Ca2+ stimulates the activity of StAR [21, 76] and is paralleled by an influx of Ca2+ into the mitochondria [144]. A rise in mitochondrial Ca2+ enhances the availability of NAPDH, a cofactor of several steroidogenic enzymes [107, 113]. As a negative feedback, an increase of intracellular Ca2+ activates Ca2+-regulated K+ channels, which hyperpolarize the membrane, thereby preventing overwhelming aldosterone secretion. Chronic stimulation of aldosterone production elicits trophic effects on the adrenal gland. Long-term treatment of rats with Ang-II or a low Na+ diet induces proliferation and hypertrophy of aldosterone-producing glomerulosa cells [88, 114].
ACTH acts at least in two ways on adrenocortical cells; it acutely stimulates cortisol (and aldosterone) secretion, and it promotes cell proliferation and differentiation of glomerulosa and fasciculata cells [131, 134]. With regard to aldosterone secretion, the acute effect of ACTH is mainly mediated via an increased supply of cholesterol, the precursor of steroid hormones [54]. In addition, ACTH facilitates voltage-gated Ca2+ channel opening probably by direct phosphorylation and indirectly via inhibition of K+ channels leading to depolarization [38, 39, 41, 44, 81, 124, 136]. In bovine and human fasciculata cells, ACTH is a negative regulator of TREK1. TREK1 is also expressed in bovine cells from zona glomerulosa and in human adrenocortical NCI-H295R cells [14, 36, 80]. Inactivation of TREK1 in NCI-H295R cells induces a depolarization [14]. By contrast, native primary cultured human glomerulosa cells do not show K+ currents resembling TREK1 [102]. Trek1 is also expressed in mouse adrenocortical cells (UniGene data), but an adrenal phenotype of the Trek1 knockout mouse has not yet been reported [56, 91, 142].
Adrenocortical cells are excitable
Cultured primary cells or adrenocortical cell lines display a very high K2P K+ channel activity which is inhibited by Ang-II. The inhibition of these K+ channels depolarizes the membrane and triggers the activation of voltage-gated Ca2+ channels and hormone synthesis. Dispersed and cultured adrenocortical cells are considered non-excitable cells; they usually do not display action potentials. However, experiments under more physiological conditions, e.g., on fresh adrenal slices, have disclosed that native adrenocortical cells are excitable with rapid oscillations of membrane voltage and intracellular Ca2+ [58, 103, 109, 115]. In slice preparations, Ang-II-induced Ca2+ waves propagate from cell to cell suggesting functional coupling of adrenocortical cells via gap junctions [103]. Most likely, oscillatory activity of K+ and Ca2+ channels and Ca2+-transporting systems is the molecular correlate of these oscillations. The absence of Ca2+ oscillations in most adrenocortical cell lines and adrenal primary cells suggests that adrenal-specific cell differentiation and electrical properties are not sufficiently preserved in these “model systems”.
What is the possible role of K+ channels without oscillatory activity, such as K2P, in excitable adrenocortical cells? As mentioned above, the activity of K+ channels is essential for hyperpolarizing the membrane voltage at resting conditions, and it allows glomerulosa cells to act as sensors of extracellular K+. Constitutively, active K+ channels ensure the hyperpolarized membrane voltage that is required for hyperpolarization-dependent extrusion of Ca2+, e.g., via Na+/Ca2+ exchangers. Moreover, these K+ channels determine the level of excitability of adrenocortical cells and the frequency of action potentials, reminiscent of the role of KATP K+ channels in insulin-secreting cells. In pancreatic beta cells, KATP channel inhibition is induced by rises of the ATP/ADP ratio and results in membrane depolarization, action potentials, and oscillations of intracellular Ca2+ [60]. In a similar way, Ca2+ oscillations in adrenal glomerulosa cells are triggered by Ang-II-induced inhibition of K2P channels. This function of adrenal K2P channels is important for the regulation of aldosterone synthesis under physiological and pathophysiological conditions [58].
Other factors controlling aldosterone secretion
Besides the main regulators (Ang-II, high plasmatic K+ concentrations, ACTH), a variety of other mediators modulate aldosterone secretion, e.g., serotonin, endothelin-1 [129]. Over the last decades, the pathways stimulating aldosterone secretion have been in the focus of research activities. Conversely, mechanisms that inhibit the synthesis of aldosterone and prevent excessive secretion are also important e.g., atrial natriuretic peptide (ANP)-induced inhibition of StAR expression [20], ANP-mediated activation of Ca2+-dependent MaxiK (KCNMA1) K+ channels [46], activation of voltage-gated K+ channels such as KCNQ1, as well as Na+- and G-protein-dependent activation of Kir3.4 (KCNJ5) K+ channels. These K+ channels have in common that their activation hyperpolarizes the membrane thereby reducing Ca2+ influx and aldosterone secretion. Other regulatory pathways involve the control of Na+/K+ ATPase activity and of transport systems lowering intracellular Ca2+, e.g., Na+/Ca2+ exchangers (NCX and NCKX) and Ca2+ ATPases [11, 133]. In addition to acute effects, a complex network of regulating factors controls cell proliferation, centripetal migration, and differentiation as well as apoptosis of adrenocortical cells [129].
Regulation of cortisol/corticosterone synthesis
Glucocorticoids affect a plethora of physiological functions, e.g., lipid and glucose metabolism and the immune system. Inappropriately, high cortisol production is a characteristic for Cushing’s syndrome (hypercortisolism) [126]. Cortisol synthesis in fasciculata cells is mainly controlled by ACTH. Binding of ACTH to the Gs-protein-coupled melanocortin 2 receptor (MC2R) activates adenylate cyclase leading to increased cAMP synthesis. Activated by cAMP, protein kinase A (PKA) phosphorylates several target proteins. Two of these target proteins improve the supply with cholesterol: cholesterol ester hydrolase (CEH) releases cholesterol from esterified cholesterol in intracellular lipid droplets [125], and StAR shuttles cholesterol into the inner mitochondrial membrane. Moreover, PKA phosphorylates transcription factors that stimulate expression of the 11ß-hydroxylase (CYP11B1), the enzyme catalyzing the final step of glucocorticoid synthesis [47, 127]. The central role of PKA signaling for cortisol synthesis was recently underlined by the observation that a somatic gain-of-function mutation of PKA is present in about 50 % of patients with cortisol-producing adrenal adenomas [12, 15, 47, 117]. Another target of PKA is the K2P channel TREK1 that is inhibited by phosphorylation [39]. Due to inhibition of TREK1, ACTH stimulation leads to depolarization of fasciculata cells and activation of voltage-dependent Ca2+ channels [39]. One might speculate that chronic TREK1 inhibition contributes to the pathophysiology of cortisol-producing adenoma cells carrying the gain-of-function mutation of PKA. Interestingly, the Trek1 knockout mouse does not present with obvious signs of Cushing’s syndrome suggesting that Trek1 inhibition increases glucocorticoid secretion only in the presence of other stimulating factors.
Why are such mutations enhancing cAMP-dependent pathways not detected in aldosterone-producing adenomas? Adrenocortical cells undergo a centripetal migration and differentiation starting from capsular and subcapsular stem cells and ending up by apoptosis at the border between the adrenal cortex and medulla. The subcapsular-medullar migration is accompanied by a shift of differentiation from the glomerulosa to the fasciculata cell type [43]. ACTH influences these important processes: ACTH stimulates proliferation and accelerates centripetal differentiation of adrenocortical cells [131]. On the other hand, suppression of ACTH by dexamethasone decreases proliferation and differentiation [131]. Thus, alterations of the cAMP/PKA pathway will most likely effect proliferation, migration, and differentiation of adrenocortical cells. One might speculate that in glomerulosa cells, mutational activation of PKA will probably cause an accelerated differentiation. Thus, adenomas originating from glomerulosa cells with constitutively active PKA might be phenotypical classified as cortisol secreting tumors. Further studies are needed to test this hypothesis.
Besides activating aldosterone secretion, Ang-II also stimulates synthesis of cortisol in bovine and human fasciculata cells [39, 40]. Fasciculata cells display similar oscillations of the membrane potential as it was described for glomerulosa cells [8, 58, 94, 108]. Via inhibition of K2P channels, Ang-II depolarizes fasciculata cells resulting in activation of voltage-dependent Ca2+ channels. These synergistic effects of ACTH and Ang-II on cortisol secretion might be of particular importance under stress conditions when a strong increase of cortisol secretion is needed.
Mutations of ion-transporting membrane proteins are associated with aldosterone-producing adenomas
The regulation of membrane voltage and cytosolic Ca2+ activity is central for the physiological control of aldosterone secretion. Disturbed function of proteins controlling membrane voltage and Ca2+ homeostasis can cause adrenal diseases. The importance of the control of membrane voltage and intracellular ion composition is exemplified by somatic mutations of K+ channels, Ca2+ channels, the Na+/K+-ATPase, and a plasma membrane Ca2+-ATPase found in aldosterone-producing adenomas [3]. The most frequently mutated gene is the inwardly rectifying K+ channel Kir3.4 (KCNJ5). About 40 % of adrenal adenomas show somatic mutations of Kir3.4 [13, 42, 122]. These mutations confer a pathological Na+ conductance (“gain-of-function”) to the channel that depolarizes the cells and activates autonomous aldosterone synthesis and proliferation [17, 22, 70, 92, 93, 99, 123, 133]. Although these results clearly established the link between Kir3.4 mutations and hyperaldosteronism, the physiological role of non-mutated Kir3.4 in the adrenal cortex is still largely elusive. The wild-type Kir3.4 channel seems to be inactive in human adrenal cells under resting conditions [66, 70, 133]. Probably, Kir3.4 modulates the membrane potential of human glomerulosa cells after stimulation of aldosterone synthesis and prevents excess secretion of aldosterone [98]. Unfortunately, Kir3.4 function cannot be studied in mice because the channel is not expressed in the mouse adrenal gland (unpublished data).
Adrenal phenotype of Task channel knockout mice
Different knockout mouse models were used to investigate the relevance of Task1 and Task3 K+ channels for the adrenal gland [7, 28, 34, 51, 55, 103]. Mice with single deletions of the Task1 [55] or Task3 gene [7, 51, 103] as well as Task1/Task3 double knockout mice displayed disturbances of the steroid hormone homeostasis [28]. A common feature of all these models was the partially autonomous aldosterone synthesis. The severity of the phenotype of the mice was dependent on the inactivated gene. Detailed analysis of the different phenotypes revealed that both K+ channels, Task1 and Task3, are necessary for normal control of aldosterone synthesis. Moreover, each Task K+ channel seems to play a specific role for the sex- and age-dependent regulation of adrenocortical cell function.
Adrenal phenotype of Task1−/− mice
In adrenal glands of rats and mice, Task1 is expressed in cells of zona glomerulosa and zona fasciculata [27, 28, 55]. Besides this adrenal localization, Task1 is also expressed in the brain, the heart, and vascular tissue. The neurological phenotype of Task1 −/− mice is rather mild [2, 32, 77, 78, 90]. In addition, Task1 is expressed in the carotid bodies where it is involved in the chemosensory control of breathing [135]. The characterization of the cardiac phenotype of Task1 −/− mice [29, 31] as well as genetic studies identifying genetic TASK1 variations associated with arrhythmia [73] reveals the regulatory role of TASK1 channels in the cardiac conduction system. In humans, several mutations of TASK1 are associated with autosomal dominant pulmonary hypertension [85].
In mice, aldosterone secretion is strongly altered by deletion of the Task1 gene [55]. Interestingly, the adrenal phenotype of adult mice is restricted to females, which present a severe primary hyperaldosteronism with low plasma renin and hypokalemia. Female Task1 −/− mice are not able to adapt their remarkably high aldosterone levels to different salt diets, which normally increase (low Na+ or high K+ diet) or decrease (high Na+ diet) plasma aldosterone. Similar to patients with hyperaldosteronism, female Task1 −/− mice develop arterial hypertension. Treatment with the mineralocorticoid receptor blocker canrenoate leads to normalization of the blood pressure corroborating the link between hyperaldosteronism and hypertension in these animals.
According to the model for the regulation of aldosterone synthesis, the deletion of Task1 leads to cell membrane depolarization, increased cytosolic Ca2+ activity, and increased transcription of aldosterone synthase (Cyp11b2). Indeed, female Task1 −/− mice show increased messenger RNA (mRNA) and protein expression of aldosterone synthase. The histomorphological basis for the sex-specific hyperaldosteronism, however, is surprising. Glomerulosa cells of female Task1 −/− mice are devoid of aldosterone synthase (as measured by immunofluorescence). Instead, female Task1 −/− show a strong expression of aldosterone synthase in zona fasciculata cells. The pathological localization of aldosterone synthase suggests a profoundly disturbed zonation of the adrenal cortex. But surprisingly, the localization of the glomerulosa marker Dab2 is preserved, and corticosterone synthesis is also normal in Task1 −/− mice. Apparently, the “dezonation” is restricted to specific cellular properties such as the ectopic expression of the aldosterone synthase and does not reflect a totally disturbed adrenocortical zonal architecture. Moreover, treatment of female Task1 −/− mice with the synthetic glucocorticoid dexamethasone strongly suppressed the hyperaldosteronism. This suppression of aldosterone secretion might be caused by direct effects of dexamethasone on fasciculata cells [52, 67] or via suppression of ACTH. In Task1 −/− fasciculata cells, ACTH might act as a permissive factor for the abnormal expression of the aldosterone synthase. This is reminiscent of a glucocorticoid-remediable form of familial hyperaldosteronism (FH-I), which is caused by a CYP11B1/CYP11B2 chimeric gene expressed under the control of ACTH [75]. In which way could ACTH modulate the phenotype of female Task1 −/− mice? Via inhibition of Trek1, ACTH probably depolarizes the plasma membrane [38, 39, 41, 81]. In mice lacking Task1, the ACTH-induced depolarization could be more pronounced and sufficient to elicit ectopic expression of aldosterone synthase. In addition, effects of ACTH on cell proliferation and differentiation could influence the severity of the phenotype in female Task1 −/− mice.
Age-dependent phenotype of Task1−/− mice
Before puberty, the mislocalization of aldosterone synthase in the zona fasciculata was observed in Task1 −/− mice of both sexes. After puberty, male Task1 −/− mice restored the normal glomerulosa-specific localization of aldosterone synthase and normal plasma aldosterone levels, while female Task1 −/− mice maintained the ectopic expression of the aldosterone synthase and the hyperaldosteronism phenotype. The compensation of the Task1 invalidation in male mice after puberty was probably driven by androgen-dependent mechanisms. Castration of young male Task1 −/− mice prevented restoration of glomerulosa-specific localization of aldosterone synthase as seen in adult male Task1 −/− mice. Accordingly, treatment of female Task1 −/− mice with testosterone led to the disappearance of aldosterone synthase from fasciculata cells and to a normal expression in glomerulosa cells [55]. Different factors possibly contribute to the compensation of the Task1 deletion in male mice or testosterone treated female mice. Adult male mice exhibit a higher expression of Task3 [55], Trek1, and Kcnq1 (unpublished data) K+ channels than female mice. In addition, Task3 protein expression in male mice was found in zona glomerulosa and zona fasciculata, while it seems to be largely restricted to zona glomerulosa in female mice [103].
Adrenal phenotype of Task1−/−/Task3−/− double knockout mice
The possible role of Task3 as a compensatory factor for Task1 deletion in male mice was tested by analysis of the adrenal phenotype of mice with a double knockout of the Task1 and Task3 genes [28]. However, male Task1 −/−/Task3 −/− mice do not develop ectopic expression of aldosterone synthase as observed in female Task1 −/− mice. Although those male double knockout mice display hyperaldosteronism, while male Task1 −/− mice do not, the normal glomerulosa-specific localization of the aldosterone synthase is retained. Apparently, Task3 is not the sole androgen-dependent factor that establishes a normal distribution of aldosterone synthase in the adrenal cortex of mice lacking Task1.
In patch-clamp measurements, native glomerulosa cells from male Task1 −/−/Task3 −/− mice have no Task-like currents and are severely depolarized. As a consequence, plasma aldosterone levels in male Task1 −/−/Task3 −/− mice are increased. Normally, high Na+ diet suppresses plasma renin levels and, thereby, aldosterone secretion. In Task1 −/−/Task3 −/− mice, high Na+ diet does not lead to the physiological suppression of aldosterone synthesis. In these mice, plasma renin levels are already suppressed under normal diet, and a further suppression by high Na+ intake is not possible. These results indicate that Task3 K+ channels are needed for a normal control of aldosterone synthesis in glomerulosa cells, but they are not essential for the suppression of aldosterone synthase expression in zona fasciculata.
Effect of acidosis on aldosterone secretion of Task knockout mice
Besides Ang-II and high plasma K+, acidosis is known to stimulate aldosterone synthesis [53, 110, 119, 120]. Task1 and Task3 K+ channels are inhibited by extracellular acidification [26, 27, 33]. Therefore, Guagliardo et al. hypothesized that Task1 −/−/Task3 −/− mice exhibit an altered response of aldosterone secretion upon NH4Cl-induced acidosis [50]. Interestingly, stimulation of aldosterone production by mild acidosis, as observed in wild-type mice, was not completely abrogated in Task1 −/−/Task3 −/− mice. Apparently, Task1 and Task3 channels are not essential for the stimulatory effect of acidosis on aldosterone secretion. Most likely, acidosis has a dual effect; it stimulates the renin/Ang-II system, and it has a direct effect on adrenal K+ channels.
Expression of Dkk3 modulates the adrenal phenotype of male Task1−/− mice
The androgen-dependent compensatory mechanism in male Task1 −/− mice is presumably complex and probably involves several factors on different levels of the signaling cascade. In order to identify those factors, El Wakil et al. performed a gene chip analysis [34] to investigate potential changes of adrenal mRNA expression in Task1 −/− mice with ectopic aldosterone expression. The most appealing differentially regulated factor was dickkopf-3 (Dkk3). Dkk3 is a member of the dickkopf family and modulates the Wnt/ß-catenin pathway, which is involved in the control of glomerulosa cell function and differentiation in mouse adrenal glands [35]. Dkk3 is expressed in the zona glomerulosa of humans and mice [132], and its expression is stimulated by cytosolic Ca2+ [34]. The function of Dkk3 is to inhibit aldosterone synthesis [18], and therefore, it could be a factor counterbalancing the hyperaldosteronism of Task1 −/− mice. A possible role of Dkk3 for the compensation of the Task1 deletion in male mice was verified by phenotyping Task1 −/−/Dkk3 −/− double knockout mice [34]. Similar to female Task1 −/− mice [55], male Task1 −/−/Dkk3 −/− mice showed increased plasma aldosterone levels, which were not further stimulated by a K+-rich diet. The expression of Cyp11b2 mRNA was increased, but the localization of the aldosterone synthase was still restricted to the zona glomerulosa. Obviously, Dkk3 functions as a repressor of Cyp11b2 expression in glomerulosa cells, but it is not essential for suppression of aldosterone synthase expression in zona fasciculata in male Task1 −/− mice.
The adrenal phenotype of Task3−/− mice
The specific role of Task3 K+ channels for adrenocortical function was investigated using two different Task3 −/− mouse models [51, 103]. Under high Na+ diet, adult Task3 −/− animals do not show the physiological suppression of aldosterone secretion and develop salt-sensitive arterial hypertension [51, 103]. What is the explanation for the lack of adaptation to high dietary Na+ intake? Normally, high Na+ intake leads to a decrease of the renin and Ang-II levels and to a suppression of aldosterone. In Task3 −/− mice, aldosterone secretion is partially autonomous and does not require stimulation by renin/Ang-II. Under normal diet, the autonomous component of aldosterone secretion is masked by a compensatory suppression of renin/Ang-II and a reduction of Ang-II-driven aldosterone secretion. At high Na+ diet, a further suppression of renin/Ang-II is not possible. With regard to the high Na+ intake, aldosterone stays inappropriately high, Na+ is retained in a pathological way, and arterial hypertension develops. Accordingly, the aldosterone/renin ratio, a clinical indicator for autonomous aldosterone production, is strongly increased under a control diet and a high Na+ diet in Task3 −/− mice. In contrast to the ectopic expression of aldosterone synthase in female Task1 −/− mice, Task3 −/− mice of both sexes display normal localization of aldosterone synthase in zona glomerulosa [103]. Obviously, invalidation of the Task3 gene affects the physiological control of aldosterone production, but functional differentiation and zonation of the adrenal cortex are maintained.
Interestingly, Guagliardo et al. observed a hyperpolarized membrane voltage in glomerulosa cells of fresh adrenal slices of Task3 −/− mice, although Task3 is believed to be an important K+ channel of these cells [51]. How can this surprising observation be explained? In contrast to Guagliardo et al., we found primary cultured adrenocortical cells of Task3 −/− mice depolarized to −50 mV compared to −80 mV in wild-type cells. However, after stimulation with Ang-II, primary cells of Task3 −/− mice did not show the expected depolarization; they hyperpolarized transiently, probably due to enhanced activity of Ca2+-activated K+ channels (unpublished data). Most likely, the increased activity of Ca2+-activated K+ channels in glomerulosa cells of Task3 −/− mice compensates for the loss of Task3 and masks the electrical phenotype under certain experimental conditions.
To gain further insights into the role of Task3 for adrenal signaling, intracellular Ca2+ was measured in freshly prepared adrenal slices [103]. Slices of wild-type mice showed a spontaneous Ca2+ oscillation only in a small number of cells, most of the cells were silent. After stimulation with Ang-II or high extracellular K+, most of the wild-type cells showed high frequency Ca2+ oscillations. By contrast, in slices of Task3 −/− mice, glomerulosa cells often showed spontaneous Ca2+ oscillations under control conditions, but the stimulatory effects of Ang-II and high extracellular K+ were attenuated [103].
From these Ca2+ measurements on adrenal slices, we expected impaired aldosterone response of Task3−/− mice in vivo at high K+ diet and low Na+ diet (the latter increases renin and Ang-II). Surprisingly, glomerulosa cell of Task3 -/- mice still showed a normal increase of aldosterone production under low Na+ and high K+ diets [51, 103]. Despite the impaired effects of Ang-II and high K+ in the slice preparation, the adrenal responsiveness towards major stimulatory pathways appears to be preserved in Task3 −/− mice, allowing aldosterone to increase normally in response to these strong stimuli. Probably, several compensatory mechanisms act in concert to counterbalance the impaired membrane and Ca2+ signaling. For instance, Ca2+-independent signaling pathways (e.g., via lipoxygenase and activation of p38-MAPK [49, 100]) could contribute to the preserved Ang-II effect on aldosterone production. Moreover, an increase of the plasma K+ concentration could activate K+-sensitive adrenomedullary cells which stimulate glomerulosa cells via paracrine factors [10] or via nerve fibers projecting into the adrenal cortex [24].
Severe hyperaldosteronism in newborn Task3−/− mice
The adrenal phenotype of Task3 −/− mice is age-dependent [7]. Newborn Task3 −/− mice have a more severe hyperaldosteronism than adult mice. In addition, plasma concentrations of other steroid hormones such as corticosterone and progesterone are increased and transcription of steroidogenic enzymes, e.g., aldosterone synthase and hydroxy-β-5-steroiddehydrogenase, 3 β-and steroid β-isomerase 6 (Hsd3b6, an enzyme needed for glomerulosa-specific progesterone synthesis), is enhanced.
A gene chip analysis was performed to identify transcriptionally regulated potential factors and pathways underlying the transient hyperaldosteronism of neonatal Task3 −/− mice. This analysis revealed a strong but transient upregulation of renin mRNA in adrenal glands of 1-day-old Task3 −/− mice; in 12-day-old animals, renin expression was back to control values. The renin expressing cells were localized in zona fasciculata. Local renin expression in the adrenal gland and in other extra-renal tissues (e.g., in the heart and the eye) is known for a long time [101]. The exact function of the local adrenal renin/Ang-II system is not well understood. It was suggested that local renin has a role for the regulation of tissue function independently of or synergistically with the systemic (renal) renin signaling [104]. In mice, adrenal renin is normally detected during fetal development, but it disappears at the time of birth [64, 68]. Interestingly, adrenal renin expression can be activated under several conditions. Aldosterone synthase knockout mice show abnormal renin expression in the adrenal gland [71]. In adult rats, renin can be found in glomerulosa cells and appears to be involved in the regulation of aldosterone synthesis. Local renin is upregulated after nephrectomy and after stimulation with Ang-II, high K+, and ACTH [30, 61, 105, 141]. The cellular signaling mechanisms translating these conditions and stimuli into increased renin expression, however, are not known. Moreover, it is not clear which pathways are involved to link the loss of Task3 channels to the abnormal renin expression in fasciculata cells and how local renin stimulates steroid hormone secretion.
Why is the hyperaldosteronism phenotype of neonatal Task3 −/− mice transient in nature? To address this question, gene chip analyses of 1- and 12-day-old Task3 −/− mice were used. The comparison of the results at the two time points revealed several age-dependently expressed genes that are known modulators of adrenal function [7]. For instance, the expression of the nicotinamide nucleotide transhydrogenase, which produces NAPDH as a cofactor for Cyp-enzymes, decreased over time [89, 128]. Similarly, the expression of the store-operated Ca2+ channel Trpc5, which is possibly involved in the generation of the Ang-II dependent Ca2+ signal [57, 74], was decreased in 12-day-old Task3 −/− mice. Other factors such as galanin, a neuropeptide stimulating glucocorticoid secretion, showed enhanced expression with age. Most likely, a complex network of factors and pathways counterbalance the cellular deficit induced by the inactivation of Task3, and apparently, this compensatory mechanisms take time to fully develop.
Task1 and Task3 channels serve distinct functions
Task1 and Task3 are related K+ channels and probably assemble to form Task1-Task3 heterodimers [25]. One might assume that the two channels serve a similar cellular function. However, the tissue distribution of mRNA expression is not identical and genetic inactivation of each of these channels in mice led to different adrenal phenotypes (Table 2). It appears that an important function of Task1 is to prevent the expression of the aldosterone synthase in fasciculata cells, thereby restricting aldosterone synthase expression to glomerulosa cells. In female Task1 −/− mice, the most striking phenotype is the abnormal expression of aldosterone synthase in fasciculata cells that causes severe hyperaldosteronism [55]. In adult Task1 −/− males, the presence of Task3 in fasciculata cells may contribute to the correction of this phenotype after puberty, but it is certainly not essential, because adult male Task1 −/−/Task3 −/− mice also display a normal Cyp11b2 expression pattern [28]. Interestingly, glomerulosa cells of female Task1 −/− mice are still sensitive for negative feedback mechanisms in the presence of high plasma aldosterone: they completely shut off aldosterone synthase expression [55].
Table 2.
Comparison of the different adrenal phenotypes of Task1 −/− [55], Task1 −/−/Task3 −/− double knockout [28, 50], Task3 −/− [51, 103], and Task1 −/−/Dkk3 −/− double knockout mice [34]
| Parameter | Task1−/− ♀+♂ [55] | Task1 −/−/Task3 −/− ♂ [28, 50]} | Task3−/− ♀+♂ [103] | Task3−/− ♂ [51] | Newborn Task3 −/− [7] | Task1 −/−/Dkk3 −/− ♀+♂ [34] |
|---|---|---|---|---|---|---|
| Membrane potential of adrenocortical cells under control conditions (compared to wild-type cells; only cells from male mice were analyzed) | Depolarized primary cultured cells | Depolarized glomerulosa cells in adrenal slices | Depolarized primary cultured cells | Glomerulosa cells in adrenal slices were not depolarized | n.a. | Depolarized primary cultured cells; no difference between Task1 −/− and Task1 −/−/Dkk3 −/− cells |
| Cytosolic Ca2+ | n.a. | n.a. | Spontaneous oscillations in glomerulosa cells (adrenal slices) under control, which were silenced by Ang-II; smaller increase under high K+ | n.a. | n.a. | n.a. |
| Aldosterone | Severe hyperaldosteronism in female mice was independent of the salt intake, but normalized by dexamethasone; male mice were normal | Under control conditions hyperaldosteronism in male mice in one study [50]; no hyperaldosteronism in another study [28]; no suppression (even an increase) under high Na+ and normal increase under low Na+ [28] | Normal aldosterone under control; no suppression under high Na+, smaller decrease under low K+ in female mice, and normal increase under high K+ and low Na+; increased aldosterone secretion in perifused adrenal glands (with absent Ang-II) | Mild hyperaldosteronism under control; no suppression under high Na+, normal increase under high K+ and low Na+; higher in vivo Ang-II-dependent aldosterone secretion | Severe hyperaldosteronism; additional increase of corticosterone and progesterone | Hyperaldosteronism in male Task1 −/−/Dkk3 −/− mice, while male Task1-/- had normal aldosterone levels; no further increase by high K+ |
| Renin | Decreased plasma renin in female mice under control condition, but not in male mice | Decreased plasma renin in male mice under control [50] or no change [28], low Na+, and under high Na+ [28] | Decreased plasma renin under control and under high Na+; increase under low Na+ similar in both genotypes | Decreased plasma renin under control, low Na+, and under high Na+ | Normal plasma renin, but decreased renin in renal lysates; abnormal adrenal renin production | Decreased plasma renin levels in Task1 −/−/Dkk3 −/− mice of both sexes |
| Aldosterone/renin ratio (ARR) | Increased in female mice | Increased | Increased | Increased | Increased | Increased in male Task1 −/−/Dkk3 −/− mice, while male Task1 −/− had normal ARR |
| Blood pressure (SBP: systolic blood pressure, DBP: diastolic blood pressure) | SBP increased in female mice, but not in male mice; canrenoate normalized SBP | n.a. | SBP was normal under control but increased under high Na+ | SBP and DBP increased under control; candesartan normalized DBP; SBP increased under high Na+ | n.a. | n.a. |
| Cyp11b2 (aldosterone synthase) expression | Increased Cyp11b2 mRNA and protein in female mice under control; no further increase of protein under high K+ | n.a. | Cyp11b2 mRNA not altered under control | Increased Cyp11b2 mRNA under control and high Na+, protein levels were not increased | Increased Cyp11b2 mRNA in newborn mice, but not in 12-day-old mice | Increased Cyp11b2 mRNA in male Task1 −/−/Dkk3 −/− mice, but normal levels in male Task1 −/− mice |
| Aldosterone synthase localization (“zonation”) | Female mice: ectopic expression in zona fasciculata, suppressed expression in zona glomerulosa; normal zonation in male mice | Normal zonation in male mice with glomerulosa-specific expression | Normal zonation in male and female mice with glomerulosa-specific expression | Normal zonation in male mice with glomerulosa-specific expression | Normal zonation with glomerulosa-specific expression | Normal zonation with glomerulosa-specific expression in male Task1 −/−/Dkk3 −/− mice |
| Plasma electrolytes | Hypokalemia in female mice, normal plasma Na+ | Hypokalemia | No difference in plasma K+ and Na+ | Hypokalemia | Hypernatremia in 4-day-old mice | n.a. |
| Additional data | Increased amiloride-sensitive current in distal colon of female mice | – | Sex-dependent Task3 expression in wt mice (♂: higher than ♀ ZG+ZF; ♀: ZG) | Decreased heart rate at night | Increased expression of Hsd3b6 | No effect of Dkk3 knockout in ♀ Task1 −/− |
Please note, that the phenotype of Task3 −/− mice was analyzed by two different groups using independent Task3 −/− models. The phenotype of Task1 −/−/Task3 −/− mice and of one of the Task3 −/− strains [51] was only analyzed in male mice. Aldosterone levels were measured in plasma or in 24 h urine samples. If not stated otherwise, the phenotype of knockout mice is compared with the one of wild-type mice
n.a. not analyzed, ZG zona glomerulosa, ZF zona fasciculata
Probably, the major role of Task3 is setting the resting potential of mouse glomerulosa cells [103]. Also, in rat glomerulosa cells, Task3 homomers seem to be the dominant channel type ensuring the high resting potential [25, 26]. In Task3 −/− mice, aldosterone secretion becomes largely autonomous from the renin-angiotensin-axis, because the glomerulosa cells are depolarized even in the absence of Ang-II. Suppression of aldosterone secretion by low K+ diet is also compromised, probably due to the inability of Task3 −/− cells to hyperpolarize appropriately when extracellular K+ is low [103]. In both Task1 −/− and Task3 −/− mice, the adrenal phenotypes are age-dependent with regard to the ectopic expression of aldosterone synthase and the severe hyperaldosteronism, respectively. In both knockout models, compensation becomes more effective with age and the severity of the symptoms decreases although an abnormal depolarization of the adrenocortical cells is still detectable in cells from adults. In the light of the genetic defects of the K+ channel KCNJ5, Ca2+ channels, and ion-transporting ATPases that are causative factors for the formation of aldosterone-producing adenomas [42], it is surprising that the depolarized adrenal cortex of Task1 and Task3 knockout animals doesn’t show adenomas or overt adrenal hyperplasia. Maybe the adrenal cell biology of Task1 and Task3 knockout animals is sufficiently dynamic to allow effective adaptation and prevention of hyperplasia - or the consequences of Task channel inactivation are not strong enough to cause obvious hyperplasia during the short lifespan of mice.
Outlook: role of TASK channels for human adrenal pathology
Na+-permeable gain-of-function mutations of KCNJ5 are causative for some 40 % of aldosterone-producing adenomas [17, 22, 70, 92, 93, 123]. Are TASK channels also candidate genes for an increased risk of adrenal hyperplasia or adenoma formation in humans? Interestingly, TASK channels were reported to change their ion selectivity and to become permeable to Na+ upon extracellular acidification [86]. However, up to now, no mutations of TASK channels have been found that increase the Na+ permeability and cause aldosterone-producing adenomas. Perhaps, such permeability-changing TASK channel mutations, even if they occur, do not induce proliferation that is a prerequisite for adenoma formation. Loss-of-function mutations of TASK3 have been linked to Birk Barel mental retardation dysmorphism syndrome [9, 140]. It is, however, not known if these patients have an adrenal phenotype. Interestingly, TASK3 is a genomically imprinted gene showing paternal silencing [9]. Therefore, a mutation in the maternal copy of TASK3 can lead to a disease, while a mutation in the paternal allele will have no effect. In a genome-wide association study, a correlation of SNPs in the TASK3 gene with aldosterone levels and the risk for hypertension was found, but no difference between males and females was reported [65]. Also for TASK1, a human disease was linked to gene mutations. Loss-of-function mutations can cause pulmonary hypertension [85]. In addition, a single nucleotide polymorphism nearby the TASK1 gene was found to be associated with blood pressure [45], but no adrenal phenotype was reported so far. It is possible that mutations of TASK genes can be compensated and do not cause phenotypes strong enough to provoke monogenetic human adrenal diseases. Further studies are required to investigate a potential role of TASK channels as modifier genes of adrenocortical disorders.
Acknowledgments
Disclosure statement
The authors have nothing to disclose.
Funding
The study was supported by the Deutsche Forschungsgemeinschaft (FOR1086 to R.W. and S.B.), by the French Agence Nationale pour la Recherche (ANR) BeyondTASKs grant (JB and EL), and LabEx Ion Channel Science and Therapeutics grant (ANR-11-LABX-0015-01, to JB).
References
- 1.Akizuki O, Inayoshi A, Kitayama T, Yao K, Shirakura S, Sasaki K, Kusaka H, Matsubara M. Blockade of T-type voltage-dependent Ca2+ channels by benidipine, a dihydropyridine calcium channel blocker, inhibits aldosterone production in human adrenocortical cell line NCI-H295R. Eur J Pharmacol. 2008;584:424–434. doi: 10.1016/j.ejphar.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Salameh A, Cohen R, Desailloud R. Overview of the genetic determinants of primary aldosteronism. Appl Clin Genet. 2014;7:67–79. doi: 10.2147/TACG.S45620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrighi I, Bloch-Faure M, Grahammer F, Bleich M, Warth R, Mengual R, Drici MD, Barhanin J, Meneton P. Altered potassium balance and aldosterone secretion in a mouse model of human congenital long QT syndrome. Proc Natl Acad Sci U S A. 2001;98:8792–8797. doi: 10.1073/pnas.141233398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:E819–E829. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- 6.Bandulik S, Penton D, Barhanin J, Warth R. TASK1 and TASK3 potassium channels: determinants of aldosterone secretion and adrenocortical zonation. Horm Metab Res. 2010;42:450–457. doi: 10.1055/s-0029-1243601. [DOI] [PubMed] [Google Scholar]
- 7.Bandulik S, Tauber P, Penton D, Schweda F, Tegtmeier I, Sterner C, Lalli E, Lesage F, Hartmann M, Barhanin J, Warth R. Severe hyperaldosteronism in neonatal Task3 potassium channel knockout mice is associated with activation of the intraadrenal renin-angiotensin system. Endocrinology. 2013;154:2712–2722. doi: 10.1210/en.2013-1101. [DOI] [PubMed] [Google Scholar]
- 8.Barbara JG, Takeda K. Voltage-dependent currents and modulation of calcium channel expression in zona fasciculata cells from rat adrenal gland. J Physiol. 1995;488(Pt 3):609–622. doi: 10.1113/jphysiol.1995.sp020994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barel O, Shalev SA, Ofir R, Cohen A, Zlotogora J, Shorer Z, Mazor G, Finer G, Khateeb S, Zilberberg N, Birk OS. Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am J Hum Genet. 2008;83:193–199. doi: 10.1016/j.ajhg.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belloni AS, Malendowicz LK, Rucinski M, Guidolin D, Nussdorfer GG. Galanin stimulates cortisol secretion from human adrenocortical cells through the activation of galanin receptor subtype 1 coupled to the adenylate cyclase-dependent signaling cascade. Int J Mol Med. 2007;20:859–864. [PubMed] [Google Scholar]
- 11.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–444. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 12.Beuschlein F, Fassnacht M, Assie G, Calebiro D, Stratakis CA, Osswald A, Ronchi CL, Wieland T, Sbiera S, Faucz FR, Schaak K, et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med. 2014;370:1019–1028. doi: 10.1056/NEJMoa1310359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulkroun S, Beuschlein F, Rossi GP, Golib-Dzib JF, Fischer E, Amar L, Mulatero P, Samson-Couterie B, Hahner S, Quinkler M, Fallo F, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. 2012;59:592–598. doi: 10.1161/HYPERTENSIONAHA.111.186478. [DOI] [PubMed] [Google Scholar]
- 14.Brenner T, O’Shaughnessy KM. Both TASK-3 and TREK-1 two-pore loop K channels are expressed in H295R cells and modulate their membrane potential and aldosterone secretion. Am J Physiol Endocrinol Metab. 2008;295:E1480–E1486. doi: 10.1152/ajpendo.90652.2008. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, He M, Gao Z, Peng Y, Li Y, Li L, Zhou W, Li X, Zhong X, Lei Y, Su T, et al. Activating hotspot L205R mutation in PRKACA and adrenal Cushing’s syndrome. Science. 2014;344:913–917. doi: 10.1126/science.1249480. [DOI] [PubMed] [Google Scholar]
- 16.Chai W, Danser AH. Why are mineralocorticoid receptor antagonists cardioprotective? Naunyn Schmiedebergs Arch Pharmacol. 2006;374:153–162. doi: 10.1007/s00210-006-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charmandari E, Sertedaki A, Kino T, Merakou C, Hoffman DA, Hatch MM, Hurt DE, Lin L, Xekouki P, Stratakis CA, Chrousos GP. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J Clin Endocrinol Metab. 2012;97:E1532–E1539. doi: 10.1210/jc.2012-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Hornsby PJ. Adenovirus-delivered DKK3/WNT4 and steroidogenesis in primary cultures of adrenocortical cells. Horm Metab Res. 2006;38:549–555. doi: 10.1055/s-2006-950500. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, Viana F, Garrison JC, Bayliss DA. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci U S A. 2006;103:3422–3427. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherradi N, Brandenburger Y, Rossier MF, Vallotton MB, Stocco DM, Capponi AM. Atrial natriuretic peptide inhibits calcium-induced steroidogenic acute regulatory protein gene transcription in adrenal glomerulosa cells. Mol Endocrinol. 1998;12:962–972. doi: 10.1210/mend.12.7.0132. [DOI] [PubMed] [Google Scholar]
- 21.Cherradi N, Brandenburger Y, Capponi AM. Mitochondrial regulation of mineralocorticoid biosynthesis by calcium and the StAR protein. Eur J Endocrinol. 1998;139:249–256. doi: 10.1530/eje.0.1390249. [DOI] [PubMed] [Google Scholar]
- 22.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark BJ, Pezzi V, Stocco DM, Rainey WE. The steroidogenic acute regulatory protein is induced by angiotensin II and K+ in H295R adrenocortical cells. Mol Cell Endocrinol. 1995;115:215–219. doi: 10.1016/0303-7207(95)03683-0. [DOI] [PubMed] [Google Scholar]
- 24.Costa JJ, Averill S, Ching YP, Priestley JV. Immunocytochemical localization of a growth-associated protein (GAP-43) in rat adrenal gland. Cell Tissue Res. 1994;275:555–566. doi: 10.1007/BF00318824. [DOI] [PubMed] [Google Scholar]
- 25.Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002;277:5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- 26.Czirjak G, Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol. 2002;16:621–629. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- 27.Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- 28.Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U S A. 2008;105:2203–2208. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decher N, Wemhoner K, Rinne S, Netter MF, Zuzarte M, Aller MI, Kaufmann SG, Li XT, Meuth SG, Daut J, Sachse FB, et al. Knock-out of the potassium channel TASK-1 leads to a prolonged QT interval and a disturbed QRS complex. Cell Physiol Biochem. 2011;28:77–86. doi: 10.1159/000331715. [DOI] [PubMed] [Google Scholar]
- 30.Doi Y, Atarashi K, Franco-Saenz R, Mulrow PJ. Effect of changes in sodium or potassium balance, and nephrectomy, on adrenal renin and aldosterone concentrations. Hypertension. 1984;6:I124–I129. doi: 10.1161/01.hyp.6.2_pt_2.i124. [DOI] [PubMed] [Google Scholar]
- 31.Donner BC, Schullenberg M, Geduldig N, Huning A, Mersmann J, Zacharowski K, Kovacevic A, Decking U, Aller MI, Schmidt KG. Functional role of TASK-1 in the heart: studies in TASK-1-deficient mice show prolonged cardiac repolarization and reduced heart rate variability. Basic Res Cardiol. 2011;106:75–87. doi: 10.1007/s00395-010-0128-x. [DOI] [PubMed] [Google Scholar]
- 32.Du G, Chen X, Todorovic MS, Shu S, Kapur J, Bayliss DA. TASK channel deletion reduces sensitivity to local anesthetic-induced seizures. Anesthesiology. 2011;115:1003–1011. doi: 10.1097/ALN.0b013e3182343660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Wakil A, Bandulik S, Guy N, Bendahhou S, Zennaro MC, Niehrs C, Mari B, Warth R, Barhanin J, Lalli E. Dkk3 is a component of the genetic circuitry regulating aldosterone biosynthesis in the adrenal cortex. Hum Mol Genet. 2012;21:4922–4929. doi: 10.1093/hmg/dds333. [DOI] [PubMed] [Google Scholar]
- 35.El Wakil A, Lalli E. The Wnt/beta-catenin pathway in adrenocortical development and cancer. Mol Cell Endocrinol. 2011;332:32–37. doi: 10.1016/j.mce.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Enyeart JJ, Danthi SJ, Liu H, Enyeart JA. Angiotensin II inhibits bTREK-1 K+ channels in adrenocortical cells by separate Ca2+- and ATP hydrolysis-dependent mechanisms. J Biol Chem. 2005;280:30814–30828. doi: 10.1074/jbc.M504283200. [DOI] [PubMed] [Google Scholar]
- 37.Enyeart JA, Danthi S, Enyeart JJ. Corticotropin induces the expression of TREK-1 mRNA and K+ current in adrenocortical cells. Mol Pharmacol. 2003;64:132–142. doi: 10.1124/mol.64.1.132. [DOI] [PubMed] [Google Scholar]
- 38.Enyeart JA, Danthi SJ, Enyeart JJ. TREK-1 K+ channels couple angiotensin II receptors to membrane depolarization and aldosterone secretion in bovine adrenal glomerulosa cells. Am J Physiol Endocrinol Metab. 2004;287:E1154–E1165. doi: 10.1152/ajpendo.00223.2004. [DOI] [PubMed] [Google Scholar]
- 39.Enyeart JJ, Enyeart JA. Ca2+ and K+ channels of normal human adrenal zona fasciculata cells: properties and modulation by ACTH and AngII. J Gen Physiol. 2013;142:137–155. doi: 10.1085/jgp.201310964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enyeart JA, Liu H, Enyeart JJ. Curcumin inhibits ACTH- and angiotensin II-stimulated cortisol secretion and Ca(v)3.2 current. J Nat Prod. 2009;72:1533–1537. doi: 10.1021/np900227x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enyeart JJ, Xu L, Danthi S, Enyeart JA. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J Biol Chem. 2002;277:49186–49199. doi: 10.1074/jbc.M207233200. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64:354–361. doi: 10.1161/HYPERTENSIONAHA.114.03419. [DOI] [PubMed] [Google Scholar]
- 43.Freedman BD, Kempna PB, Carlone DL, Shah MS, Guagliardo NA, Barrett PQ, Gomez-Sanchez CE, Majzoub JA, Breault DT. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26:666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallo-Payet N, Grazzini E, Cote M, Chouinard L, Chorvatova A, Bilodeau L, Payet MD, Guillon G. Role of Ca2+ in the action of adrenocorticotropin in cultured human adrenal glomerulosa cells. J Clin Invest. 1996;98:460–466. doi: 10.1172/JCI118812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganesh SK, Chasman DI, Larson MG, Guo X, Verwoert G, Bis JC, Gu X, Smith AV, Yang ML, Zhang Y, Ehret G, et al. Effects of long-term averaging of quantitative blood pressure traits on the detection of genetic associations. Am J Hum Genet. 2014;95:49–65. doi: 10.1016/j.ajhg.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganz MB, Nee JJ, Isales CM, Barrett PQ. Atrial natriuretic peptide enhances activity of potassium conductance in adrenal glomerulosa cells. Am J Physiol. 1994;266:C1357–C1365. doi: 10.1152/ajpcell.1994.266.5.C1357. [DOI] [PubMed] [Google Scholar]
- 47.Goh G, Scholl UI, Healy JM, Choi M, Prasad ML, Nelson-Williams C, Kuntsman JW, Korah R, Suttorp AC, Dietrich D, Haase M, et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nat Genet. 2014;46:613–617. doi: 10.1038/ng.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci U S A. 2009;106:11800–11805. doi: 10.1073/pnas.0904635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu J, Wen Y, Mison A, Nadler JL. 12-lipoxygenase pathway increases aldosterone production, 3′,5′-cyclic adenosine monophosphate response element-binding protein phosphorylation, and p38 mitogen-activated protein kinase activation in H295R human adrenocortical cells. Endocrinology. 2003;144:534–543. doi: 10.1210/en.2002-220580. [DOI] [PubMed] [Google Scholar]
- 50.Guagliardo NA, Yao J, Bayliss DA, Barrett PQ. TASK channels are not required to mount an aldosterone secretory response to metabolic acidosis in mice. Mol Cell Endocrinol. 2011;336:47–52. doi: 10.1016/j.mce.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guagliardo NA, Yao J, Hu C, Schertz EM, Tyson DA, Carey RM, Bayliss DA, Barrett PQ. TASK-3 channel deletion in mice recapitulates low-renin essential hypertension. Hypertension. 2012;59:999–1005. doi: 10.1161/HYPERTENSIONAHA.111.189662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gummow BM, Scheys JO, Cancelli VR, Hammer GD. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol Endocrinol. 2006;20:2711–2723. doi: 10.1210/me.2005-0461. [DOI] [PubMed] [Google Scholar]
- 53.Gyorke ZS, Sulyok E, Guignard JP. Ammonium chloride metabolic acidosis and the activity of renin-angiotensin-aldosterone system in children. Eur J Pediatr. 1991;150:547–549. doi: 10.1007/BF02072203. [DOI] [PubMed] [Google Scholar]
- 54.Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350:151–162. doi: 10.1016/j.mce.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heitzmann D, Derand R, Jungbauer S, Bandulik S, Sterner C, Schweda F, El Wakil A, Lalli E, Guy N, Mengual R, Reichold M, et al. Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J. 2008;27:179–187. doi: 10.1038/sj.emboj.7601934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong C, Kim J, Jeon JP, Wie J, Kwak M, Ha K, Kim H, Myeong J, Kim SY, Jeon JH, So I. Gs cascade regulates canonical transient receptor potential 5 (TRPC5) through cAMP mediated intracellular Ca2+ release and ion channel trafficking. Biochem Biophys Res Commun. 2012;421:105–111. doi: 10.1016/j.bbrc.2012.03.123. [DOI] [PubMed] [Google Scholar]
- 58.Hu C, Rusin CG, Tan Z, Guagliardo NA, Barrett PQ. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J Clin Invest. 2012;122:2046–2053. doi: 10.1172/JCI61996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibrahim HN, Hostetter TH. Aldosterone in renal disease. Curr Opin Nephrol Hypertens. 2003;12:159–164. doi: 10.1097/00041552-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 61.Inagami T, Mizuno K, Naruse M, Nakamaru M, Naruse K, Hoffman LH, McKenzie JC. Active and inactive renin in the adrenal. Am J Hypertens. 1989;2:311–319. doi: 10.1093/ajh/2.4.311. [DOI] [PubMed] [Google Scholar]
- 62.Inoue M, Harada K, Matsuoka H, Naakamura J and Warashina A (2012) Mechanisms and roles of muscarinic activation in guinea-pig adrenal medullary cells. Am J Physiol Cell Physiol [DOI] [PMC free article] [PubMed]
- 63.Inoue M, Harada K, Matsuoka H, Sata T, Warashina A. Inhibition of TASK1-like channels by muscarinic receptor stimulation in rat adrenal medullary cells. J Neurochem. 2008;106:1804–1814. doi: 10.1111/j.1471-4159.2008.05521.x. [DOI] [PubMed] [Google Scholar]
- 64.Jones CA, Sigmund CD, McGowan RA, Kane-Haas CM, Gross KW. Expression of murine renin genes during fetal development. Mol Endocrinol. 1990;4:375–383. doi: 10.1210/mend-4-3-375. [DOI] [PubMed] [Google Scholar]
- 65.Jung J, Barrett PQ, Eckert GJ, Edenberg HJ, Xuei X, Tu W, Pratt JH. Variations in the potassium channel genes KCNK3 and KCNK9 in relation to blood pressure and aldosterone production: an exploratory study. J Clin Endocrinol Metab. 2012;97:E2160–E2167. doi: 10.1210/jc.2012-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kienitz MC, Mergia E, Pott L. The NCI-H295R cell line as in vitro model of hyperaldosteronism lacks funktional KCNJ5 (GIRK4; Kir3.4) channels. Acta Physiol. 2014;210:213–213. doi: 10.1016/j.mce.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 67.Kim AC, Hammer GD. Adrenocortical cells with stem/progenitor cell properties: recent advances. Mol Cell Endocrinol. 2007;265–266:10–16. doi: 10.1016/j.mce.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kon Y, Hashimoto Y, Kitagawa H, Sugimura M, Murakami K. Renin immunohistochemistry in the adrenal gland of the mouse fetus and neonate. Anat Rec. 1990;227:124–131. doi: 10.1002/ar.1092270114. [DOI] [PubMed] [Google Scholar]
- 69.Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 70.Kuppusamy M, Caroccia B, Stindl J, Bandulik S, Lenzini L, Gioco F, Fishman V, Zanotti G, Gomez-Sanchez C, Bader M, Warth R et al. (2014) A novel KCNJ5-insT149 somatic mutation close to, but outside, the selectivity filter causes resistant hypertension by loss of selectivity for potassium. J Clin Endocrinol Metab 20141927: [DOI] [PMC free article] [PubMed]
- 71.Lee G, Makhanova N, Caron K, Lopez ML, Gomez RA, Smithies O, Kim HS. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology. 2005;146:2650–2656. doi: 10.1210/en.2004-1102. [DOI] [PubMed] [Google Scholar]
- 72.Lenzini L, Caroccia B, Campos AG, Fassina A, Belloni AS, Seccia TM, Kuppusamy M, Ferraro S, Skander G, Bader M, Rainey WE, et al. Lower expression of the TWIK-related acid-sensitive K+ channel 2 (TASK-2) gene is a hallmark of aldosterone-producing adenoma causing human primary aldosteronism. J Clin Endocrinol Metab. 2014;99:E674–E682. doi: 10.1210/jc.2013-2900. [DOI] [PubMed] [Google Scholar]
- 73.Liang B, Soka M, Christensen AH, Olesen MS, Larsen AP, Knop FK, Wang F, Nielsen JB, Andersen MN, Humphreys D, Mann SA, et al. Genetic variation in the two-pore domain potassium channel, TASK-1, may contribute to an atrial substrate for arrhythmogenesis. J Mol Cell Cardiol. 2014;67:69–76. doi: 10.1016/j.yjmcc.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 74.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC: Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci U S A. 2009;106:3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lifton RP, Dluhy RG, Powers M, Rich GM, Gutkin M, Fallo F, Gill JR, Jr, Feld L, Ganguly A, Laidlaw JC. Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase. Nat Genet. 1992;2:66–74. doi: 10.1038/ng0992-66. [DOI] [PubMed] [Google Scholar]
- 76.Lin D, Sugawara T, Strauss JF, III, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 77.Linden AM, Aller MI, Leppa E, Rosenberg PH, Wisden W, Korpi ER. K+ channel TASK-1 knockout mice show enhanced sensitivities to ataxic and hypnotic effects of GABA(A) receptor ligands. J Pharmacol Exp Ther. 2008;327:277–286. doi: 10.1124/jpet.108.142083. [DOI] [PubMed] [Google Scholar]
- 78.Linden AM, Aller MI, Leppa E, Vekovischeva O, Aitta-Aho T, Veale EL, Mathie A, Rosenberg P, Wisden W, Korpi ER. The in vivo contributions of TASK-1-containing channels to the actions of inhalation anesthetics, the alpha(2) adrenergic sedative dexmedetomidine, and cannabinoid agonists. J Pharmacol Exp Ther. 2006;317:615–626. doi: 10.1124/jpet.105.098525. [DOI] [PubMed] [Google Scholar]
- 79.Lindner M, Leitner MG, Halaszovich CR, Hammond GR, Oliver D. Probing the regulation of TASK potassium channels by PI4,5P(2) with switchable phosphoinositide phosphatases. J Physiol. 2011;589:3149–3162. doi: 10.1113/jphysiol.2011.208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu H, Enyeart JA, Enyeart JJ. Angiotensin II inhibits native bTREK-1 K+ channels through a PLC-, kinase C-, and PIP2-independent pathway requiring ATP hydrolysis. Am J Physiol Cell Physiol. 2007;293:C682–C695. doi: 10.1152/ajpcell.00087.2007. [DOI] [PubMed] [Google Scholar]
- 81.Liu H, Enyeart JA, Enyeart JJ. ACTH inhibits bTREK-1 K+ channels through multiple cAMP-dependent signaling pathways. J Gen Physiol. 2008;132:279–294. doi: 10.1085/jgp.200810003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu H, Enyeart JA and Enyeart JJ (2009) N6-substituted cAMP Analogs Inhibit bTREK-1 K+ channels and stimulate cortisol secretion by a PKA-Independent mechanism. Mol Pharmacol [DOI] [PMC free article] [PubMed]
- 83.Lotshaw DP. Biophysical and pharmacological characteristics of native two-pore domain TASK channels in rat adrenal glomerulosa cells. J Membr Biol. 2006;210:51–70. doi: 10.1007/s00232-005-7012-x. [DOI] [PubMed] [Google Scholar]
- 84.Lu HK, Fern RJ, Luthin D, Linden J, Liu LP, Cohen CJ, Barrett PQ. Angiotensin II stimulates T-type Ca2+ channel currents via activation of a G protein, Gi. Am J Physiol. 1996;271:C1340–C1349. doi: 10.1152/ajpcell.1996.271.4.C1340. [DOI] [PubMed] [Google Scholar]
- 85.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Tregouet DA, Borczuk A, Rosenzweig EB, Girerd B, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma L, Zhang X, Zhou M, Chen H. Acid-sensitive TWIK and TASK two-pore domain potassium channels change ion selectivity and become permeable to sodium in extracellular acidification. J Biol Chem. 2012;287:37145–37153. doi: 10.1074/jbc.M112.398164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McEwan PE, Vinson GP, Kenyon CJ. Control of adrenal cell proliferation by AT1 receptors in response to angiotensin II and low-sodium diet. Am J Physiol. 1999;276:E303–E309. doi: 10.1152/ajpendo.1999.276.2.E303. [DOI] [PubMed] [Google Scholar]
- 89.Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, Nurnberg P, Mann NP, Banerjee R, Saka HN, Chapple JP, et al. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet. 2012;44:740–742. doi: 10.1038/ng.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meuth SG, Aller MI, Munsch T, Schuhmacher T, Seidenbecher T, Meuth P, Kleinschnitz C, Pape HC, Wiendl H, Wisden W, Budde T. The contribution of TWIK-related acid-sensitive K+-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol Pharmacol. 2006;69:1468–1476. doi: 10.1124/mol.105.020594. [DOI] [PubMed] [Google Scholar]
- 91.Mirkovic K, Palmersheim J, Lesage F, Wickman K. Behavioral characterization of mice lacking Trek channels. Front Behav Neurosci. 2012;6:60. doi: 10.3389/fnbeh.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monticone S, Hattangady NG, Penton D, Isales C, Edwards MA, Williams TA, Sterner C, Warth R, Mulatero P and Rainey WE (2013) A novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab Epub ahead of print: [DOI] [PMC free article] [PubMed]
- 93.Murthy M, Azizan EA, Brown MJ, O′Shaughnessy KM. Characterization of a novel somatic KCNJ5 mutation delI157 in an aldosterone-producing adenoma. J Hypertens. 2012;30:1827–1833. doi: 10.1097/HJH.0b013e328356139f. [DOI] [PubMed] [Google Scholar]
- 94.Natke E, Jr, Kabela E. Electrical responses in cat adrenal cortex: possible relation to aldosterone secretion. Am J Physiol. 1979;237:E158–E162. doi: 10.1152/ajpendo.1979.237.2.E158. [DOI] [PubMed] [Google Scholar]
- 95.Nishi H, Arai H, Momiyama T. NCI-H295R, a human adrenal cortex-derived cell line, expresses purinergic receptors linked to Ca(2)(+)-mobilization/influx and cortisol secretion. PLoS One. 2013;8:e71022. doi: 10.1371/journal.pone.0071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nogueira EF, Gerry D, Mantero F, Mariniello B, Rainey WE. The role of TASK1 in aldosterone production and its expression in normal adrenal and aldosterone-producing adenomas. Clin Endocrinol (Oxf) 2010;73:22–29. doi: 10.1111/j.1365-2265.2009.03738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nussberger J. Investigating mineralocorticoid hypertension. J Hypertens Suppl. 2003;21:S25–S30. doi: 10.1097/00004872-200305002-00005. [DOI] [PubMed] [Google Scholar]
- 98.Oki K, Plonczynski MW, Lam ML, Gomez-Sanchez EP, Gomez-Sanchez CE. The potassium channel, Kir3.4 participates in angiotensin II-stimulated aldosterone production by a human adrenocortical cell line. Endocrinology. 2012;153:4328–4335. doi: 10.1210/en.2012-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oki K, Plonczynski MW, Luis LM, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology. 2012;153:1774–1782. doi: 10.1210/en.2011-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Otis M, Gallo-Payet N. Role of MAPKs in angiotensin II-induced steroidogenesis in rat glomerulosa cells. Mol Cell Endocrinol. 2007;265–266:126–130. doi: 10.1016/j.mce.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 101.Paul M, Poyan MA, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 102.Payet MD, Durroux T, Bilodeau L, Guillon G, Gallo-Payet N. Characterization of K+ and Ca2+ ionic currents in glomerulosa cells from human adrenal glands. Endocrinology. 1994;134:2589–2598. doi: 10.1210/endo.134.6.7515004. [DOI] [PubMed] [Google Scholar]
- 103.Penton D, Bandulik S, Schweda F, Haubs S, Tauber P, Reichold M, Cong LD, El WA, Budde T, Lesage F, Lalli E, et al. Task3 potassium channel gene invalidation causes low renin and salt-sensitive arterial hypertension. Endocrinology. 2012;153:4740–4748. doi: 10.1210/en.2012-1527. [DOI] [PubMed] [Google Scholar]
- 104.Peters J. Local renin-angiotensin systems in the adrenal gland. Peptides. 2012;34:427–432. doi: 10.1016/j.peptides.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 105.Peters J, Obermuller N, Woyth A, Peters B, Maser-Gluth C, Kranzlin B, Gretz N. Losartan and angiotensin II inhibit aldosterone production in anephric rats via different actions on the intraadrenal renin-angiotensin system. Endocrinology. 1999;140:675–682. doi: 10.1210/endo.140.2.6489. [DOI] [PubMed] [Google Scholar]
- 106.Philipp S, Trost C, Warnat J, Rautmann J, Himmerkus N, Schroth G, Kretz O, Nastainczyk W, Cavalie A, Hoth M, Flockerzi V. TRP4 (CCE1) protein is part of native calcium release-activated Ca2+-like channels in adrenal cells. J Biol Chem. 2000;275:23965–23972. doi: 10.1074/jbc.M003408200. [DOI] [PubMed] [Google Scholar]
- 107.Pralong WF, Hunyady L, Varnai P, Wollheim CB, Spat A. Pyridine nucleotide redox state parallels production of aldosterone in potassium-stimulated adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1992;89:132–136. doi: 10.1073/pnas.89.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Quinn SJ, Cornwall MC, Williams GH. Electrical properties of isolated rat adrenal glomerulosa and fasciculata cells. Endocrinology. 1987;120:903–914. doi: 10.1210/endo-120-3-903. [DOI] [PubMed] [Google Scholar]
- 109.Quinn SJ, Williams GH, Tillotson DL. Calcium oscillations in single adrenal glomerulosa cells stimulated by angiotensin II. Proc Natl Acad Sci U S A. 1988;85:5754–5758. doi: 10.1073/pnas.85.15.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Radke KJ, Schneider EG, Taylor RE, Jr, Kramer RE. Effect of hydrogen ion concentration on corticosteroid secretion. Am J Physiol. 1986;250:E259–E264. doi: 10.1152/ajpendo.1986.250.3.E259. [DOI] [PubMed] [Google Scholar]
- 111.Remuzzi G, Perico N, Macia M and Ruggenenti P (2005) The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int SupplS57–S65 [DOI] [PubMed]
- 112.Rohacs T, Bago A, Deak F, Hunyady L, Spat A. Capacitative Ca2+ influx in adrenal glomerulosa cells: possible role in angiotensin II response. Am J Physiol. 1994;267:C1246–C1252. doi: 10.1152/ajpcell.1994.267.5.C1246. [DOI] [PubMed] [Google Scholar]
- 113.Rohacs T, Nagy G, Spat A. Cytoplasmic Ca2+ signalling and reduction of mitochondrial pyridine nucleotides in adrenal glomerulosa cells in response to K+, angiotensin II and vasopressin. Biochem J. 1997;322(Pt 3):785–792. doi: 10.1042/bj3220785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Romero DG, Yanes LL, de Rodriguez AF, Plonczynski MW, Welsh BL, Reckelhoff JF, Gomez-Sanchez EP, Gomez-Sanchez CE. Disabled-2 is expressed in adrenal zona glomerulosa and is involved in aldosterone secretion. Endocrinology. 2007;148:2644–2652. doi: 10.1210/en.2006-1509. [DOI] [PubMed] [Google Scholar]
- 115.Rossig L, Zolyomi A, Catt KJ, Balla T. Regulation of angiotensin II-stimulated Ca2+ oscillations by Ca2+ influx mechanisms in adrenal glomerulosa cells. J Biol Chem. 1996;271:22063–22069. doi: 10.1074/jbc.271.36.22063. [DOI] [PubMed] [Google Scholar]
- 116.Sarzani R, Pietrucci F, Francioni M, Salvi F, Letizia C, D’Erasmo E, Dessi FP, Rappelli A. Expression of potassium channel isoforms mRNA in normal human adrenals and aldosterone-secreting adenomas. J Endocrinol Invest. 2006;29:147–153. doi: 10.1007/BF03344088. [DOI] [PubMed] [Google Scholar]
- 117.Sato Y, Maekawa S, Ishii R, Sanada M, Morikawa T, Shiraishi Y, Yoshida K, Nagata Y, Sato-Otsubo A, Yoshizato T, Suzuki H, et al. Recurrent somatic mutations underlie corticotropin-independent Cushing’s syndrome. Science. 2014;344:917–920. doi: 10.1126/science.1252328. [DOI] [PubMed] [Google Scholar]
- 118.Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, et al. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112:60–68. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- 119.Scandling JD, Ornt DB. Mechanism of potassium depletion during chronic metabolic acidosis in the rat. Am J Physiol. 1987;252:F122–F130. doi: 10.1152/ajprenal.1987.252.1.F122. [DOI] [PubMed] [Google Scholar]
- 120.Schambelan M, Sebastian A, Katuna BA, Arteaga E. Adrenocortical hormone secretory response to chronic NH4Cl-induced metabolic acidosis. Am J Physiol. 1987;252:E454–E460. doi: 10.1152/ajpendo.1987.252.4.E454. [DOI] [PubMed] [Google Scholar]
- 121.Schiekel J, Lindner M, Hetzel A, Wemhoner K, Renigunta V, Schlichthorl G, Decher N, Oliver D, Daut J. The inhibition of the potassium channel TASK-1 in rat cardiac muscle by endothelin-1 is mediated by phospholipase C. Cardiovasc Res. 2013;97:97–105. doi: 10.1093/cvr/cvs285. [DOI] [PubMed] [Google Scholar]
- 122.Scholl UI, Lifton RP. New insights into aldosterone-producing adenomas and hereditary aldosteronism: mutations in the K+ channel KCNJ5. Curr Opin Nephrol Hypertens. 2013;22:141–147. doi: 10.1097/MNH.0b013e32835cecf8. [DOI] [PubMed] [Google Scholar]
- 123.Scholl UI, Nelson-Williams C, Yue P, Grekin R, Wyatt RJ, Dillon MJ, Couch R, Hammer LK, Harley FL, Farhi A, Wang WH, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci U S A. 2012;109:2533–2538. doi: 10.1073/pnas.1121407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 125.Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech. 2003;61:300–307. doi: 10.1002/jemt.10339. [DOI] [PubMed] [Google Scholar]
- 126.Silver S. The Cushing syndrome; neoplasms of the adrenal gland. Bull N Y Acad Med. 1940;16:368–380. [PMC free article] [PubMed] [Google Scholar]
- 127.Simpson ER, Waterman MR. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol. 1988;50:427–440. doi: 10.1146/annurev.ph.50.030188.002235. [DOI] [PubMed] [Google Scholar]
- 128.Spat A, Fulop L, Szanda G. The role of mitochondrial Ca(2+) and NAD(P)H in the control of aldosterone secretion. Cell Calcium. 2012;52:64–72. doi: 10.1016/j.ceca.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 129.Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84:489–539. doi: 10.1152/physrev.00030.2003. [DOI] [PubMed] [Google Scholar]
- 130.Spyroglou A, Manolopoulou J, Wagner S, Bidlingmaier M, Reincke M, Beuschlein F. Short term regulation of aldosterone secretion after stimulation and suppression experiments in mice. J Mol Endocrinol. 2009;42:407–413. doi: 10.1677/JME-08-0167. [DOI] [PubMed] [Google Scholar]
- 131.Stachowiak A, Nussdorfer GG, Malendowicz LK. Proliferation and distribution of adrenocortical cells in the gland of ACTH- or dexamethasone-treated rats. Histol Histopathol. 1990;5:25–29. [PubMed] [Google Scholar]
- 132.Suwa T, Chen M, Hawks CL, Hornsby PJ. Zonal expression of dickkopf-3 and components of the Wnt signalling pathways in the human adrenal cortex. J Endocrinol. 2003;178:149–158. doi: 10.1677/joe.0.1780149. [DOI] [PubMed] [Google Scholar]
- 133.Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C, Beuschlein F, Reincke M, Barhanin J, Bandulik S, Warth R. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology. 2014;155:1353–1362. doi: 10.1210/en.2013-1944. [DOI] [PubMed] [Google Scholar]
- 134.Thomas M, Keramidas M, Monchaux E, Feige JJ. Dual hormonal regulation of endocrine tissue mass and vasculature by adrenocorticotropin in the adrenal cortex. Endocrinology. 2004;145:4320–4329. doi: 10.1210/en.2004-0179. [DOI] [PubMed] [Google Scholar]
- 135.Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tremblay E, Payet MD, Gallo-Payet N. Effects of ACTH and angiotensin II on cytosolic calcium in cultured adrenal glomerulosa cells. Role of cAMP production in the ACTH effect. Cell Calcium. 1991;12:655–673. doi: 10.1016/0143-4160(91)90036-e. [DOI] [PubMed] [Google Scholar]
- 137.Uebele VN, Nuss CE, Renger JJ, Connolly TM. Role of voltage-gated calcium channels in potassium-stimulated aldosterone secretion from rat adrenal zona glomerulosa cells. J Steroid Biochem Mol Biol. 2004;92:209–218. doi: 10.1016/j.jsbmb.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 138.Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, Gerlach U, Rong Q, Pfeifer K, Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci U S A. 2005;102:17864–17869. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Varnai P, Hunyady L, Balla T. STIM and Orai: the long-awaited constituents of store-operated calcium entry. Trends Pharmacol Sci. 2009;30:118–128. doi: 10.1016/j.tips.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Veale EL, Hassan M, Walsh Y, Al-Moubarak E, Mathie A. Recovery of current through mutated TASK3 potassium channels underlying Birk Barel syndrome. Mol Pharmacol. 2014;85:397–407. doi: 10.1124/mol.113.090530. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y, Yamaguchi T, Franco-Saenz R, Mulrow PJ. Regulation of renin gene expression in rat adrenal zona glomerulosa cells. Hypertension. 1992;20:776–781. doi: 10.1161/01.hyp.20.6.776. [DOI] [PubMed] [Google Scholar]
- 142.Westphalen RI, Krivitski M, Amarosa A, Guy N, Hemmings HC., Jr Reduced inhibition of cortical glutamate and GABA release by halothane in mice lacking the K+ channel, TREK-1. Br J Pharmacol. 2007;152:939–945. doi: 10.1038/sj.bjp.0707450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.White RE, Lee AB, Shcherbatko AD, Lincoln TM, Schonbrunn A, Armstrong DL. Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature. 1993;361:263–266. doi: 10.1038/361263a0. [DOI] [PubMed] [Google Scholar]
- 144.Wiederkehr A, Szanda G, Akhmedov D, Mataki C, Heizmann CW, Schoonjans K, Pozzan T, Spat A, Wollheim CB. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 2011;13:601–611. doi: 10.1016/j.cmet.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 145.Wilke BU, Lindner M, Greifenberg L, Albus A, Leitner MG, Oliver D. TASK1/3 inhibition by Gq-protein coupled receptors is mediated by diacylglycerol. Acta Physiol. 2014;210:81–81. [Google Scholar]
- 146.Williams DLJ, Katz GM, Roy-Contancin L, Reuben JP. Guanosine 5′-monophosphate modulates gating of high-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1988;85:9360–9364. doi: 10.1073/pnas.85.23.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu H, Garver H, Galligan JJ, Fink GD. Large-conductance Ca2+-activated K+ channel beta1-subunit knockout mice are not hypertensive. Am J Physiol Heart Circ Physiol. 2011;300:H476–H485. doi: 10.1152/ajpheart.00975.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Young MJ, Rickard AJ. Mechanisms of mineralocorticoid salt-induced hypertension and cardiac fibrosis. Mol Cell Endocrinol. 2012;350:248–255. doi: 10.1016/j.mce.2011.09.008. [DOI] [PubMed] [Google Scholar]