Abstract
The effect of CD on human health is an emerging issue. Many records link CD with diseases such as cancer, cardiovascular, cognitive impairment and obesity, all of them conducive to premature aging. The amount of sleep has declined by 1.5 h over the past century, accompanied by an important increase in obesity. Shift work, sleep deprivation and exposure to bright light at night increase the prevalence of adiposity. Animal models have shown that mice with Clock gene disruption are prone to developing obesity and MetS. This review summarizes the latest developments with regard to chronobiology and obesity, considering (1) how molecular clocks coordinate metabolism and the specific role of the adipocyte; (2) CD and its causes and pathological consequences; (3) the epidemiological evidence of obesity as a chronobiological illness; and (4) theories of circadian disruption and obesity. Energy intake and expenditure, relevance of sleep, fat intake from a circadian perspective and psychological and genetic aspects of obesity are examined. Finally, ideas about the use of chronobiology in the treatment of obesity are discussed. Such knowledge has the potential to become a valuable tool in the understanding of the relationship between the chronobiology, etiology and pathophysiology of obesity.
Keywords: metabolic syndrome, chronobiology, circadian, clock genes
Introduction
Correct timing of the endogenous circadian clock enables organisms to predict and anticipate daily environmental changes, and temporally adjust behavioral and physiological functions accordingly. In humans, current societal habits, including high snacking frequency, a reduction in the time spent sleeping and increased exposure to bright light during the night, act on the brain to induce a loss of ‘feeling’ for internal and external rhythms. Consequently, the environment sensed by the brain has become metabolically flattened and arrhythmic. Among the well-known consequences of a disrupted circadian function are altered metabolism and even life span, which may be all adversely affected when the circadian time-keeping system is altered.1
Obesity has emerged as one of the most serious public health concerns in the 21st century and the morbidity and mortality associated with obesity continue to increase. Endogenous (that is, genetic) and exogenous factors (that is, diet and physical activity) have an important role in the assessment and management of obesity.2 Recently, studies have suggested that disruption of the circadian system (chronodisruption (CD)) may lead to obesity.3 CD can also be produced by alteration of the core machinery of the molecular circadian clock. Brain- and muscle ANRT-like protein-1 (BMAL1), Period Circadian Protein-2 (PER2) and Circadian Locomotor Output Cycles Kaput (CLOCK), among others clock proteins, have a specific role in the organism’s physiology in addition to their role in the circadian molecular clock. Animal models have shown that mice with Clock gene disruption are prone to developing obesity and a phenotype resembling metabolic syndrome (MetS).4
Complications of obesity include cardiovascular disease, hypertension, dyslipidemia, endothelial dysfunction, type-2 diabetes mellitus and impaired glucose tolerance, among others. Interestingly, chronobiology is implicated in most of these alterations.5 Circadian control of the cardiovascular function is firmly established. Furthemore, it is known that many hormones involved in metabolism, such as insulin, glucagon, growth hormone and cortisol, show circadian oscillation with different daily patterns. Current studies are illustrating the particular role of different clock gene variants and their predicted haplotypes in obesity. This review will summarize recent findings concerning the relationship between the chronobiology, etiology and pathophysiology of obesity.
How molecular clocks coordinate metabolism
The mammalian circadian time system is composed of a network of hierarchically organized structures responsible for the generation of circadian rhythms and their synchronization with the environment. This circadian system can be conceptualized as having three sets of components: the inputs, the 24-h oscillators and the outputs (Figure 1). It is essential to note that the most important circadian oscillator is a central pacemaker, located in the suprachiasmatic nucleus (SCN) of the hypothalamus.
Figure 1.
A general overview of the functional organization of the circadian system in mammals. Inputs: Environmental periodical cues can reset the phase of the central pacemaker so that the period and phase of circadian rhythms became coincident with the timing of the external cues. Central pacemakers: The SCN is considered the major pacemaker of the circadian system, driving circadian rhythmicity in other brain areas and peripheral tissues by sending them neural and humoral signals. Peripheral oscillators: Most peripheral tissues and organs contain circadian oscillators. Usually they are under the control of the SCN; however, under some circumstances (that is, restricted feeding, jet-lag and shift work.), they can desynchronize from the SCN. Outputs: Central pacemakers and peripheral oscillators are responsible for the daily rhythmicity observed in most physiological and behavioral functions. Some of these over-rhythms (physical exercise, core temperature, sleep–wake cycle and feeding time), in turn, provide a feedback, which can modify the function of SCN and peripheral oscillators. SCN, suprachiasmatic nucleus.
Because the endogenous SCN oscillation period is not exactly 24 h, circadian rhythms free-run with a period slightly different from 24 h when the subject is maintained under artificially constant environmental conditions. However, under natural environmental conditions, the SCN is reset every day by the periodical light/dark signal through a non-visual pathway consisting of the melanopsin ganglionar cells and the retinohypothalamic tract. Although the photic input is the main SCN entraining signal, other periodical cues, such as feeding time, social contacts and regular physical activity, can also entrain the mammalian circadian system.6,7
When food availability is limited to short time periods (restricted feeding) many circadian rhythms can phase-shift to adapt the organism’s physiology to the time of scheduled food (food-anticipatory activity, FAA). However, under unrestricted access to food, when light becomes the dominant synchronizing signal, these circadian rhythms return to their previous phase.8 Evidence from SCN lessoned animals, which are able to maintain circadian rhythmicity entrained to restricted feeding, suggests that in addition to the light-entrainable pacemaker (SCN) another circadian oscillator may supersedes the SCN.8 This putative clock, called the feeding-entrainable oscillator, could be located in the dorsomedial nucleus of the hypothalamus (DMH), as only insertion of functional Bmal1, an essential clock component, in the DMH neurons restores the ability of Bmal1-defective animals to entrain to food but not light. Similarly, insertion of Bmal1 in the SCN was able to restore the light but not food-entrained rhythms.9 However, the persistence of FAA in DMH lesioned rats9 suggests that feeding-entrainable oscillator could be dependent on a network of oscillators, rather than be located in one place. In intact animals, different circadian oscillators located in the digestive tract, liver or cerebral areas other than the SCN might contribute to entraining the circadian rhythms of feeding times, raising strong expectations that one or more structures that underlie FAA.10 Recently, a new discovery has increased the present controversy.11 Mutant mice lacking known circadian clock function in all tissues show normal FAA both in a light–dark cycle and in constant darkness, regardless of whether the mutation disables the positive or negative limb of the clock feedback mechanism. FAA seems to be independent of the known circadian clock. The authors concluded that either FAA is not the output of an oscillator or that it is the output of a circadian oscillator different from known circadian clocks. New research is needed to confirm these results and to show the existence of a real feeding-entrainable oscillator.
In addition to the SCN, other brain areas have been proposed as self-sustained circadian oscillators and challenge the traditional uniclock model. Among these areas, the retina and the olfactory bulb are master oscillators, which are capable of self-sustained circadian output in isolation.12
A crucial step toward a better understanding of circadian time system physiology was the discovery that circadian clocks are present in most peripheral tissues13 (Figure 1). Self-sustained oscillations can be observed in some organs and tissues such as the heart, lung, liver, intestine and adrenal and adipose tissue (AT). These peripheral oscillators must receive periodical inputs from the SCN to prevent the spontaneous dampening of their rhythmical activity with time. However, they are also sensitive to their own synchronizers such as feeding time, local temperature, glucocorticoids, retinoic acid, prostaglandins, adrenaline, noradrenaline, glucose and angiotensin-II.
In contrast to the relatively well-known structure and function of photic synchronizing inputs and the master circadian pacemaker, the pathways acting downstream from the SCN remain poorly understood. These pathways are used by the SCN to exert their circadian control on behavioral and physiological processes. Both, the direct output of the SCN (prokineticin-2, transforming growth factor-a, γ-aminobutyric acid and vasopressin) and the brain-mediated output (selective activation of parasympathetic and sympathetic nerves, nocturnal secretion of pineal melatonin) have been reported as mediators in the effects of the SCN on the organism (Figure 1).5,12
The biological clock at a molecular level
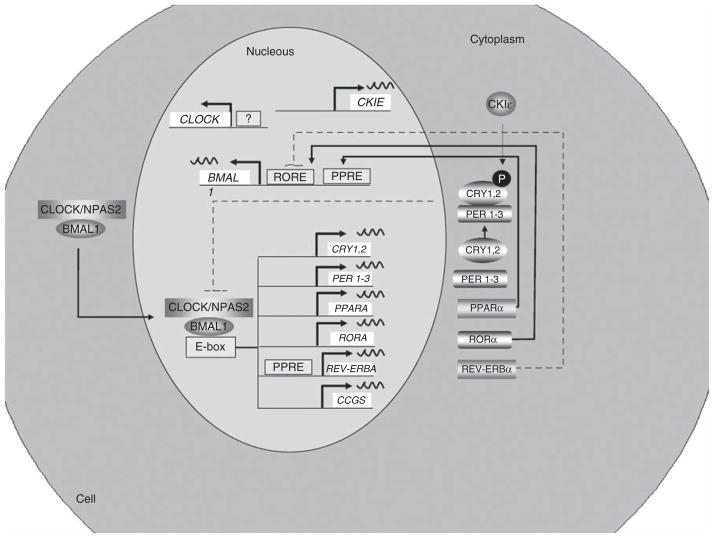
Circadian clocks are composed of a set of proteins that gene-rate self-sustained circadian oscillations through positive and negative transcriptional/translational feedback loops. Although the molecular clock model is continuously evolving, the current view suggests that the core of the molecular clock is composed of two basic helix–loop–helix PER-ARNT-SIM (Period circadian protein–Aryl hydrocarbon Receptor Nuclear Translocator protein–SIngle-Minded protein) transcription factors, CLOCK (circadian locomotor output cycles kaput) and BMAL1 (brain- and muscle- ANRT-like protein), which act as positive elements in the feed back loop (Figure 2). Recently, it has been proposed that the CLOCK homolog neuronal PAS domain (name derived from PER-ARNT-SIM) protein-2 (NPAS2) could functionally compensate for the lack of CLOCK. The heterodimer CLOCK–BMAL1 binds to E-boxes in the promoter of some target genes, driving the transcription of six repressor encoding genes: three period genes (Per1, Per2 and Per3), two cryptochrome (Cry1 and Cry2) and the transcription factor reverse erythroblastosis virus-α (Rev-Erbα) gene, and one promoting gene, Ror-α (retinoic acid receptor-related orphan receptor-α) (Figure 2). PER and cryptochrome circadian protein (CRY) dimerize and are then translocated into the nucleus. The transcription of the repressor-encoding genes is stopped when PER–CRY dimers counteract the positive effect of CLOCK–BMAL1 and thereby inhibit their own transcription. In addition, the Bmal1 rhythm is regulated by rhythmical changes in RORE occupancy by REV-ERBα and RORα (Figure 2). Other nuclear receptors and coactivators, such as peroxisome proliferator-activated receptors (PPARs) and PPARγ-coactivator-1α (PGCα), have been proposed as modulators of BMAL1 and CLOCK (Figure 2).
Figure 2.
Organization of the mammalian circadian intracellular oscillator. The cellular oscillator is composed of a positive (CLOCK and BMAL1) and a negative (PER1–3 and CRY1, 2) limb. CLOCK–BMAL1 heterodimeres, through binding to E-box elements, drive the transcription of several genes: Cry1, Cry2, Per1, Per2, Per3, Rev-Erbα, Rorα and multiple CCGs. After dimerization PERs and CRYs undergo nuclear translocation inhibiting CLOCK–BMAL1-mediated transcription. Once the levels of PERs and CRYs fall, the negative repression is lifted and CLOCK–BMAL1 binds again to the E-box. A secondary stabilizing loop is established by the negative, REV-ERBα, and positive, RORα, effect on Bmal1 transcription through their activity on RORE. In addition, the PPARα, a CCG, induces Bmal1 and Rev-Erbα transcription through its action on PPAR-response elements located in their respective promoters. The molecular circadian clock in linked to metabolism through several mechanisms. RORα and Rev-Erbα regulates lipid metabolism and adipogenesis. BMAL1, brain- and muscle ANRT-like protein-1; CCG, Clock-controlled gene; CLOCK, Circadian Locomotor Output Cycles Kaput; CRY, cryptochrome circadian protein; PER, Period Circadian Protein; PPAR, peroxisome proliferator-activated receptor; REV-ERBα, erythroblastosis virus-α; RORα, retinoic acid receptor-related orphan receptor-α.
It has been shown that PPARα can modify BMAL1 and CLOCK expression and activity by its union to PPAR-response elements located in the Bmal1 promoter.14–17 Similarly, PPARα induces the expression of rev-ERα by its union to PPAR-response elements in its promoter.18,19
Conversely, PPARγ-coactivator-1α (PGC-1α) induces the transcription of Bmal1 and Rev-Erbα through coactivation of de RORα.20 PGC-1α is the key factor in the homeostasis of glucose, lipids, energy and it is also modulated by stress and nutrients.21,22 Indeed, PGC-1α-knockout mice show metabolic alterations (disrupted weight control, muscle dysfunction and liver steatosis) and impaired circadian rhythms (lengthened period of locomotor activity, body temperature and metabolic rate).20,21
It is known that chromatin remodeling is a key process evolved in CLOCK-regulated gene expression;23 in fact, the CLOCK protein has a significant activity as a histone acetyltransferase facilitating gene transcription;24 this activity is counteracted by Sirtuin-1, a histone deacetylase25,26 that is related to energy metabolism and aging.27
Recent evidence show that clock genes also act as sensors of the cellular metabolic status through changes in the redox state.28 This dependence of the molecular clock on energy uptake could explain why alterations in metabolic cues, such as restricted feeding or conflicting synchronizers, are able to modulate the activity of the circadian timing system.29 It is known that increased levels of the reduced cofactors NADH and NADPH consequence of energy supply by feeding or fat depots mobilization, induce an increase in the affinity of CLOCK–BMAL1 and NPAS2–BMAL1 heterodimers for their E-box targets in vitro.28,30 In addition, NAD+ is required by Sirtuin-1 as a co-substrate for their deacetylation activity, providing an additional link between energy cellular status and molecular clock.31 The balance between NAD+/NADH is regulated by a key enzyme, the nicotinamide phosphoribosyl-transferase, also called visfatin. It is sensitive to cellular energy supply, being induced in hepatocytes of rats after 48 h of fasting.32 It is noteworthy that its plasma values increase with obesity and type-2 diabetes.33,34
In addition to these core clock genes, other genes of SCN neurons, which are not components of the circadian mechanisms, but whose expression is regulated by clock genes, oscillate with a periodicity close to 24 h. These are the so-called clock-controlled genes (CCGs) or circadian output genes. A large percentage (3–20%) of the mammalian transcriptome shows circadian rhythms, including both direct CCGs and the downstream output of these CCGs3,35,36 (Figure 2).
What about the adipocyte?
Although the clock mechanism is considered universal, tissue-specific differences have been observed. Such differences within cell types may provide important insight into the association between alterations in the circadian clock and different diseases. Among peripheral tissues, AT has taken on a new importance given the current rise in obesity epidemic.
Clock genes in AT from experimental animal models
Most molecular analyses of clock genes in AT have involved rodent models13,37–41 and evidence suggests that a fully functional circadian clock mechanism exists within AT. In isolated brown AT and white AT, clock genes were seen to display an oscillatory profile, with anti-phase expression of the positive (Bmal1, Clock) and negative (Cry, Per) arms, respectively.13,37,39,41 When food access was temporally restricted, the phase of clock genes shifted accordingly.13,42 Several groups have evaluated clock genes in the AT of obese mice4,37,43,44 and, while some observed that obesity had little effect on the clock gene machinery,44 others found that it attenuated and/or disrupted clock gene expression.37,43 This discrepancy may result from the murine strains used and the fact that the mechanism of diet-induced obesity44 differs from that of obesity due to single mutations.37
In addition, some clock genes, especially Bmal1 and Rev-Erbα, may play a part in adipocyte differentiation and lipogenesis.45 This role of clock genes seems to be important in the development of obesity.
Clock genes in human AT
Clock gene expression has been shown in human AT. Furthermore, their expression has been associated with different components of the MetS.46 It has also been shown that clock genes can oscillate accurately and independently of the SCN in human AT explants47 and that this intrinsic oscillatory mechanism may participate in regulating the timing of other CCGs such as PPAR-γ and glucocorticoid metabolism genes.47,48 Moreover, these circadian patterns differ between visceral and subcutaneous AT depots.47,48
Circadian rhythmicity in AT metabolism
AT metabolism has a strong correlation with circadian system.49,50 Over the course of a 24-h period, the adipocyte must reciprocally adjust the rates of triglyceride synthesis (lipogenesis) and storage with the rates of triglyceride breakdown (lipolysis). Diurnal variations in adipose metabolism are influenced by neurohumoral factors; for example by sympathetic activity (adrenaline). However, it has been shown that such lipolysis persists ex vivo, showing that the circadian clock within the adipocyte has a significant role by altering the sensitivity of the adipocyte to specific stimuli (for example, insulin, adrenaline) and showing the intrinsic nature of adipocyte diurnal variations.39
A number of adipocyte-specific factors show rhythmic expression.5 Some examples are leptin, adipsin, resistin, adiponectin and visfatin, all of them showing circadian rhythmicity. Glucocorticoids have also shown circadian rhythmicity in human AT.47 Adiponectin, a protector against MetS disturbances,51 shows both ultradian pulsatility and a diurnal variation.52 The daily pattern of this adipocytokine is out of phase with leptin, showing a significant decline at night and reaching a nadir in the early morning.53
CD: pathological consequences
Circadian disruption or CD could be defined as a relevant disturbance of the internal temporal order of physiological, biochemical and behavioral circadian rhythms. It is also a breakdown of the normal phase relationship between the internal circadian rhythms and 24-h environmental cycles.54 In our modern 24/7 society, CD may result from several conditions such as jet-lag, shift work, nocturnal light pollution or nocturnal leisure activities (Figure 3).
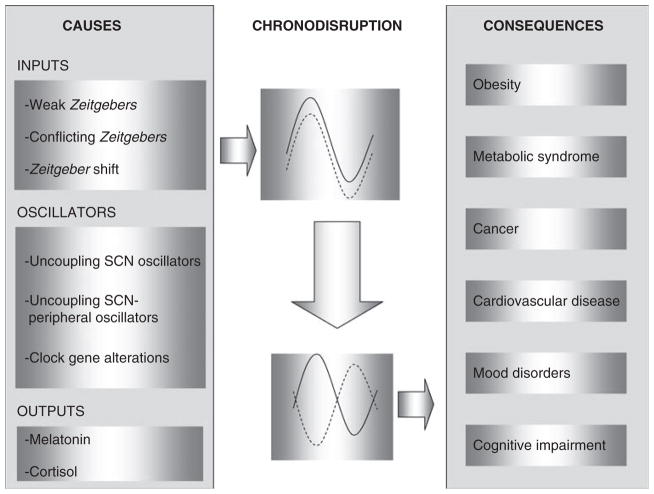
Figure 3.
The causes and consequences of circadian disruption. CD is the result of a phase shift in the oscillation of the circadian (solid line) and activity-controlled physiological processes (dotted line). This circadian pathology can be induced by factors related to the following: Inputs: low contrast between day and night synchronizing signals (continuous light, frequent snacking, low physical exercise…); by Zeitgebers with different periods or unusual phasing (that is, light at night, nocturnal eating, nocturnal physical activity…) or by Zeitgeber shifts (that is, daylight-saving time, crossing time zones, shift work…). Oscillators: The uncoupling between the different oscillators inside the SCN caused by aging, the uncoupling between the central and peripheral oscillators or clock gene functional alterations result in circadian disruption. Outputs: Nocturnal melatonin suppression and loss of cortisol rhythmicity are also chronodisrupters. Many pathological states can be promoted or impaired as consequence of CD. CD, chronodisruption.
In the recent years the effect of CD on human health has become an important issue. Multiple records link CD with the increased risk of developing certain diseases and with an impairment of pre-existing pathologies: premature aging, cancer, cardiovascular diseases, cognitive impairment and mood disorders.55,56 Obesity and metabolic syndrome are also closely related to CD.
Premature aging
Similar to that observed in many physiological processes, the functioning of the circadian system changes with age. Phase advance, reduced amplitude, circadian fragmentation, impaired ability to resynchronize after a time shift and internal desynchronization among different rhythms are the major characteristics of aged rhythms.57 However, less well known is the fact that CD has a direct role in inducing accelerated aging. For example, a 6-h shift of the light–dark cycle every week induced a significant reduction in the lifespan of aged mice.58 Longevity in hamsters decreased with circadian disruption and increased in older animals given fetal SCN implants that restore higher amplitude circadian rhythms.59 Similarly, chronic reversal of the light–dark cycle decreases the median lifespan in cardiomyophatic hamsters.60 Therefore, it is generally believed that disruptions in circadian rhythms lead to reduced life expectancy, whereas their appropriate resetting leads to well-being and increased longevity.
Cancer
The incidence and progression of some types of cancer, such as breast, endometrial, prostate, colorectal and melanoma, can be promoted by CD.54,61 Both human epidemiological and experimental studies of animals have documented that a potential negative consequence of CD is cancer initiation and development.62
In addition, a relation between CD and cancer prognosis has been observed in humans. In metastatic colorectal cancer patients, marked rest–activity rhythms are associated with a better quality of life, better response to chemotherapy and longer survival.63
Cardiovascular diseases
In people with normal blood pressure (BP) and uncomplicated essential hypertension, BP declines to its lowest levels during night-time sleep, rises abruptly with morning awakening and attains a maximum during diurnal activity. It has been shown that night-time BP is the best predictor of stroke and myocardial infarction risk.64 Thus, hypertensive patients with a normal reduction in nocturnal BP (dipper) had a relative hazard of cardiovascular mortality similar to that in non-dipper normotensives.65 It is noteworthy that the non-dipper circadian pattern is more frequent among shift workers and elderly people.
Obesity and MetS
As will be discussed in forthcoming sections, a well-known effect of CD on human health is the development of obesity and MetS. Many epidemiological studies show that CD induced by shift work, sleep deprivation or by shifting the normal feeding time to night hours, is associated with high risk of developing obesity and many characteristics of metabolic syndrome.5 All the different metabolism-related activities of the circadian system, such as regulation of lipid and glucose metabolism, or insulin response, which are impaired by CD, may contribute to the pathophysiology of obesity.
Psychological diseases
CD is also associated with an increased frequency of affective disorders such as seasonal affective disorder, major depression and insomnia.56 Finally, cognitive impairment associated with Alzheimer’s disease, vascular dementia or Parkinson’s disease can be aggravated by CD, as is observed when such sufferers spend too much time indoors or in a constant environment such as hospital wards or in intensive care units.66
What causes CD?
CD can be induced by any impairment of the functioning of the inputs, of the 24-h oscillators or of the outputs of the circadian system (Figure 3).
Inputs
Light–dark
Light–dark cycle is the main synchronizing input to the central circadian pacemaker, the SCN. Light activation by blue-rich lights of the ganglion-containing melanopsin cells in the retina is sufficient for circadian synchronization. However, circadian photoreception decreases with normal aging as a result of age-related pupillary miosis and reduced crystalline lens transmission, particularly for blue light.66 Thus, people in their eighties retain only 10% of the circadian photoreception of a 10-year-old child. In addition, contemporary artificial light sources do not provide more that 1% of the brightness of outdoor natural light, with spectra shifted to longer wavelengths, which are less effective for SCN synchronization. Light deficiency, whether due to improper timing, suboptimal spectrum or insufficient intensity, may contribute to medical conditions associated to CD.66
Feeding time
Feeding time is considered one of the most important external synchronizers or Zeitgebers for peripheral oscillators. Thus, unusual feeding times can also contribute to the effects of CD. For example, when nocturnal (characterized by late awakening, omitting breakfast and late dinner) and diurnal (early awakening and early dinner) lifestyles were compared among healthy young people, plasma glucose and insulin response were found to be well regulated in the diurnal group, whereas a sustained hyperglycemia during the night and hypoglycemia in the morning were observed in the case of the nocturnal lifestyle.67 In addition, nocturnal leptin and melatonin were reduced with the nocturnal lifestyle. Therefore, nocturnal lifestyle is likely to be one of the risk factors to the health of modern people, including night eating syndrome, obesity and diabetes.
Pacemakers
Different rates of synchronization
As regards the pacemaker contribution to CD, classically, different rates of synchronization of biological variables have been suggested as the cause of jet-lag and shift-work-induced CD. This may be the result of the different coupling strength between the SCN and the peripheral oscillators. For example, following a 6-h phase delay, the acrophase of adrenocorticotropic hormone and cortisol rhythms take 7 days to resynchronize, whereas the acrophase of the sleep–wake cycle resynchronizes in 3 days. Thus, for several days and because each organ shifts at a different rate, the organism is sub-optimally organized to efficiently accomplish its functions.
Circadian clock alteration
In addition to this classical hypothesis, CD can also be produced by alteration of the core machinery of the molecular circadian clock. BMAL1, PER2 and CLOCK, among others clock proteins, have a specific role in an organism’s physiology in addition to their role in the circadian molecular clock.55 A deficiency in a particular clock protein results in two conditions: disruption of circadian rhythms and development of a primary pathology. Alterations in particular clock genes have been associated with specific diseases, some examples of which are mentioned below.
BMAL1 and premature aging
Bmal1-knockout mice, for example, are arrhythmic and represent one of the best models of premature aging. The mean lifespan of knockout animals was 37 weeks as compared with 120 weeks for wild-type animals.68 Other characteristics of this premature aging model are sarcopenia, alteration of the percentage of lymphocytes and impaired vision.
PER2 and cancer
Although the mechanism through which CD promotes cancer development is not well known, disruption of the core clockwork by mutations in Per2 increases the susceptibility of mice to spontaneous and irradiation-induced tumors.69 This non-functional clock gene, Per2, was associated with changes in two genes, the proto-oncogene c-myc and the tumor repressor p53. Stimulation of the proto-oncogene, together with repression of the oncostatic p53 gene, could lead to genomic instability, cellular proliferation and cancerogenesis.
CLOCK gene and obesity
CLOCK protein is a key protein involved in the synchronization of metabolic processes with the environment and in the control of the mammalian energy balance. Circadian rhythms of both food intake and metabolic rate in Clock-mutant mice are abolished or reduced. Clock-mutant mice are obese, show adipocyte hypertrophy, hepatic steatosis and alteration in the blood levels of leptin.4
However, significant controversy exists in these aspects. Kennaway et al.70 shown that Clock (Delta 19) mutation with arrhythmic Clock gene expression in the liver and skeletal muscle did not cause obesity and increased insulin sensitivity. While serum triglycerides and free fatty acids (FFAs) were significantly lower in circadian Clock-mutant mice.71 The lack of reports for some other circadian mutants apart from Clock and Bmal1 suggests that obesity and MetS are not a result from general disruption of rhythmicity and synchronization between different processes in tissues. Probably some circadian proteins have, in addition to their role in the circadian system, specific non-circadian functions important to the regulation of body fat.
Outputs
The third element, which can cause CD, is the alteration of the outputs of the circadian system. Impairment of the melatonin rhythm seems to be relatively well known. Melatonin is produced in response to a sympathetic output of the SCN with a relay in the upper cervical ganglion. Because melatonin is produced during the night in all species studied, it is frequently called the ‘chemical darkness’. However, in addition to being controlled by the pacemaker, light at night can induce an acute suppression of melatonin secretion and its nocturnal plasma level shows progressive reduction with aging. Melatonin provides a potential connection between CD and the pathological states induced by it.55,62 Melatonin is a well-known anti-oxidant, which directly scavenges free radicals. In addition, the antioxidant activity of melatonin is connected with induction of the expression/activity of major antioxidant enzymes and with the improvement of the function of the electro-transport chain in the mitochondria.72,73
Besides its antiaging effects, melatonin is considered as an anticarcinogenic and oncostatic substance, particularly against, breast, colorectal, prostate and melanoma cancers. Melatonin has been also related to obesity alterations, acting as a protective hormone. It reduces BP and improves glucose metabolism.74
Obesity as a chronobiological illness: epidemiological evidence
There is epidemiological evidence of a relationship between obesity and chronobiological aspects. One of the most interesting findings is that shift work is an independent risk factor in the development of obesity.75 Industrialization has given rise to the widespread adoption of 24-h continuous operations in a number of industries. This has resulted in an increase in the proportion of the population routinely engaged in shift work, reaching >20% of the industrialized world. Epidemiological studies show that shift work is associated with obesity, hypertriglyceridemia, low high-density lipoprotein, abdominal obesity, diabetes and cardiovascular disease.76 Furthermore, increased glucose, insulin and triglyceride postprandial metabolic response is observed in shift workers with disrupted circadian rhythmicity of the melatonin profile.77
Together with shift work, a similar situation underlies the sleep ‘disorders’ of jet lag, which is not in itself a disease but rather reflects a normal function of circadian timing in the context of extraordinary demands on sleep–wake scheduling. Both jet lag and shift work insomnia are related to obesity and represent important social problems, which deserve public health and medical attention.78
Interesting results have arisen from studies relating sleep duration and metabolic risk. The time we sleep has declined by 1.5 h over the past century, accompanied by an important increase in obesity. Moreover, a third of adults sleep less than six hours a night.79 Clinical studies show that healthy subjects restricted to 4 h of sleep for six consecutive nights showed impaired glucose tolerance and reduced insulin responsiveness after a glucose challenge.80 These metabolic changes are rather concerning children, in whom short sleep duration, affected by factors such as the day of the week, season and having younger siblings, has been described as an independent risk factor for obesity.80
Circadian disruption and obesity: different theories
The precise mechanisms linking obesity to CD are not well known. It has been hypothesized that current habits, such as high snacking frequency, a reduction in total daily sleep and increased exposure to bright light during the night, induce brain to lose its ‘feeling’ for internal and external rhythms. The lack of day–night environmental contrast may lead to CD and metabolic disturbances, including obesity. Conversely, studies performed using experimental models have shown that developing obesity and diabetes itself disrupts the molecular clock system.37 Both, as a cause or as a consequence, CD is closely linked to obesity. Some of the following theories have been put forward:
The autonomic nervous system
As the brain uses the autonomic nervous system to implement internal rhythmicity, an unbalanced arrhythmic autonomic nervous system has been proposed as a major cause of obesity.
One of the crucial components of metabolic syndrome in obesity is visceral fat. Many studies show that CD is more frequent among obese subjects with a central distribution of fat than in those with a gluteofemoral pattern. Furthermore, the chronotype of the subject has been shown to be related to obesity, with ‘evening type’ subjects being more likely to display central obesity than morning types.
The high specialization of autonomic nervous system neurons allows them to project either to the intra-abdominal or subcutaneous body compartment, and this depot-specific effect could also vary depending on the time of the day.81 As a consequence, intra-abdominal organs, such as visceral fat, liver and pancreas, receive input from the same neurons and, therefore, the same stimulus will increase insulin sensitivity at the same time in the three locations. It has been hypothesized that as meals consumed in the evening are known to lead to enhanced insulin secretion, a shift of feeding toward the end of the day will result in a more efficient uptake of glucose in visceral fat and will result in easier accumulation of intra-abdominal fat. However, further studies should confirm this hypothesis considering that insulin resistance is also increased during night hours.
The pineal–hypothalamic–adipocyte pathway
In the same way, but related to the pineal–hypothalamic–adipocyte pathway, Scott and Grant82 proposed a similar hypothesis on the basis of the fact that hibernators are a good model of human obesity. In these animals the patterns of fat acquisition follow endogenous circannual rhythms entrained by ambient light. In preparation for winter, during the period of fat storage, there is an insulin resistance state during which weight almost doubles through storage of vast depots of fat. Thus, it can be hypothesized that modern westernized man are always getting ready for a food deprivation state (winter) that never comes.
Internal environment challenges within the organism
Most hypotheses to explain the relationship between obesity and chronobiology point to internal desynchronization between the different circadian rhythms involved in metabolism as a key factor. Some examples are the following:
Food intake signals
Excess energy intake produces a progressive derangement in temporal communication between different food intake signals by modifying the strength, duration and frequency in their rhythmicities. An example is the well-known reciprocal circadian and ultradian rhythmicities in anorexigenic leptin and orexigenic ghrelin, which encode a corresponding release pattern of appetite stimulating neuropeptide-Y (NPY) in the hypothalamus.
With obesity, hyperleptinemia leads to a central leptin insufficiency and breakdown in this coordinated rhythmic interplay. Similar theories have been proposed for histamine and H1 receptors, apoprotein A-IV and other peptides highly implicated in food intake regulation.
LPL and meal times
On the basis of its ability to cleave circulating triglycerides, lipoproteinlipase (LPL) was originally identified as a clearing factor as it releases FFAs and facilitates their uptake across the cell’s plasma membrane.83 Under physiological conditions FFAs are transported to the adipocytes where they are stored for subsequent use. Gimble et al. recently described that ‘If AT expressed LPL continuously, the time that an individual ingested a fatty meal would be irrelevant. However, LPL mRNA and protein levels show circadian oscillations, and, as long as LPL peaks at meal times, the FFAs will be properly stored within the adipocytes. By contrast, if meal times do not coincide with LPL peaks, the organism may store circulating FFAs in ectopic tissues, resulting in lipotoxicity and, as a consequence, hepatic, muscular or pancreatic comorbidities’.83
Most of the hypotheses proposed to date point to the same: perhaps obesity is on the rise not only because we consume too much food, but also because we eat it at the wrong times. In this line an interesting study has shown that nocturnal mice fed a high-fat diet only during the 12-h light phase gain significantly more weight than mice fed only during the 12-h dark phase.84
Energy intake and expenditure: the relevance of sleep
Classical lesion studies performed more than 50 years ago showed that distinct regions of the hypothalamus control hunger and satiety. A relevant aspect in those studies was the cloning of leptin, which decreases food intake and body weight. Leptin decreases hunger and increases energy expenditure through stimulation of the melanocortin system.85–89 The fact that leptin is expressed in a circadian manner and fluctuates in response to fasting and feeding89 reinforces the importance of chronobiology for controlling food intake. An important implication arising from these results is that ‘an internal desynchronization of the circadian rhythms of peptides involved in food intake control, especially of leptin, could be involved in the imbalance between energy intake and expenditure. Conversely, high energy intake, constant snacking or alteration in meal times could be affecting this internal circadian machinery.
Sleep alterations are also related to food intake. For example, several studies have shown that short sleepers have significantly reduced circulating levels of the anorectic hormone leptin and increased levels of ghrelin, an orexigenic stomach-derived hormone,90 suggesting that sleep deprivation may affect the peripheral regulators of hunger. Furthermore, leptin-deficient mice show impaired sleep and altered diurnal rhythmicity,91 whereas ghrelin has been shown to increase non-rapid eye movement sleep in both rodents and humans.92,93
The circadian pacemaker and cytokine systems seem to overlap with respect to their influence on energy intake patterns and sleep–wake regulation.94 Indeed, there is growing evidence that interleukin-6 and several other proinflammatory cytokines are ‘sleep factors’.95 In addition, it has been shown that these cytokines also influence energy intake by enhancing insulin and leptin sensitivity.96 However, any possible role they might have in coordinating sleep/wakefulness with food-motivated behavior is still unclear. There is increasing evidence that the neurophysiological and metabolic mechanisms responsible for controlling food-seeking behavior and sleep/wakefulness are coordinated. Indeed, hunger and vigilance are paired during daylight hours, whereas satiety and sleep are paired during the dark.94 Recently we have reported that CLOCK gene polymorphisms are significantly associated with food intake and that the cytokine system, particularly interleukin-6, has a significant role in this connection.97
Other neuromodulators involved in feeding and alertness, including histaminergic and serotoninergic transmitters, may also jointly affect alertness, circadian rhythmicity and metabolism.
A physiological explanation of this relationship
Experimental studies of sleep deprivation98 suggest that a number of causal pathways link short sleep duration and obesity (Figure 4). One mechanism by which sleep deprivation might contribute to weight gain is by increasing caloric intake. Experiments in animals have consistently found that total sleep deprivation produces hyperphagia,99 whereas partial sleep deprivation experiments in humans suggest a similar effect especially as regards high-fat and high-carbohydrate foods. Such changes in sleep patterns are accompanied by increased serum ghrelin levels, increased evening concentrations of cortisol and decreased levels of leptin. Alternatively, in an environment where food is readily available, reduced sleep may simply represent an increased opportunity to eat. Chronic partial sleep deprivation also clearly leads to a feeling of fatigue,100 which could lead to a reduction in physical activity. Indeed, studies of children have found short sleep duration to be associated with increased television viewing and reduced participation in organized sports.90,101 Sleep loss may also affect energy expenditure through thermoregulation, and studies of acute sleep deprivation in humans have pointed to a reduction in the core body temperature.102
Figure 4.
A schematic representation of the putative pathways leading from sleep loss to obesity and the MetS. MetS, metabolic syndrome.
Fat intake and obesity
One of the most important aspects of the interaction between chronobiology and obesity is the effect that high-fat feeding has on both. Epidemiological studies have shown that fat intake is related to CLOCK gene polymorphisms.97 However, data are still contradictory. Although experimental studies have shown alterations in clock gene expression in AT and liver, they failed to show any attenuation of clock gene expression in the hypothalamus.44 This suggests that high-fat feeding leads to obesity and affects the molecular clock function in peripheral tissues but not in the central nervous system. Such changes are associated with altered diurnal profiles of leptin, glucose, insulin, FFAs and corticosterone, as well as impaired regulation of nuclear receptors, suggesting that this disruption of peripheral clocks directly alters the metabolic function.43 Recent experimental models have shown that high-fat feeding, particularly high intake of saturated fat, modifies circadian synchronization to light and leads to metabolic abnormalities, which mimic human MetS, including obesity and insulin resistance.103
It is of note that of the genes that show circadian rhythmicity in mouse liver,104 2.5% also showed altered expression in rat liver when 40% of the energy in the diet was fat (lard, olive oil, fish oil or coconut). It is assumed that habitual high-fat meals perturb the circadian rhythm of these genes, either by flattening the rhythm or by magnifying it.
Further studies are necessary to confirm the particular roles of the different types of fat in the circadian system. Olive oil as a source of monounsaturated fatty acids may affect circadian rhythmicity in a different way to saturated fat. In this line our results in a US white population support the notion that the genetic effects of CLOCK on the insulin resistance and obesity phenotypes could be modulated by dietary intake of monounsaturated fatty acids. Indeed, the protective effect of the minor allele on insulin sensitivity was only present when monounsaturated fatty acid intake was higher than the mean value (13.2% of energy).105
Psychological aspects in obesity and chronobiology
Obesity is highly related to emotional disorders. It has long been known that the frequency of overweight and obese people is higher among depressed and bipolar patients than in the general population. Marked alteration of body weight (and appetite) is one of the most frequent symptoms of major depressive episodes. In the case of obesity, unipolar or bipolar depression is frequently observable (20–45%).106 In depressed patients, obesity is related not only to depressive episodes but also to lifestyle factors, to comorbid bulimia and probably to genetic–biological factors.106 As melancholic depression is known to be associated with elevated plasma cortisol levels, depressed patients are prone to excess visceral fat storage, with the subsequent risk of developing associated metabolic disturbances.107 At the same time, according to certain studies, circadian symptoms of depression give rise to metabolic processes in the body, which eventually led to obesity and insulin resistance.
Night eating syndrome, defined as ingestion of >25% of daily calories after dinner and nocturnal awakenings with ingestion, is also more frequent among obese subjects. Indeed, such a disorder occurs at an incidence of 1.5% in the general public but at 9% in the obese and >25% in the morbidly obese populations.108,109
There is increasing evidence that a dysfunctional circadian system could be a primary cause of altered emotional behavior. Particularly in the case of night eating syndrome, it has been reported that subjects show a 1- to 3-h phase shift in the acrophase of their circulating leptin and corticosterone levels.108,109
Experimental studies show that Clock homozygous mutants have a spectrum of behavioral abnormalities, including low anxiety, mania and hyperactivity.110,111 The hypothesis that certain genetic susceptibility factors are shared across the psychosis spectrum112 has led several groups to investigate clock gene associations in a number of mood disorders and psychoses. The most evident example is Seasonal Affective Disease, which was associated with single-nucleotide polymorphism (SNP) variants in PER2 and BMAL1.
Different research groups are currently investigating associations between clock genes and bipolar disorders, schizoaffective disorder, schizophrenia and autism. Conflicts between internal biological clocks and environmental (solar) and social clocks may well be evident in individuals with Seasonal Affective Disease and other emotional alterations.
Particularly in the case of the CLOCK polymorphism rs1801260 (3111T>C), it has been shown that it is related to clinical features of mood disorders that influence diurnal preference in healthy humans and that cause sleep phase delay and insomnia in patients with different physiological illnesses.113–115 These alterations could be related to obesity, but also to weight loss. In this line, we have previously shown that most alterations involved in the obstacles of weight loss are related to the subject’s eating behavior and psychological characteristics, and that these obstacles are directly related to the difficulty in losing weight.116 Furthermore, previous works have related rs1801260 (3111T>C) with eating disorders and obesity.117,118 This idea seems to be supported by findings in animals, which suggest a direct involvement of the CLOCK gene in the regulation of body weight, as homozygous Clock-mutant mice developed obesity, hyperphagia and also suffered from changes in eating behavior, sleeping andmood.4 Our own group has shown that CLOCK gene polymorphisms are implicated in the effectiveness of a behavioral treatment of weight loss.119
In this line, a missense mutation in the human PER2 gene has previously been linked to psycho-behavioral factors such as diurnal preference,120 advanced sleep phase syndrome,121 seasonal variations in mood and behavior and winter depression.122 Very recently, Yang et al.123 have shown that Per2−/− mice showed a feeding abnormality that resembles that of the night eating syndrome, which has the combined features of a circadian rhythm disorder and an eating disorder.123 Most of the psychological alterations associated with PER2, such as depression or seasonal affective disorder, were often accompanied by alterations in sleep patterns and eating behavior, including overeating and craving for carbohydrates.124 Such results suggest that a study of different PER2 polymorphisms could be of interest in obesity. Furthermore, our group has shown that PER2 is implicated in attrition in weight loss treatment and may modulate eating behavior-related phenotypes.125
Genetic aspects
The circadian biology of AT has been evaluated in multiple gene-deficient or mutated rodent models (Table 1). Defects in some clock genes lead to irregular circadian and adipose biology. AT volume is reduced in Bmal-deficient mice but increased in murine Clock mutants. It was the study by Turek et al.4 that provided the evidence of a molecular interaction between clock genes and obesity. This study showed that mice with a disrupted Clock gene were prone to developing a phenotype resembling MetS.
Table 1.
Gene-deficient or mutated rodent models to evaluate the circadian biology of AT
| Genea | Adipose biology | Circadian biology |
|---|---|---|
| Agouti | Obesity | |
| Bmal1−/− | Reduced adipose volume | |
| Clock (mutant) | Obesity | Arrhythmic |
| HSF1−/− | Reduced adipose volume | Arrhythmic |
| Leptin−/− | Obesity | Attenuated sleep–wake cycle, reduced body temperature |
| Melanocortin receptor-3−/− | Obesity | Disrupted clock genes expression profile, altered food anticipatory activity |
| Non-obese diabetic rat | Non-obese | No clock gene attenuation with exception of Per2 in adipose tissue |
| Nocturnin−/− | Resistance to diet-induced obesity and hepatic steatosis | |
| PGC1β−/− | Defective triglyceride storage, defective BAT fatty acid oxidation and thermogenesis | Decreased physical activity |
| PPARα−/− | Defective BAT fatty acid oxidation and thermogenesis | Altered food entrainment of clock genes in brown adipose tissue and regulation of downstream target genes |
Abbreviations: AT, adipose tissue; BAT, brown adipose tissue; Bmal, brain- and muscle ANRT-like protein. Table adapted from Gimble et al.59
Unless otherwise stated, all models are murine.
Adipokine and receptor gene deficiencies associated with obesity (leptin, melacortin receptor) lead to abnormal circadian rhythms. Furthermore, clock disruption in leptin-deficient mice (ob/ob) led to greater weight gain, increased triglyceride, cholesterol and adipocyte hypertrophy than leptin deficiency alone.45
Consistent with these findings, genetic polymorphisms in human clock genes have been associated with increased incidence of obesity in epidemiological studies. Different authors are currently investigating the role of clock gene variants and their predicted haplotypes in human obesity and MetS alterations.99,105,117,119,126,127 From studies of our own group and others we deduce that CLOCK gene SNPs rs3749474, rs4580704 and rs1801260 (3111T>C) are particularly interesting as they seem to be associated with body mass index (BMI), energy intake and different obesity-related variables.97,119 The PER2 SNPs rs2304672C>G and rs4663302C>T are also remarkable because have been related to abdominal obesity.125
Chronobiology in the treatment of obesity
To date, most studies have focused on the pathophysiological consequences of CD. However, very few papers have been published about how chronobiology can be used in the effective treatment of obesity and MetS. As CD can be considered a risk factor in the development of obesity, we hypothesize that the appropriate resetting of the circadian system (chronoenhancement) may lead to reduced incidence of obesity.
To study the effect of CD and chronoenhancement on morbidity and mortality with a high degree of accuracy, it is important to quantitatively characterize the human circadian system status. To this end, several tests and diaries have been extensively used. However, because of the poor accuracy of such procedures, some circadian marker rhythms, which are under the control the SCN, have become widely used. For example, core temperature actimetry, salivary melatonin and cortisol rhythm are the most frequently used methods. Recently, we proposed the use of wrist skin temperature, recorded by means of a wireless data logger, as a reliable circadian index for assessing the human circadian system status.128 Molecular markers, consisting of serial determination of clock genes in peripheral blood cells and in oral mucose cells,129,130 also have been proposed to evaluate human circadian system; however, both tissues contain peripheral clocks and, thus, they can be subjected easily to desynchronization from the central pacemaker and its determination interferes with the sleep–wake cycle. Only with the use of such objective techniques can the effect of CD and chronoenhancement on obesity be accurately assessed.
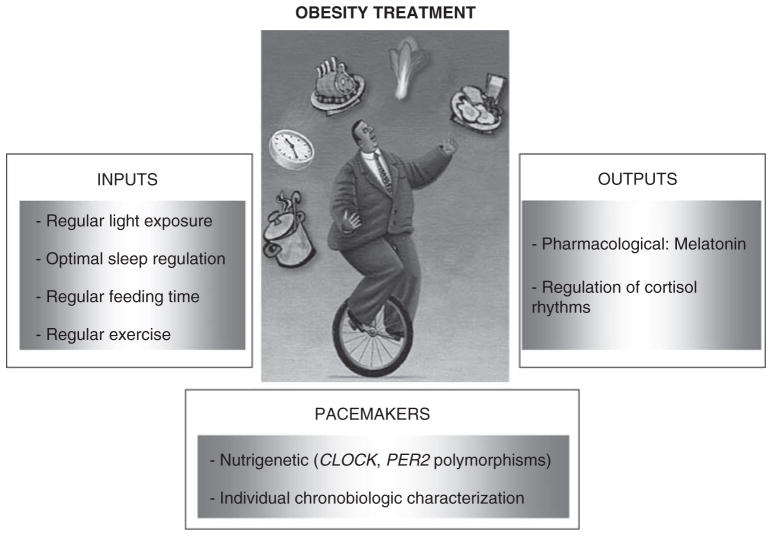
To establish a chronobiological-based therapy for obesity, three levels of potential therapeutic interventions, on the basis of the functional organization of the circadian system, can be considered: the inputs to the pacemakers, the pacemakers themselves and the output from the pacemakers (Figure 5).
Figure 5.
Different strategies in the treatment of obesity from a chronobiological perspective.
Inputs
The adequate functioning of the circadian system, which in turn is highly important for health, is dependent on regularly timed exposure to Zeitgeber stimuli.
Although no data about the influence of regular light exposure on obesity have been published, several papers show that increase in bright light exposure and its regularity improves function in a number of clinical conditions closely related to obesity, including bulimia,131 anorexia,131 insomnia and depression.56
Furthermore, sleep loss and sleep instability have also been related to impaired glucose and lipid metabolism, and to MetS development. To achieve optimal sleep–wake rhythm regulation, adherence to a regular sleep schedule and, particularly, to a regular getting up time is fundamental. The regularization of sleep–wake schedules for 4 weeks in young adults led to a significant improvement in daytime alertness and nocturnal sleep efficiency.132
Feeding rhythms
Feeding rhythms have a strong influence on peripheral oscillator synchronization, and feeding regularity and the timing of nutrient intake should be taken into consideration. To date, no studies of humans have been published evaluating the influence of a sustained more regular 24-h pattern of food intake on the circadian system. However, animal studies show that regular restricted feeding availability improves the synchronization of the circadian system.6 It is likely that regular feeding times also affect the synchronization of the circadian timing system through their effect on cortisol and body temperature changes due to diet-induced thermogenesis.
Better known is the effect of the timing of nutrient intake on metabolism. For example, in young healthy people, the results of the oral glucose tolerance test worsened when glucose was ingested during the evening as compared with that in the morning. Circadian variation in glucose tolerance in rats was related with a reduction in insulin sensitivity.133 Furthermore, when the total daily caloric intake was ingested as one meal (dinner), an increase in body weight as compared with the same meal ingested in the morning was observed.134
Besides light exposure, sleep–wake pattern and feeding time, physical exercise is a relevant Zeitgeber for the circadian system and is a key factor in the prevention and treatment of obesity. Epidemiological studies indicate that prolonged and regular exercise is associated with better nocturnal sleep and lower daytime tiredness.134 Prolonged regular exercise in elderly people also counteracts the age-related fragmentation of rest–activity rhythms,135 and in sedentary elderly subjects, exercise improves performance in executive control tasks136 and prefrontal activation.137 However, the objective effect of exercise through the circadian system may develop only slowly. For example in a study performed in rats concerning the effect of exercise on obesity induced by periodical phase shifts, the significant effect of regular exercise on body weight control appeared only after 3 months.134
Pacemakers
Contrasting with the relatively known therapeutic effects of input manipulation, direct interventions on pacemakers are difficult to perform and scarce. Knowledge is looking to achieve pharmacological success in the effective manipulation of SCN without causing adverse effects on this and other systems. Nevertheless, on the basis of recent epidemiological studies showing a link between some clock gene polymorphisms and obesity, a new chronobiology treatment strategy could be proposed in the near future.
The success of obesity therapy is, at least in part, dependant on the genetic background of a patient.138 The identification of clock gene polymorphisms could be useful in the diagnosis of obesity and may predict the outcome of body weight reduction strategies on the basis of low-energy diets. In this sense our research group has shown that the CLOCK rs1801260 SNP may predict the outcome of body weight reduction strategies on the basis of low-energy diets.119 Such new strategies could represent a step toward personalized health care and nutrition on the basis of a combination of genotyping and chronobiological characterization.
Outputs
As regards pacemaker outputs, melatonin and cortisol rhythms have been relatively well studied and are a known link between CD and metabolic disturbances. Much evidence supports the ability of melatonin to resynchronize the sleep–wake rhythm in blind people, as well in jet-lag and shift-work. Melatonin is an example of a chemical output from the SCN acting simultaneously as an input to the SCN. Melatonin induces sleep and therefore enhances circadian robustness by binding to membrane receptors MT1 and MT2 in the SCN. Animal studies pointed to the positive health consequences of prolonged regular melatonin administration. 138 Most studies indicate that regular melatonin treatment increases the lifespan of animals, probably by means of its antioxidant effects and through induction of an enhanced circadian robustness. New, recently synthesized melatonin agonists such as Ramelteon and Agomelatine can improve circadian rhythmicity through promotion of sleep and improving depressive states, respectively, without the stimulation of the immune system by melatonin administration.
Circadian rhythms in glucocorticoids have been considered as a key factor in the signaling pathway involved in peripheral clock synchronization. The cortisol level starts to increase immediately after the usual awakening time and peaks within an hour of awakening, diminishing during the rest of the day. According to van Someren et al. (2007),134 a regular cortisol rhythm could be promoted by a regular wakeup time, regular early morning light exposure and a regular feeding time and physical exercise. These behavioral interventions could have a direct effect on obesity and particularly abdominal obesity, both of which have been related to alteration of glucocorticoid rhythmicity.139
In summary, although we are not aware of experimental studies specifically addressing the chronobiological treatment of obesity, the close relationship between obesity, metabolic syndrome and CD suggests that all pharmacological, dietary and behavioral treatments that improve the circadian system status may help to reduce the risk of obesity and to improve the success of treatment.
Acknowledgments
This work was supported by the Government of Education, Science and Research of Murcia (Project BIO/FFA 07/01-0004 and PI/05700/07) and by The Spanish Government of Science and Innovation (projects AGL2008-01655/ALI and BFU2007-60658/BFI), by Instituto de Salud Carlos III (RETICEF, RD06/0013/0019), by NIDDK Grant DK075030 and by contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research Service.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. Review. [DOI] [PubMed] [Google Scholar]
- 2.Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A. 2007;143A:3016–3034. doi: 10.1002/ajmg.a.32035. [DOI] [PubMed] [Google Scholar]
- 3.Froy O. The relationship between nutrition and circadian rhythms in mammals. Front Neuroendocrinol. 2007;28:61–71. doi: 10.1016/j.yfrne.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol. 2009;20:127–134. doi: 10.1097/MOL.0b013e3283292399. [DOI] [PubMed] [Google Scholar]
- 6.Lax P, Zamora S, Madrid JA. Coupling effect of locomotor activity on the rat’s circadian system. Am J Physiol. 1998;275:R580–R587. doi: 10.1152/ajpregu.1998.275.2.R580. [DOI] [PubMed] [Google Scholar]
- 7.Lax P, Zamora S, Madrid JA. Food-entrained feeding and locomotor circadian rhythms in rats under different lighting conditions. Chronobiol Int. 1999;16:281–291. doi: 10.3109/07420529909116858. [DOI] [PubMed] [Google Scholar]
- 8.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;230:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- 11.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci USA. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- 13.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, et al. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 14.McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, et al. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 15.Inoue I, Shinoda Y, Ikeda M, Hayashi K, Kanazawa K, et al. CLOCK/BMAL1 is involved in lipid metabolismvia transactivation of the peroxisome proliferator-activated receptor (PPAR) response element. J Atheroscler Thromb. 2005;12:169–174. doi: 10.5551/jat.12.169. [DOI] [PubMed] [Google Scholar]
- 16.Oishi K, Amagai N, Shirai H, Kadota K, Ohkura N, et al. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Res. 2005;12:191–202. doi: 10.1093/dnares/dsi003. [DOI] [PubMed] [Google Scholar]
- 17.Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol. 2006;20:1715–1727. doi: 10.1210/me.2006-0052. [DOI] [PubMed] [Google Scholar]
- 18.Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, et al. The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation. J Biol Chem. 2003;278:37672–37680. doi: 10.1074/jbc.M304664200. [DOI] [PubMed] [Google Scholar]
- 19.Laitinen S, Fontaine C, Fruchart JC, Staels B. The role of the orphan nuclear receptor Rev-Erb alpha in adipocyte differentiation and function. Biochimie. 2005;87:21–25. doi: 10.1016/j.biochi.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 21.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 23.Nakahata Y, Grimaldi B, Sahar S, Hirayama J, Sassone-Corsi P. Signaling to the circadian clock: plasticity by chromatin remodeling. Curr Opin Cell Biol. 2007;19:230–237. doi: 10.1016/j.ceb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 26.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 29.Bechtold DA. Energy-responsive timekeeping. J Genet. 2008;87:447–458. doi: 10.1007/s12041-008-0067-6. [DOI] [PubMed] [Google Scholar]
- 30.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 31.Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. Review. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Yang T, Baur JA, Perez E, Matsui T, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 34.Retnakaran R, Youn BS, Liu Y, Hanley AJ, Lee NS, et al. Correlation of circulating full-length visfatin (PBEF/NAMPT) with metabolic parameters in subjects with and without diabetes: a cross-sectional study. Clin Endocrinol (Oxf) 2008;69:885–893. doi: 10.1111/j.1365-2265.2008.03264.x. [DOI] [PubMed] [Google Scholar]
- 35.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Letters. 2008;582:142–151. doi: 10.1016/j.febslet.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 37.Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, et al. Rhythmic mRNA expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 38.Aoyagi T, Shimba S, Tezuka M. Characteristics of circadian gene expressions in mice white adipose tissue and 3T3-L1 adipocytes. J Health Sci. 2005;51:21–32. [Google Scholar]
- 39.Bray MS, Young ME. Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes Rev. 2007;8:169–181. doi: 10.1111/j.1467-789X.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 40.Goh BC, Wu X, Evans AE, Johnson ML, Hill MR, Gimble JM. Food entrainment of circadian gene expression altered in PPARalpha−/− brown fat and heart. Biochem Biophys Res Commun. 2007;360:828–833. doi: 10.1016/j.bbrc.2007.06.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, Renard P, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology. 2003;144:5347–5352. doi: 10.1210/en.2003-0693. [DOI] [PubMed] [Google Scholar]
- 42.Endo T, Ohno M, Kotani S, Gunji K, Onaya T. Thyrotropin receptor in non-thyroid tissues. Biochem Biophys Res Commun. 1993;190:774–779. doi: 10.1006/bbrc.1993.1116. [DOI] [PubMed] [Google Scholar]
- 43.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Yanagihara H, Ando H, Hayashi Y, Obi Y, Fujimura A. High-fat feeding exerts minimal effects on rhythmic mRNA expression of clock genes in mouse peripheral tissues. Chronobiol Int. 2006;23:905–914. doi: 10.1080/07420520600827103. [DOI] [PubMed] [Google Scholar]
- 45.Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Gómez-Abellán P, Hernández-Morante JJ, Luján JA, Madrid JA, Garaulet M. Clock genes are implicated in the human metabolic syndrome. Int J Obes. 2008;32:121–128. doi: 10.1038/sj.ijo.0803689. [DOI] [PubMed] [Google Scholar]
- 47.Gómez-Santos C, Gómez-Abellán P, Madrid JA, Hernández-Morante JJ, Lujan JA, Ordovas JM, et al. Circadian rhythm of clock genes in human adipose explants. Obesity. 2009;17:1481–1485. doi: 10.1038/oby.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernandez-Morante JJ, Gomez-Santos C, Milagro F, Campión J, Martínez JA, Zamora S, et al. Expression of cortisol metabolism-related genes shows circadian rhythmic patterns in human adipose tissue. Int J Obes. 2009;33:473–480. doi: 10.1038/ijo.2009.4. [DOI] [PubMed] [Google Scholar]
- 49.Bartness TJ, Wade GN. Photoperiodic control of body weight and energy metabolism in Syrian hamsters (Mesocricetus auratus): role of pineal gland, melatonin, gonads, and diet. Endocrinology. 1984;114:492–498. doi: 10.1210/endo-114-2-492. [DOI] [PubMed] [Google Scholar]
- 50.Zvonic S, Floyd ZE, Mynatt RL, Gimble JM. Circadian rhythms and the regulation of metabolic tissue function and energy homeostasis. Obesity (Silver Spring) 2007;15:539–543. doi: 10.1038/oby.2007.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garaulet M, Hernández-Morante JJ, de Heredia FP, Tébar FJ. Adiponectin, the controversial hormone. Public Health Nutr. 2007;10:1145–1150. doi: 10.1017/S1368980007000638. [DOI] [PubMed] [Google Scholar]
- 52.Gómez-Abellán P, Gómez-Santos C, Madrid JA, Milagro FI, Campion J, Martinez JA, et al. Circadian expression of adiponectin and its receptors in human adipose tissue. Endocrinology. 2010;151:115–122. doi: 10.1210/en.2009-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnea M, Madar Z, Froy O. High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology. 2009;150:161–168. doi: 10.1210/en.2008-0944. [DOI] [PubMed] [Google Scholar]
- 54.Erren TC, Reiter RJ. Defining chronodisruption. J Pineal Res. 2009;46:245–247. doi: 10.1111/j.1600-079X.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- 55.Kondratov RV. A role of the circadian system and circadian proteins in aging. Ageing Res Rev. 2007;6:12–27. doi: 10.1016/j.arr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Wirz-Justice A. Biological rhythm disturbances in mood disroders. Int Clin Psychopharmacol. 2006;(Suppl 1):S11–S15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- 57.Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Davidson AJ, Sellik MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- 60.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- 61.Otálora BB, Madrid JA, Alvarez N, Vicente V, Rol MA. Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J Pineal Res. 2008;44:307–315. doi: 10.1111/j.1600-079X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 62.Erren TC, Reiter RJ. A generalized theory of carcinogenesis due to chronodisruption. Neuro Endocrinolo Lett. 2008;29:815–821. [PubMed] [Google Scholar]
- 63.Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, et al. Marked 24-h rest-activity rhythms are associated with better quality of life, better response and longer survival in patients with meatastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6:3038–3045. [PubMed] [Google Scholar]
- 64.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 65.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 66.Turner PL, Mainster MA. Circadian photoreception: ageing and eye’s important role in systemic health. Br J Ophtalmol. 2008;92:1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian pattern in healthy subjects. Life Sci. 2003;73:2467–2475. doi: 10.1016/s0024-3205(03)00628-3. [DOI] [PubMed] [Google Scholar]
- 68.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 70.Kennaway DJ, Owens JA, Voultsios A, Boden MJ, Varcoe TJ, et al. Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. Am J Physiol Regul Integr Comp Physiol. 293:R1528–R1537. doi: 10.1152/ajpregu.00018.2007. [DOI] [PubMed] [Google Scholar]
- 71.Oishi K, Atsumi G, Sugiyama S, Kodomari I, Kasamatsu M, Machida K, et al. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 580:127–130. doi: 10.1016/j.febslet.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 72.Leon J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res. 2005;38:1–9. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 73.Hardeland R, Coto-Montes A, Poeggeler B. Circadian rhythms, oxidative stress, and antioxidant defense mechanisms. Chronobiol Int. 2003;20:921–962. doi: 10.1081/cbi-120025245. [DOI] [PubMed] [Google Scholar]
- 74.Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44:26–40. doi: 10.1111/j.1600-079X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 75.Croce N, Bracci M, Ceccarelli G, Barbadoro P, Prospero E, Santarellia L. Body mass index in shift workers: relation to diet and physical activity. G Ital Med Lav Ergon. 2007;29:488–489. [PubMed] [Google Scholar]
- 76.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol. 2001;171:557–564. doi: 10.1677/joe.0.1710557. [DOI] [PubMed] [Google Scholar]
- 78.Ptácek LJ, Jones CR, Fu YH. Novel insights from genetic and molecular characterization of the human clock. Cold Spring Harb Symp Quant Biol. 2007;72:273–277. doi: 10.1101/sqb.2007.72.017. [DOI] [PubMed] [Google Scholar]
- 79.Bonnet MH, Arand DL. We are chronically sleep deprived. Sleep. 1995;18:908–911. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- 80.Van Cauter E, Knutson K. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159:S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perez-Tilve D, Stern JE, Tschöp M. The brain and the metabolic syndrome: not a wireless connection. Endocrinology. 2006;147:1136–1139. doi: 10.1210/en.2005-1586. [DOI] [PubMed] [Google Scholar]
- 82.Scott EM, Grant PJ. Neel revisited: the adipocyte, seasonality and type 2 diabetes. Diabetologia. 2006;49:1462–1466. doi: 10.1007/s00125-006-0280-x. [DOI] [PubMed] [Google Scholar]
- 83.Gimble JM, Floyd ZE. Fat circadian biology. J Appl Physiol. 2009;107:1629–1637. doi: 10.1152/japplphysiol.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 86.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 87.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 89.Ramsey KM, Marcheva B, Kohsaka A, Bass J. The clockwork of metabolism. Annu Rev Nutr. 2007;27:219–240. doi: 10.1146/annurev.nutr.27.061406.093546. [DOI] [PubMed] [Google Scholar]
- 90.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–R903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 92.Obal FJ, Alt J, Taishi P, Gardi J, Krueger JM. Sleep in mice with nonfunctional growth hormone-releasing hormone receptors. Am J Physiol Regul Integr Comp Physiol. 2003;284:R131–R139. doi: 10.1152/ajpregu.00361.2002. [DOI] [PubMed] [Google Scholar]
- 93.Weikel JC, Wichniak A, Ising M, Brunner H, Friess E, Held K, et al. Ghrelin promotes slow-wave sleep in humans. Am J Physiol Endocrinol Metab. 2003;284:E407–E415. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- 94.Vanitallie TB. Sleep and energy balance, interactive homeostatic systems. Metabolism. 2006;55 (10 Suppl 2):S30–S35. doi: 10.1016/j.metabol.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 95.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmuno-modulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 96.Cintra DE, Ropelle ER, Pauli JR. Brain regulation of food intake and expenditure energy, molecular action of insulin, leptin and physical exercise. Rev Neurol. 2007;45:672–682. [PubMed] [Google Scholar]
- 97.Garaulet M, Lee YC, Shen J, Parnell LD, Arnett DK, Tsai MY, et al. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population) Eur J Hum Genet. 2010;18:364–369. doi: 10.1038/ejhg.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuriyan R, Bhat S, Thomas T, Vaz M, Kurpad AV. Television viewing and sleep are associated with overweight among urban and semi-urban South Indian children. Nutr J. 2007;6:25. doi: 10.1186/1475-2891-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69:55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- 100.Burke V, Beilin LJ, Simmer K, Oddy WH, Blake KV, Doherty D, et al. Predictors of body mass index and associations with cardiovascular risk factors in Australian children: a prospective cohort study. Int J Obes. 2005;29:15–23. doi: 10.1038/sj.ijo.0802750. [DOI] [PubMed] [Google Scholar]
- 101.Davison KK, Marshall SJ, Birch LL. Cross-sectional and longitudinal associations between TV viewing and girls’ body mass index, overweight status, and percentage of body fat. J Pediatr. 2006;149:32–37. doi: 10.1016/j.jpeds.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shaw PJ. Thermoregulatory changes. In: Kushida CA, editor. Sleep Deprivation Basic Science Physiology, and Behavior. Marcel Dekker; New York: 2005. pp. 319–338. [Google Scholar]
- 103.Mendoza J, Pevet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol. 2008;586 (Pt 24):5901–5910. doi: 10.1113/jphysiol.2008.159566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garaulet M, Lee YC, Shen J, Parnell LD, Arnett DK, Tsai MY, et al. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am J Clin Nutr. 2009;90:1466–1475. doi: 10.3945/ajcn.2009.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rihmer Z, Purebl G, Faludi G, Halmy L. Association of obesity and depression. Neuropsychopharmacol Hung. 2008;10:183–189. Review. [PubMed] [Google Scholar]
- 107.Mann JN, Thakore JH. Melancholic depression and abdominal fat distribution: a mini-review. Stress. 1999;3:1–15. doi: 10.3109/10253899909001108. Review. [DOI] [PubMed] [Google Scholar]
- 108.Allison KC, Lundgren JD, O’Reardon JP, Martino NS, Sarwer DB, Wadden TA, et al. The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the Night Eating Syndrome. Eat Behav. 2008;9:62–72. doi: 10.1016/j.eatbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Goel N, Stunkard AJ, Rogers NL, Van Dongen HP, Allison KC, O’Reardon JP, Ahima RS, et al. Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythms. 2009;24:85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2:11–19. doi: 10.1034/j.1601-183x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 111.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry. 2000;48:531–538. doi: 10.1016/s0006-3223(00)00883-0. [DOI] [PubMed] [Google Scholar]
- 113.Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, et al. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- 114.Lee HJ, Paik JW, Kang SG, Lim SW, Kim L. Allelic variants interaction of CLOCK gene and G-protein beta3 subunit gene with diurnal preference. Chronobiol Int. 2007;24:589–597. doi: 10.1080/07420520701534632. [DOI] [PubMed] [Google Scholar]
- 115.Benedetti F, Radaelli D, Bernasconi A, Dallaspezia S, Falini A, Scotti G, et al. Clock genes beyond the clock: CLOCK genotype biases neural correlates of moral valence decision in depressed patients. Genes Brain Behav. 2008;7:20–25. doi: 10.1111/j.1601-183X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 116.Corbalán MD, Morales EM, Canteras M, Espallardo A, Hernández T, Garaulet M. Effectiveness of cognitive-behavioral-therapy based on the Mediterranean diet for the treatment of obesity. Nutrition. 2009;25:861–869. doi: 10.1016/j.nut.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 117.Sookoian S, Gemma C, Gianotti TF, Burgueño A, Castaño G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 118.Monteleone P, Tortorella A, Docimo L, Maldonato MN, Canestrelli B, De Luca L, et al. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: association with higher body mass index. Neurosci Lett. 2008;435:30–33. doi: 10.1016/j.neulet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 119.Garaulet M, Corbalán MD, Madrid JA, Morales E, Baraza JC, Lee YC, et al. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based in Mediterranean Diet. Int J Obes (Lond) 2010;34:516–523. doi: 10.1038/ijo.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carpen JD, Archer SN, Skene DJ, Smits M, von Schantz M. A single-nucleotide polymorphism in the 5′-untranslated region of the hPER2 gene is associated with diurnal preference. J Sleep Res. 2005;14:293–297. doi: 10.1111/j.1365-2869.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 121.Hamet P, Tremblay J. Genetics of the sleep–wake cycle and its disorders. Metabolism. 2006;55 (10 Suppl 2):S7–12. doi: 10.1016/j.metabol.2006.07.006. Review. [DOI] [PubMed] [Google Scholar]
- 122.Partonen T, Treutlein J, Alpman A, Frank J, Johansson C, Depner M, et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med. 2007;39:229–238. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- 123.Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythm. Endocrinology. 2009;150:2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Partonen T, Lönnqvist J. Seasonal affective disorder. Lancet. 1998;352:1369–1374. doi: 10.1016/S0140-6736(98)01015-0. [DOI] [PubMed] [Google Scholar]
- 125.Garaulet M, Corbalán MD, Madrid JA, Baraza JC, Parnell LD, Lee YC, et al. PER2 variants are associated with abdominal obesity psycho-behavioral factors and attrition in the dietary treatment of obesity. J Am Dietetic Assoc. 2010;110:917–921. doi: 10.1016/j.jada.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 127.Woon PY, Kaisaki PJ, Bragança J, Bihoreau MT, Levy JC, Farrall M, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sarabia JA, Rol MA, Mendiola P, Madrid JA. Circadian rhythm of wrist temperature in normal-living subjects. A candidate of new index of the circadian system. Physiol Behav. 2008;95:570–580. doi: 10.1016/j.physbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 129.Teboul M, Barrat-Petit MA, Li XM, Claustrat B, Formento JL, Delaunay F, et al. Atypical patterns of circadian clock gene expression in human peripheral blood mononuclear cells. J Mol Med. 2005;83:693–699. doi: 10.1007/s00109-005-0697-6. [DOI] [PubMed] [Google Scholar]
- 130.Bjarnason GA, Jordan RC, Wood PA, Li Q, Lincoln DW, Sothern RB, et al. Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am J Pathol. 2001;158:1793–1801. doi: 10.1016/S0002-9440(10)64135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamamotova A, Papezova H, Vevera J. Normalizing effect of bright light therapy on temperature circadian rhythm in patients with eating disorders. Neuroendocrinology Letters. 2008;29:1–5. [PubMed] [Google Scholar]
- 132.Manber R, Bootzin RR, Acebo C, Carskadon MA. The effects of regularizing sleep–wake schedules on daytime sleepiness. Sleep. 1996;19:432–441. doi: 10.1093/sleep/19.5.432. [DOI] [PubMed] [Google Scholar]
- 133.La Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance. Diabetes. 2001;50:1237–1243. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 134.Van Someren EJ, Riemersma-Van Der Lek RF. Live to the rhythm, slave to the rhythm. Sleep Med Rev. 2007;11:465–484. doi: 10.1016/j.smrv.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 135.Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4:387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- 136.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 137.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vivanco P, Ortiz V, Rol MA, Madrid JA. Looking for the keys of diurnality downstream from the circadian clock: role of melatonin in a dual-phasing rodent, Octodon degus. J Pineal Res. 2007;42:280–290. doi: 10.1111/j.1600-079X.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 139.García-Prieto MD, Tébar FJ, Nicolás F, Larqué E, Zamora S, Garaulet M. Cortisol secretary pattern and glucocorticoid feedback sensitivity in women from a Mediterranean area: relationship with anthropometric characteristics, dietary intake and plasma fatty acid profile. Clin Endocrinol (Oxf) 2007;66:185–191. doi: 10.1111/j.1365-2265.2006.02705.x. [DOI] [PubMed] [Google Scholar]