Abstract
Considering that anxiety disorders frequently begin before adulthood and often result in chronic impairment, it is important to characterize the developmental pathways leading to the onset of clinical anxiety. Identifying neural biomarkers that can predict the onset of anxiety in childhood may increase our understanding of the etiopathogenesis of anxiety, as well as inform intervention and prevention strategies. An event-related potential (ERP), the error-related negativity (ERN) has been proposed as a biomarker of risk for anxiety and has previously been associated with concurrent anxiety in both adults and children. However, no previous study has examined whether the ERN can predict the onset of anxiety disorders. In the current study, ERPs were recorded while 236 healthy children, approximately 6 years of age, performed a Go/No-Go task to measure the ERN. Three years later, children and parents came back to the lab and completed diagnostic interviews regarding anxiety disorder status. Results indicated that enhanced error-related brain activity at age 6 predicted the onset of new anxiety disorders by age 9, even when controlling for baseline anxiety symptoms and maternal history of anxiety. Considering the potential utility of identifying early biomarkers of risk, this is a novel and important extension of previous work.
Introduction
Anxiety disorders are one of the most common forms of psychopathology in both children and adults, and are associated with substantial impairment (Beesdo, Knappe, & Pine, 2009; Beesdo 2010; Kessler et al., 2005; Last, Perrin, Hersen, & Kazdin, 1996). Both retrospective and prospective studies demonstrate that anxiety disorders frequently begin before adulthood and are moderately stable across the lifespan (Bittner et al., 2007; Copeland, Angold, Shanahan, & Costello, 2014; Kessler et al., 2005; Last et al., 1996; Pine, Cohen, Gurley, Brook, & Ma, 1998; Wittchen, Lieb, Pfister, & Schuster, 2000). Considering that anxiety disorders often begin early in life and result in chronic impairment, it is important to map and understand the developmental pathways leading to anxiety disorders.
There is increasing research focus on core neural systems that might underlie clinical anxiety, and elucidating their development (Pine, 2007). Identifying neural biomarkers that not only correlate with anxiety, but can predict the subsequent onset of anxiety disorders in childhood may be particularly important for understanding the etiopathogenesis of clinical anxiety. Reliable biomarkers of risk would have considerable implications for the implementation and development of intervention and prevention strategies.
The error-related negativity (ERN) has been proposed as a biomarker of risk for anxiety (Hajcak, 2012; Proudfit, Inzlicht, & Mennin, 2013). The ERN is a negative-going deflection in the event-related potential that occurs when participants make mistakes during a speeded response task (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). Within adult populations, many studies have documented an increased ERN in obsessive-compulsive disorder (OCD; Endrass, Klawohn, Schuster, & Kathmann, 2008; Gehring, Himle, & Nisenson, 2000; Stern et al., 2010; Xiao et al., 2011) and generalized anxiety disorder (Weinberg, Klein, & Hajcak, 2012; Weinberg, Olvet, & Hajcak, 2010; Xiao et al., 2011). In one study, children with OCD continued to have an increased ERN even after successful treatment when symptoms scores were below the clinical range (Hajcak, Franklin, Foa, & Simons, 2008). Additionally, unaffected individuals who have first-degree relatives with OCD are characterized by an increased ERN (Riesel, Endrass, Kaufmann, & Kathmann, 2011), and this same pattern has recently been found in children (Carrasco et al., 2013). Also, the ERN appears to be trait-like – characterized by excellent test-retest reliability of up to two years in adults (Weinberg & Hajcak, 2011) and children (Meyer, Bress, & Proudfit, 2014). Considering that the ERN has been shown to be between 45% and 60% heritable (Anokhin, Golosheykin, & Heath, 2008), these findings collectively suggest an increased ERN may be a heritable biomarker, or endophenotype, for anxiety (Hajcak, 2012; Manoach & Agam, 2013; Proudfit et al., 2013), and may be useful in tracing developmental trajectories of risk.
Consistent with work in adults, previous cross-sectional research in developmental populations has found an increased ERN within a heterogeneous group of clinically anxious children and adolescents (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006), children and adolescents with obsessive-compulsive disorder (Carrasco et al., 2013; Hajcak et al., 2008; Hanna et al., 2012), and children as young as 6 years old with anxiety disorders (Meyer et al., 2013). Additionally, the relationship between the ERN and anxiety appears to be specific - for example, depression has been related to a blunted ERN in children (Ladouceur et al., 2012).
Evidence from multiple source localization studies (Dehaene, Posner, & Don, 1994; Mathalon, Whitfield, & Ford, 2003; van Veen & Carter, 2002), as well as studies that combine ERP and fMRI (Debener et al., 2005), suggest that the ERN is generated in the anterior cingulate cortex (ACC). The ACC is a region of the medial frontal cortex where information about pain, threat, and punishment is integrated to modify subsequent behavior (Shackman et al., 2011). FMRI studies have also found increased error-related ACC activity among anxious individuals (Fitzgerald et al., 2005; Paulus, Hozack, Frank, & Brown, 2002; Ursu, Stenger, Shear, Jones, & Cameron, 2003), and one study found that adolescents with generalized anxiety disorder showed greater activation in a network including the ACC in response to fearful faces (McClure, Monk, & Nelson, 2007). We have proposed that a larger ERN among anxious individuals – and among those at risk for anxiety – may reflect the increased threat value of errors; that is, a larger ERN suggests that errors are more aversive for these individuals (Proudfit et al., 2013). This proposal is consistent with data in which punishing errors potentiates the ERN in the lab (Riesel, Weinberg, Endrass, Kathmann, & Hajcak, 2012), and recent work linking harsh parenting to a larger ERN in children (Meyer, Proudfit, et al., 2014).
We conceptualize errors as a specific type of threat – indeed, errors prompt a cascade of physiological and neural responses consistent with defensive mobilization (i.e., skin conductance response, heart rate deceleration, potentiated startle reflex, pupil dilation, corrugator muscle contraction, amygdala activation, for review, see Weinberg, Riesel, & Hajcak, 2012). This is consistent with the notion that error-related ACC activity reflects the need to exert control in the face of threat, punishment, or pain (Shackman et al., 2011). Thus, we view variability in the ERN as reflecting individual differences in reactivity to an internal source of threat. During the transition from early to late childhood, normative anxiety tends to transition from fear of external threat (e.g., the dark, spiders, monsters) to self-conscious shyness and worry about behavioral competence and evaluation (i.e., internal threat and performance monitoring; Copeland et al., 2014; Gullone, 2000; Spence & McCathie, 1993; Spence, Rapee, McDonald, & Ingram, 2001; Vasey, Crnic, & Carter, 1994). Similarly, the magnitude of the ERN begins to increase during the transition into late childhood (Tamnes, Walhovd, Torstveit, Sells, & Fjell, 2013). A recent study found that negative life events specifically related to children’s own behavior (e.g., conflicts with peers) have a greater impact on the likelihood of developing an anxiety disorder than external life events (e.g., natural disasters) in youth (Broeren, Newall, Dodd, Locker, & Hudson, 2014), suggesting that internal sources of threat may be particularly relevant in the development of anxiety. Thus, we expect that children who are on a developmental trajectory towards the onset of an anxiety disorder may display an increased sensitivity to errors as an internal source of threat, reflected in a larger ERN. These children, characterized by an increased neural response to errors, may be more focused on their own competence and more sensitive to performance-based failures than typically developing youth, and thereby have a greater probability of developing clinical anxiety.
To our knowledge there are only two prospective studies that have examined the relation of the ERN to subsequent anxiety, both finding that among children high in early temperamental behavioral inhibition, an increased ERN predicted anxiety symptoms later in development (Lahat et al., 2014; McDermott et al., 2009). Importantly, however, no study has yet controlled for baseline anxiety or examined whether the ERN can predict the onset of anxiety disorders.
In the current study, we sought to address this question and test whether an increased ERN would confer risk for subsequent anxiety disorders. ERPs were recorded while 236 healthy children, approximately 6 years of age, performed a Go/No-Go task to measure the ERN. Previous reports suggest that anxiety disorder status at age 6 related to a larger ERN (Meyer et al., 2013). The current study focuses on only those six-year olds without an anxiety disorder, and examined whether ERN would prospectively predict new anxiety diagnoses three years later. The ability of the ERN to predict anxiety disorder onset was examined in relation to, and controlling for, variables that have been previously linked to the ERN, especially current anxiety symptoms (Meyer, Weinberg, Klein, & Hajcak, 2012; Santesso, Segalowitz, & Schmidt, 2006) and maternal history of anxiety disorder (Meyer et al., 2013; Torpey et al., 2013). We hypothesized that an enhanced ERN at age 6 would predict the onset of an anxiety disorder 3 years later. To examine specificity, we also investigated whether the ERN predicted new onsets of externalizing disorders.
Method
Participant Recruitment and Study Overview
Originally, participants were identified through a commercial mailing list. Eligible families had a child with no significant medical conditions or developmental disability, and at least one English-speaking biological parent. Of families who were eligible, 66.4% entered the study. Families who agreed and declined to participate did not differ significantly on child sex and race/ethnicity and parental marital status, education, and employment status. Census data suggest the sample is reasonably representative of the surrounding county (Bufferd, Dougherty, Carlson, & Klein, 2011; Olino, Klein, Dyson, Rose, & Durbin, 2010). An initial assessment was completed when the children were approximately 3 years of age during which a parent (generally mothers) completed a diagnostic interview regarding their child, the Preschool Age Psychiatric Assessment, Version 1.4 (PAPA; Egger, Ascher, & Angold, 1999). Mothers were also interviewed regarding their own histories of psychopathology using the Structured Clinical Interview for DSM-IV (SCID; First et al., 1995).
Children returned to the lab 3 years later (i.e., around 6 years of age) and completed EEG tasks. During the age 6 assessment, the parent was re-administered the PAPA and completed the Child Behavior Checklist (CBCL; Achenbach & Edelbrock, 1981). Three years later, when children were approximately 9 years of age, parents and children completed a diagnostic interview, the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997). At all assessments, after a description of the study, written informed consent was obtained; all procedures were approved by the University Institutional Review Board.
Demographics
The original sample included 328 children with adequate ERP data collected when the children were approximately 6 years of age (Torpey, Hajcak, Kim, Kujawa, & Klein, 2011). Of these children, 85 met criteria for a current DSM diagnosis at the age 6 assessment, and were excluded from all subsequent analyses: 48 for any anxiety disorder, 18 for attention deficit/hyperactivity disorder, 31 for oppositional defiant disorder, and 17 for a depressive disorder (Meyer et al., 2013).
Of the remaining 243 children, 236 had adequate diagnostic data from the age 9 assessment. Of these 236 children (109 female), 26 met criteria for the onset of a new anxiety disorder between the ages of 6 and 9 (ANX) and 210 did not develop an anxiety disorder (No ANX). Overall, 95.8% of the children were Caucasian, 2.3% Asian, 0.5 % Hispanic, and 1.4% identified as Other. The average age during the age 6 assessment was 6.11, SD = .43; during the age 9 assessment, it was 9.21, SD = .45.
Diagnostic and Symptom Measures
PAPA
The PAPA assesses a range of disorders from the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders in preschool-aged children (Egger et al., 1999). DSM-IV diagnoses were derived using algorithms made by the developers of the instrument. Symptoms occurring within the 3 months prior to the interview are rated to maximize recall. The interview has been shown to have good psychometric properties (Egger et al., 2006). Interviews were conducted by M.A. level psychologists, over the telephone at age 3 and face-to-face with parents at age 6. Based on 35 audiotaped interviews, randomly selected: at age 3, Kappas were 1.00 for all diagnostic categories, and at age 6, Kappas were 0.64 for depression, 0.89 for any anxiety disorder, 0.64 for attention deficit/hyperactivity disorder, and 0.87 for oppositional defiant disorder. More detailed information on the rates of psychiatric disorders in this sample have been reported elsewhere (Bufferd, Dougherty, Carlson, Rose, & Klein, 2012).
K-SADS-PL
The K-SADS-PL, modified for DSM-IV, was administered by an M.A. level interviewer with extensive clinical experience and clinical psychology graduate students in in video-recorded face-to-face interviews with both a parent (generally the mother) and the child. The K-SADS is designed to assess a range of psychopathology in children and adolescents (Kaufman et al., 1997). Lifetime DSM-IV diagnoses were derived based on a combination of the parent and child report and if discrepancies arose, the interviewer attempted to reconcile them with both the parent and child at the end of the interview. All diagnoses were reviewed in case conferences led by an experienced child psychiatrist and a clinical psychologist. Reliability ratings were performed by the interviewers based on 74 randomly selected videotaped interviews, the kappa for any anxiety disorder was .67. At the age 9 assessment, 26 children met criteria for a new anxiety disorder (with an onset between the ages of 6 and 9): 6 for social phobia, 12 for specific phobia, 5 for generalized anxiety disorder, and 3 for anxiety disorder NOS.1 Additionally, 19 children met criteria for a new externalizing disorder: 3 for oppositional defiant disorder, 8 for attention deficit hyperactivity disorder, 6 for attention deficit hyperactivity disorder NOS, and 2 for co-morbid oppositional defiant disorder and attention deficit hyperactivity disorder.
SCID
Lifetime maternal anxiety was assessed with the SCID by M.A. level psychologists during the age 3 assessment. Interviews were conducted over the telephone and interrater reliabilities for the presence or absence of a lifetime anxiety disorder was 0.91 (For details, see: Torpey et al., 2013). Consistent with lifetime prevalence rates from the National Comorbidity Survey (Kessler et al., 2005), in the current sample, 73 mothers (30.9%) had a history of an anxiety disorder.
CBCL
Previous studies have found an association between anxiety symptoms and the ERN in children (Meyer, Weinberg, et al., 2012; Santesso et al., 2006). In order to control for children’s prior anxiety symptoms at age 6, we used the DSM-IV Anxiety Problems scale derived from the CBCL. The Anxiety Problems subscale consists of 6 items that are rated for the past 6 months on a scale from 0 (not true) to 2 (very or often true). In the current sample, coefficient alpha was .65, and the mean score was .79, SD = 1.07, with a range of 0 – 6. From here on, anxiety symptoms refer to those measured by the CBCL at age 6. For follow-up analyses, we also used the age 6 CBCL externalizing scale, ranging from 0 to 21, with a mean of 3.74, SD = 3.91, as well as the age 9 CBCL anxiety and externalizing scales, M = .95, SD = 1.4, and M = 3.39, SD = 3.82, respectively.
EEG Task and Materials
As previously reported (Meyer et al., 2013; Torpey et al., 2011), a Go/NoGo task was administered using Presentation software (Neurobehavioral Systems, Inc.). The stimuli were green equilateral triangles in four orientations: during 60% of trials, the triangles were vertically aligned and pointed up, on 20% of the trials, the triangles were vertically aligned and pointed down, on 10% of the trials, the triangles were titled slight to the left, and 10% of the trials, the triangles were titled slightly to the right. Children were instructed to respond to upward-pointing triangles by pressing a mouse button, and to withhold a response to all other triangles.
Psychophysiological Recording
The Active Two system (Biosemi, Amsterdam, Netherlands) was used to acquire EEG data. Thirty-two electrodes were used with a small amount of electrolyte, Signa Gel; BioMedical Instruments Inc., Warren, Michigan, applied at each electrode position. Data were processed offline with Brain Vision Analyzer, Brain Products, Gilching, Germany. EEG data were referenced to the nose, and high and low pass filtered at 0.1 Hz and 30 Hz, respectively. Segments were extracted from the continuous EEG, beginning 500 ms prior to responses. ERP data were corrected for eye-movements and blinks using the Gratton, Coles, and Donchin method (Gratton, Coles, & Donchin, 1983). Artifacts were automatically rejected if a voltage step of more than 50 microvolts between data points occurred, or if a voltage difference of less than 0.5 microvolts within a 100 ms interval occurred. After this, data were visually inspected for remaining artifacts. ERP averages were created for each trial type (error and correct) and a baseline of the average activity from -500 to -300 ms prior to the response was subtracted from each data point.
ERP and behavioral results have been previously reported in the full sample (Meyer et al., 2013; Torpey et al., 2011; Torpey et al., 2013). The error-related negativity (ERN) and correct-related negativity (CRN) were scored as the average voltage in the window from 0 to 100 ms after the response, at Fz, Cz, and Pz. The ΔERN was calculated by subtracting the CRN from the ERN. Behavioral measures include the number of errors of omission and commission, as well as average reaction times (RTs) on error and correct trials. RTs on correct trials that followed error trials were calculated to evaluate post-error RT slowing.
All statistical analyses were conducted using SPSS (Version 17.0) General Linear Model Software, with Greenhouse-Geisser correction applied to p values with multiple-df when necessitated by violation of the assumption of sphericity. Repeated measures ANOVAs and t-tests were used to examine behavioral data. A repeated measures ANOVA was conducted with response type (error vs. correct) and electrode (Fz, Cz, and Pz) as within-subject variables and child anxiety disorder at age 9 (ANX or No ANX) as the between-subject variable, controlling for age 6 CBCL DSM-IV Anxiety Problems, maternal history of anxiety (lifetime disorder or no disorder), and age 9 externalizing disorders (disorder or no disorder). Based on these results, we conducted a follow-up simultaneous binary logistic regression to examine whether the ΔERN uniquely predicted the onset of anxiety disorders when considering maternal history of anxiety and child anxiety symptoms at age 6. Additionally, as a follow-up analysis, we conducted a simultaneous multiple regression wherein age 6 ΔERN, maternal history of anxiety, and age 6 CBCL anxiety symptoms were all entered predicting age 9 CBCL anxiety symptoms.
Results
Behavioral Data
Accuracy and RT data are presented in Table 1 for the ANX and No ANX groups. Overall, children were faster on error trials than on correct trials, F(1, 232) = 97.03, p < .001, ηp2 = .30. The ANX and No ANX groups did not differ in RT, F(1, 232) = .50, p = .48, nor did the effect of trial type vary by diagnostic status, F(1, 232) = .22, p = .22. Also, the ANX and No ANX groups made a comparable number of errors, t(234) = −.83, p = .41 and correct responses, t(234) = .93, p = .36.
Table 1.
Means and standard deviations of error-related brain activity, clinical variables, and behavioral data for children who did (ANX) and did not (No ANX) develop anxiety disorders.
| ANX (N = 26) | No ANX (N = 210) | |
|---|---|---|
| Clinical Variables | ||
| Age 6 anxiety symptoms | 1.32 (1.24)** | 0.72 (1.03)** |
| % with maternal history of anxiety | 50.0%* | 31.43%* |
| Reaction time (ms) | ||
| Error trials | 491.55 (75.88) | 503.25 (81.09) |
| Correct trials | 627.04 (68.53) | 625.97 (68.37) |
| Post-error trials | 644.03 (95.18) | 654.16 (117.68) |
| Accuracy | ||
| No. of errors | 27.27 (12.68) | 25.00 (13.27) |
| No. of correct trials | 210.69 (14.54) | 213.50 (14.52) |
| % correct | 86.59% (7.92) | 87.76% (8.02) |
| ERPs (μV) | ||
| ERN at Cz | −0.87 (8.15) | 0.16 (8.98) |
| CRN at Cz | 9.30 (6.42) | 8.89 (5.45) |
| ΔERN at Cz | −10.16 (8.96) | −8.74 (8.55) |
| ERN at Fz | −1.76 (8.21) | −0.38 (7.87) |
| CRN at Fz | 5.33 (4.95) | 3.79 (4.21) |
| ΔERN at Fz | −7.09 (8.75)* | −4.17 (8.07)* |
| ERN at Pz | 3.94 (7.81) | 1.47 (8.70) |
| CRN at Pz | 9.50 (6.64) | 9.09 (6.02) |
| ΔERN at Pz | −5.56 (7.68) | −7.62 (8.59) |
Post-error RT are also presented in Table 1. Overall, children were slower on Go trials that occurred after an error trial than the average of all Go trials, F(1, 232) = 7.34, p < .05, ηp2 = .03, but this effect did not vary by diagnostic group, F(1, 232) = .45, p = .50.
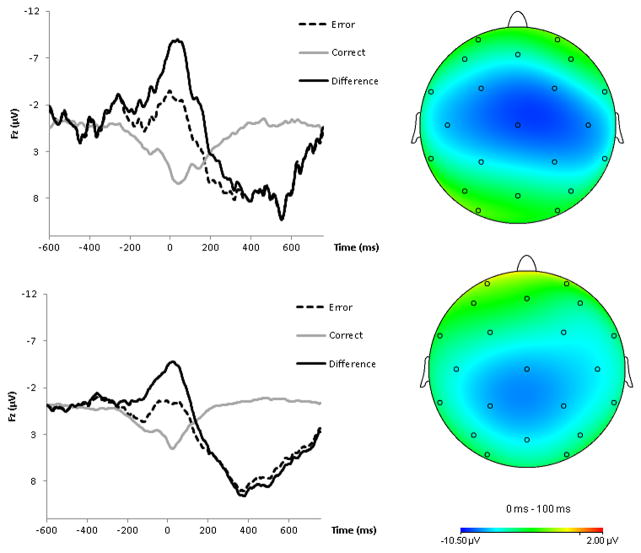
Error-Related Brain Activity
Figure 1 presents topographic maps on the right for the ANX (top) and No ANX (bottom) groups, depicting voltage differences (in μV) across the scalp for the ΔERN at Age 6. Grand average response-locked ERPs are also presented in Figure 1 (left). Average ERN, CRN, and Δ ERN values for the ANX and No ANX group are presented in Table 1.
Figure 1.
On the left, response-locked ERP waveforms for correct and error trials, as well as the difference waves (top = ANX group, bottom = no ANX group). On the right, topographic maps of activity (error minus correct; top = ANX group, bottom = no ANX group).
Overall, the ERP response was more negative following errors than correct responses, F(1, 231) = 84.42, p < .001, ηp2 = .27. There was no overall difference between the ANX and No ANX groups, F(1, 231) = .08, p = .77. However, there was a significant three-way interaction between response, electrode, and anxiety group, F(2, 462) = 4.51, p < .05, ηp2 = .02. In a follow-up repeated-measures ANOVA examining only the ERN, there was a significant interaction between electrode and anxiety group, F(2, 460) = 3.26, p < .05. However, none of the follow-up ANOVAs suggested that the ERN alone differed between the ANX and no ANX groups at any electrode (all ps > .1). In a repeated-measures ANOVA using only the CRN, the interaction between electrode and anxiety was not significant, F(2, 460) = 1.25, p = .28.
To probe the response by condition interaction, we utilized the ΔERN. Confirming the impression from Figure 1, a follow-up ANOVA suggested that children who would develop an anxiety disorder by age 9 had a larger ΔERN at Fz than children who would not develop any anxiety disorder, F(1, 231) = 4.14, p < .05, ηp2 = .02. The ΔERN did not differ between the ANX and No ANX group at Pz or Cz, all ps > .30.
Alternatively, we used pairwise t-tests to compare the ΔERN at each electrode within the ANX and No ANX group. In the No ANX group, the ΔERN was more negative at Cz compared to both Pz, t(209) = −2.41, p < .05, and Fz, t(209) = −10.11, p < .001. Moreover, the ΔERN was more negative at Pz than Fz, t(209) = −5.55, p < .001. Similar to the No ANX group, in the ANX group the ΔERN was more negative at Cz compared to both Pz, t(24) = −4.04, p < .001, and Fz, t(24) = −3.15, p < .01. However, in contrast to the No ANX group, the ΔERN was not more negative at Pz compared to Fz, t(24) = 1.29, p = .21 within the ANX group.
Consistent with previous findings in the larger sample (Meyer et al., 2013; Torpey et al., 2013), offspring of mothers with a history of anxiety disorder were characterized by a blunted ΔERN, F(1, 232) = 2.49, p = .09, ηp2 = .02, at a trend level. However, child anxiety symptoms at age 6 did not relate to the ΔERN, F(1, 232) = 2.46, p = .12. Additionally, children who developed any externalizing disorder by age 9 were not characterized by an enhanced or blunted ΔERN at age 6, F(1, 231) = 1.01, p = .32. Levene’s test indicated equal variances in the ΔERN at Fz between the ANX and No ANX group, F = .49, p = .49.
As a follow-up analysis, to further investigate the specificity of the ΔERN to anxiety, we conducted a repeated measures ANOVA with response type (error vs. correct) and electrode (Fz, Cz, and Pz) as within-subject variables and child externalizing disorder at age 9 (externalizing diagnosis or not) as the between-subject variable, controlling for age 6 CBCL Externalizing symptoms. Again, the results suggested that age 6 ΔERN was not related to the onset of externalizing disorders, F(2, 442) = 1.11, p = .32.
Based on the findings from Lahat et al. (2014) and McDermott et al. (2009) regarding temperamental BI, we examined whether the ΔERN might interact with anxiety symptoms at age 6 to predict the onset of anxiety disorders by age 9. The interaction reached a trend level of significance: among children with a larger ΔERN, anxiety symptoms predicted the onset of an anxiety disorder, and this same pattern was not present in children with a relatively smaller Δ ERN, B = −.054, Wald = 3.48, OR[95% CI] = .95[.90, 1.00], p = .06.
Predicting the Onset of Anxiety Disorders
The results of a logistic regression suggested that enhanced child anxiety symptoms at age 6 predicted the onset of a child anxiety disorder by age 9, R2 = .05, χ2 = 6.17, OR = 1.52 [1.11, 2.10], p < .01. Furthermore, there was an association between maternal lifetime anxiety disorder and the onset of child anxiety by age 9, χ2 (1) = 3.58, p = .06, at a trend level. To examine the unique ability of these factors to predict new anxiety disorder diagnoses by age 9, maternal anxiety disorder, child anxiety symptoms, and the ΔERN (at Fz) were all entered as predictors in a logistic regression. Results confirmed that maternal anxiety was a predictor at a trend level, and both child anxiety symptoms and the ΔERN uniquely predicted the onset of an anxiety disorder by age 9 (see Table 2).
Table 2.
Logistic Regression predicting the onset of child anxiety disorders between the ages of 6 and 9.
| Onset of anxiety disorder between the ages of 6 and 9 | |||
|---|---|---|---|
| B | Wald | OR[95% CI] | |
| Constant | −3.17 | 55.35 | |
| Maternal Anxiety Disorder | .67 | 2.18 | 1.93 [0.81, 4.62] |
| Child Anxiety Symptoms at Age 6 | 0.44 | 6.49 | 1.55 [1.11, 2.17]** |
| ΔERN at Age 6 (Fz) | −0.60 | 4.30 | 0.94 [0.89, 1.00]* |
p < .05,
p < .01
Note: R2 = .10 (Nagelkerke). Model χ2 = 12.47, p < .01; +p < .09, * p < .05, ** p < .01
In order to examine anxiety symptoms, rather than disorders, as an outcome we entered age 6 ΔERN, maternal history of anxiety, and age 6 CBCL anxiety symptoms into a simultaneous regression predicting age 9 CBCL anxiety symptoms. Results indicated that the ΔERN did not predict an increase in symptoms, p > .5.
Discussion
This is the first study to demonstrate that enhanced error-related brain activity precedes the onset of anxiety disorders. Increased error-related brain activity at age 6 predicted the onset of new anxiety disorders by age 9, even when controlling for baseline anxiety symptoms and maternal history of anxiety. There were no observable behavioral differences between the children who would become anxious and the rest of the sample, suggesting that enhanced error processing in the ANX group did not reflect performance-related differences. Moreover, the ERN did not predict the onset of externalizing disorders, suggesting that the ERN may specifically predict anxious trajectories—although future work is needed to examine the specificity of the ERN with respect to internalizing disorders (e.g., anxiety versus depression). Follow-up analyses suggested that the pattern of results was the same after controlling for age 6 and 9 depression symptoms, and that the ERN did not predict a change in depression symptoms. Considering the potential utility of identifying early biomarkers of risk, this is a novel and important extension of previous work that has linked concurrent anxiety and an increased ERN (Carrasco et al., 2013; Hajcak et al., 2008; Ladouceur et al., 2006; Meyer et al., 2013; Meyer, Weinberg, et al., 2012).
Considering that the ΔERN is elevated in children with OCD after successful treatment (Hajcak et al., 2008), increased in unaffected first-degree relatives (Carrasco et al., 2013; Riesel et al., 2011), relatively stable across development (Meyer, Bress, et al., 2014), and, in the present study, increased in children before the onset of anxiety disorders, the ΔERN may be a biomarker of risk for anxiety. Additionally, because the ΔERN has been shown to be heritable (Anokhin et al., 2008), and linked to a number of specific genetic polymorphisms (Manoach & Agam, 2013; in this sample: Meyer, Klein, et al., 2012), it has been proposed as a potential endophenotype for anxiety disorders (Manoach & Agam, 2013; Olvet & Hajcak, 2008; Proudfit et al., 2013). To our knowledge, these are the first data to suggest that an increased ΔERN predicts later anxiety disorders, controlling for initial symptoms. The ΔERN may facilitate early identification of at-risk youth, contribute to the detection of further susceptibility genes, increase our understanding of the mechanisms of illness, and aid in the development of novel targets for early intervention (Gottesman & Gould, 2003; Manoach & Agam, 2013).
Additionally, results suggested there were topographical differences in the distribution of the ΔERN between the ANX and No ANX groups. While the overall ΔERN was largest at Cz, the ΔERN at Fz was larger among children who would become anxious compared to those who did not. And, in the No ANX group, the ΔERN had a more parietal distribution (i.e., more negative at Pz than Fz) compared to the ΔERN in the ANX group. Overall, children in the ANX group had a more frontally distributed ΔERN, and this is where risk differentiated error-related brain activity.
The results from the current investigation are consistent with the notion that the ERN may index sensitivity to internal threat that is enhanced before the onset of an anxiety disorder. Children who were characterized by an increased ERN relative to peers may be developmentally advanced in terms of transitioning from external fears to anxiety and concern about behavioral competence and increased performance monitoring. In line with previous work suggesting that error-related ACC activity increases normatively across development (Tamnes et al., 2013), children who will go on to develop anxiety disorders may be characterized by greater (i.e., large and more frontal) sensitivity to errors. And, while many of the children in the current study where characterized by phobic disorders at age 9, previous developmental work found that 73% of children diagnosed with a specific phobia met criteria for an anxiety or depressive disorder 10 years later (Emmelkamp & Wittchen, 2009), suggesting that childhood phobias may mark pathological trajectories. Future work is needed to further examine these developmental trajectories.
To our knowledge, only one other study has reported on a biomarker that predicted risk for anxiety, finding that elevated startle during a safe condition predicted the onset of anxiety disorders over the course of 3 – 4 years in adolescents and young adults (Craske et al., 2012). In the current study, 10% of the variance in the onset of anxiety between the ages of 6 and 9 was accounted for by age 6 symptoms, ΔERN, and maternal history of anxiety. However, the children are still very young. As more cases accumulate over time, effect sizes may increase. Also, future work could investigate these risk factors in conjunction with other EEG or fMRI neural markers, parenting style, life stress, genes, cognitive vulnerabilities, executive functioning, cortisol reactivity, startle, attentional biases, socioeconomic status, temperament, fear learning, peer functioning, or family conflict to identify risk signatures (i.e., patterns or constellations of risk markers) that may increase our ability to predict the onset of clinical anxiety.
In addition to the ΔERN, maternal history of anxiety predicted the onset of anxiety disorders in children at a trend level. However, maternal history of anxiety was related at a trend level to a smaller ΔERN, consistent with previous findings in this sample (Meyer et al., 2013). Thus, maternal history of anxiety appears to relate to child anxiety risk via different mechanisms than error-related brain activity. This may be due in part to maternal anxiety being a non-specific risk factor that also predicts disorders associated with decreased error processing, i.e., depression and externalizing disorders (Beidel & Turner, 1997; Bijl, Cuijpers, & Smit, 2002; Merikangas, Dierker, & Szatmari, 1998). Future work might further explore the mechanisms underlying these associations. Additionally, the ΔERN did not predict age 9 CBCL anxiety symptoms. This finding is generally consistent with our previous work which suggests dimensional anxiety symptoms in non-clinical populations only begin to relate to an increased ERN in later childhood (Meyer et al., 2013; Torpey et al., 2013; Meyer et al., 2012). Previous work suggests that among children high in early temperamental behavioral inhibition, an increased ERN predicted anxiety symptoms later in development (Lahat et al., 2014; McDermott et al., 2009). Results from the current study are consistent with this – among children with an increased ΔERN at age 6, anxiety symptoms predicted the onset of anxiety disorders, at a trend level. Future work is needed to further examine the relationship between normative levels of anxiety and the ERN across development.
A limitation of the current investigation is that anxiety disorders at age 9 were heterogeneous (i.e., included specific phobia, separation anxiety disorder, social phobia, generalized anxiety disorder, and anxiety NOS). Because the number of children with any particular disorder was small, we were unable to examine whether the ΔERN predicted the onset of specific anxiety disorders. Along with this, we attempted to examine specificity by conducting analyses regarding the onset of externalizing disorders. While examining the onset of near-neighbor disorders such as depression would provide the strongest test of specificity (Garber & Hollon, 1991), the incidence of depression during this developmental period is quite low. Indeed, only one child in our sample developed a new depressive disorder between the ages of 6 and 9. Additionally, the current study was limited in age range (i.e., only 5 to 7 year-olds were assessed for ERN). It is possible that the relationship between ΔERN and risk for anxiety changes across development. For example, it is possible that increased error-related brain activity precedes the onset of anxiety disorders in early childhood, but not in adolescence or adulthood. We are continuing to collect longitudinal data in the current sample to investigate this issue. Additionally, given that 96% of the sample was Caucasian, future studies should explore whether the current findings are generalizable to more diverse populations. A final limitation to consider is that the PAPA at age 6 assessed symptoms within the preceding 3 months. While children with anxiety disorders occurring before 6 years of age, as reported at the age 9 K-SADS-PL, were excluded from the analyses, it is possible that retrospective reporting was not entirely accurate. Therefore, some of the children with an enhanced ERN at age 6 may have previously had anxiety disorders. Future longitudinal work should replicate and extend the current findings by assessing lifetime anxiety diagnoses at baseline.
An intriguing possibility is that error-related brain activity may be a novel target for treatment and prevention efforts. Among children who will become anxious, the ΔERN is enhanced early in development, before children meet criteria for clinical anxiety disorders —thereby providing a novel and potentially malleable target for prevention. While anxiety treatments that target symptoms (i.e., cognitive behavioral therapy) do not seem to impact the ERN (Hajcak et al., 2008), findings suggest that environmental influences that more directly relate to error sensitivity (e.g., punishment) may impact the ERN. A previous study found that punishment for errors in the lab increases the ERN – an effect that persists after punishment ends (Riesel et al., 2012). Similarly, both observed and self-reported harsh parenting is related to an increased ΔERN in children, and the magnitude of the ΔERN mediated the relationship between harsh parenting and anxiety disorders in children (Meyer et al., 2014b). Thus, interventions that decrease harsh or critical parenting (or even more general success or failure experiences) may impact the ΔERN and decrease the likelihood of anxiety disorders. Future studies might explore other ways in which the ΔERN can be modified, and the degree to which modifying the ERN reduces the likelihood of developing anxiety disorders in early childhood.
Footnotes
During the age 9 K-SADS-PL assessment, 2 children reported having phobias that developed before the age of 6 who had not previously received a diagnosis during the age 6 PAPA assessment. These children were excluded from the current study.
References
- Achenbach TM, Edelbrock C. Child behavior checklist. Burlington, Vt: 1981. [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45(4):524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatric Clinics of North America. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo PDSLRWH. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Archives of General Psychiatry. 2010;67(1):47–57. doi: 10.1001/archgenpsychiatry.2009.177. [DOI] [PubMed] [Google Scholar]
- Beidel DC, Turner SM. At Risk for Anxiety: I. Psychopathology in the Offspring of Anxious Parents. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):918–924. doi: 10.1097/00004583-199707000-00013. doi: http://dx.doi.org/10.1097/00004583-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Bijl RV, Cuijpers P, Smit F. Psychiatric disorders in adult children of parents with a history of psychopathology. Social Psychiatry And Psychiatric Epidemiology. 2002;37(1):7–12. doi: 10.1007/s127-002-8208-8. [DOI] [PubMed] [Google Scholar]
- Bittner A, Egger HL, Erkanli A, Jane Costello E, Foley DL, Angold A. What do childhood anxiety disorders predict? J Child Psychol Psychiatry. 2007;48(12):1174–1183. doi: 10.1111/j.1469-7610.2007.01812.x. [DOI] [PubMed] [Google Scholar]
- Broeren S, Newall C, Dodd HF, Locker R, Hudson JL. Longitudinal investigation of the role of temperament and stressful life events in childhood anxiety. Development and psychopathology. 2014;26(02):437–449. doi: 10.1017/S0954579413000989. [DOI] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Carlson GA, Klein DN. Parent-reported mental health in preschoolers: findings using a diagnostic interview. Comprehensive Psychiatry. 2011;52(4):359–369. doi: 10.1016/j.comppsych.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN. Psychiatric disorders in preschoolers: continuity from ages 3 to 6. American Journal of Psychiatry. 2012;169(11):1157–1164. doi: 10.1176/appi.ajp.2012.12020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased Error-Related Brain Activity In Youth With Obsessive-Compulsive Disorder And Unaffected Siblingseased Error-Related Brain Activity In Youth With Obsessive-Compulsive Disorder And Unaffected Siblings. Depression and Anxiety. 2013;30(1):39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Angold A, Shanahan L, Costello EJ. Longitudinal patterns of anxiety from childhood to adulthood: The great smoky mountains study. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(1):21–33. doi: 10.1016/j.jaac.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Wolitzky–Taylor KB, Mineka S, Zinbarg R, Waters AM, Vrshek–Schallhorn S, Ornitz E. Elevated responding to safe conditions as a specific risk factor for anxiety versus depressive disorders: Evidence from a longitudinal investigation. Journal of Abnormal Psychology. 2012;121(2):315. doi: 10.1037/a0025738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger Ascher B, Angold A. Unpublished Interview Schedule. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; 1999. The preschool age psychiatric assessment: Version 1.1. [Google Scholar]
- Egger, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA) Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(5):538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Emmelkamp PMG, Wittchen HU. Specific Phobias. In: Andrews G, Charney DS, Sirovatka PJ, editors. Stress-induced and fear circuitry disorders. Refining the research agenda for DSM-V. Arlington, VA: APA; 2009. pp. 77–101. [Google Scholar]
- Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46(7):1877–1887. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Davies M, Borus J, Rounsaville B. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part II: Multi-site test-retest reliability study. Journal of personality disorders. 1995;9(2):92–104. [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A Neural System for Error Detection and Compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-Monitoring dysfunction in Obsessive-Compulsive Disorder. Psychological Science. 2000;11(1):1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gullone E. The development of normal fear: A century of research. Clinical Psychology Review. 2000;20(4):429–451. doi: 10.1016/s0272-7358(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Hajcak G. What we’ve learned from mistakes. Current Directions in Psychological Science. 2012;21(2):101–106. doi: 10.1177/0963721412436809. [DOI] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased Error-Related Brain Activity in Pediatric Obsessive-Compulsive Disorder Before and After Treatment. Am J Psychiatry. 2008;165(1):116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Carrasco M, Harbin SM, Nienhuis JK, LaRosa CE, Chen P, Gehring WJ. Error-Related Negativity and Tic History in Pediatric Obsessive-Compulsive Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2012 doi: 10.1016/j.jaac.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2014 doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last CG, Perrin S, Hersen M, Kazdin AE. A prospective study of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 1996;35(11):1502–1510. doi: 10.1097/00004583-199611000-00019. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Agam Y. Neural markers of errors as endophenotypes in neuropsychiatric disorders. Frontiers in human neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Dierker LC, Szatmari P. Psychopathology among offspring of parents with substance abuse and/or anxiety disorders: a high-risk study. Journal Of Child Psychology And Psychiatry, And Allied Disciplines. 1998;39(5):711–720. [PubMed] [Google Scholar]
- Meyer, Klein DN, Torpey DC, Kujawa AJ, Hayden EP, Sheikh HI, Hajcak G. Additive effects of the dopamine D2 receptor and dopamine transporter genes on the error-related negativity in young children. Genes, Brain and Behavior. 2012 doi: 10.1111/j.1601-183X.2012.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress J, Proudfit GH. Psychometric Properties of the Error-Related Negativity in Children and Adolescents. Psychophysiology. 2014 doi: 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, Klein DN. Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of Abnormal Child Psychology. 2013:1–10. doi: 10.1007/s10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Proudfit GH, Bufferd SJ, Kujawa AJ, Laptook RS, Torpey DC, Klein DN. Self-reported and observed punitive parenting prospectively predicts increased error-related negativity in six-year-old children. Journal of Abnormal Child Psychology. 2014 doi: 10.1007/s10802-014-9918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample. Journal of Abnormal Psychology. 2010;119(3):468. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008;28(8):1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology & Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Proudfit GH, Inzlicht M, Mennin DS. Anxiety and error monitoring: the importance of motivation and emotion. Frontiers in human neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for Obsessive-Compulsive Disorder: Evidence from unaffected first-degree relatives. Am J Psychiatry. 2011;168(3):317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49(2):239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Developmental neuropsychology. 2006;29(3):431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SH, McCathie H. The Stability of Fears in Children: a Two-Year Prospective Study: a Research Note. Journal of Child Psychology and Psychiatry. 1993;34(4):579–585. doi: 10.1111/j.1469-7610.1993.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Spence SH, Rapee R, McDonald C, Ingram M. The structure of anxiety symptoms among preschoolers. Behaviour Research and Therapy. 2001;39(11):1293–1316. doi: 10.1016/s0005-7967(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Stern ER, Liu Y, Gehring WJ, Lister JJ, Yin G, Zhang J, Taylor SF. Chronic medication does not affect hyperactive error responses in obsessive-compulsive disorder. Psychophysiology. 2010;47(5):913–920. doi: 10.1111/j.1469-8986.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Torstveit M, Sells VT, Fjell AM. Performance monitoring in children and adolescents: A review of developmental changes in the error-related negativity and brain maturation. Developmental Cognitive Neuroscience. 2013;6(0):1–13. doi: 10.1016/j.dcn.2013.05.001. doi: http://dx.doi.org/10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa A, Klein DN. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Developmental Psychobiology. 2011:n/a–n/a. doi: 10.1002/dev.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa AJ, Dyson MW, Olino TM, Klein DN. Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. Journal of Child Psychology and Psychiatry. 2013:no–no. doi: 10.1111/jcpp.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey M, Crnic K, Carter W. Worry in childhood: A developmental perspective. Cognitive Therapy and Research. 1994;18(6):529–549. doi: 10.1007/bf02355667. [DOI] [Google Scholar]
- Weinberg A, Hajcak G. Longer term test–retest reliability of error-related brain activity. Psychophysiology. 2011;48(10):1420–1425. doi: 10.1111/j.1469-8986.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes Generalized Anxiety Disorder with and without Comorbid Major Depressive Disorder. Journal of Abnormal Psychology. 2012 doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological psychology. 2010;85(3):472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Hajcak G. Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion. 2012:1–17. [Google Scholar]
- Wittchen HU, Lieb R, Pfister H, Schuster P. The waxing and waning of mental disorders: evaluating the stability of syndromes of mental disorders in the population. Compr Psychiatry. 2000;41(2 Suppl 1):122–132. doi: 10.1016/s0010-440x(00)80018-8. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, Fromson JA. Error-related negativity abnormalities in generalized anxiety disorder and obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(1):265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]