Functional neurovascular changes reflecting alterations in brain function and cognition and/or originating primarily from abnormalities localized to the cerebrovascular system have been described in many neurological disorders and during normal brain aging. However, the relationship between vascular and neuronal dysfunction, and how they relate to each other and contribute to cognitive impairment and dementia due to Alzheimer’s disease (AD), vascular cognitive impairment and dementia (VCID), and/or other neurological disorders, still remains controversial1,2. An obvious place to look for neurovascular and cognitive changes is in the hippocampus, a region involved with learning and memory that is particularly susceptible to changes in oxygen and blood supply and is damaged early in AD.
Using a variety of magnetic resonance imaging (MRI) techniques3,4 including cerebral blood volume (CBV)-fMRI with gadolinium contrast5, it was found that hypometabolism coupled with diminished CBV in the hippocampus is associated with cognitive impairment in the elderly and early stages of AD. Employing an advanced protocol and post-processing analysis of the CBV-fMRI maps in the hippocampus, a recent study interrogated whether functional changes in the dentate gyrus drive hippocampus-specific cognitive dysfunction in cognitively normal older adults who were enrolled in a randomized trial with cocoa flavanols, an ingredient in cocoa, red wine, berries and dark chocolate6. Interestingly, this recent study6 showed that high-flavanol dietary intake increases the CBV in the dentate gyrus and enhances performance on a modified version of the Benton Visual Retention Test (ModBent) that is dependent on pattern separation in the hippocampus, specifically localized to the dentate gyrus.
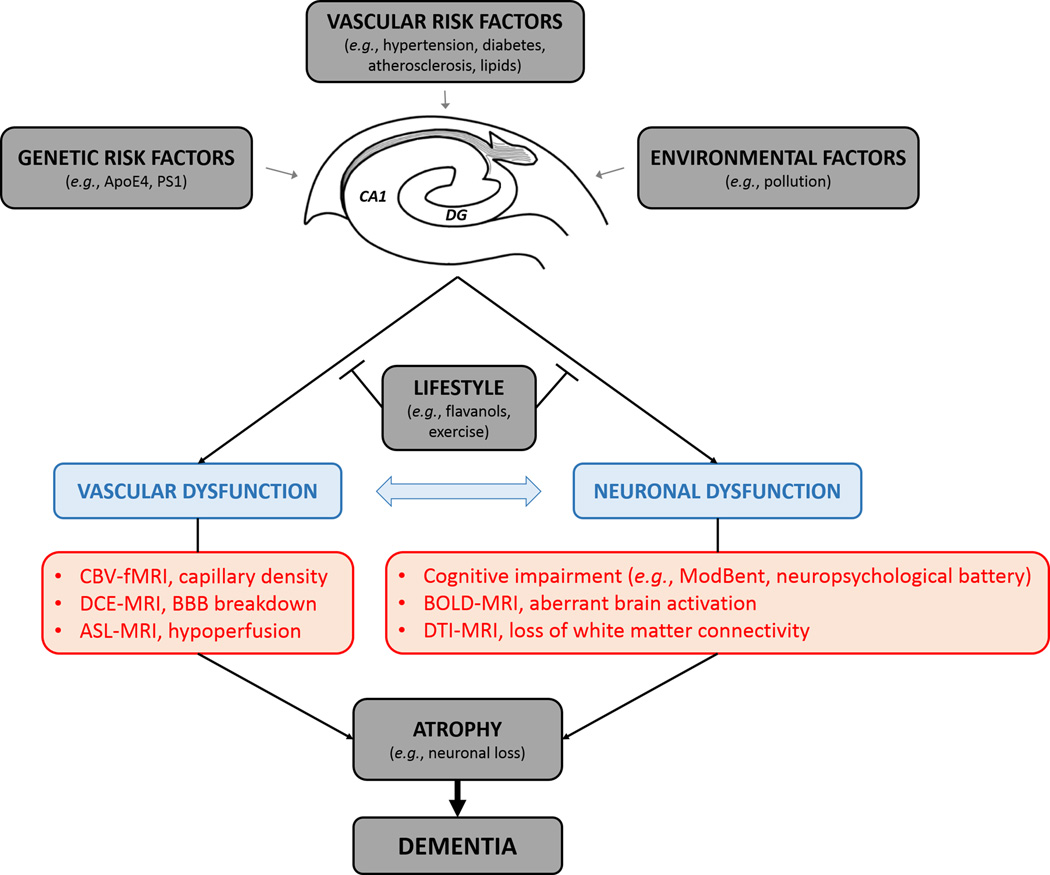
These recent findings raise a possibility that during normal aging the human hippocampus retains significant vasculoplastic reserve that is likely mediated via enhanced angiogenesis and/or new blood vessel formation. The question persists, however, whether changes in basal blood flow, blood-brain barrier (BBB) permeability and/or brain activation that all can be studied by imaging neurovascular function in the living human brain with different techniques such as arterial spin labeling (ASL)-MRI, dynamic contrast-enhanced (DCE)-MRI7 and/or BOLD-fMRI, respectively, can also play a role in the observed adaptive cognition responses during normal aging (Figure 1). Additionally, major genetic risk factors such as apolipoprotein E4 allele for late onset AD, or presenilin 1 gene mutations for familial AD, as well as environmental factors and vascular risk factors might also affect formation of these adaptive responses. These questions warrant future investigations. Some other interesting and timely questions are when and whether cortical or subcortical brain regions or white matter connections become involved to cause cognitive impairment and/or accelerate progressive cognitive decline and hippocampal atrophy associated with dementia, which all can be studied in the living human brain by the diffusion tensor imaging (DTI)-MRI (Figure 1).
Figure 1. Modern neuroimaging techniques may provide new insights into the vascular and neural plasticity of the hippocampus in aging and disease.
Imaging vascular and neuronal functions in the living human brain in the hippocampus during normal aging, mild cognitive impairment (MCI) and dementia due to Alzheimer’s disease (AD) and related disorders, and vascular cognitive impairment and dementia (VCID).
As CBV-fMRI is a neurovascular-dependent outcome measure of hippocampal function in the absence of brain activation, it is worth noting that increasingly recognized alterations in the neurovascular functions in many neurological disorders associated with cognitive impairment1,2 might potentially influence presently used fMRI measurements. It would be interesting to know, for example, how the CBV-fMRI findings relate to changes in BBB integrity that have been reported in AD and VCID1,2 and more recently during normal aging in the hippocampus that worsen with MCI, as suggested by a recent study7. Use of the DCE-MRI approach to determine regional BBB integrity7 and the effects of lifestyle modifiers such as flavanols, exercise and/or the role of vascular risk factors and their treatment during cognitively normal aging and aging associated with MCI, AD and/or VCID may help us better understand the emerging role of the cerebral vascular system in maintaining overall cognitive health.
Future studies using multiple imaging biomarkers to access neurovascular function in relation to cognition and brain function (Figure 1), and combining imaging biomarkers with analysis of molecular cerebrospinal fluid biomarkers of the cell-specific injury within the neurovascular unit are needed to establish whether vascular dysfunction can precede neuronal dysfunction and cognitive impairment during normal aging, dementia due to AD and related disorders, or VCID, and ultimately how these changes are influenced by lifestyle, genetics and environment.
Acknowledgments
We would like to thank the National Institutes of Health for support: R37NS34467 (B.V.Z.), R37AG23084 (B.V.Z.), R01AG039452 (B.V.Z.), Zilkha Senior Scholar support (B.V.Z.), K01-AG034175 (J.P.), and Alzheimer’s Association NIRP-12-259277 (J.P.). We thank Dr. Arthur W. Toga for reading the manuscript and his very helpful comments.
Footnotes
Conflict of Interest Disclosures
None reported.
Contributor Information
Axel Montagne, Zilkha Neurogenetic Institute, Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, 1501 San Pablo Street, Los Angeles, CA 90089, USA..
Judy Pa, Institute for Neuroimaging & Informatics and Department of Neurology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90089, USA..
Berislav V. Zlokovic, Zilkha Neurogenetic Institute, Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, CA 90089, USA..
References
- 1.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature Reviews Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol. 1999;45(4):466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 2002;51(3):290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- 5.Reitz C, Brickman AM, Brown TR, et al. Linking hippocampal structure and function to memory performance in an aging population. Arch Neurol. 2009;66(11):1358–1892. doi: 10.1001/archneurol.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brickman AM, Khan UA, Provenzano FA, et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014;17(12):1798–1803. doi: 10.1038/nn.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]