Abstract
There remains limited research on cardiovascular disease (CVD) risk factors in Puerto Rican adults. We compared lifestyle and CVD risk factors in Puerto Rican men and women with normal fasting glucose (NFG), impaired fasting glucose (IFG), or type 2 diabetes (T2D), and investigated achievement of American Diabetes Association (ADA) treatment goals in those with T2D. Baseline data from the Boston Puerto Rican Health Study were analyzed, which included 1,287 adults aged 45–75 years. Obesity, hyperglycemia, and dyslipidemia were prevalent and increased from NFG to IFG and T2D. In individuals without T2D, fasting insulin correlated significantly with body mass index. Achievement of ADA goals was poor; LDL cholesterol was most achieved (59.4%), followed by blood pressure (27.2%) and glycosylated hemoglobin (27.0%). Poverty, female sex, current alcohol use, and diabetes or anti-hypertensive medication use were associated with not meeting goals. Puerto Rican adults living in the Boston area showed several metabolic abnormalities and high CVD risk, likely due to pervasive obesity and socio-economic disparities.
Keywords: Cardiovascular disease risk factors, Type 2 diabetes, Impaired fasting glucose, Hispanics, Puerto Ricans
Introduction
The obesity epidemic in the United States (US) has led to high rates of insulin resistance and type 2 diabetes (T2D). Both conditions are particularly prevalent among Hispanics [1], the largest and fastest growing ethnic minority group [2], and prevalence continues to escalate [3]. Puerto Ricans are reported to have the second-highest age-adjusted prevalence of T2D (12.6%) of all ethnic groups in the US [4], after the American Indian and Alaska Native population (16.5%) [5], which coincides with greater overall and central obesity than other ethnic groups [6]. Impaired fasting glucose (IFG), an insulin-resistant state of pre-diabetes, also occurs more frequently in US Hispanics than in non-Hispanic whites or non-Hispanic blacks [1], but prevalence has not been reported in Puerto Ricans. The progression from normal fasting glucose (NFG) to IFG and T2D is associated with deterioration in metabolic control, including hyperglycemia, dyslipidemia, and hypertension, leading to increased risk of cardiovascular disease (CVD) [7]. To date, few studies have focused on metabolic control and CVD risk among Puerto Rican adults predisposed to T2D. This study captures prevalence of IFG, T2D, and CVD risk factors across the spectrum of glucose control, ranging from normal to impaired fasting glucose to T2D, in Puerto Rican adults living in Massachusetts.
In individuals with T2D, dysregulation in CVD risk factors constitutes the typical “ABCs of diabetes”. The American Diabetes Association (ADA) has provided treatment recommendations for glycemic (glycosylated hemoglobin, HbA1c), blood pressure, and low-density lipoprotein cholesterol (LDL-C) control [8]; however, only 27–52% of US adults achieve these goals [9–12]. Although data on ADA goal achievement among Hispanics are sparse, Hispanics are more likely than non-Hispanic whites to exhibit poor glycemic control [13]. A probable contributor is inadequate self-management of disease, which has been linked to lower education [14], higher perceived stress [15], and other socio-economic and health disparities [16], in this ethnic group.
Few observational studies have reported CVD risk factors in Puerto Rican adults. In the Massachusetts Hispanic Elders Study (MAHES, 1993–1997), Puerto Ricans had high prevalence of T2D [17] across all body mass index (BMI) and waist circumference categories [18], which was associated with poor glycemic control [17] and dyslipidemia [19]. Moreover, prevalence and characteristics of IFG in Puerto Ricans have not previously been described. The main objective of this study was to compare lifestyle and CVD risk factors in Puerto Rican adults with NFG, IFG, or T2D. A secondary objective was to investigate factors associated with achievement of ADA treatment goals in this population.
Methods
Study Population
We used baseline data from the Boston Puerto Rican Health Study, the central study of the Boston Puerto Rican Center on Population Health and Health Disparities. Briefly, Puerto Rican adults were recruited from Boston and surrounding urban areas, primarily (77%) through door-to-door enumeration using a sampling scheme derived from year 2000 US Census data, as well as through community events (10%), referrals (7%), and calls to the study office (6%) [20]. Eligible individuals were of Puerto Rican origin based on self-report, aged 45–75 years, scored >10 points on the Mini-Mental State Examination, and were planning to stay in Massachusetts for at least 2 years. Only one eligible person per household was interviewed. Of the 2,084 eligible individuals invited to participate between June 2004 and October 2009, 1,500 (72%) completed the baseline interview. Boston Puerto Rican Health Study participants were recently found to be representative of Puerto Rican adults residing in high-density urban areas in the US, and additional details on study methodology have been published [20]. For these analyses, baseline data from the first 1,287 participants (i.e., 379 [29.5%] men and 908 [70.6%] women) with complete data on fasting plasma glucose (FPG) concentration were included. Study protocol and procedures were approved by the Institutional Review Board of Tufts Medical Center. All participants provided written informed consent.
Data Collection
Bilingual interviewers administered a 3–4 h questionnaire in the participant’s home, on medical history, socio-demographic characteristics, and health behaviors, performed anthropometric and blood pressure measurements, and recorded use of all medications. Questions included highest grade completed in school, and past or current use of alcohol and smoking. Physical activity was assessed using a modified version of the Paffenbarger Harvard Alumni Questionnaire [21], and a summary score captured time spent in different activities and metabolic equivalents. Poverty was defined using US Department of Health & Human Services poverty guidelines [22], in which each participant’s total household income was compared with threshold values based on year of study interview and family size. Anthropometric and blood pressure measurements were performed following standard procedures from NHANES [23] and the MacArthur Studies of Successful Aging [24]. Height, weight, and waist circumference were measured in duplicate, and the average measure was used. Seated blood pressure was obtained, in duplicate, three times during the interview, using a digital, automated sphygmomanometer, and the average of the last two measurements was taken [25]. On a separate day, participants fasted for 12 h overnight, and venipuncture was performed in their home by a certified phlebotomist. All study interviewers were thoroughly trained by experienced staff on performing standardized interview procedures, and they attended retraining and review sessions periodically [20]. Further, each interview questionnaire was self- and peer-reviewed before data entry.
Laboratory Methods
Serum glucose was measured using an enzymatic, kinetic reaction on the Olympus AU400e with Olympus Glucose Reagents (OSCR6121) (Olympus America Inc., Melville, NY), with an intra-assay coefficient of variation (CV) of 2%. Serum insulin was measured using the Immulite 1000 Insulin Kit (LKIN1) on the Immulite 1000 (Seimens Medical Solutions Diagnostics, Los Angeles, CA), with intra-assay CVs of 5.2–6.4%. HbA1c was analyzed using a latex-enhanced immunoturbimetric method, on the Cobas FARA using the Roche Unimate HbA1c kit (Roche Diagnostics, Indianapolis, Indiana). Blood lipids were obtained from EDTA plasma used for the lipoprotein profile, with an enzymatic endpoint reaction on the Olympus AU400e with Olympus Cholesterol (OSR6116), High-Density Lipoprotein Cholesterol (HDL-C) (OSR 6156), and Triglyceride (OSR6033) Reagents (Olympus America Inc., Melville, NY); intra-assay CVs for cholesterol and triacylglycerols were 1.8 and 2.8%, respectively. LDL-C was calculated using the Friedewald equation: [LDL-C] = [total cholesterol] − [HDL-C] − [triacylgly-cerols/5].
Explanatory Variables and Outcome Measures
We classified study participants into the following FPG categories: NFG if FPG was <100 mg/dl (5.6 mmol/l), IFG if FPG was between 100 mg/dl (5.6 mmol/l) and 125 mg/dl (6.9 mmol/l), or T2D if FPG was ≥126 mg/dl (7.0 mmol/l) and/or the individual was using oral diabetes medication and/or insulin [26]. Of those with T2D, 91 (18%) were not taking diabetes medication and were classified based on FPG concentration alone; these individuals may represent “undiagnosed” T2D [27]. Another 282 (56%) were on oral medication only, and 129 (26%), insulin and oral medication. Agreement between our T2D classification and self-reported “ever” medical diagnosis was good; only 59 (12%) of those we classified did not report the disease. Of these, only four were on diabetes medication, indicating that nearly all of those on medication knew about the disease.
Cardiovascular disease risk factors were treated both as continuous measures to obtain adjusted mean concentrations, and as categorical variables to denote level of CVD risk. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting plasma insulin (μIU/ml) * fasting plasma glucose (mmol/l)/22.5. We used published guidelines to define categories of BMI and waist circumference [28], high HbA1c [8], sex-specific low HDL-C [29], borderline and high triacylglycerols [30], and high systolic and diastolic blood pressure [31]. Among individuals with T2D, ADA goals were considered met for HbA1c <7.0%, blood pressure <130/80 mmHg, and LDL-C <100 mg/dl (2.6 mmol/l) [8].
Statistical Analyses
Among individuals with NFG, IFG, or T2D, lifestyle and CVD risk factors were compared, separately in men and women. Before analysis, a natural logarithmic transformation was applied to glucose, HbA1c, insulin, HOMA-IR, HDL-C, and triacylglycerols, to better approximate normal distributions. Adjusted means of CVD risk factors were obtained by sex and compared using ANCOVA, adjusting for the following: BMI and waist circumference—age; glycemia measures, blood lipids, and blood pressure—age, waist circumference, and use of respective medications. We tested for interaction between FPG category and age, medication use, or waist circumference, but no interaction was significant. In individuals with NFG or IFG, age- and sex-adjusted means of fasting insulin by BMI category were compared using ANCOVA with Tukey’s HSD test and tests for linear trend. Among those with T2D, we calculated percentage of individuals achieving each ADA goal, and conducted multivariate logistic regression analyses to investigate factors associated with goal achievement. Analyses were conducted using SAS for WINDOWS, version 9.1 (SAS Institute, Cary, NC, USA), and all P values were two-sided.
Results
In the total sample, prevalence of NFG, IFG, and T2D were 37, 24, and 39%, respectively. In women only, those with IFG or T2D were more likely to have ≤8th grade education (Table 1). For both men and women, physical activity levels were low, but no significant difference was observed across FPG categories. Trends for smoking and alcohol use differed by sex; men with T2D were less likely to currently smoke, and women with T2D less likely to consume alcohol, compared to those with NFG. Medication use was widespread, particularly in those with T2D. Adjusted means of all obesity and glycemia measures increased progressively across FPG categories. However, individuals with T2D had lower total and LDL cholesterol concentrations than those with NFG or IFG, after controlling for lipid-lowering medication use. Among women, those with IFG or T2D had lower HDL-C and higher triacylglycerols than those with NFG.
Table 1.
Lifestyle characteristics and cardiovascular disease (CVD) risk factors in Puerto Rican men and women with normal fasting glucose (NFG), impaired fasting glucose (IFG), or type 2 diabetes (T2D)
| Men (n = 379)
|
Women (n = 908)
|
|||||
|---|---|---|---|---|---|---|
| NFG (37.2%) | IFG (23.8%) | T2D (39.1%) | NFG (37.1%) | IFG (23.8%) | T2D (39.1%) | |
| Lifestyle characteristics | ||||||
| Age (years) | 55.2 (53.9, 56.5)x | 57.2 (55.6, 58.8)xy | 57.9 (56.6, 59.2)y | 55.7 (54.9, 56.5)x | 57.2 (56.2, 58.2)y | 59.2 (58.5, 60.0)z |
| Education ≤ 8th grade (%) | 37.9 | 44.9 | 50.0 | 40.4x | 54.6y | 54.9y |
| Physical activity (%) | ||||||
| Sedentary/light | 88.7 | 88.9 | 91.9 | 96.1 | 97.2 | 98.6 |
| Moderate/heavy | 11.4 | 11.1 | 8.1 | 3.9 | 2.8 | 1.4 |
| Current smoking (%) | 39.9x | 41.6x | 24.5y | 22.0 | 21.3 | 19.3 |
| Current alcohol use (%) | 50.0xy | 61.8x | 43.8y | 42.6x | 34.7xy | 29.0y |
| Medication use (%) | ||||||
| Lipid-lowering | 20.1x | 32.6x | 53.7y | 24.0x | 28.4x | 63.9y |
| Anti-hypertensive | 30.9x | 48.3y | 71.4z | 35.3x | 47.4y | 78.6z |
| CVD risk factors | ||||||
| Obesity | ||||||
| Body mass index (kg/m2) | 27.6 (26.8, 28.4)x | 29.3 (28.3, 30.3)y | 31.6 (30.8, 32.4)z | 30.8 (30.0, 31.5)x | 33.1 (32.1, 34.0)y | 34.7 (34.0, 35.5)z |
| Waist circumference (cm) | 96.8 (94.6, 98.9)x | 101 (98.2, 103)y | 107 (104, 109)z | 97.0 (95.4, 98.7)x | 102 (99.6, 104)y | 106 (105, 108)z |
| Glycemia measures | ||||||
| Glucose (mg/dl)a | 87.9 (84.4, 91.6)x | 104 (99.5, 109)y | 151 (147, 154)z | 84.2 (82.0, 86.4)x | 101 (98.2, 104)y | 152 (150, 154)z |
| HbA1c (%)a | 6.4(6.2, 6.5)x | 6.9 (6.7, 7.1)y | 8.0 (7.9, 8.1)z | 6.5 (6.4, 6.7)x | 6.9 (6.7, 7.0)y | 8.2 (8.1, 8.2)z |
| Insulin (μIU/ml)a | 9.1 (8.2, 10.0)x | 12.0 (10.8, 13.4)y | 16.4 (15.6, 17.4)z | 9.7 (9.1, 10.4)x | 13.0 (12.1, 14.0)y | 16.9 (16.2, 17.5)z |
| HOMA-IRa | 1.9 (1.7, 2.2)x | 3.1 (2.7, 3.4)y | 6.1 (5.7, 6.5)z | 2.0 (1.8, 2.1)x | 3.2 (2.9, 3.4)y | 6.2 (5.9, 6.4)z |
| Blood lipids | ||||||
| Total cholesterol (mg/dl) | 176 (169, 184)x | 180 (171, 189)x | 162 (155, 169)y | 193 (188, 198)x | 196 (190, 201)x | 178 (174, 183)y |
| HDL-C (mg/dl)a | 38.5 (37.4, 39.5) | 40.4 (39.2, 41.7) | 37.4 (36.4, 38.3) | 47.9 (47.2, 48.6)x | 45.0 (44.2, 45.7)y | 43.7 (43.2, 44.3)y |
| LDL-C (mg/dl) | 104 (97.4, 110)x | 103 (95.5, 110)x | 89.7 (83.9, 95.6)y | 116 (112, 120)x | 116 (112, 121)x | 100 (96.8, 104)y |
| Triacylglycerols (mg/dl)a | 137 (130, 144) | 158 (149, 168) | 155 (147, 162) | 129 (125, 132)x | 149 (144, 154)y | 143 (139, 147)y |
| Blood pressure | ||||||
| Systolic (mmHg) | 136 (133, 140) | 138 (134, 142) | 139 (135, 142) | 133 (131, 135) | 136 (134, 139) | 134 (131, 136) |
| Diastolic (mmHg) | 83.7 (81.8, 85.7) | 84.4 (82.1, 86.7) | 81.1 (79.2, 83.0) | 81.0 (79.8, 82.1)x | 81.4 (80.0, 82.8)x | 78.4 (77.3, 79.6)y |
NFG was defined as fasting plasma glucose (FPG) concentration < 100 mg/dl (5.6 mmol/l), IFG as FPG 100–125 mg/dl (5.6–6.9 mmol/l), and T2D as FPG ≥ 126 mg/dl (7.0 mmol/l) and/or diabetes medication use
HbA1c glycosylated hemoglobin, HOMA-IR homeostasis model assessment of insulin resistance, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol
Adjustments were as follows: lifestyle characteristics and obesity measures—age; glycemia measures, blood lipids, and blood pressure—age, waist circumference, and use of respective medications. Data are presented as mean (95% confidence interval) except where indicated. Separately by sex, differences were assessed using ANCOVA with Tukey’s adjustment for continuous variables, and logistic regression for categorical variables. Within sexes, values with different superscript letters in a row (x, y or z) are significantly different at P < 0.05
Data were log-transformed prior to statistical analysis and are presented as geometric means (95% confidence interval)
There was high prevalence of overweight and obesity, and both overall obesity and central obesity showed clear upward trends across FPG categories (Table 2). Glycemic control in those with T2D was poor, with more than 70% of men and women having HbA1c ≥7%. Dyslipidemia was common, and was primarily driven by low HDL-C; across sexes and FPG categories, 11–26% of individuals had both low HDL-C and high triacylglycerols.
Table 2.
Prevalence (%) of cardiovascular disease (CVD) risk factors among Puerto Rican men and women with normal fasting glucose (NFG), impaired fasting glucose (IFG), or type 2 diabetes (T2D)
| CVD risk factor | Men
|
Women
|
||||
|---|---|---|---|---|---|---|
| NFG (n = 141) | IFG (n = 90) | T2D (n = 148) | NFG (n = 337) | IFG (n = 216) | T2D (n = 355) | |
| Obesity | ||||||
| Body mass index (kg/m2) | ||||||
| Normal ( < 25) | 25.7 | 14.6 | 11.5**** | 15.7 | 10.7 | 7.0**** |
| Overweight (25–29) | 45.0 | 41.6 | 30.4 | 34.7 | 25.1 | 20.6 |
| Obese class I (30–34) | 25.7 | 34.8 | 32.4 | 28.5 | 28.8 | 30.1 |
| Obese class II (35–39) | 2.1 | 9.0 | 18.9 | 12.5 | 21.4 | 22.3 |
| Obese class III (≥40) | 1.4 | 0.0 | 6.8 | 8.6 | 14.0 | 20.0 |
| Waist circumference (cm) | ||||||
| Central obesity (men > 102, women > 88) | 30.5 | 43.3 | 59.5**** | 74.5 | 81.5 | 87.6**** |
| Glycemia | ||||||
| High HbA1c ≥ 7% | 5.0 | 14.4 | 71.6**** | 4.2 | 12.5 | 73.5**** |
| Blood lipids | ||||||
| Low HDL-C (men < 40, women < 50 mg/dl) | 50.4 | 53.3 | 61.5 | 58.5 | 66.2 | 73.0*** |
| Triacylglycerols (TG, mg/dl) | ||||||
| Borderline high (150–199) | 19.9 | 26.7 | 16.2* | 18.7 | 14.4 | 22.3*** |
| High (≥200) | 17.7 | 24.4 | 33.1 | 14.5 | 26.9 | 22.5 |
| Dyslipidemia: low HDL-C and high TG | 12.8 | 20.0 | 25.7* | 10.7 | 24.1 | 19.2*** |
| Blood pressure (BP, mmHg) | ||||||
| High systolic BP (≥140) | 35.3 | 39.8 | 49.0† | 29.6 | 37.8 | 42.4*** |
| High diastolic BP (≥90) | 23.0 | 22.7 | 21.4 | 19.5 | 20.7 | 13.8† |
NFG was defined as fasting plasma glucose (FPG) concentration < 100 mg/dl (5.6 mmol/l), IFG as FPG 100–125 mg/dl (5.6–6.9 mmol/l), and T2D as FPG ≥ 126 mg/dl (7.0 mmol/l) and/or diabetes medication use
HbA1c glycosylated hemoglobin, HDL-C high-density lipoprotein cholesterol
CVD risk cut-points were obtained from the following sources: obesity measures—National Institutes of Health [28]; HbA1c—American Diabetes Association [8]; HDL-C—International Diabetes Federation [29]; TG—National Cholesterol Education Program Adult Treatment Panel III [30]; systolic and diastolic BP—Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [31]
Differences in percentage of individuals in CVD risk categories across FPG categories were tested using Pearson’s Chi-square test
P < 0.1;
P < 0.05;
P < 0.001;
P < 0.0001
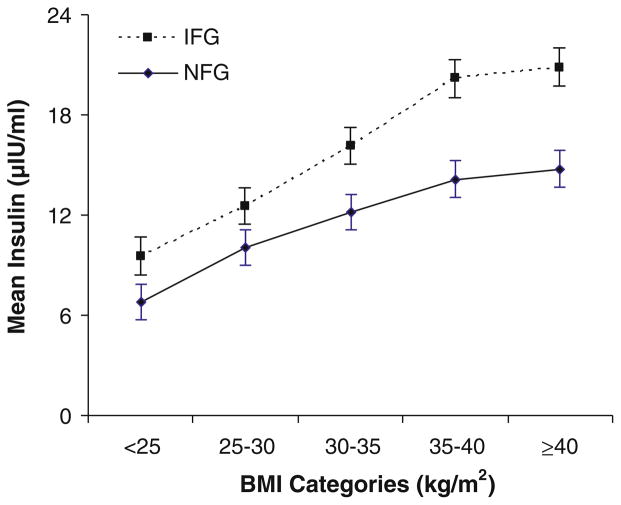
In those with NFG or IFG, we examined associations between BMI and fasting insulin, a surrogate measure of insulin resistance (Fig. 1). In both groups, we observed significant positive trends in adjusted mean insulin concentrations across BMI categories. Regardless of BMI category, individuals with IFG had higher insulin concentrations than those with NFG.
Fig. 1.
Association between BMI and fasting insulin in Puerto Rican adults with normal fasting glucose (NFG, n = 478) or impaired fasting glucose (IFG, n = 306), adjusted for age and sex. NFG was defined as a fasting plasma glucose (FPG) concentration <100 mg/dl (5.6 mmol/l), and IFG as FPG 100–125 mg/dl (5.6–6.9 mmol/l). Values are means with their standard errors represented by vertical bars. At each BMI category, those with IFG had significantly higher mean insulin concentrations (P <0.05) than those with NFG. Tests for linear trend of mean insulin across BMI categories were also conducted, separately by NFG or IFG; P < 0.0001 for each
Among individuals with T2D, achievement of ADA treatment goals was poor. Specifically, 21% of individuals did not meet any goal, 48% met one, 27% met two, and only 4% met all three goals (data not shown). The goal most met was that of LDL-C (59.4%), followed by blood pressure (27.2%) and HbA1c (27.0%). For each goal, an association was seen between medication use and goal achievement (Table 3). However, use of diabetes and anti-hypertensive medications were each strongly associated with not achieving respective goals, while the reverse was true for lipid-lowering medication. Other factors that correlated with not meeting goals included poverty (P = 0.06) for HbA1c, current alcohol use (P = 0.001) for blood pressure, and being female (P = 0.04) or current alcohol use (P = 0.08) for LDL-C.
Table 3.
Factors associated with achievement of American Diabetes Association treatment goals in Puerto Rican adults with type 2 diabetes (n = 503)
| Outcome variable | Explanatory variable | OR | 95% CI | P value |
|---|---|---|---|---|
| Glycosylated hemoglobin (HbA1c) <7% (n = 136, 27%) | Diabetes medication use | |||
| Oral only | 0.36 | (0.21, 0.59) | < 0.0001 | |
| Insulin and oral | 0.11 | (0.06, 0.23) | < 0.0001 | |
| Poverty | 0.66 | (0.43, 1.02) | 0.06 | |
| Blood pressure < 130/80 mm Hg (n = 132, 27%) | Anti-hypertensive medication use | 0.46 | (0.28, 0.75) | 0.002 |
| Alcohol use | ||||
| Past vs. never | 0.93 | (0.56, 1.6) | 0.11 | |
| Current vs. never | 0.42 | (0.23, 0.74) | 0.001 | |
| LDL-cholesterol < 100 mg/dl (2.6 mmol/l) (n = 291, 59%) | Lipid-lowering medication use | 3.3 | (2.2, 4.9) | < 0.0001 |
| Female | 0.61 | (0.38, 0.97) | 0.04 | |
| Alcohol use | ||||
| Past vs. never | 0.75 | (0.45, 1.2) | 0.90 | |
| Current vs. never | 0.59 | (0.36, 0.99) | 0.08 | |
Multivariate logistic regression analyses. Only significant or borderline significant results are shown. All models included age, sex, poverty, alcohol use, waist circumference, and respective medication use
Discussion
Characteristics of individuals with NFG, IFG, and T2D and their relationships with CVD risk factors have not previously been well characterized in Puerto Rican adults. Our findings show consistently greater CVD risk across FPG categories in Boston area Puerto Ricans aged 45–75 years. Progressive elevations were observed in both overall and central obesity, as well as glycemia measures. Across FPG categories, lipid and blood pressure control was poor, despite medication use. In those with T2D, achievement of ADA treatment goals was low.
Prevalence of T2D was high in the present study, with 39% of Puerto Ricans classified with the condition. This estimate is substantially higher than the national, age-adjusted estimate for Puerto Rican adults aged ≥20 years in 2004–2006 (12.6%), which was the second highest prevalence of all ethnic groups studied [4]. However, our prevalence was comparable to self-reported estimates for similarly aged (≥45 years) Puerto Ricans in Chicago (34–35%) [32], and New York City (33–39%) [33]. Previous research in the Boston area confirms these findings [17], and suggests that T2D prevalence in Puerto Ricans has increased over the last 10 years. In MAHES, 38% of those aged ≥60 years were diagnosed with diabetes, in contrast to 48% in the present study.
To our knowledge, this is the first study to report prevalence of IFG among Puerto Ricans. IFG prevalence in our sample (24%) was similar to Hispanics in the New York City Health and Nutrition Examination Survey, which included Puerto Ricans (25%) [27], but lower than for Mexican–Americans in NHANES 1999–2002 (33%) [1]. In comparison with other Hispanic groups, T2D was more prevalent than IFG in the present sample, demonstrating greater frequency of overt glucose dysregulation.
Overall obesity prevalence across FPG categories in men and women in the present study (29–58 and 50–72%, respectively) surpassed recent NHANES population estimates in similarly aged US adults (32–41% of men and women) [34]. However, prevalence was similar to that observed in non-Hispanic black (53–61%) and Mexican American (37–51%) adults [34], highlighting the severity of the obesity epidemic in ethnic minorities. The epidemic is a particular problem in Puerto Ricans, with evidence suggesting an increase in both overall and central obesity in the past decade [18]. Further, a cross-sectional study in New York City adults aged ≥18 years revealed that Puerto Ricans had the largest BMI, percent body fat, and waist-to-hip ratios relative to any other ethnic group studied, which included non-Hispanic whites, non-Hispanic blacks, and Asians [6]. The high prevalence of obesity in Puerto Ricans is likely due, in part, to lower physical activity, as has been reported for Hispanics, relative to other ethnic groups [35]. However, our sample’s prevalence of sedentary/light physical activity far surpassed age-adjusted estimates for “no leisure-time physical activity” among Hispanics in the US (34.6%) and in Massachusetts (40.2%), which possibly relates to both lower education and older age in our sample, correlates of reduced physical activity among Hispanics [36]. Consequently, obesity is of major concern in the Puerto Rican population, and an important mediator of the metabolic abnormalities observed.
Although individuals with T2D had the worst CVD risk profile, those with NFG or IFG were also at considerable risk. Beginning with the 2010 guidelines, the ADA now recommends the use of HbA1c to identify increased risk for diabetes (5.7–6.4%), and a cutoff point of HbA1c ≥6.5% for diabetes diagnosis [8]. Therefore, the adjusted mean HbA1c in Puerto Rican men and women with NFG (6.4–6.5%) or IFG (6.9%) in the present study may, in fact, be indicative of clinical disease. In the US, mean HbA1c has decreased significantly over 5 years in all ethnic groups except Hispanics [37]. This translates into a widening gap in glycemic control between non-Hispanic white and His-panic adults with T2D [13], which is troubling given direct associations between HbA1c and diabetes complication rates [8]. Consistent with studies on adults without T2D [38], we observed a direct relationship between BMI and fasting insulin in both NFG and IFG, and higher insulin in those with IFG, which suggests increasing insulin resistance with increasing obesity. In addition, many of those with NFG or IFG exhibited low HDL-C, high triacylglycerols, and high systolic blood pressure. Those with IFG showed similar dyslipidemia as those with T2D—elevated triacylglycerol concentrations, and, for 20–24% of individuals, both low HDL-C and high triacylglycerols.
Among individuals with T2D in the present study, medication use was comparable to adults with T2D in NHANES 1999–2000 [10]. Nonetheless, only 4% met all three ADA goals, which was considerably lower than observed in an ethnically and economically diverse cohort of patients at 30 US academic medical centers (10.0%) [11], or in Hispanics enrolled in the Look AHEAD randomized lifestyle intervention trial (7.9%) [12]. However, this estimate was similar to that observed in patients with both diabetes and hypertension at two major urban medical centers in Brooklyn and Detroit (3.2%) [9]. Glycemic control among those with T2D was less frequent in the present study (27%) than in many study samples (34–46%) [10–12], but was identical to that observed in the Brooklyn and Detroit patients (27%) [9]. The most common goal achieved was that of LDL-C, which is consistent with a review indicating that US ethnic minorities with T2D experience the smallest disparity in LDL-C control [39]. Nonetheless, the lower LDL-C in this population was accompanied by low HDL-C, which may be of greater importance to CVD risk [40]. Further, this differs from the general US population, where low HDL-C prevalence has decreased over time [41].
Factors associated with meeting ADA treatment goals were similar to other studies. Medication use was associated with a higher likelihood of meeting the LDL-C goal, but with a lower likelihood of meeting the HbA1c and blood pressure goals, as reported elsewhere [12]. One explanation could be a more targeted medical approach to lowering LDL-C [9–11]. Alternatively, Caribbean-origin Hispanics may tend to have lower total and LDL-C concentrations than other ethnic groups [19].
Another factor that likely contributed to poor metabolic control in this population relates to socio-economic disparities. Individuals with IFG or T2D were more likely to have ≤8th grade education, and poverty was associated with a 34% lower likelihood of meeting the HbA1c goal. Similar to our findings, the multi-ethnic Look AHEAD study found that lower education and fewer economic resources were related to not meeting the HbA1c goal [12]. Research in Hispanics has shown that low educational attainment [14, 42] and language or cultural barriers [43] could lead to an incomplete understanding of T2D and poor self-management. Compounding the negative effects of low socio-economic status, cumulative physiological stress [44], and high prevalence of depression, high self-reported stress, and low acculturation levels [45, 46] have been associated with poorer health in the Boston Puerto Rican Health Study. In the present study, prevalence of undiagnosed T2D was relatively low (18%), likely because, as US citizens, low-income Puerto Ricans in Massachusetts receive health insurance coverage through the state Medicaid program, MassHealth. Thus, we hypothesize that individuals may have an inadequate understanding of how to manage the disease, and this may be occurring in combination with suboptimal medical management related to the “ABCs” of diabetes. Additional research on this topic is warranted.
This study has some limitations. First, the cross-sectional nature of this study precludes determination of causality. Second, CVD risk factors were ascertained by a single fasting blood measure, which could contribute to misclassification. Third, given that we classified all individuals prescribed diabetes medication as having T2D, we may have underestimated the prevalence of IFG and overestimated the prevalence of T2D. However, there was good agreement between our classification of T2D and self-reported “ever” diagnosis.
Despite these limitations, this is the first large-scale study in Puerto Rican adults that characterizes CVD risk profiles across levels of impaired glucose regulation. The high prevalence of obesity, poor glycemic control, and dyslipidemia observed in this study emphasize the need for greater attention on the health status of this understudied ethnic group. In conclusion, CVD risk factors appear to be much more prevalent than previously seen in this population of Puerto Rican adults with prevalent diabetes [47]. It is therefore critical that they be followed more closely to prevent increases in CVD in the future. Comprehensive assessment of CVD risk profiles in individuals with NFG or IFG provides the opportunity to interrupt progression to T2D, and ADA goal achievement serves as an important gauge of CVD risk. Additional culturally sensitive, longitudinal research in Puerto Rican adults is needed.
Acknowledgments
This work was supported by National Institutes of Health P01AG023394, and P50HL105185, and by the US Department of Agriculture, Agricultural Research Service agreement number 58-1950-7-707.
Contributor Information
Maria I. Van Rompay, Nutrition and Genomics Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA
Carmen Castaneda-Sceppa, Department of Health Sciences, Northeastern University, 316 Robinson Hall, 360 Huntington Avenue, Boston, MA 02115, USA.
Nicola M. McKeown, Nutritional Epidemiology Program, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA. Gerald J. and Dorothy R. Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA
José M. Ordovás, Nutrition and Genomics Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA. Gerald J. and Dorothy R. Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA
Katherine L. Tucker, Email: kl.tucker@neu.edu, Department of Health Sciences, Northeastern University, 316 Robinson Hall, 360 Huntington Avenue, Boston, MA 02115, USA
References
- 1.Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. J Diabetes Complicat. 2007;21(6):363–70. doi: 10.1016/j.jdiacomp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Census Bureau. U.S. Hispanic population surpasses 45 million. [Accessed 17 Nov 2009];Now 15 percent of total (news release) 2008 Available at: http://www.census.gov/Press-Release/www/releases/archives/population/011910.html.
- 3.Davidson JA. Insulin resistance syndrome: implications for the Latino/Hispanic population. Endocr Pract. 2003;9(Suppl 2):26–7. doi: 10.4158/EP.9.S2.26. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Diabetes and Digestive and Kidney Diseases. [Accessed 27 Sept 2008];National diabetes statistics. 2007 Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf.
- 5.Barnes PM, Adams PF, Powell-Griner E. Health characteristics of the American Indian or Alaska native adult population: United States, 2004–2008. Natl Health Stat Rep. 2010;(20):1–22. [PubMed] [Google Scholar]
- 6.Wang J, Thornton JC, Burastero S, Shen J, Tanenbaum S, Heymsfield SB, et al. Comparisons for body mass index and body fat percent among Puerto Ricans, blacks, whites and Asians living in the New York City area. Obes Res. 1996;4(4):377–84. doi: 10.1002/j.1550-8528.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 7.Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The task force on diabetes and cardiovascular diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 8.ADA. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarlane SI, Jacober SJ, Winer N, Kaur J, Castro JP, Wui MA, et al. Control of cardiovascular risk factors in patients with diabetes and hypertension at urban academic medical centers. Diabetes Care. 2002;25(4):718–23. doi: 10.2337/diacare.25.4.718. [DOI] [PubMed] [Google Scholar]
- 10.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 11.Grant RW, Buse JB, Meigs JB. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28(2):337–442. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertoni AG, Clark JM, Feeney P, Yanovski SZ, Bantle J, Montgomery B, et al. Suboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the Look AHEAD Study. J Diabetes Complicat. 2008;22(1):1–9. doi: 10.1016/j.jdiacomp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: U.S. trends from 1999 to 2006 and effects of medicare coverage. Ann Intern Med. 2009;150(8):505–15. doi: 10.7326/0003-4819-150-8-200904210-00005. [DOI] [PubMed] [Google Scholar]
- 14.Brunt MJ, Milbauer MJ, Ebner SA, Levenson SM, Millen BE, Quatromoni P, et al. Health status and practices of urban Caribbean Latinos with diabetes mellitus. Ethn Dis. 1998;8(2):158–66. [PubMed] [Google Scholar]
- 15.Laugero KD, Falcon LM, Tucker KL. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite. 2010 doi: 10.1016/j.appet.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker KL. Stress and nutrition in relation to excess development of chronic disease in Puerto Rican adults living in the Northeastern USA. J Med Invest. 2005;52(Suppl):252–8. doi: 10.2152/jmi.52.252. [DOI] [PubMed] [Google Scholar]
- 17.Tucker KL, Bermudez OI, Castaneda C. Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. Am J Public Health. 2000;90(8):1288–93. doi: 10.2105/ajph.90.8.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bermudez OI, Tucker KL. Total and central obesity among elderly Hispanics and the association with type 2 diabetes. Obes Res. 2001;9(8):443–51. doi: 10.1038/oby.2001.58. [DOI] [PubMed] [Google Scholar]
- 19.Bermudez OI, Velez-Carrasco W, Schaefer EJ, Tucker KL. Dietary and plasma lipid, lipoprotein, and apolipoprotein profiles among elderly Hispanics and non-Hispanics and their association with diabetes. Am J Clin Nutr. 2002;76(6):1214–21. doi: 10.1093/ajcn/76.6.1214. [DOI] [PubMed] [Google Scholar]
- 20.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10(1):107. doi: 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paffenbarger RS, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328(8):538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health & Human Services. [Accessed 9 July 2009];The 2009 HHS poverty guidelines. Available at: http://aspe.hhs.gov/poverty/09poverty.shtml.
- 23.Najjar MF, Kuczmarski RJ. Anthropometric data and prevalence of overweight for Hispanics: 1982–84. Vital Health Stat. 1989;11(239):1–106. [PubMed] [Google Scholar]
- 24.Seeman TE, Charpentier PA, Berkman LF, Tinetti ME, Guralnik JM, Albert M, et al. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. J Gerontol. 1994;49(3):M97–108. doi: 10.1093/geronj/49.3.m97. [DOI] [PubMed] [Google Scholar]
- 25.Variability of blood pressure and the results of screening in the hypertension detection and follow-up program. J Chronic Dis. 1978;31(11):651–67. doi: 10.1016/0021-9681(78)90069-3. [DOI] [PubMed] [Google Scholar]
- 26.ADA. Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorpe LE, Upadhyay UD, Chamany S, Garg R, Mandel-Ricci J, Kellerman S, et al. Prevalence and control of diabetes and impaired fasting glucose in New York City. Diabetes Care. 2009;32(1):57–62. doi: 10.2337/dc08-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NHLBI Obesity Education Initiative. [Accessed 10 Nov 2008];Practical guide to the identification, evaluation and treatment of overweight and obesity in adults. 2000 Available at: http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf.
- 29.International Diabetes Federation. [Accessed 10 Nov 2008];The IDF consensus worldwide definition of the metabolic syndrome. 2006 Available at: http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf.
- 30.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 31.National High Blood Pressure Education Program. [Accessed 10 Nov 2008];Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. 2004 Available at: http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf.
- 32.Whitman S, Silva A, Shah AM. Disproportionate impact of diabetes in a Puerto Rican community of Chicago. J Community Health. 2006;31(6):521–31. doi: 10.1007/s10900-006-9023-7. [DOI] [PubMed] [Google Scholar]
- 33.Melnik TA, Hosler AS, Sekhobo JP, Duffy TP, Tierney EF, Engelgau MM, et al. Diabetes prevalence among Puerto Rican adults in New York City, NY, 2000. Am J Public Health. 2004;94(3):434–7. doi: 10.2105/ajph.94.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogden C, Carroll M, McDowell M, Flegal K. NCHS data brief. Hyattsville, MD: National Center for Health Statistics; 2007. Obesity among adults in the United States—no statistically significant change since 2003–2004. [PubMed] [Google Scholar]
- 35.CDC Division of Nutrition, Physical Activity and Obesity, and National Center for Chronic Disease Prevention and Health Promotion. [Accessed 11 Apr 2010];US physical activity statistics. 2010 Available at: http://apps.nccd.cdc.gov/PASurveillance/DemoComparev.asp.
- 36.Ham SA, Yore MM, Kruger J, Heath GW, Moeti R. Physical activity patterns among Latinos in the United States: putting the pieces together. Prev Chronic Dis. 2007;4(4):A92. [PMC free article] [PubMed] [Google Scholar]
- 37.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31(1):81–6. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 38.Palaniappan L, Carnethon M, Fortmann S. Heterogeneity in the relationship between ethnicity, BMI, and fasting insulin. Diabetes Care. 2002;25(8):1351–7. doi: 10.2337/diacare.25.8.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirk JK, Bell RA, Bertoni AG, Arcury TA, Quandt SA, Goff DC, Jr, et al. Ethnic disparities: control of glycemia, blood pressure, and LDL cholesterol among US adults with type 2 diabetes. Ann Pharmacother. 2005;39(9):1489–501. doi: 10.1345/aph.1E685. [DOI] [PubMed] [Google Scholar]
- 40.Greenfeder S. Emerging strategies and agents to lower cardiovascular risk by increasing high density lipoprotein cholesterol levels. Curr Med Chem. 2009;16(2):144–56. doi: 10.2174/092986709787002754. [DOI] [PubMed] [Google Scholar]
- 41.Kim JK, Alley D, Seeman T, Karlamangla A, Crimmins E. Recent changes in cardiovascular risk factors among women and men. J Womens Health (Larchmt) 2006;15(6):734–46. doi: 10.1089/jwh.2006.15.734. [DOI] [PubMed] [Google Scholar]
- 42.von Goeler DS, Rosal MC, Ockene JK, Scavron J, De Torrijos F. Self-management of type 2 diabetes: a survey of low-income urban Puerto Ricans. Diabetes Educ. 2003;29(4):663–72. doi: 10.1177/014572170302900412. [DOI] [PubMed] [Google Scholar]
- 43.Wagner J, Abbott G, Lacey K. Knowledge of heart disease risk among Spanish speakers with diabetes: the role of interpreters in the medical encounter. Ethn Dis. 2005;15(4):679–84. [PubMed] [Google Scholar]
- 44.Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker K. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Soc Sci Med. 2010;70(12):1988–96. doi: 10.1016/j.socscimed.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henkin S, Tucker KL, Gao X, Falcon LM, Qawi I, Brugge D. Association of depression, psycho-social stress and acculturation with respiratory disease among Puerto rican adults in Massachusetts. J Immigr Minor Health. 2009 doi: 10.1007/s10903-009-9307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falcon LM, Todorova I, Tucker K. Social support, life events, and psychological distress among the Puerto Rican population in the Boston area of the United States. Aging Ment Health. 2009;13(6):863–73. doi: 10.1080/13607860903046552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon T, Garcia-Palmieri MR, Kagan A, Kannel WB, Schiffman J. Differences in coronary heart disease in Framingham, Honolulu and Puerto Rico. J Chronic Dis. 1974;27(7–8):329–44. doi: 10.1016/0021-9681(74)90013-7. [DOI] [PubMed] [Google Scholar]