Abstract
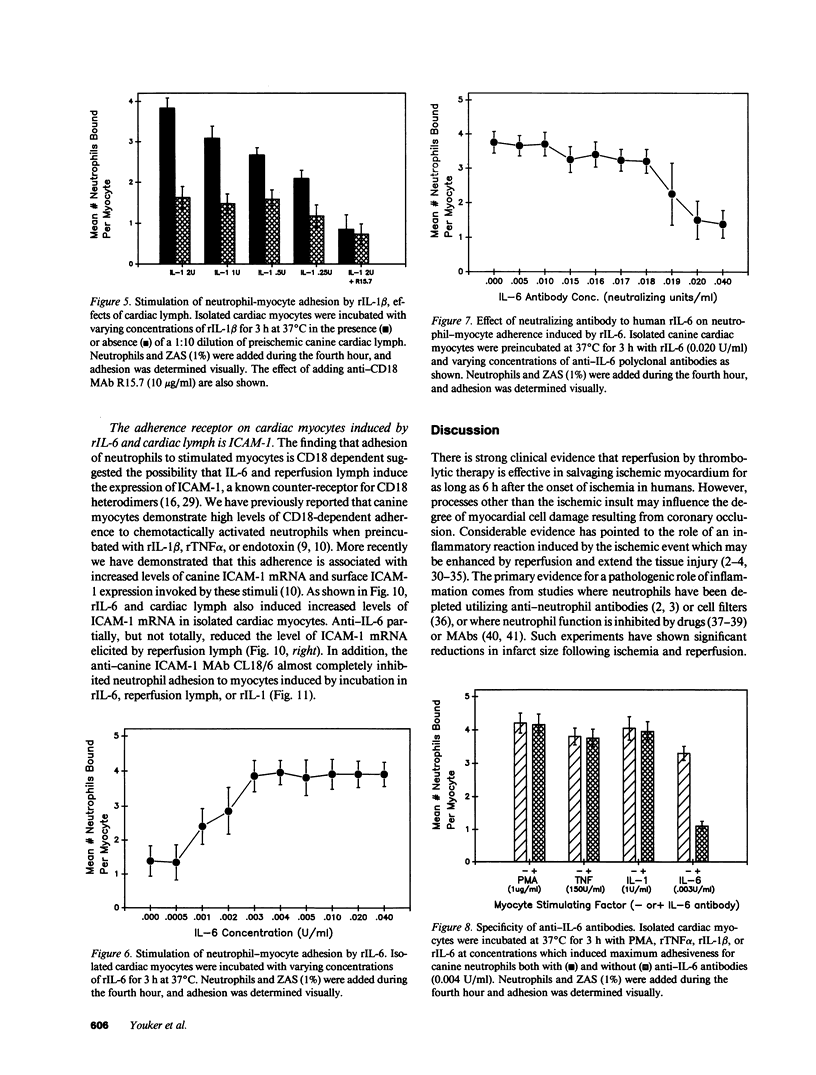
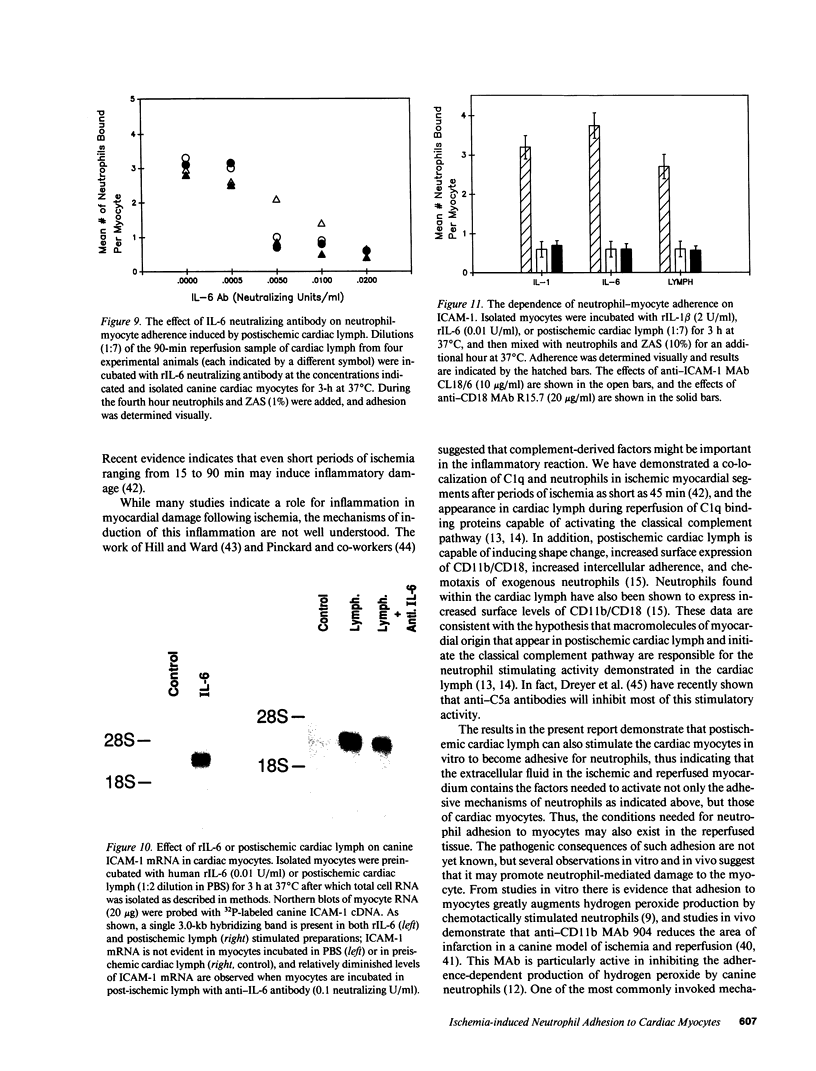
Canine neutrophils can be induced to adhere in vitro to isolated adult cardiac myocytes by stimulation of the neutrophils with chemotactic factors such as zymosan-activated serum (ZAS) only if the myocytes have been previously exposed to cytokines such as interleukin 1 (IL-1) or tumor necrosis factor-alpha. These cytokines induce synthesis and surface expression of intercellular adhesion molecule-1 (ICAM-1) on the myocyte, and neutrophil adhesion is almost entirely CD18 and ICAM-1 dependent. The present study examines cardiac-specific lymph collected from awake dogs during 1-h coronary occlusion and 3 d of reperfusion for its ability to induce both ICAM-1 expression in cardiac myocytes, and neutrophil-myocyte adherence. Reperfusion lymph induced ICAM-1 expression in isolated myocytes, and myocyte adherence to ZAS-stimulated neutrophils that was completely inhibited by anti-CD18 and anti-ICAM-1 monoclonal antibodies. This activity peaked at 90 min of reperfusion and persisted for up to 72 h. Preischemic lymph was not stimulatory. IL-1 appeared not to be a stimulating factor in lymph in that dilutions of lymph were found to inhibit the stimulatory effects of recombinant IL-1 beta. However, investigation of interleukin 6 (IL-6) revealed that recombinant IL-6 stimulated myocyte adhesiveness for ZAS-stimulated neutrophils (ED50 = 0.002 U/ml) and expression of ICAM-1 by isolated myocytes. IL-6 neutralizing antibody markedly reduced the ability of reperfusion lymph to stimulate adhesion and ICAM-1 expression, and estimates of levels of IL-6 in reperfusion lymph ranged from 0.035 to 0.14 U/ml. These results indicate that cytokines capable of promoting neutrophil-myocyte adhesion occur in extracellular fluid during reperfusion of ischemic myocardium, and that one of these cytokines is IL-6. Neutrophil-myocyte adhesion may be of pathogenic significance because it may enhance the cytotoxic activity of the neutrophil.
Full text
PDF



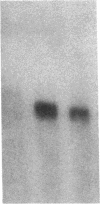
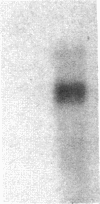
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter D. B., Deibel M. R., Jr, Dunn C. J., Tomich C. S., Laborde A. L., Slightom J. L., Berger A. E., Bienkowski M. J., Sun F. F., McEwan R. N. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990 Apr 12;344(6267):633–638. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer W. J., Smith C. W., Michael L. H., Rossen R. D., Hughes B. J., Entman M. L., Anderson D. C. Canine neutrophil activation by cardiac lymph obtained during reperfusion of ischemic myocardium. Circ Res. 1989 Dec;65(6):1751–1762. doi: 10.1161/01.res.65.6.1751. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Eisenberg S. P., Evans R. J., Arend W. P., Verderber E., Brewer M. T., Hannum C. H., Thompson R. C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990 Jan 25;343(6256):341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schönbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Peterson M. A., Dobbs A., Schmid-Schönbein G. W. Accumulation of polymorphonuclear leukocytes during 3-h experimental myocardial ischemia. Am J Physiol. 1986 Jul;251(1 Pt 2):H93–100. doi: 10.1152/ajpheart.1986.251.1.H93. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Entman M. L., Youker K., Shappell S. B., Siegel C., Rothlein R., Dreyer W. J., Schmalstieg F. C., Smith C. W. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J Clin Invest. 1990 May;85(5):1497–1506. doi: 10.1172/JCI114596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Hannum C. H., Wilcox C. J., Arend W. P., Joslin F. G., Dripps D. J., Heimdal P. L., Armes L. G., Sommer A., Eisenberg S. P., Thompson R. C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990 Jan 25;343(6256):336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hill J. H., Ward P. A. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971 Apr 1;133(4):885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. R., Kane W. J., Hook B. G., Abrams G. D., Kunkel S. L., Lucchesi B. R. Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. Am Heart J. 1986 Oct;112(4):682–690. doi: 10.1016/0002-8703(86)90461-8. [DOI] [PubMed] [Google Scholar]
- Kagiyama A., Savage H. E., Michael L. H., Hanson G., Entman M. L., Rossen R. D. Molecular basis of complement activation in ischemic myocardium: identification of specific molecules of mitochondrial origin that bind human C1q and fix complement. Circ Res. 1989 Mar;64(3):607–615. doi: 10.1161/01.res.64.3.607. [DOI] [PubMed] [Google Scholar]
- Lombardo A., Caimi L., Marchesini S., Goi G. C., Tettamanti G. Enzymes of lysosomal origin in human plasma and serum: assay conditions and parameters influencing the assay. Clin Chim Acta. 1980 Dec 22;108(3):337–346. doi: 10.1016/0009-8981(80)90339-3. [DOI] [PubMed] [Google Scholar]
- Lucchesi B. R., Mullane K. M. Leukocytes and ischemia-induced myocardial injury. Annu Rev Pharmacol Toxicol. 1986;26:201–224. doi: 10.1146/annurev.pa.26.040186.001221. [DOI] [PubMed] [Google Scholar]
- Mehta J. L., Nichols W. W., Mehta P. Neutrophils as potential participants in acute myocardial ischemia: relevance to reperfusion. J Am Coll Cardiol. 1988 Jun;11(6):1309–1316. doi: 10.1016/0735-1097(88)90297-5. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane K. M., Kraemer R., Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985 Nov;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Mullane K. M., Westlin W., Kraemer R. Activated neutrophils release mediators that may contribute to myocardial injury and dysfunction associated with ischemia and reperfusion. Ann N Y Acad Sci. 1988;524:103–121. doi: 10.1111/j.1749-6632.1988.tb38534.x. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinckard R. N., Olson M. S., Kelley R. E., DeHeer D. H., Palmer J. D., O'Rourke R. A., Goldfein S. Antibody-independent activation of human C1 after interaction with heart subcellular membranes. J Immunol. 1973 May;110(5):1376–1382. [PubMed] [Google Scholar]
- Rokita H., Mackiewicz M., Koj A. Acute phase response to recombinant interleukin-6 and macrophage- and fibroblast-derived crude cytokine preparations in primary cultures of mouse hepatocytes. Cell Biochem Funct. 1989 Oct;7(4):257–262. doi: 10.1002/cbf.290070404. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Rigot V. H., Schork M. A., Swanson D. P., Lucchesi B. R. The effect of ibuprofen on accumulation of indium-111-labeled platelets and leukocytes in experimental myocardial infarction. Circulation. 1982 Nov;66(5):1002–1011. doi: 10.1161/01.cir.66.5.1002. [DOI] [PubMed] [Google Scholar]
- Rossen R. D., Michael L. H., Kagiyama A., Savage H. E., Hanson G., Reisberg M. A., Moake J. N., Kim S. H., Self D., Weakley S. Mechanism of complement activation after coronary artery occlusion: evidence that myocardial ischemia in dogs causes release of constituents of myocardial subcellular origin that complex with human C1q in vivo. Circ Res. 1988 Mar;62(3):572–584. doi: 10.1161/01.res.62.3.572. [DOI] [PubMed] [Google Scholar]
- Rossen R. D., Swain J. L., Michael L. H., Weakley S., Giannini E., Entman M. L. Selective accumulation of the first component of complement and leukocytes in ischemic canine heart muscle. A possible initiator of an extra myocardial mechanism of ischemic injury. Circ Res. 1985 Jul;57(1):119–130. doi: 10.1161/01.res.57.1.119. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Engler R. L. Granulocytes as active participants in acute myocardial ischemia and infarction. Am J Cardiovasc Pathol. 1987 Jan;1(1):15–30. [PubMed] [Google Scholar]
- Sehgal P. B., Helfgott D. C., Santhanam U., Tatter S. B., Clarick R. H., Ghrayeb J., May L. T. Regulation of the acute phase and immune responses in viral disease. Enhanced expression of the beta 2-interferon/hepatocyte-stimulating factor/interleukin 6 gene in virus-infected human fibroblasts. J Exp Med. 1988 Jun 1;167(6):1951–1956. doi: 10.1084/jem.167.6.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shappell S. B., Toman C., Anderson D. C., Taylor A. A., Entman M. L., Smith C. W. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990 Apr 1;144(7):2702–2711. [PubMed] [Google Scholar]
- Simpson P. J., Mickelson J., Fantone J. C., Gallagher K. P., Lucchesi B. R. Iloprost inhibits neutrophil function in vitro and in vivo and limits experimental infarct size in canine heart. Circ Res. 1987 May;60(5):666–673. doi: 10.1161/01.res.60.5.666. [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Fantone J. C., Mickelson J. K., Griffin J. D., Lucchesi B. R. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988 Feb;81(2):624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Mickelson J. K., Fantone J. C., Gallagher K. P., Lee K. A., Tamura Y., Cronin M., Lucchesi B. R. Sustained limitation of myocardial reperfusion injury by a monoclonal antibody that alters leukocyte function. Circulation. 1990 Jan;81(1):226–237. doi: 10.1161/01.cir.81.1.226. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Entman M. L., Lane C. L., Beaudet A. L., Ty T. I., Youker K., Hawkins H. K., Anderson D. C. Adherence of neutrophils to canine cardiac myocytes in vitro is dependent on intercellular adhesion molecule-1. J Clin Invest. 1991 Oct;88(4):1216–1223. doi: 10.1172/JCI115424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. F., 3rd, Egan J. W., Bugelski P. J., Hillegass L. M., Hill D. E., Griswold D. E. Temporal relation between neutrophil accumulation and myocardial reperfusion injury. Am J Physiol. 1988 Nov;255(5 Pt 2):H1060–H1068. doi: 10.1152/ajpheart.1988.255.5.H1060. [DOI] [PubMed] [Google Scholar]
- Worthen G. S., Schwab B., 3rd, Elson E. L., Downey G. P. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989 Jul 14;245(4914):183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Lorgeril M., Basmadjian A., Lavallée M., Clément R., Millette D., Rousseau G., Latour J. G. Influence of leukopenia on collateral flow, reperfusion flow, reflow ventricular fibrillation, and infarct size in dogs. Am Heart J. 1989 Mar;117(3):523–532. doi: 10.1016/0002-8703(89)90724-2. [DOI] [PubMed] [Google Scholar]