Abstract
Although it is well established that human adipose tissue (AT) shows circadian rhythmicity, published studies have been discussed as if tissues or systems showed only one or few circadian rhythms at a time. To provide an overall view of the internal temporal order of circadian rhythms in human AT including genes implicated in metabolic processes such as energy intake and expenditure, insulin resistance, adipocyte differentiation, dyslipidemia, and body fat distribution. Visceral and subcutaneous abdominal AT biopsies (n = 6) were obtained from morbid obese women (BMI ≥ 40 kg/m2). To investigate rhythmic expression pattern, AT explants were cultured during 24-h and gene expression was analyzed at the following times: 08:00, 14:00, 20:00, 02:00 h using quantitative real-time PCR. Clock genes, glucocorticoid metabolism-related genes, leptin, adiponectin and their receptors were studied. Significant differences were found both in achrophases and relative-amplitude among genes (P <0.05). Amplitude of most genes rhythms was high (>30%). When interpreting the phase map of gene expression in both depots, data indicated that circadian rhythmicity of the genes studied followed a predictable physiological pattern, particularly for subcutaneous AT. Interesting are the relationships between adiponectin, leptin, and glucocorticoid metabolism-related genes circadian profiles. Their metabolic significance is discussed. Visceral AT behaved in a different way than subcutaneous for most of the genes studied. For every gene, protein mRNA levels fluctuated during the day in synchrony with its receptors. We have provided an overall view of the internal temporal order of circadian rhythms in human adipose tissue.
The survival of any organism exposed to a strictly periodic environment, relies on the appropriate timing of its responses (Grodins, 1963). Therefore, the optimal synchronization of behavioral and physiological events is a crucial mechanism performed in humans and other species by the circadian system.
Within an individual subject, there is a highly specific temporal order of the circadian system (Moore-Ede and Sulzman, 1981) manifested by various rhythms playing an intricate counterpoint, reaching their peaks and troughs at different phases of the day. These rhythms provide a glimpse of the elaborate timekeeping that underlies physiological processes.
The adipose tissue is a metabolically active organ presenting a highly rhythmic behavior (Garaulet and Madrid, 2009). Each cytokine has to be secreted at the right time and order in order to achieve a concerted function. The relevance of this orchestration is supported by the finding of a peripheral clock in murine adipose tissue. Moreover, in humans, we demonstrated the expression of clock genes in adipose tissue, and that their expression was correlated with the metabolic syndrome (MetS) (Zvonic et al., 2006). It is estimated that about 20% of the genes expressed in adipose tissue, show circadian rhythmicity in murine species (Gómez-Santos et al., 2009; Loboda et al., 2009). This is the case for PPARγ (Teboul et al., 2009), and adiponectin (Garaulet et al., 2007; Gómez-Abellán et al., 2010). Glucocorticoide metabolites, highly implicated in food intake and central accumulation of fat, also show circadian rhythm (Hernandez-Morante et al., 2009). To date, most published studies have been discussed as if organisms showed only one or few circadian rhythms at a time, however, circadian rhythmcity is exhibited by many variables simultaneously, raising the issue of how do the multiple rhythms relate to each other to generate a precise internal temporal order which is relevant to metabolic homeostasis.
Circadian phase maps have been compiled for many species, demonstrating the extent and potential physiological relevance of an internal temporal order in the circadian rhythms (Szabó et al., 1978; Benavides et al., 1998). However, to our knowledge such a phase map has not been developed in human adipose tissue. Thus, the purpose of the present work was to compile a phase map with achrophases and relative amplitudes of various genes involved in adipose tissue metabolism, with the aim of providing an overall view of the internal temporal order of circadian rhythms in human adipose tissue.
Subjects and Methods
Subjects
Visceral and subcutaneous abdominal adipose tissue (AT) biopsies were obtained from morbid obese women (n = 6), aged 51 ± 9 years and BMI: 44.1 ± 5.5 kg/m2, undergoing laparoscopic gastric bypass surgery due to obesity at the General Surgery Service of “Virgen de la Arrixaca” Hospital (Murcia, Spain). According to the International Diabetes Federation (IDF), subjects met the criteria to be defined as metabolic syndrome patients. Average values of waist, triglycerides, HDL cholesterol, glucose, and systolic pressure exceeded the cut off points proposed by the IDF (Alberti et al., 2006).
The day before surgery, all patients were synchronized having lunch at 14:30 and dinner at 21:00 h. The AT biopsies were taken as paired samples from the two AT depots (visceral and subcutaneous) at the beginning of the surgical procedure (estimated time of biopsies sampling at 13:00 h).
The protocols were approved by the Ethics Committee of the “Virgen de la Arrixaca” University Hospital, and the subjects signed a written informed consent before the biopsies were obtained.
Clinical characteristics
Arterial pressure, BMI, waist and hip circumference were assessed by standard procedures, while skinfolds (biceps, triceps, suprailiac, and subscapular) were measured with a Harpenden caliper (Holtain Ltd., Bryberian, Crymmych, Pembrokeshire, UK). Total body fat (%) was evaluated by bioimpedance with a TANITA TBF-300 (TANITA Corporation of America, Arlington Heights, IL). Sagittal diameter and coronal diameter were measured at the level of the iliac crest (L4-5) using a Holtain Kahn Abdominal Caliper. Women were classified in visceral and subcutaneous obesity calculating the index VA/SA after applying the following equation (Garaulet et al., 2006): VA/SA predicted = 0.868 + 0.064 × Sagittal diameter—0.036 × coronal diameter—0.022 × triceps skinfold. Those patients with VA/SA >0.42 were classified as having visceral obesity. Fasting plasma concentrations of glucose, triacylglycerols, total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol were determined with commercial kits (Roche Diagnostics GmbH, Mannheim, Germany). Basal metabolic rate (BMR) was calculated from the Harris and Benedict equation (Harris and Benedict, 1918).
Adipose tissue culture
Immediately after the surgery, an aliquot of the AT biopsies were frozen at −80 °C and used for analyzing the basal gene expression, and the remaining aliquot was used for culture. Thus, 800–1,000 mg AT explants (1–2 mm3 pieces to maximize contact with the medium) were transferred to cell culture bottles with membrane filter screw cap to safeguard the viability of the culture, and placed in 5 ml of Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum, and kept at 37 °C for 24 h in a humidified atmosphere containing 7% CO2.
We take the sample from surgery during the morning (around 11 h AM) and we culture it in the adequate conditions till the next day in order to allow the explants to adapt to the new environmental conditions. It is next day on which we start to study the different cycles in gene expression. To determine this circadian cycle, adipose explants were collected to perform gene expression analysis at the following times (time at 0, 6, 12, and 18 h), T0 being arbitrarily defined as 08:00 h, because this was the usual waking time for patients, T6 as 14:00 h, T12 as 20:00 h, and T18 as 02:00 h. All cultures were performed in duplicates.
Analysis of gene expression
Total RNA was extracted from AT explants using RNeasy Kit (QIAGEN, Courtabeouf, France). Reverse transcription was performed using random hexamers as primers and Thermoscript® reverse transcriptase (Invitrogen, Cergy Pontoise, France) with 1 μg total RNA for each sample. Quantitative real-time PCR was performed using an ABI PRISM 7000 HT Sequence Detection System (Applied Biosystems, Foster City, CA), using TaqMan® Universal PCR Master Mix and specific TaqMan probes (Applied Biosystems). We used 18S rRNA as internal control because of its lower variance across time compared with the GAPDH gene. In addition, we carried out a one-way (Zeitgeber time, ZT) analysis of variance (ANOVA) for 18S and observed no significant difference in any of the adipose depots studied (P >0.05). In addition, there was no significant difference in 18S Ct between subcutaneous (mean Ct = 23.3 ± 3.3) and visceral depots (mean Ct = 23.2 ± 3.3). mRNA expression levels were normalized to 18S using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Rhythm calculation and statistical analysis
The single cosinor method was used to analyze for circadian rhythm individually (Balsalobre et al., 2000). This inferential method involves fitting a curve of a predefined period by the least squares method. The rhythm characteristics and their 95% confidence intervals estimated by this method include the mesor (middle value of the fitted cosine representing a rhythm-adjusted mean), the amplitude (half the difference between the minimum and maximum of the fitted cosine function), and the temporal location of maximum value or acrophase (the time at which the peak of a rhythm occurs, expressed in hours) and the percent rhythm (PR) percentage of variability accounted for by cosine curve). Relative amplitude was expressed as a percentage of mesor values (relative amplitude = (amplitude/mesor) × 100). The significance of the rhythms was determined by rejection of the zero amplitude hypotheses with a threshold of 60%.
Differences in acrophase between subcutaneous and visceral depots were analyzed by Mann–Whitney non-parametric test for the genes studied. All statistical analyses were carried out using SPSS for windows (release 15.0; SPSS Inc., Chicago, IL). The level of significance for all statistical tests and hypotheses was set at P <0.05.
Results
Table 1 contains basal characteristics of the women studied. Body mass index (BMI) was higher than 40 kg/m2 in these patients and VA/SA higher than 0.4, indicating that women suffered from visceral and morbid obesity. All subjects were defined as metabolic syndrome patients, according to the International Diabetes Federation (IDF) criteria (Alberti et al., 2006).
TABLE 1.
General characteristics of the population studied
| Patients (n = 6) | |
|---|---|
| Age (years) | 51 ± 9 |
| Weight (kg) | 107.9 ± 11.2 |
| Height (cm) | 156 ± 5.0 |
| Body mass index (kg/m2) | 44.1 ± 5.5 |
| Body fat (%) | 43 ± 6 |
| Waist circumference (cm) | 126 ± 8 |
| Hip circumference (cm) | 139 ± 9 |
| Waist to hip ratio | 0.91 ± 0.03 |
| Sagittal diameter (cm) | 22 ± 4 |
| Coronal diameter (cm) | 31 ± 4 |
| Bicipital skinfold (mm) | 28 ± 3 |
| Tricipital skinfold (mm) | 28 ± 4 |
| Subscapular skinfold (mm) | 33 ± 4 |
| Suprailliac skinfold (mm) | 31 ± 3 |
| Basal metabolic rate (kcal) | 1,721.5 ± 119.6 |
| Visceral area/subcutaneous areapredicted | 0.54 ± 0.28 |
| Glucose (mmol/L) | 6.13 ± 0.82 |
| Cholesterol (mmol/L) | 4.53 ± 1.02 |
| Triglycerides (mmol/L) | 1.02 ± 0.42 |
| High-density lipoprotein cholesterol (mmol/L) | 1.34 ± 0.22 |
| Low-density lipoprotein cholesterol (mmol/L) | 3.14 ± 0.68 |
| Systolic pressure (mmHg) | 148.33 ± 23.17 |
| Diastolic pressure (mmHg) | 73.33 ± 13.66 |
Data are presented as means ± standard deviation.
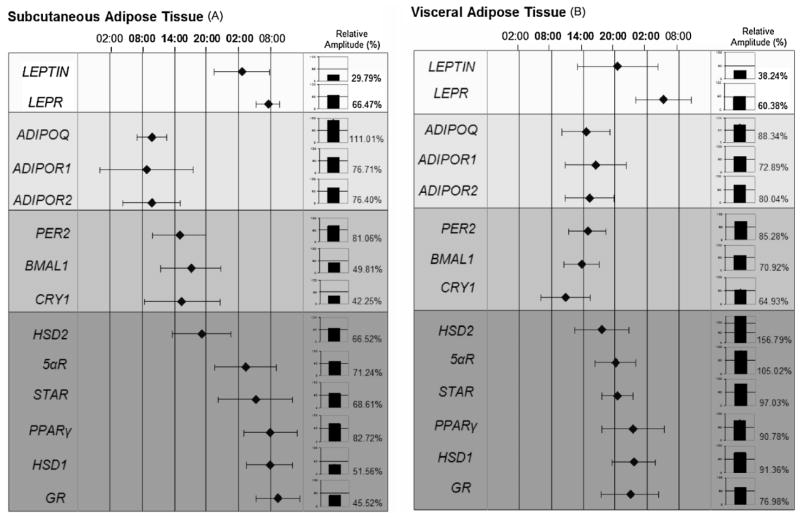
Figure 1 shows the phase map of gene expression in subcutaneous (Fig. 1A) and in visceral AT (Fig. 1B) for the following genes: adipokines, i.e., LEPTIN and adiponectin (ADIPOQ) and their receptors (LEPR, ADIPOR1, and ADIPOR2); clock genes such as PER2, BMAL1, CRY1; PPARγ and glucocorticoid metabolism-related genes such as GR, HSD1, HSD2, 5αR, and STAR. Average acrophases (the time at which the peak of a rhythm occurs, expressed in hours) imputed from cosinor analysis of every gene were plotted against 24h-time scale. Relative amplitude (%) was represented in bars. Significant differences were found both in achrophases and relative amplitude among genes (P <0.05).
Fig. 1.
A phase map of circadian rhythms of several genes (leptin and its receptor (LEPR), adiponectin and its receptors (ADIPOR1 and ADIPOR2), clock genes (PER2, BMAL1, and CRY1)and glucocorticoid metabolism-related genes(PPARγ, GR, HSD1, HSD2, STAR, and5αR))implicated in human adipose tissue metabolism subcutaneous adipose tissue (A), visceral adipose tissue (B). This figure shows the acrophase (time of occurrence of the best-fit maximum value) of numerous rhythms. The mean values of achrophases are plotted ± SEM.
In subcutaneous AT, leptin showed its achrophase (maximum expression) during the night (at 02:00 h) while for adiponectin the maximum expression was during the morning time (at 10:00 h).
PPARγ, corticosteroid receptors (GR), and the isoenzime 11β-hydroxysteroid dehydrogenase type 1 (HSD1) showed their achrophase in the morning (around 08:00 h). However, isoenzime HSD2 and STAR, and 5-alfa reductase type 1 (5αR) presented the achrophase earlier.
As expected, visceral AT behaved in a different way than subcutaneous for most of the genes studied (Fig. 1B). Considering adipokines, leptin anticipated in its expression towards afternoon and evening hours, while adiponectin and its receptors were significantly delayed in visceral AT as compared with subcutaneous. Interestingly, PPARγ, and GR both presented their achrophases at night (23:00 h) in visceral AT, while in subcutaneous reached a peak near the time of waking.
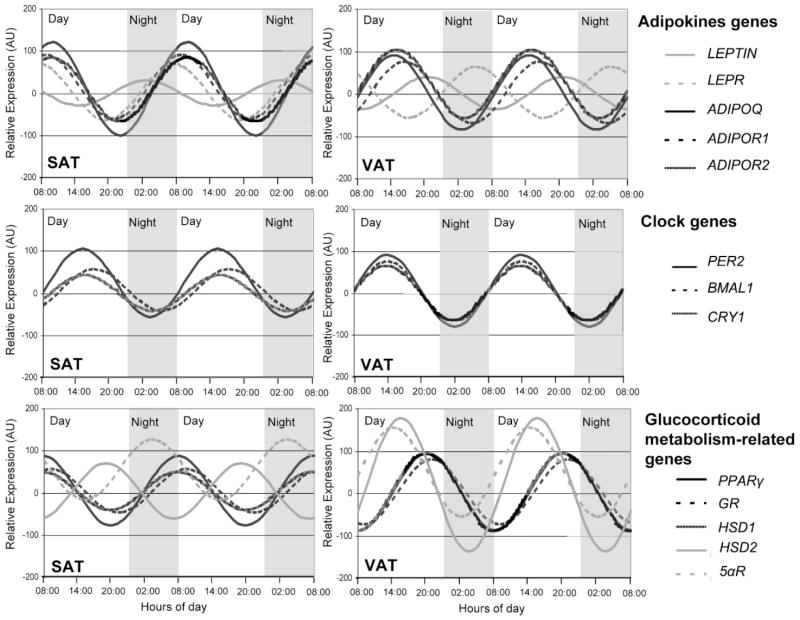
Figure 2 shows mean circadian rhythms for the examined group in subcutaneous and visceral adipose tissues. When comparing both AT depots, different circadian patterns were observed. Thus, while the subcutaneous tissue displayed a strong oscillatory trend, for adiponectin, PER2 and PPARγ, in visceral fat adiponectin was dampened, data which was confirmed with the relative amplitude values represented in Figure 1. By contrary, circadian rhythmicity of cortisol metabolism-related genes, was more robust in visceral than in subcutaneous depot. Most of these cortisol-related genes ranged in anti-phase in visceral and in subcutaneous AT (P = 0.002). Interestingly, for every gene studied protein mRNA levels fluctuated during the day in synchrony with its receptors.
Fig. 2.
Rhythmic expression of genes studied (leptin and its receptor (LEPR), adiponectin and its receptors (ADIPOR1 and ADIPOR2), clock genes (PER2, BMAL1, and CRY1) and glucocorticoid metabolism-related genes (PPARγ, GR, HSD1, HSD2, and 5αR)) in human subcutaneous (A) and visceral adipose tissue (B). Adipose depots were isolated at 6-h intervals over the course of the day from adipose tissue cultures (time at 0, 6, 12, and 18 h). Results are presented relative to the lowest basal relative expression for each gene. Data of relative expression are represented as arbitrary units (AU). Data are reported as means ± SEM (SEM of ΔCt are represented in parenthesis).
Discussion
Most mammalian display a circadian oscillation in their baseline expression; consequently, the phase and amplitude of each component of a signal transduction cascade has downstream consequences. In the current study, we represent the phase map for human adipose tissue of different proteins which regulate a number of processes that contribute to the development of obesity and MetS. These processes include energy intake and expenditure, insulin resistance, adipocyte differentiation, dyslipidemia, and body fat distribution.
When interpreting the phase map, data indicated that circadian rhythmicity of the genes studied followed a predictable physiological pattern, particularly for subcutaneous AT. Feeding is subject to circadian regulation (Garaulet and Madrid, 2010). Indeed, food intake is a major physiological function in animals and must be entrained to the circadian oscillations in food availability (Dietrich and Horvath, 2009). As expected, leptin, a peripherally synthesized hormone which acts as an anorexigenic hormone in the brain, showed its achrophase (maximum expression) during the night (at 02:00 h). Leptin and other humoral signals produced in the peripherical tissues are capable of communicating the nutritional state of the organism to the hypothalamic centers that control hunger and satiety, in a circadian-dependent manner (Kalra et al., 2003). It has been that plasma leptin levels were high during the night, when appetite decreases, and low during the day, when hunger increases (Langendonk et al., 1998). The nocturnal increase in leptin levels indicates its role as a satiety hormone, favoring fasting and nocturnal rest.
Adiponectin, the adipose tissue most abundant secreted protein, is highly implicated in glucose metabolism (Garaulet et al., 2007). It has been called the fat-burning molecule because it is able to redirect fatty acids to the muscle for their oxidation. In the present study, the expression of adiponectin (ADIPOQ) achieved its zenith (maximum) during the morning (at 10:00 h) which could be implicated in the maximal withdrawal of fatty acids, and the improvement in glucose tolerance and that time (Gómez-Abellán et al., 2010).
Of note are the relationships between ADIPOQ, LEPTIN, and glucocorticoid-related genes circadian profiles. Our current data in adipose tissue are consistent with previous findings in serum adiponectin and leptin variations showing out-of-phase 24-h profiles (Gavrila et al., 2003). With respect to cortisol receptor (GR), ADIPOQ followed similar 24-h rhythmicity. However, although ADIPOQ and GR reached peak levels around the same time, ADIPOQ reached its achrophase 2 h after GR. These results are consistent with previous data obtained in plasma from healthy men, and highlight the tightly interactions between AT proteins (Gavrila et al., 2003).
PPARγ could be also related to ADIPOQ circadian pattern. In fact, the high expression of PPARγ during the morning (08:00 h), located at the beginning of the of the daily activity, is consistent with results obtained in nocturnal mammals (Yang et al., 2006) and could be influencing the further increase in ADIPOQ expression and the increase in insulin sensitivity during this time of the day. It has been demonstrated that PPARγ ligands stimulate adipocyte differentiation, which is associated with mitigation of insulin resistance, presumably because of decreases in free fatty acids and upregulation of adiponectin. They also improve glucose uptake in insulin-resistant tissues via an increase in the glucose transporter GLUT-4 (Verreth et al., 2004).
Among the women studied, glucocorticoides related genes such as GR, and the isoenzime HSD1, showed their acrophase in the morning (around 08:00 h). It has been described that in all species the maximum of corticosteroid rhythms occurs just before or at the onset of activity (Peterson, 1957). In plasma, similarly to what happened in the current study performed in adipose tissue, glucocorticoides start to climb from baseline levels about 4–5 h prior to the time of waking, reaching a peak near the time of waking (García-Prieto et al., 2007). Over the course of the day they fall, reaching low or undetectable levels an hour or two before bedtime.
It is well-known that the corticosteroid rhythm is normally tightly synchronized to the day–night and sleep–wake cycles. The antiphase relationship between leptin and glucocorticoids shown in the current study is reasonable considering that both hormones are strongly interrelated (Henry and Clarke, 2008) and they exert opposite functions in food intake regulation. While leptin displays an anorexigenic role, glucocorticoids increase appetite (orexigenic function). In previous works performed in plasma, leptin ultradian pulses were also inversely correlated with those of ACTH and cortisol (Gavrila et al., 2003).
Regarding the existence of an internal temporal order, an interesting issue concerns causation. Currently, circadian physiologists try to elucidate which genes can be drived by the circadian molecular clock and therefore, can be considered as clock controlled genes (CCGs). This is a very difficult question to answer in the present study. However, if we observe the clock genes circadian rhythms in the phase map, the advance of phases that suffered BMAL1 and CRY1 in visceral with respect to subcutaneous AT, was accompanied with a phase advance in most of the genes studied, this situation is particularly relevant for PPARγ and glucocorticoides-related-genes. Moreover, the phase-relationship of BMAL1, and PPARγ is maintained in both AT depots. Previously, it has been described that PPARγ is a CCG, which is activated by the positive limb, the hetero-dimmer CLOCK-BMAL1. Moreover, in a study performed in mPer2−/− mice the glucocorticoid rhythms disappeared suggesting that corticosteroids could be considered as CCGs (Yang et al., 2009).
Visceral AT behaved in a different way than subcutaneous for most of the genes studied. Differences were more evident for leptin and glucocorticoids-related genes, both highly related to food intake. The unexpected lower values of leptin and higher values of glucocorticoid-related genes during night hours in visceral AT could be accounting for food intake behavioral alterations already described in subjects with a predominance of visceral fat (García-Prieto et al., 2007). Night eaters are typically abdominal obese, show anorexia in the morning, hyperphagia in the evening and insomnia at night with frequent awakenings accompanied by food intake (Qin et al., 2003).
In subcutaneous fat, from the total genes analyzed adiponectin showed the highest circadian rhythmicity, followed by PPARγ and PER2. For instance, in visceral fat, glucocorticoids-related genes were the genes with the highest amplitude. These data reinforce the particular relevance of adiponectin in subcutaneous and glucocorticoids in visceral fat, already described in previous researches (Björntorp, 1991; Mårin et al., 1992; Garaulet et al., 2007). Of note, dispersion in achrophases among subjects were higher in subcutaneous than in visceral AT which suggests that the synchronizer mechanisms are more potent in visceral AT, driving to a higher homogenicity. The different circadian behavior of both AT locations could be related to the fact that the suprachiasmatic nucleus uses the autonomic nervous system (ANS) to implement internal rhythmicity. The high specialization of ANS neurons allows them to project either to intra-abdominal or subcutaneous body compartment, and this depot-specific effect could also vary depending on the time of the day (Perez-Tilve et al., 2006).
In general the relative amplitude of the genes studied was high in this study, as compared previous work carried out in different organs or tissues (Hermida et al., 2003a, b).
From all the genes studied leptin was the one with the lowest amplitude, the obesity degree and the insulin resistance status of the women studied could be accounting for these results. Previous studies have demonstrated that MetS is characterized, not only by high absolute leptin levels but also by blunted relative diurnal excursions and dampened pulsatility (Anubhuti and Arora, 2008). Interestingly, for every gene studied protein mRNA levels fluctuated during the day in synchrony with its receptors, which demonstrates the efficacy of this circadian system in human AT.
In conclusion, “Time is of the Essence” in adipose tissue. Energy metabolism and circadian systems have evolved together over millions of years to optimize internal coordination among multiple physiological and molecular processes. An adequate temporal order in the daily pattern of the different cytokines and proteins implicated in adipose tissue metabolism could have important consequences not only in body fat distribution but also in the metabolic alterations associated to obesity.
Acknowledgments
This work was supported by the Government of Education, Science and Research of Murcia (Project BIO/FFA 07/01-0004) and by The Spanish Government of Science and Innovation (Project AGL2008-01655/ALI). National Heart, Lung, and Blood Institute grants HL-54776, National Institute of Diabetes and Digestive and Kidney Diseases, Grant Number DK075030 and by contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research and by Línea Especial of University of Navarra (LE/97).
Literature Cited
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome a new wold-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Anubhuti AS, Arora S. Leptin and its metabolic interactions: An update. Diabetes Obes Metab. 2008;10:973–993. doi: 10.1111/j.1463-1326.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Benavides A, Siches M, Llobera M. Circadian rhythms of lipoprotein lipase and hepatic lipase activities in intermediate metabolism of adult rat. Am J Physiol. 1998;275:R811–817. doi: 10.1152/ajpregu.1998.275.3.R811. [DOI] [PubMed] [Google Scholar]
- Björntorp P. Adipose tissue distribution and function. Int J Obes. 1991;15:67–81. [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur J Neurosci. 2009;30:1688–1696. doi: 10.1111/j.1460-9568.2009.06963.x. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol. 2009;20:127–134. doi: 10.1097/MOL.0b013e3283292399. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Madrid JA. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv Drug Deliv Rev. 2010;62:967–978. doi: 10.1016/j.addr.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Hernández-Morante JJ, Tébar FJ, Zamora S, Canteras M. Two-dimensional predictive equation to classify visceral obesity in clinical practice. Obesity (Silver Spring) 2006;14:1181–1191. doi: 10.1038/oby.2006.135. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Hernández-Morante JJ, de Heredia FP, Tébar FJ. Adiponectin, the controversial hormone. Public Health Nutr. 2007;10:1145–1150. doi: 10.1017/S1368980007000638. [DOI] [PubMed] [Google Scholar]
- García-Prieto MD, Tébar FJ, Nicolás F, Larqué E, Zamora S, Garaulet M. Cortisol secretary pattern and glucocorticoid feedback sensitivity in women from a Mediterranean area: Relationship with anthropometric characteristics, dietary intake and plasma fatty acid profile. Clin Endocrinol (Oxf) 2007;66:185–191. doi: 10.1111/j.1365-2265.2006.02705.x. [DOI] [PubMed] [Google Scholar]
- Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: Comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- Gómez-Abellán P, Gómez-Santos C, Madrid JA, Milagro FI, Campion J, Martínez JA, Ordovás JM, Garaulet M. Circadian expression of adiponectin and its receptors in human adipose tissue. Endocrinology. 2010;151:115–122. doi: 10.1210/en.2009-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Santos C, Gómez-Abellán P, Madrid JA, Hernández-Morante JJ, Lujan JA, Ordovás JM, Garaulet M. Circadian rhythm of clock genes in human adipose explants. Obesity (Silver Spring) 2009;17:1481–1485. doi: 10.1038/oby.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodins FS. Control theory in biological systems. New York: Columbia University Press; 1963. [Google Scholar]
- Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BA, Clarke IJ. Adipose tissue hormones and the regulation of food intake. J Neuroendocrinol. 2008;20:842–849. doi: 10.1111/j.1365-2826.2008.1730.x. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Calvo C, López JE, Fernández JR, Mojón A, Domínguez MJ, Covelo M. Administration-time dependent effects of acetyl-salicylic acid on blood pressure in patients with mild essential hypertension. Med Clin (Barc) 2003;120:686–692. doi: 10.1016/s0025-7753(03)73813-5. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Calvo C, Ayala DE, López JE, Fernández JR, Mojón A, Domínguez MJ, Covelo M. Seasonal variation in plasma fibrinogen in dipper and non-dipper patients with mild-moderate essential hypertension. Med Clin (Barc) 2003;121:6–11. doi: 10.1016/s0025-7753(03)74111-6. [DOI] [PubMed] [Google Scholar]
- Hernandez-Morante JJ, Gomez-Santos C, Milagro F, Campión J, Martínez JA, Zamora S, Garaulet M. Expression of cortisol metabolism-related genes shows circadian rhythmic patterns in human adipose tissue. Int J Obes (Lond) 2009;33:473–480. doi: 10.1038/ijo.2009.4. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Bagnasco M, Otukonyong EE, Dube MG, Kalra PS. Rhythmic, reciprocal ghrelin and leptin signaling: New insight in the development of obesity. Regul Pept. 2003;111:1–11. doi: 10.1016/s0167-0115(02)00305-1. [DOI] [PubMed] [Google Scholar]
- Langendonk JG, Pijl H, Toornvliet AC, Burggraaf J, Frölich M, Schoemaker RC, Doornbos J, Cohen AF, Meinders AE. Circadian rhythm of plasma leptin levels in upper and lower body obese women: Influence of body fat distribution and weight loss. J Clin Endocrinol Metab. 1998;83:1706–1712. doi: 10.1210/jcem.83.5.4717. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loboda A, Kraft WK, Fine B, Joseph J, Nebozhyn M, Zhang C, He Y, Yang X, Wright C, Morris M, Chalikonda I, Ferguson M, Emilsson V, Leonardson A, Lamb J, Dai H, Schadt E, Greenberg HE, Lum PY. Diurnal variation of the human adipose transcriptome and the link to metabolic disease. BMC Med Genomic. 2009;2:7. doi: 10.1186/1755-8794-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårin P, Darin N, Amemiya T, Andersson B, Jern S, Björntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41:882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM. Biological rhythms. Vol. 4. New York: Plenum; 1981. Internal: Temporal order. In: Asschoff J, editor. Handbook of behavioral neurobiology; pp. 215–241. [Google Scholar]
- Perez-Tilve D, Stern JE, Tschöp M. The brain and the metabolic syndrome: Not a wireless connection. Endocrinology. 2006;147:1136–1139. doi: 10.1210/en.2005-1586. [DOI] [PubMed] [Google Scholar]
- Peterson RE. Plasma cortiscosterone and Hydrocortisone levelsin man. J Clin Endocrinol Mertab. 1957;17:1150–1157. doi: 10.1210/jcem-17-10-1150. [DOI] [PubMed] [Google Scholar]
- Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci. 2003;73:2467–2475. doi: 10.1016/s0024-3205(03)00628-3. [DOI] [PubMed] [Google Scholar]
- Szabó I, Kovats TG, Halberg F. Circadian rhythm in murine reticuloendothelial function. Chronobiologia. 1978;5:137–143. [PubMed] [Google Scholar]
- Teboul M, Gréchez-Cassiau A, Guillaumond F, Delaunay F. How nuclear receptors tell time. J Appl Physiol. 2009;107:1965–1971. doi: 10.1152/japplphysiol.00515.2009. [DOI] [PubMed] [Google Scholar]
- Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK, Mertens A, Quarck R, Benhabilès N, Marguerie G, Mackness B, Mackness M, Ninio E, Herregods MC, Balligand JL, Holvoet P. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation. 2004;110:3259–3269. doi: 10.1161/01.CIR.0000147614.85888.7A. [DOI] [PubMed] [Google Scholar]
- Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]