Abstract
The Two-Process theory of psychopathy posits distinct etiological mechanisms contribute to the disorder: 1) a weakness in defensive (fear) reactivity related to affective-interpersonal features, and 2) impaired cognitive-executive functioning, marked by reductions in brain responses such as P3, related to impulsive-antisocial features. The current study examined relations between psychopathy factors and electrocortical response to emotional and neutral pictures in male offenders (N=139) assessed using the Psychopathy Checklist-Revised (PCL-R). Impulsive-antisocial features of the PCL-R (Factor 2) were associated with reduced amplitude of earlier P3 brain response to pictures regardless of valence, whereas the affective-interpersonal dimension (Factor 1) was associated specifically with reductions in late positive potential response to aversive pictures. Findings provide further support for the Two-Process theory and add to a growing body of evidence linking the impulsive-antisocial facet of psychopathy to the broader construct of externalizing proneness. Findings are discussed in terms of current initiatives directed at incorporating neuroscientific concepts into psychopathology classification.
Keywords: psychopathy, Two-Process theory, LPP, P3, event-related potential
Psychopathy is a multifaceted condition characterized by distinctive features in the domains of affective-interpersonal functioning (low fear/anxiety, deficient empathy, exploitativeness) and impulsive-antisocial traits/behaviors (lack of restraint, irresponsibility, aggression, persistent rule- and/or law-breaking). The Two-Process (Patrick & Bernat, 2009; or Dual-Pathway [Fowles & Dindo, 2009]) theory of psychopathy posits that separate neural mechanisms contribute differentially to the affective-interpersonal and impulsive-antisocial components of the condition, with deficits in defensive (fear) reactivity contributing more to the former and impaired cognitive-executive functioning contributing more to the latter (and to externalizing forms of psychopathology more broadly).
Prior research has provided support for this theoretic model, as the two components of psychopathy exhibit diverging associations with external variables across multiple domains, in a manner consistent with the theory (for reviews see Patrick, 2007; Patrick & Bernat, 2009; Skeem, Polaschek, Patrick, & Lilienfeld, 2011). Evidence for the Two-Process model has also emerged from psychophysiological studies, which have demonstrated selective negative associations for the affective-interpersonal component of psychopathy with reactivity to aversive emotional stimuli (e.g., Benning, Patrick, & Iacono, 2005; Vaidyanathan, Hall, Patrick, & Bernat, 2011), and reductions in brain response within cognitive processing tasks preferentially associated with the impulsive-antisocial component (e.g., Carlson, Thai, & McLarnon, 2009; Venables & Patrick, 2014). The current study extended this existing work by examining associations of the two factors of psychopathy as measured by the PCL-R with event-related potential (ERP) responses to emotional and neutral pictures in an incarcerated male offender sample.
Psychopathy and Deficits in Emotional Response to Aversive Stimuli
Prominent theorists have asserted that psychopathy is associated with deficient responsiveness to aversive or threatening stimuli (e.g., Fowles, 1980; Hare, 1965; Lykken, 1995). Contemporary support for this has been found for the affective-interpersonal component of psychopathy in particular—in terms of reduced skin conductance response (SCR) for aversive as compared to neutral pictures (Benning, Patrick, & Iacono, 2005), and reduced SCR during anticipation of an impending noise stressor (Dindo & Fowles, 2011; see also Patrick, 1994). The affective-interpersonal component is also associated with reduced potentiation of the startle blink reflex to abrupt noise probes occurring during viewing of aversive relative to neutral pictures (Patrick, Bradley, & Lang, 1993); this finding has been replicated across multiple laboratories and populations (e.g., Pastor, Molto, Vila, & Lang, 2003; Sutton, Vitale, & Newman, 2002; for a review, see Patrick & Bernat, 2009). Results along these lines are consistent with the notion that psychopathy, and its distinct affective-interpersonal features in particular, is associated with reduced reactivity of the brain's defensive (fear) system.
A more limited body of research has also investigated aversive response deficits in psychopathy using event-related potential (ERP) measures recorded in lab picture-viewing tasks. One key index of affective processing in this type of task is the late positive potential (LPP), a sustained low-frequency, positive-voltage ERP component with a parietal scalp distribution that typically begins 250-400 ms following picture onset, and persists for several seconds afterward. The LPP is reliably enhanced for emotional or motivationally-relevant stimuli as compared to neutral scenes, and in the context of emotional picture-viewing is thought to reflect the preferential elaborative processing of affective information (for a review, see Schupp, Flaisch, Stockburger, & Junghöfer, 2006). In a recent study of offenders assessed using a screening version of Hare's Psychopathy Checklist—Revised (PCL-R; Hare, 2003), Sadeh and Verona (2012) found that scores on the affective-interpersonal component of psychopathy were associated with reduced amplitude of LPP response to aversive versus neutral scenes, whereas the impulsive-antisocial component of psychopathy was unrelated to LPP amplitude. However, the picture-viewing paradigm employed in this study did not include pleasant scenes, raising the possibility that the observed LPP deficits might reflect a more generalized affective response deficit (Cleckley, 1941/1976; Verona, Patrick, Curtin, Bradley, & Lang, 2004) as opposed to a selective deficit in negative emotional response, as posited by the Two-Process model.
Another recent study by Anderson and Stanford (2012), involving community participants, included pleasant as well as aversive images and reported that individuals with elevated psychopathic tendencies as indexed by total scores on the self-report based Psychopathic Personality Inventory (PPI; Lilienfeld & Andrews, 1996; Lilienfeld & Widows, 2005) exhibited reduced LPP response to affective pictures as a whole. However, analyses were not presented separately for pleasant and aversive pictures conditions in this study; rather, the two affective picture conditions were combined. Additionally, analyses in this study focused on extreme groups (high vs. low) in terms of overall PPI total scores; thus, the separate contributions of affective-interpersonal versus impulsive-antisocial components of psychopathy to affective modulation of LPP amplitude were not examined.
In light of these gaps in the existing literature, one key aim of the present study was to test the hypothesis of a specific reduction in LPP response to aversive emotional stimuli, as opposed to a deficit in response to affective stimuli more broadly, for the affective-interpersonal component of psychopathy in particular.
Psychopathy and Brain Response Deficits in Cognitive Processing Tasks
The P3 (or P300) ERP component occurs in response to task-relevant or otherwise salient stimuli in a range of experimental contexts, and is hypothesized to reflect multiple cognitive processes including attention and updating of working memory arising from multiple coordinated sources within the brain (Polich, 2007). Several studies have investigated relations between psychopathy and amplitude of P3 response by comparing groups of offenders classified as psychopathic or non-psychopathic according to overall scores on Hare's (2003) Psychopathy Checklist-Revised (PCL-R; for reviews, see Gao & Raine, 2009; Patrick, Venables, & Skeem 2012). Results from these studies generally indicate (see Jutai, Hare, & Connolly, 1987 and Raine & Venables, 1987, for notable exceptions) that P3 amplitude is reduced in psychopathic offenders when compared to those classified as nonpsychopathic in the oddball experimental paradigm (Kiehl, Bates, Laurens, Hare, & Liddle, 2006; Kiehl, Hare, Liddle, & McDonald, 1999) and the Go/No-Go task (involving equally probable events, in contrast with the oddball paradigm; Kiehl, Smith, Hare, & Liddle, 2000).
While the foregoing studies have relied exclusively on PCL-R total scores and conceptions of psychopathy as a unitary clinical entity, other more recent studies have examined associations for the two factors of psychopathy separately. One such study by Carlson and colleagues (2009) examined P3 response in relation to factors of psychopathy indexed by the PPI in a mixed gender undergraduate sample. Consistent with the Two-Process theory, these authors reported a negative relationship between amplitude of P3 response to target stimuli in a visual oddball task and scores on the Self-Centered Impulsivity (or Impulsive Antisociality; Benning, Patrick, Salekin, & Leistico, 2005) factor of the PPI, with no such relationship evident for the Fearless Dominance factor of the PPI (reflecting interpersonal dominance, emotional resilience, and venturesomeness). Extending this work to incarcerated male offenders, Venables and Patrick (2014) reported a parallel negative relationship of scores on the impulsive-antisocial factor of the PCL-R in response to both target and incidental novel stimuli in a 3-stimulus visual oddball task. Notably, when examining the lower-order facets of the PCL-R (Hare & Nuemann, 2006), P3 reductions were most evident for the Antisocial facet of Factor 2 as opposed the Impulsive-Irresponsible features. Also, consistent with findings from Carlson et al., P3 amplitude in this study was unrelated to scores on the PCL-R affective-interpersonal factor.
The results of these two recent studies are consistent with findings from studies of community samples that have reported reliable reductions in P3 amplitude in participants exhibiting high levels of disinhibitory (or externalizing; Krueger et al., 2002, 2007) problems and traits (e.g., Costa et al., 2000; Iacono, Carlson, Malone, & McGue, 2002; Venables, Patrick, Hall, & Bernat, 2011). Based on these findings, Iacono and colleagues (2003) hypothesized that P3 amplitude reduction indexes the genetically transmitted vulnerability toward a spectrum of disinhibitory problems including impulsive-irresponsible behavior, aggression, other antisocial deviance, and substance abuse/dependence. Follow-up studies documenting a robust relationship between the factor in common among problems of these types (Patrick, Bernat, Malone, Iacono, Krueger, & McGue, 2006), attributable mostly to genetic influence (Hicks et al., 2007; Yancey, Venables, Hicks & Patrick, 2013), provide compelling support for this perspective.
The finding that reduced P3 in psychopathy appears to be attributable mainly to the impulsive-antisocial features of the disorder coincides in turn with evidence for deficits in cognitive-executive function in antisocial-externalizing individuals (Blair, 2005; Ishikawa & Raine, 2003; Kiehl, 2006; Morgan & Lilienfeld, 2000). This reported evidence of cognitive-executive deficits served as part of the foundation for the Two-Process theory (Patrick & Bernat, 2009), which posits deficits of this type as a neurobiological mechanism contributing preferentially to the impulsive-antisocial features of psychopathy. One aim of the current study was to expand upon the aforementioned research and evaluate whether P3 amplitude elicited in a picture-viewing paradigm would evidence effects seen in cognitive processing tasks (i.e., preferential relations with psychopathy factor 2).
Current Study Aims and Hypotheses
With the foregoing background review in mind, the current study sought to evaluate, for the first time, associations of separable components of ERP response to affective and neutral picture stimuli, consisting of P3 and LPP, with distinct factors of PCL-R psychopathy and their constituent facets (Hare & Neumann, 2006) in an offender sample. In line with the Two-Process theory, we hypothesized that (1) participants high in impulsive-antisocial features of psychopathy as indexed by PCL-R Factor 2 would show reduced overall P3 response amplitude (i.e., across picture stimuli, regardless of content) relative to participants low in such features, and (2) participants high in affective-interpersonal features of psychopathy would show reduced emotional modulation of the LPP response—for aversive scenes in particular (relative to neutral)—relative to those low in features of this type.
Method
Participants
Participants were 166 adjudicated adult male offenders from a court-ordered residential substance abuse treatment facility in Florida. Participants were randomly recruited from individuals who indicated interest via a sign-up sheet and who met the following inclusion criteria: no current major mental disorder (i.e., schizophrenia, Bipolar I) as determined from items on a screening questionnaire and information contained in program file records; competency in English; and no visual or hearing impairments. Of the 166 who participated in testing, a total of 27 were excluded from analysis due to factors including technical problems with electroencephalogram (EEG) recording (n=18), excessive artifact in the EEG recordings (n=8; described further below), and incomplete interview data (n=1), resulting in a final sample of 139 for analyses (M age = 29.6, SD = 9.4; M years of education 11.3, SD = 2.05). The racial/ethnic composition of the analysis sample was: Caucasian, 66.9%; African American, 15.8%; Hispanic, 13.7%; mixed race, 2.9%; other, .7%. Most study participants (92.1%) were currently incarcerated due to non-violent criminal offense(s) such as drug possession or selling, theft, fraud/forgery, and parole/probation violation,) with the remainder (9.4%) instead or additionally charged with at least one violent offense (e.g., robbery, assault, weapon-related, sexual); 84.9% had prior non-violent charges/convictions and 38.1% had prior violent charges/convictions.
Study procedures were approved by the Institutional Review Boards of Florida State University and the University of South Florida, were additionally approved by the residential treatment facility where data were collected. Study participants provided informed written consent prior to participation. Data reported in this manuscript were collected as part of a larger three-session assessment protocol. Participants received a payment of $10 for each session of testing, deposited into their institutional account.
Psychopathy Assessment: Psychopathy Checklist-Revised
The Psychopathy Checklist-Revised (PCL–R; Hare, 2003) was developed to assess for psychopathy in forensic settings. Its 20 items are scored on the basis of data from a semi-structured interview along with information derived from collateral sources (i.e., institutional file records). PCL-R interviews, accompanying file review, and ratings were completed by individuals with either a bachelor's or doctoral degree in psychology who underwent specialized training for administering and rating the PCL-R under the supervision of the senior investigator (Christopher J. Patrick). The items of the PCL–R are viewed as having a hierarchical organization (Hare & Neumann, 2006), in which items comprising its affective-interpersonal (Factor 1) and impulsive-antisocial deviance (Factor 2) components can be further subdivided into facets reflecting social guile and manipulativeness (Interpersonal facet), callous-unemotionality (Affective facet), disinhibitory tendencies (Impulsive-Irresponsible facet), and chronic law-breaking (Antisocial facet). Scores for the two higher-order factors (Factor 1 M = 7.6, SD = 3.9; Factor 2 M = 10.3, SD = 3.6) and four lower order PCL–R facets (Interpersonal M = 3.6, SD = 2.7; Affective M =4.0, SD = 2.2; Impulsive-Irresponsible M = 6.5, SD = 2.4; Antisocial M = 3.8, SD = 2.0) were computed for each participant and utilized in analyses.
Experiment Stimuli and Design
Participants viewed a series of 90 pictures consisting of 30 pleasant, 30 neutral, and 30 unpleasant scenes from the International Affective Picture System (IAPS; Lang et al., 2008).1 Each picture was presented for 3.5 s followed by an intertrial interval of 4 s. Pleasant pictures included action (e.g., skydiving, river rafting), erotic (e.g., opposite-sex nude individuals, intimate couples), and nurturant scenes (e.g., babies, small animals). Unpleasant pictures included scenes of physical injury (e.g., mutilated bodies, serious wounds) and direct threat scenes (e.g. pointed guns, looming attackers, and threatening animals). Neutral pictures consisted of scenes of inactive people, neutral human faces, household objects, and kitchen utensils.2
During 81 of the 90 picture stimuli, noise probes (50 ms, 105 dB, 10 μs rise time) were presented 1.5, 2.35, or 3.2 s after picture onset to elicit the startle blink reflex. For 6 of the remaining 9 unprobed pictures, probes were delivered either 1, 1.5, or 2s following picture offset. Preceding the main 90-picture test set, four initial pictures (IAPS #s 7508, 7110, 9252, 7233) were presented, with intervening noise probes in three instances, to provide familiarity with task stimuli and habituate initial large startle responses (Patrick et al., 1993); data for these trials were excluded from analyses. The main picture set was presented in eight different orders across participants. Within and between orders, pictures and noise probes were counterbalanced such that valence categories (pleasant, neutral, unpleasant) were represented equally across orders at each serial position, with the following constraints: no more than two pictures of a particular valence occurred consecutively within any stimulus order; pictures of the same content category never appeared consecutively or across orders; pictures were rotated so as to serve in both probed and unprobed trials across participants.
Physiological Data Recording and Reduction Procedures
During the experiment, participants viewed the picture stimuli on a 21” computer monitor (situated approximately 1 m away, at eye level) while seated in a comfortable recliner. Data collection was performed using two IBM compatible computers, one equipped with E-Prime presentation software (MEL software, Inc) for stimulus delivery and the other with Neuroscan Acquire software for physiological data acquisition. ERP activity was recorded from 128 scalp sites positioned according to the NSL system, using sintered Ag-AgCl electrodes embedded within an elastic head cap (Neuroscan Quik-Cap). Electrodes were positioned above and below the left eye to monitor vertical electrooculogram (VEOG) activity, and adjacent to the outer canthi of the left and right eyes to monitor horizontal electrooculogram (HEOG) activity. All electrode impedances were kept below 10 KOhms.
EEG signal activity was recorded using an on-line reference sensor placed at the vertex of the scalp and applying an analog band pass filter of .05-200 Hz prior to digitization at 1000Hz. Data were then arithmetically re-referenced off-line to the average of left and right mastoid electrodes for subsequent processing and analysis. Data epochs from -1000 ms to 2000 ms were extracted from the continuous EEG recordings using Neuroscan EDIT software (version 4.5, Neuroscan Inc.), and corrected for eye movements using the algorithm developed by Semlitsch, Anderer, Schuster, and Presslich (1986), as implemented within the EDIT software. The epoched and eye-blink corrected EEG data were then imported to Matlab (Mathworks, Inc.) for subsequent data processing, including resampling to 128 Hz using the Matlab resample command, which applies a low pass anti-aliasing filter before downsampling.
Trials in which activity exceeded ±100 μV either during the pre- (-1000 ms to 0) or poststimulus (0 to 2000 ms) intervals were excluded from further processing. Approximately 8% of trials across all participants were removed due to excessive artifact. Visual inspection of each participant's average waveforms was subsequently undertaken to evaluate the effectiveness of the aforementioned criteria. Electrodes deemed to contain excessive artifact were replaced by their nearest neighboring sites. Participants were excluded if more than 25% of trials were rejected due to excessive artifact after replacement (n = 8; 4.8% of total sample). Using the grand average across participants as a referent for window selection and considering prior published research (Foti, Hajcak, & Dien, 2009), P3 amplitude was quantified as the maximum voltage within a window of 250 ms to 500 ms post-stimulus, and LPP amplitude was calculated as the mean voltage activity occurring between 500 ms to 1500 ms, each relative to a −102 to −8 ms baseline. Prior investigations of P3 (cf. Iacono et al, 2003; Patrick et al, 2006; Yancey et al., 2013) and LPP response (cf. Hajcak & Olvet, 2008) have focused on activity at the midline parietal (Pz) recording site specifically, at which both P3 and LPP occur with maximal amplitude. Following these precedents, analyses for these ERP components were conducted on data for Neuroscan Quik-Cap NSL electrode site 66, which corresponds to electrode site Pz in standard 10-20 nomenclature.
Data Analyses
Primary statistical analyses consisted of repeated measures ANOVAs evaluating effects of picture valence category (pleasant, neutral, aversive) and continuous PCL-R psychopathy scores on either P3 or LPP amplitude as the continuous dependent measure.3 Repeated-measures ANOVA statistics were Greenhouse-Geisser adjusted were applicable, and reported p values represent the adjusted statistic. Partial eta square (ηp2) values are also reported as an index of effect size. Significant effects involving the picture valence factor were decomposed through use of orthogonal arousal (quadratic pleasant/aversive vs. neutral) and valence (linear pleasant vs. aversive) contrasts.
One-way repeated-measures ANOVAs were first conducted to evaluate effects of picture valence on ERP responses (P3, LPP) in the participant sample as a whole. This was followed by repeated measures ANOVAs in which scores on either PCL-R Factor 1 or Factor 2 were included as a continuous between-subjects variable along with picture valence as a discrete within-subject variable, in predicting either P3 or LPP amplitude. Significant effects for the two PCL-R factors were further evaluated by examining effects for constituent facets of each (i.e., Factor 1—Affective, Interpersonal; Factor 2—Impulsive-Irresponsible, Antisocial) in separate correlational analyses (in cases of significant main effects for one PCL-R factor or the other) or repeated-measures ANOVAs (in the case of significant Psychopathy Score x Valence interactions).
Results
Overall valence and arousal effects on P3 and Late Positive Potential amplitude
In the initial repeated-measures AVOVAs examining effects of picture valence (aversive, neutral, pleasant) on ERP score variables across all participants, there was (as expected based on prior work) a significant main effect of picture valence on P3 amplitude, F(2,138) = 81.59, p < .001, ηp2 = .372. Decomposition of this effect through contrasts revealed a highly robust quadratic-arousal effect, F(1,138) = 144.43, p < .001, ηp2 = .511, reflecting augmented P3 response to aversive (M = 9.76, SD = 5.2) and pleasant scenes (M = 9.33, SD = 5.1) relative to neutral (M = 6.49, SD = 4.6). The linear-valence contrast was not significant, F(1,138) = 2.68, p > .10, indicating comparable P3 response to pleasant as compared to aversive scenes. Also in line with expectation based on prior work, a significant main effect of picture valence was likewise evident for the LPP, F(2,138) = 94.43, p < .001, ηp2 = .406, with both quadratic-arousal and linear-valence contrasts significant in this case, Fs(1,138) = 172.54 and 19.75, ps < .001, ηp2s = .556 and .125—indicating augmented LPP response for both aversive (M = 4.56, SD = 4.1) and pleasant (M = 3.16, SD = 3.8) scenes relative to neutral (M = 0.56, SD = 3.4), and enhanced response for aversive as compared to pleasant scenes.
Associations of P3 amplitude with PCL-R psychopathy factors and facets
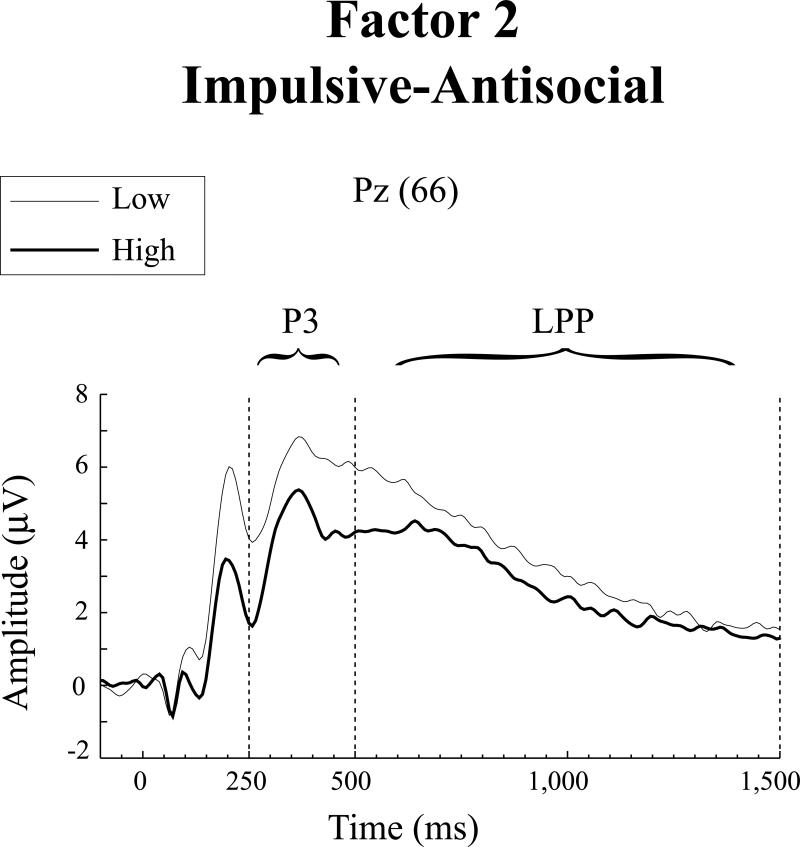
Consistent with our a priori hypothesis for the impulsive-antisocial component of psychopathy, the repeated-measures AVOVA for P3 amplitude in which scores on PCL-R Factor 2 were included as a continuous between-subjects factor along with the discrete picture valence factor yielded a significant main effect for Factor 2, F(1,137) = 6.18, p = .014, ηp2 = .043— reflecting reduced P3 amplitude as a function of increasing levels of PCL-R Factor 2 (r = -.21, p = .014)—but no Factor 2 x Valence interaction, F(2,137) = 1.42, p > .25. Consistent with the hypothesis that P3 amplitude would be selectively related to disinhibitory features of PCL-R psychopathy, there was no main effect of Factor 1, and no Factor 1 x Valence interaction (Fs <= 2, ps > .14). To illustrate the main effect for Factor 2, Figure 1 depicts waveform plots (collapsed across valence categories) for participants scoring high versus low on this factor of the PCL-R (upper and lower quartile, respectively). As evident in the figure, participants scoring high in Factor 2 exhibited markedly reduced P3 amplitude.
Figure 1.
Average ERP waveforms at electrode site 66 (Pz), collapsed across pleasant, neutral, and aversive picture categories. High and Low PCL-R Factor 2 groups consist of study participants falling within the upper and lower quartiles, respectively, of scores on the Impulsive-Antisocial factor of the Psychopathy Checklist-Revised (Hare, 2003).
In the follow-up correlational analyses evaluating effects for constituent facets of PCL-R Factor 2, P3 amplitude (collapsed across valence categories) showed a significant negative association with scores on the Antisocial facet (r = −.22, p = .009), as compared to only a modest association with the Impulsive-Irresponsible facet (r = −.13, p > .12). These results indicate that the inverse association between P3 amplitude and Factor 2 of the PCL-R was attributable more to elevations on the Antisocial facet, than to the Impulsive-Irresponsible facet.
Associations of Late Positive Potential amplitude with PCL-R psychopathy factors and facets
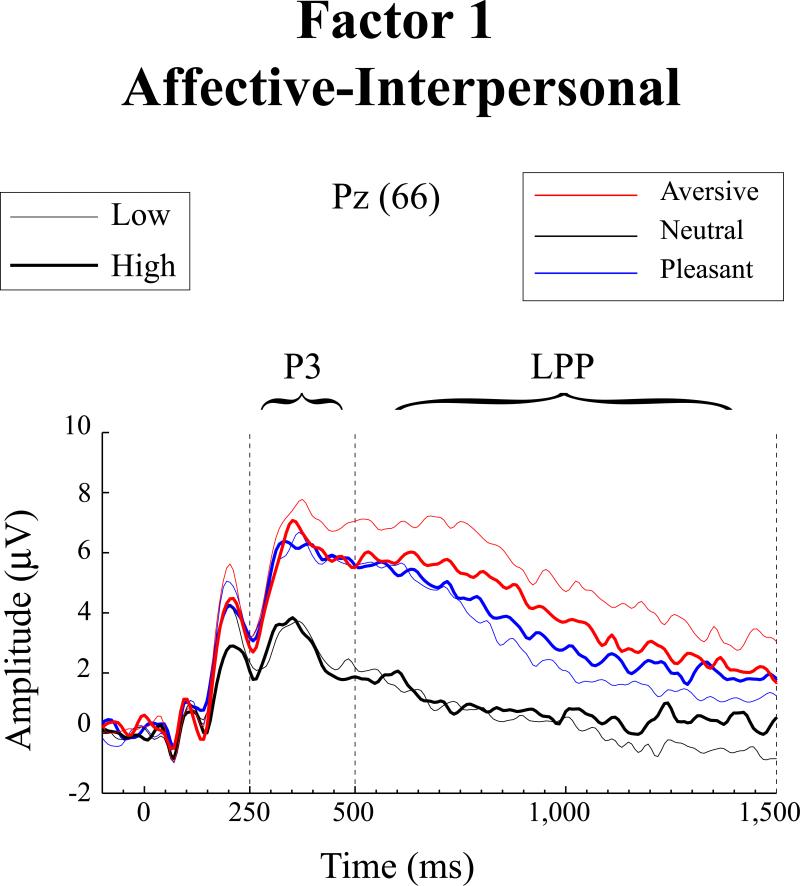
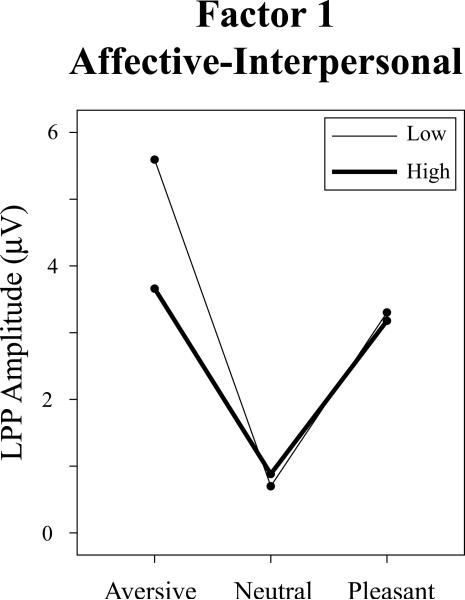
For the LPP response variable, a significant Factor 1 x Valence interaction was evident, F(2,137) = 5.93, p = .003, ηp2 = .041, with no accompanying main effect for either Factor 1 or Factor 2, Fs >1.21, ps > .27, and no evidence of a Factor 2 x Valence interaction, F = 2.5, p > .08. Consistent with our hypothesis for the affective-interpersonal component of psychopathy, decomposition of the Factor 1 x Valence interaction revealed a significant effect for the linear-valence contrast, F(1,137) = 6.26, p = .014, ηp2 = .044, reflecting lesser augmentation of LPP for aversive versus pleasant pictures with increasing levels of PCL-R Factor 1. A somewhat less robust significant effect was also evident for the quadratic-arousal contrast, F(1,137) = 5.59, p = .019, ηp2 = .039, reflecting lesser augmentation of LPP for pleasant and aversive scenes conjointly as compared to neutral scenes. Figure 2 depicts waveform plots for participants scoring high versus low on Factor 1 of the PCL-R (upper vs. lower quartile) and Figure 3 depicts mean LPP amplitudes by picture valence for each subgroup. It can be seen from the line plot in particular that participants scoring high on Factor 1, relative to those scoring low, exhibited reduced LPP amplitude for aversive scenes in particular.
Figure 2.
Average ERP waveforms at electrode site 66 (Pz), presented separately for pleasant, neutral, and aversive picture categories. High and Low PCL-R Factor 1 groups consist of study participants falling within the upper and lower quartiles, respectively, of scores on the Affective-Interpersonal factor of the Psychopathy Checklist-Revised (Hare, 2003).
Figure 3.
Mean LPP amplitude (electrode site = 66 [Pz]; time window = 500 – 1,500 ms) elicited during processing of pleasant, neutral, and aversive pictures. High and Low PCL-R Factor 1 groups = scorers in upper and lower quartiles, respectively.
Follow-up repeated measures ANOVAs for the two facets of PCL-R Factor 1 revealed significant interaction effects for each: Affective Facet x Valence F(2,137) = 4.33, p = .014, ηp2 = .031; Interpersonal Facet x Valence F(2,137) = 4.80, p = .009, ηp2 = .034. For the Affective facet, only the linear-valence contrast was significant, F(1,137) = 6.01, p = .015, ηp2 = .042 (F for quadratic contrast = 2.61, p > .11); for the Interpersonal facet, a trend-level linear valence contrast was also evident, F(1,137) = 3.64, p = .058, ηp2 = .026, along with a significant quadratic-valence contrast, F(1,137) = 6.02, p = .015, ηp2 = .042. These findings clarify results for Factor 1 scores as a whole by showing that both of its facets contributed to the finding of blunted LPP for aversive versus pleasant scenes, albeit with the Affective facet contributing somewhat more, whereas the finding of attenuated LPP for affective scenes as a whole relative to neutral was attributable mainly to the Interpersonal facet.
Discussion
Hypotheses for the current study were generated from the Two-Process theory of psychopathy (Fowles & Dindo, 2009; Patrick & Bernat, 2009), which posits deficient reactivity to aversive stimuli is primarily related to the affective-interpersonal (Factor 1) features of psychopathy and cognitive-executive processing deficits in relation to the impulsive-antisocial (Factor 2) features. As such, this study sought to expand upon a growing body of evidence linking P3 amplitude reductions in psychopathy specifically to the impulsive-antisocial features (Carlson et al., 2009; Venables & Patrick, 2014), and in turn, corroborate prior work tying this component of psychopathy to the broader psychopathology construct of externalizing proneness (Patrick, Hicks, Krueger, & Lang, 2005; Venables & Patrick, 2012; see also Patrick et al., 2013).
In line with study hypotheses, reduced amplitude of P3 response to picture stimuli in general (regardless of valence) was evident in the current offender sample as a function of higher scores on Factor 2 of the PCL-R. By contrast, P3 amplitude was unrelated to scores on Factor 1. Further, analyses focusing on narrower symptomatic facets of PCL-R Factor 2 revealed that P3 amplitude reduction was more evident in relation to the Antisocial facet than the Impulsive-Irresponsible facet. This latter finding is consistent with recent work by Venables and Patrick (2014), employing a separate male prisoner sample, that showed reduced amplitude of P3 response to target and novel stimuli in an oddball task to be associated with scores on the Antisocial facet of Factor 2 in particular.
In contrast with the foregoing results for P3, deficits in emotional processing as indexed by amplitude of the later LPP component of brain response were related to scores on Factor 1 of the PCL-R but not Factor 2. More specifically, and consistent with hypothesis, we found evidence for a selective deficit in modulation of LPP response to aversive as compared to neutral picture stimuli in relation to this factor of the PCL-R; modulation of LPP response to pleasant versus neutral pictures was unrelated to scores on Factor 1. Further, analyses focusing on the narrower Affective and Interpersonal facets of PCL-R Factor 1 indicated contributions of each to this deficit in reactivity to aversive picture stimuli.
Implications for Understanding Neural Mechanisms Underlying Cognitive and Affective Deficits in Psychopathy
Results from the present study indicate that P3 amplitude reductions in criminal psychopathy are selectively related to impulsive-antisocial features of the disorder and largely unrelated to affective-interpersonal features. This core finding is consistent with the Two-Process theory of psychopathy (Fowles & Dindo, 2009; Patrick & Bernat, 2009), which posits that impairments in neural indicators of cognitive function such as P3 amplitude should be related to the features of psychopathy that overlap most with externalizing tendencies. An interesting result emerging from analyses in which the Impulsive-Irresponsible and Antisocial Behavior facets of Factor 2 were examined separately as predictors of P3 response was that the Antisocial facet accounted for the general reduction in P3 response to affective and neutral stimuli. This result indicates that, within the current offender sample, a history of antisocial behaviors reflecting criminality and violence/aggression was predictive of brain deficits as reflected by reduced P3 amplitude. In turn, this finding coincides with theory and research pointing to a role for dysfunction in cognitive-executive systems in the salient affective and behavioral control problems exhibited by antisocial-aggressive individuals (e.g., Blair, 2005; Davidson, Putnam, & Larson, 2000; Ishikawa & Raine, 2003; Kiehl, 2006). Consistent with these perspectives, findings from the current study provide further evidence linking reduced P3 response in criminal psychopathy to the nomological network of externalizing psychopathology, which includes P3 amplitude as an endophenotypic indicator (Gottesman & Gould, 2003) of the heritable propensity toward disinhibitory problems and traits (Iacono et al., 2003; Hicks et al., 2007; Yancey et al., 2013).
Results for the LPP component in the current study indicate impairment in sustained neural processing of aversive stimulus events among individuals high in core affective-interpersonal (Factor 1) features of psychopathy. That this impairment was evident for aversive but not pleasurable picture stimuli suggests a more focal (Lykken, 1995; Patrick, 1994) as opposed to global weakness in affective capacity (Cleckley, 1941/1976)—consistent with the Two-Process theory, which posits a weakness in sensitivity of the brain's defensive system as a distinct etiological substrate for the affective-interpersonal symptoms of psychopathy. Our findings are also consistent with theory and research indicating that dispositional fearlessness, associated with reduced responsiveness to aversive or threatening stimuli (Benning et al., 2005; Dvorak-Bertsch, Curtin, Rubinstein, & Newman, 2009; Dindo & Fowles, 2011; Vaidyanathan, Bernat, & Patrick, 2009), is a hallmark element of psychopathy that distinguishes it from the more prevalent DSM diagnosis of antisocial personality disorder (Drislane, Vaidyanathan, & Patrick, 2013; Patrick, 1994; Vaidyanathan et al., 2011; see also Venables, Hall, & Patrick, 2014). From these perspectives, individuals exhibiting the affective-interpersonal features of psychopathy would exhibit reduced levels of fear reactivity, which may be advantageous in certain situations (e.g., firefighters entering burning buildings) but potentially harmful in others (e.g., dangerous thrill seeking).
Taken together, our findings also suggest that individuals scoring high on the impulsive-antisocial component of psychopathy (i.e., high externalizers; Patrick et al., 2005) exhibit diminished cognitive-elaborative processing of stimuli (i.e., reduced sustained attention and memorial processing) as indexed by amplitude of the P3 brain potential response (cf. Polich, 2007). As such, the impulsive-antisocial features appear to be associated with impaired cognitive-elaborative processing following initial perceptual registration of picture stimuli, whether neutral or affective. Conversely, individuals high on the affective-interpersonal dimension of psychopathy exhibited selective reductions in sustained neural processing of aversive visual images—as evidenced by reduced LPP amplitude, which is hypothesized to index motivationally-driven engagement with affectively-arousing stimuli, associated with activation of defensive/withdrawal and appetitive/approach systems of the brain (Lang, Bradley, & Cuthbert, 1997; Ferrari, Codispoti, Cardinale, & Bradley, 2008). From this perspective, our current findings are consistent with the Two-Process theory of psychopathy and suggest that amplitude of the LPP in response to aversive stimuli is reflective of neural deficits in reactivity to aversive or threatening stimuli that are specific to the affective-interpersonal features of psychopathy.
Connecting Psychopathy Research to Broader Psychopathology-Neuroscience Initiatives
The Two-Process perspective on the etiology of psychopathy provides a point of contact with current initiatives directed at reconceptualizing mental disorder conditions in neurobiological terms. In particular, the National Institute of Mental Health's Research Domain Criterion (RDoC) initiative (Cuthbert & Kozak, 2013; Insel et al. 2010) incorporation of neuroscientific concepts and methods into psychopathology classification through a focus on transdiagnostic “process” constructs that can be studied across multiple levels of analysis (i.e., from genetic-cellular through neural circuitry and physiology to observable behavior). The RDoC research initiative calls for shift in the focus of research from traditional categorical diagnoses, which represent complex, multi-determined clinical phenotypes, toward more basic symptom dimensions and systems-oriented (e.g., cognitive, emotional, arousal, regulatory) constructs for understanding them. In line with this perspective, the Two-Process theory suggests that antisocial-psychopathic behavior in some individuals reflects high dispositional externalizing proneness involving generalized cognitive-elaborative processing deficits (e.g., as evidenced by reduced P3 amplitude to task stimuli), whereas behavior of this type in other individuals reflects low dispositional fear entailing reduced sensitivity to aversive cues or events (e.g., as evidenced by reduced affective modulation of the LPP to unpleasant visual images).
From this perspective, the current study serves to link findings on P3 response amplitude in criminal psychopathy to the construct-network (Patrick et al., 2013) of externalizing psychopathology (Krueger et al., 2002, 2007; Venables & Patrick, 2012; Patrick et al., 2013), which includes known referents across multiple levels as described in the RDoC framework (Beauchaine & McNulty, 2013; Dick, 2007; Krueger et al., 2002; Hicks et al., 2007; Young et al., 2009). In particular, current findings for the P3 response component corroborate (cf. Carlson et al., 2009; Venables & Patrick, 2014) a neurophysiological link between the impulsive-antisocial factor of psychopathy and the dispositional liability to externalizing proneness that has been termed “disinhibition” (Patrick, Kramer, Krueger, & Markon, 2013; Yancey et al., 2013). This dispositional liability can be considered the counterpart, in trait terms, of the construct of “response inhibition” within the Cognitive Systems domain of the RDoC framework (Nelson, Strickland, Krueger, & Patrick, 2014; Venables et al., 2014; see also Young et al., 2009). Viewed in this way, studies of the neurobiology of impulsive-antisocial tendencies in psychopathy can contribute to a systematic, multi-level analysis of the role of inhibitory control deficits in a much broader range of clinical problems.
Regarding the affective-interpersonal (Factor 1) component of psychopathy, our finding of reduced cortical-LPP reactivity to aversive picture stimuli coincides with prior research demonstrating reductions in aversive startle potentiation (Patrick & Bernat, 2009) and noise-probe P3 response (Drislane et al., 2013) in relation to this symptomatic component. Reductions of these kinds have also been reported for individuals low in dispositional fear (Benning et al., 2005; Patrick, Durbin, & Moser, 2012; Vaidyanathan et al., 2009). The implication, in line with the Two-Process theory, is that the affective-interpersonal component of psychopathy reflects a weakness in dispositional defensive (fear) reactivity. In turn, defensive (fear) reactivity can be considered the counterpart, in dispositional terms, of the construct of “acute threat” within the Negative Valence Systems domain of RDoC. Viewed in this way, studies of the neurobiology of core affective-interpersonal tendencies in psychopathy can contribute to a multi-level analysis of the role of dispositional threat sensitivity in a broad scope of clinical conditions—including those marked by excessive threat sensitivity (e.g., Specific and Social Phobia, Panic Disorder, Avoidant Personality Disorder; Nelson et al., 2014; Patrick & Bernat, 2010) as well as those entailing deficits in threat sensitivity.
Limitations and Future Directions
Some limitations of the present study are noteworthy and highlight important directions for future research. One limitation is that the specific nature of the participant sample—i.e., male offenders—may limit the generalizability of the reported findings. However, this concern is mitigated somewhat by reports of parallel findings in studies that have examined brain reactivity in relation to scores on the two factors of psychopathy in non-offender (student) participants (Anderson & Stafford, 2012; Carlson et al., 2009). Results from these prior studies suggest that the current study effects may well extend to other samples. Nonetheless, further research is needed evaluate whether the current results replicate in mixed-gendered community or other offender samples (e.g., female prisoners).
Another limitation concerns the use of the PCL-R to operationalize distinguishable affective-interpersonal and impulsive-antisocial features of psychopathy. Whereas the Two-Process theory posits two independent neurobiological processes underlying psychopathy, scores on the two factors of the PCL-R are moderately correlated (r ~ 5; Hare, 2003). To further clarify how processes of the model contribute to tendencies considered psychopathic, it will be important in future work to utilize alternative assessment instruments that operationalize the affective-interpersonal and impulsive-antisocial components of psychopathy as more distinctive, less correlated dimensions (e.g., Lilienfeld's PPI—see, e.g.: Benning, Patrick & Iacono, 2005; Carlson et al., 2009). It will also be important to evaluate how varied phenotypic expressions of psychopathy, as embodied in alternative assessment instruments (cf. Patrick, Fowles, & Krueger, 2009), are reflective of mechanisms in the Two-Process theory, which posits distinctive etiological influences contributing to differing symptomatic features of psychopathy (Patrick & Bernat, 2009; see also: Patrick & Drislane, in press; Skeem et al., 2011).
A further limitation concerns our exclusive reliance on ERP responses elicited by affective and neutral picture stimuli to index cognitive and emotional processing deficits in psychopathy. It will be valuable in future studies to include additional measures representing different domains/levels of analysis (e.g., genetic, neuroanatomical, self-experiential, behavioral) in neuroscientific and psychophysiological studies of psychopathy and its components. Studies of this kind would contribute to a more in-depth “construct-network” analysis of psychopathy (cf. Patrick et al., 2013), entailing systematic delineation of patterns of covariation among multiple variables of interest, within- and across alternative domains of measurement representing differing levels of empirical analysis. More broadly, we encourage further systematic research on core dispositional liabilities as a basis for understanding the nature and etiology of psychopathy, and for connecting research on this important condition to work on other problems of clinical concern as well as work on healthy adaptive function.
Acknowledgments
The research reported here was supported by grants MH089727 and MH072850 from the National Institute of Mental Health. We are grateful to the residents and staff of the Drug Abuse Comprehensive Coordinating Office - Men's Department of Corrections Residential Treatment Center, in Tampa, FL.
Footnotes
The 90 affective pictures, listed by their IAPS identification numbers, were as follows: pleasant: 1440, 1463, 1721, 1722, 1750, 2071, 2150, 2160, 2311, 2340, 2530, 4180, 4210, 4232, 4250, 4607, 4652, 4659, 4664, 4670, 5470, 5621, 8030, 8050, 8080, 8170, 8180, 8185, 8370, 8400; neutral: 2210, 2214, 2215, 2372, 2393, 2495, 2499, 2870, 2890, 5731, 7000, 7002, 7009, 7010, 7020, 7034, 7038, 7041, 7050, 7090, 7100, 7130, 7180, 7233, 7490, 7491, 7500, 7510, 7595, 9070; and unpleasant: 1220, 1525, 3010, 3053, 3060, 3064, 3069, 3071, 3080, 3102, 3130, 3180, 3280, 3350, 3400, 3500, 3530, 3550, 6210, 6241, 6242, 6250, 6300, 6313, 6350, 3670, 6510, 6530, 6370, 6510, 6530, 6830, 9040.
Mean valence and arousal normative ratings (Lang et al., 2008) for each valence category were as follows: pleasant: valence (mean = 7.43, SD = 1.50); arousal (mean = 5.92, SD = 2.01); neutral: valence (mean = 5.06, SD = 1.16); arousal (mean = 2.96, SD = 1.90); and unpleasant: valence (mean = 2.65, SD = 1.56); arousal (mean = 6.13, SD = 2.22).
We tested for possible effects of demographic variables (age, race/ethnicity, education level) in supplemental analyses and did not find evidence that these variables accounted for associations between psychopathy scores and brain response. A detailed account of these supplemental analyses is available from the authors upon request.
Contributor Information
Noah C. Venables, Department of Psychology, Florida State University, Tallahassee, FL, USA.
Jason R. Hall, Department of Mental Health Law and Policy, University of South Florida, Tampa, FL, USA
James R. Yancey, Department of Psychology, Florida State University, Tallahassee, FL, USA
Christopher J. Patrick, Department of Psychology, Florida State University, Tallahassee, FL, USA.
References
- Anderson NE, Stanford MS. Demonstrating emotional processing differences in psychopathy using affective ERP modulation. Psychophysiology. 2012;49:792–806. doi: 10.1111/j.1469-8986.2012.01369.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, McNulty T. Comorbidities and continuities as ontogenic processes: Toward a developmental spectrum model of externalizing psychopathology. Development and Psychopathology. 2013;25:1505–1528. doi: 10.1017/S0954579413000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Iacono WG. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005;42:753–762. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Salekin RT, Leistico AR. Convergent and discriminant validity of psychopathy factors assessed via self-report: A comparison of three instruments. Assessment. 2005;12:270–289. doi: 10.1177/1073191105277110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development and Psychopathology. 2005;17:865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Thái S, McLaron ME. Visual P3 amplitude and self-reported psychopathic personality traits: Frontal reduction is associated with self-centered impulsivity. Psychophysiology. 2009;46:100–113. doi: 10.1111/j.1469-8986.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- Cleckley H. The mask of sanity: an attempt to reinterpret the so-called psychopathic personality. Mosby; Oxford, UK: 1941. [Google Scholar]
- Cleckley H. The mask of sanity. 5th ed. Mosby; St. Louis, MO: 1976. [Google Scholar]
- Cooke DJ, Michie C. Refining the construct of psychopathy: Towards a hierarchical model. Psychological Assessment. 2001;13(2):171–188. [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O'Connor S, Hesselbrock V, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Cuthbert NB, Kozak MJ. Constructing constructs for psychopathology: The NIMH research domain criteria. Journal of Abnormal Psychology. 2013;122:928–937. doi: 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation: a possible prelude to violence. Science. 2000;298:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dick DM. Identification of genes influencing a spectrum of externalizing psychopathology. Current Directions in Psychological Science. 2007;16(6):331–335. [Google Scholar]
- Dindo L, Fowles D. Dual temperamental risk factors for psychopathic personality: evidence from self-report and skin conductance. Journal of Personality and Social Psychology. 2011;100:557–566. doi: 10.1037/a0021848. [DOI] [PubMed] [Google Scholar]
- Drislane LE, Vaidyanathan U, Patrick CJ. Reduced cortical call to arms differentiates psychopathy from antisocial personality disorder. Psychological Medicine. 2013;43:825–835. doi: 10.1017/S0033291712001547. [DOI] [PubMed] [Google Scholar]
- Dvorak-Bertsch JD, Curtin JJ, Rubinstein TJ, Newman JP. Psychopathic traits moderate the interaction between cognitive and affective processing. Psychophysiology. 2009;46(5):913–921. doi: 10.1111/j.1469-8986.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC. The three arousal model: Implications of Gray's two- factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;17(2):87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Dindo L. Temperament and psychopathy: A dual-pathway model. Current Directions in Psychological Science. 2009;18:179–183. [Google Scholar]
- Gao Y, Raine A. P3 event-related potential impairments in antisocial and psychopathic individuals: A meta-analysis. Biological Psychology. 2009;82:199–210. doi: 10.1016/j.biopsycho.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion. 2008;8:250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hare RD. Temporal gradient of fear arousal in psychopaths. Journal of abnormal psychology. 1965;70:442–445. doi: 10.1037/h0022775. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised. 2nd ed. Multi-Health Systems; Toronto, Ontario, Canada: 2003. [Google Scholar]
- Hare RD, Neumann CS. The PCL-R assessment of psychopathy: Development, structural properties, and new directions. In: Patrick CJ, editor. Handbook of psychopathy. The Guilford Press; New York: 2006. pp. 58–88. [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WJ, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria: Toward a new classification framework for research on mental disorders. The American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Ishikawa SS, Raine A. Prefrontal deficits and antisocial behavior: A causal model. In: Lahey BB, Moffitt TE, Caspi A, editors. Causes of conduct disorder and juvenile delinquency. Guilford; New York: 2003. pp. 277–304. [Google Scholar]
- Jutai JW, Hare RD, Connolly JF. Psychopathy and event-related potentials (ERPs) associated with attention to speech stimuli. Personality and Individual Differences. 1987;8:175–184. [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Bates AT, Laurens KR, Hare RD, Liddle PF. Brain potentials implicate temporal lobe abnormalities in criminal psychopaths. Journal of Abnormal Psychology. 2006;115:443–453. doi: 10.1037/0021-843X.115.3.443. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Harem RD, Liddle PF, McDonald JJ. Reduced P300 responses in criminal psychopaths during a visual oddball task. Biological Psychiatry. 1999;45:1498–1507. doi: 10.1016/s0006-3223(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biological Psychiatry. 2000;48:210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson S, Iacono WG, McGue M. Etiological connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116(4):645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida; Gainesville, FL.: 2008. [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Widows MR. Psychopathic Personality Inventory—Revised (PPI R) professional manual. Psychological Assessment Resources; Odessa, FL: 2005. [Google Scholar]
- Lykken DT. The antisocial personalities. Lawrence Erlbaum Associates; Hillsdale, N.J.: 1995. [Google Scholar]
- Nelson LD, Strickland C, Krueger RF, Patrick CJ. Research domain criteria (RDoC) constructs as transdiagnostic predictors of clinical problems. 2014 Manuscript submitted for publication. [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: Startling new insights. Psychophysiology. 1994;31:319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Getting to the heart of psychopathy. In: Hervé H, Yuille JC, editors. The psychopath: Theory, research, and social implications. Lawrence Erlbaum Associates; Hillsdale, NJ: 2007. pp. 207–252. [Google Scholar]
- Patrick CJ, Bernat EM. Neurobiology of psychopathy: A two-process theory. In: Berntson GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioral sciences. John Wiley & Sons; New York: 2009. pp. 1110–1131. [Google Scholar]
- Patrick CJ, Bernat EM. Neuroscientific foundations of psychopathology. In: Millon T, Krueger RF, Simonsen E, editors. Contemporary directions in psychopathology: Scientific foundations of the DSM-V and ICD-11. Guilford; New York, NY: 2010. pp. 419–452. [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: startle reflex modulation. Journal of Abnormal Psychology. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Drislane LE. Triarchic model of psychopathy: Origins, operationalizations, and observed linkages with personality and general psychopathology. Journal of Personality. doi: 10.1111/jopy.12119. (in press) [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Durbin CE, Moser JS. Reconceptualizing antisocial deviance in neurobehavioral terms. Development and Psychopathology. 2012;24:1047–1071. doi: 10.1017/S0954579412000533. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology. 2009;21:913–938. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Hicks BM, Krueger RF, Lang AR. Relations between psychopathy facets and exetrnalizing in a criminal offender sample. Journal of Personality Disorders. 2005;19:339–356. doi: 10.1521/pedi.2005.19.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Kramer MD, Krueger RF, Markon KE. Optimizing efficiency of psychopathology assessment through quantitative modeling: Development of a brief form of the externalizing spectrum inventory. Psychological Assessment. 2013;25:1332–1348. doi: 10.1037/a0034864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Skeem JL. Psychopathy and brain function: Empirical findings and legal implications. In: Häkkänen-Nyholm H, Nyholm J, editors. Psychopathy and law: A Practitioner’s Guide. Wiley; New York: 2012. pp. 39–78. [Google Scholar]
- Pastor MC, Moltó J, Vila J, Lang PJ. Startle reflex modulation, affective ratings and autonomic reactivity in incarcerated Spanish psychopaths. Psychophysiology. 2003;40:934–938. doi: 10.1111/1469-8986.00111. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating the P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Venables PH. Enhanced P3 evoked potentials and longer P3 recovery times in psychopaths. Psychophysiology. 1988;25:30–38. doi: 10.1111/j.1469-8986.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Sadeh N, Verona E. Visual complexity attenuates emotional processing in psychopathy: Implications for fear-potentiated startle deficits. Cognitive, Affective, & Behavioral Neuroscience. 2012;12:346–360. doi: 10.3758/s13415-011-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: event-related brain potential studies. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction for ocular artifacts, applied to the P300. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Skeem JL, Ploaschek DL, Patrick CJ, Lilienfeld SO. Psychopathic personality: Bridging the gap between scientific evidence and public policy. Psychological Science in the Public Interest. 2011;12:95–162. doi: 10.1177/1529100611426706. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Vitale JE, Newman JP. Emotion among women with psychopathy during picture perception. Journal of Abnormal Psychology. 2002;111:610–619. doi: 10.1037//0021-843x.111.4.610. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Hall JR, Patrick CJ, Bernat EM. Clarifying the role of defensive reactivity deficits in psychopathy and antisocial personality using startle reflex methodology. Journal of Abnormal Psychology. 2011;120:253–258. doi: 10.1037/a0021224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat EM. Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology. 2009;46:75–85. doi: 10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E, Patrick CJ, Curtin JJ, Bradley MM, Lang PJ. Psychopathy and physiological response to emotionally evocative sounds. Journal of abnormal psychology. 2004;113:99–108. doi: 10.1037/0021-843X.113.1.99. [DOI] [PubMed] [Google Scholar]
- Venables NC, Hall JR, Patrick CJ. Differentiating psychopathy from antisocial personality disorder: A triarchic model perspective. Psychological Medicine. 2014;44:1005–1014. doi: 10.1017/S003329171300161X. [DOI] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ. Validity of the Externalizing Spectrum Inventory in a criminal offender sample: Relations with disinhibitory psychopathology, personality, and psychopathic features. Psychological Assessment. 2012;24:88–100. doi: 10.1037/a0024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ. Reconciling discrepant findings for P3 brain response in criminal psychopathy through reference to the concept of externalizing proneness. Psychophysiology. 2014;51:427–436. doi: 10.1111/psyp.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ, Hall JR, Bernat EM. Clarifying relations between dispositional aggression and brain potential response: Overlapping and distinct contributions of impulsivity and stress reactivity. Biological Psychology. 2011;86:279–288. doi: 10.1016/j.biopsycho.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Sellbom M, Sourander A, Kendler KS, Joiner TE, Drislane LE, Sillanmäki L, Elonheimo H, Parkkola K, Multimaki P, Patrick CJ. Separate and Interactive Contributions of Weak Inhibitory Control and Threat Sensitivity to Prediction of Suicide Risk. 2014 doi: 10.1016/j.psychres.2015.01.018. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey JR, Venables NC, Hicks BM, Patrick CJ. Evidence for a heritable brain basis to deviance-promoting deficits in self-control. Journal of Criminal Justice. 2013;41:309–317. doi: 10.1016/j.jcrimjus.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]