Abstract
Limitations of current treatment options for critical size bone defects create a significant clinical need for tissue engineered bone strategies. This review describes how control over the spatiotemporal delivery of growth factors, nucleic acids, and drugs and small molecules may aid in recapitulating signals present in bone development and healing, regenerating interfaces of bone with other connective tissues, and enhancing vascularization of tissue engineered bone. State-of-the-art technologies used to create spatially controlled patterns of bioactive factors on the surfaces of materials, to build up 3D materials with patterns of signal presentation within their bulk, and to pattern bioactive factor delivery after scaffold fabrication are presented, highlighting their applications in bone tissue engineering. As these techniques improve in areas such as spatial resolution and speed of patterning, they will continue to grow in value as model systems for understanding cell responses to spatially regulated bioactive factor signal presentation in vitro, and as strategies to investigate the capacity of the defined spatial arrangement of these signals to drive bone regeneration in vivo.
1. Need for bone tissue engineering
Over two million bone grafts are performed each year, at a cost of over $2.5 billion [1]. These procedures are used to heal acute injuries such as non-union fractures, critical size defects caused by injury or tumor resection, as well as chronic conditions such as congenital malformations. Treating these defects in the craniofacial region and extremities is important as bone serves as mechanical support, sites of muscle attachment, a barrier to protect vital organs, a framework for bone marrow, and a reservoir for ions [2]. Despite bone’s capacity for self-repair, existing treatments for large defects or non-union fractures all show limited success and/or are associated with complications [3]. For example, the utility of autografts, the gold standard for treatment, is limited by the supply of healthy tissue to graft as well as donor site morbidity, and alternatives such as allografts carry the risk of immune rejection or disease transmission [4]. Distraction osteogenesis comes with problems including prolonged treatment time, pain to the patient and potential infections at the pin sites [3]. Synthetic materials that simply act as void fillers may have limited integration with host tissue, and can exhibit minimal resorption, which inhibits replacement by new healthy bone tissue [4]. The limitations of these current treatments motivate bone regeneration using tissue engineering. Bone tissue engineering typically involves presenting physical and/or biochemical signals to transplanted or host cells which are capable of then responding to these signals, and forming new, functional bone tissue that can integrate with surrounding host tissue. Biochemical signals can be in the form of soluble bioactive factors, such as growth factors, genetic material, and drugs and small molecules, and they can be delivered to cells from a variety of biomaterials, with both temporal and spatial control.
Bone has been a tissue of much research and clinical interest since the early days of tissue engineering [5, 6]. Researchers worked to understand how to leverage bone’s capacity for self-repair of smaller defects when designing systems to heal larger ones. It quickly became apparent that it would be valuable to harness biochemical signaling molecules present during natural bone healing, either by delivering these molecules themselves or other factors that can drive bone regeneration. For example, bone morphogenetic proteins (BMPs) released from collagen sponges have been used for clinical treatment of femur and tibia fracture non-unions [7-9] and spinal fusions [10-12]. While these sponges have demonstrated success in their approved applications, the systems provide minimal control over the growth factor release in time and space, and the surrounding tissue is exposed to much higher BMP concentrations than present during natural healing [13, 14]. As a result, these systems have led to vertebral body bone resorption [15], and swelling that causes significant side effects when used in the anterior cervical spine [16]. These limitations inspired the development of systems to better control the delivery of bioactive factors in time and space. A number of excellent papers review progress in the controlled delivery of bioactive factors for bone regeneration, predominantly describing accomplishments in temporal control of their release profiles [17-19]. Recently, a great deal of exciting new research has been performed to develop systems that are not only capable of temporal control, but also able to spatially direct the presentation of desired bioactive factors. This review focuses on thoroughly exploring strategies for the controlled spatial presentation of therapeutic molecules for tissue engineering, with an emphasis on bone regeneration.
2. Motivation for spatial control of bioactive factor delivery
While most early bioactive factor delivery work was done from homogeneous, bulk materials [20], recent research has focused on tailoring the spatiotemporal presentation of these factors. Temporal control is advantageous to allow the bioactive factor to be released over the time course necessary to achieve the desired cellular responses without the need for repeated dosing. Some efforts have been made to recapitulate the timing of signal presentation to match that of bone development and healing [17, 21]. Similarly, during these processes cellular gene expression and extracellular matrix production are all tightly controlled in space [22], motivating the development of scaffolds with control over spatial presentation of bioactive factors that direct these behaviors.
2.1 Recapitulating bone development and healing
Microenvironmental signals presented during bone development and healing, including soluble factors, are highly regulated, motivating the control of bioactive factor presentation in biomimetic approaches for bone tissue engineering. Efforts to regenerate bone tissue often seek to recapitulate one of the two main pathways for bone development: intramembranous ossification or endochondral ossification [23]. In intramembranous ossification, mesenchymal stem cells (MSCs) respond to a growth-factor laden microenvironment and some differentiate directly into osteoblasts, or bone-building cells, laying down randomly oriented bone matrix that is later remodeled to form mature bone [24]. In contrast, during endochondral ossification, bone is laid down following development of a cartilage template [25]. Here, MSCs differentiate into chondrocytes that hypertrophy, calcify their extracellular matrix (ECM), and secrete specific matrix metalloproteases and growth factors triggering vascular invasion; this brings progenitor cells that can become osteoblasts, again to form immature bone tissue that will be remodeled over time [26]. Bone tissue engineering efforts have attempted to recapitulate both intramembranous and endochondral ossification, but the bioactive factors used and their spatiotemporal distribution will depend on which process is being pursued to drive bone formation.
Since bone maintains a unique capacity for self-repair of small defects throughout life [27], this healing process can also serve as a roadmap for bone regeneration by tissue engineering. Briefly, an acute inflammatory response occurs as a reaction to a bone fracture, with a hematoma, or localized collection of blood at the injury site, bringing immune cells that secrete highly regulated pro-inflammatory cytokines, fibroblasts that form granulation tissue, and growth factors and progenitor cells that will participate in the repair. This acute inflammation peaks at 24 hours after injury, and is complete within 7 days [28]. Growth factors produced by cells at the fracture site, including stromal-derived growth factor-1 (SDF-1) and vascular endothelial growth factor (VEGF), are key for recruiting MSCs and inducing vascular formation at the location of injury. These cells then differentiate into osteoblasts to form a bony collar around the fracture site, and into chondrocytes which deposit cartilaginous matrix in the interior, also under the influence of growth factors, especially the transforming growth factor-β (TGF-β) superfamily which includes the BMPs [29]. As the cartilaginous template is replaced by a mineralized bone matrix, vascular morphogenetic proteins, especially VEGF and angiopoietins, are responsible for regulating neoangiogenesis to provide a vascular supply to the newly formed bone [30]. After a structure of immature woven bone is formed to bridge the fracture gap, it is remodeled by the same process used to maintain all bones in the body to achieve the architecture and biomechanical properties of mature lamellar bone. Similar to bone development, this process is regulated by growth factors with very controlled spatiotemporal presentation [27], and some excellent reviews describe it in detail [27, 31-33]. These bioactive factors can be presented from tissue engineering scaffolds in biomimetic approaches to tissue regeneration aimed at recapitulating the native presentation of these signals to cells in both time and space [34].
2.2. Bone interfaces
Another biological motivation for creating bone tissue engineering scaffolds with spatially controlled presentation of bioactive factors is the presence of interfaces between bone and other tissues, including cartilage, ligament and tendon. Given the importance of bone’s connections to its associated musculoskeletal connective tissues for restoring movement, when these interfaces are damaged, their repair is essential to the success of tissue engineered bone. At bone transitions to the aforementioned other tissues, calcified cartilage or fibrocartilage interface directly with the bone [35]. In vivo, these interfaces are not discrete zones with sharp transitions in properties, but instead composed of physical and biochemical gradients. ECM molecule, growth factor and cell type, composition and organization, as well as mechanical properties, all shift gradually between the different tissues [36]. Notably, the presence of mechanical property gradients facilitates continuous load transfer between two different tissue types [36]. Recapitulating such gradients of bioactive factors in scaffolds for bone tissue engineering may influence cell phenotype, which can in turn affect their differentiation state and ECM production and organization. These changes may then lead to differences in resultant tissue mechanical properties, mimicking those seen in vivo. Biomimetic approaches aimed at recreating these transitions zones can utilize spatially restricted bioactive factor presentation from biomaterials, often in addition to spatial variation in scaffold physical parameters such as stiffness and porosity [37, 38].
2.3 Vascularization
Vascularization is not only important for bringing oxygen and nutrients and removing waste products from adult bone, but is also essential to regulation of bone development and remodeling bone development [22, 39]. In fact, bone formation is impaired in mice lacking VEGF, a key vasculogenic signaling molecule [40]. In regenerating bone, osteoblasts produce VEGF, among other factors, to induce local angiogenesis [41], but this growth factor also promotes differentiation of progenitor cells into osteoblasts [42]. Similarly, smooth muscle and endothelial cells produce growth factors during bone formation, including BMP-2 and platelet-derived growth factor (PDGF), to enhance osteogenic differentiation of osteoblast progenitors and mineralization by mature osteoblasts [43-45]. Because the location of cells that secrete these growth factors is tightly regulated in vivo [22], it may be desirable to develop biomaterial systems to control the delivery of bioactive factors in space, specifically providing local angiogenic signals to encourage vascular development alongside osteogenesis. Early work has shown that combined delivery of BMP-2 and VEGF led to improved osteogenesis by human MSCs (hMSCs) in an ectopic mouse model [46]. Other studies also support dual delivery of BMPs and VEGF for enhancing osteogenesis, but addition of VEGF did not lead to enhanced vascular networks compared to BMP-2 alone [47-49]. However, a combination of growth factors uniformly distributed throughout a scaffold may not be ideal for vascularized bone tissue engineering. Systems allowing spatiotemporally controlled delivery of multiple factors could segregate the osteogenic from the angiogenic signals, potentially resulting in improved vasculature in engineered bone.
3. Important Bioactive Factors for Bone Tissue Engineering
Bone tissue engineering is a broad field: in addition to a variety of cell types and biomaterial scaffolds explored, a large number of technologies have been developed to deliver bioactive factors including growth factors, genetic material, and drugs or small molecules. Understanding the structure and function of these factors is important in engineering the systems for their delivery.
3.1 Growth factors
Growth factors are soluble signaling proteins secreted by cells to induce specific biological responses such as cell survival, migration, differentiation and proliferation [50]. They act by binding to cell surface receptors, and the complex may or may not be internalized by the cell. The binding event can affect gene expression when, for example, the receptor is then phosphorylated which induces receptor conformational changes that sets off signaling cascades within the cell [51]. Alternatively, internalized growth factor-receptor complexes can go on to phosphorylate intracellular signal transduction proteins, including transcription factors that when activated can translocate to the nucleus and regulate gene activation [52] . Growth factor production follows a distinct time course throughout osteoprogenitor cell differentiation and maturation [53]. These growth factors tend to diffuse only short distances through the ECM, and act on cells near the site of their production. They are subject to proteolytic degradation, and the half-life for their biological activity is on the order of hours [54]. Additionally, they only act on cells expressing their receptors, which are highly regulated in vivo, allowing for additional specificity in their biological effects [55, 56]. As an example, one growth factor, fibroblast growth factor-2 (FGF-2), causes MSCs in various states of differentiation to upregulate other growth factors, but the magnitude of the effect and the relative increases in expression are dependent on the cell differentiation state [57].
While the BMPs have been most frequently used in bone tissue engineering, the range of growth factors used, alone and in various combinations, is extensive. These include BMP-2, BMP-4, BMP-7, FGF-2, TGF-β1, TGF-β2, TGF-β3, VEGF, insulin-like growth factor (IGF-1), PDGF and SDF-1 [58, 59]. While these growth factors are found at very low concentrations at fracture sites, on the order of pg/mL to single digit ng/mL [60], current clinical therapies often require much greater quantities of growth factor to positively impact bone formation: for example, Osigraft® contains 3.5 mg of BMP-7 per package, with some surgeons using more than one package to treat a bone defect [61]. To produce these large quantities of growth factor for laboratory research and clinical applications, recombinant DNA technology permits the synthesis of human growth factors in hosts including bacteria and mammalian cell lines. These recombinant human growth factors have been safely used in patients for decades [62]. However, synthesizing growth factors in quantities sufficient for clinical use comes at high cost: a 2008 study found that when the BMP-7 system described above was used to treat tibial fractures, the cost of the growth factor alone was £3000 (~$6000) [63]. New production techniques have the potential to reduce the cost of recombinant human growth factors, which could facilitate more clinical translation [64]. Another alternative is synthetic peptides that mimic growth factor activity. These shorter peptide sequences still activate the growth factor receptors, but are smaller molecules that can easily be modified with chemical groups to control their presentation. A number of BMP-2 mimicking peptide sequences have been used to stimulate osteogenic behavior in vitro [65, 66] and in vivo [65, 67, 68]. Peptide sequences that mimic other growth factors important for bone formation, such as analogues for FGF-2 [69] and VEGF [70], have also been identified and shown to have bioactivity.
Sustained presentation of BMP-2, the growth factor most often used for bone regeneration, is important: in vivo delivery of the growth factor over four weeks led to significantly improved ectopic bone formation compared to burst release of the same amount of BMP-2 [71]. This effect is likely because the BMP-2 presentation more closely mimics the signaling cascade after a bone fracture: osteoprogenitor cells upregulate BMP-2 expression for approximately 21 days at the site of injury [32]. More recently, work has been done studying the combinatorial effects of growth factors and the time course of their presentation. In one case, BMP-2 and IGF-1 delivered together did not lead to osteogenic differentiation of mouse pluripotent stem cells, but early delivery of BMP-2 alone followed by increased release of both growth factors led to matrix mineralization [72]. Research has also demonstrated that growth factor-induced blood vessel formation may also benefit from controlled release. For example, in one study early release of a vasculogenic growth factor combined with a more sustained presentation of an osteogenic growth factor improved in vivo ectopic bone formation [73]. In contrast, others reported that osteogenic growth factor release kinetics was critical to ectopic bone formation, and the timing of vasculogenic growth factor presentation was less important [74]. These discrepancies warrant further investigation, which can be undertaken with the many synthetic and natural polymers, as well as ceramics, that have been explored as carrier materials for growth factor delivery in bone engineering systems [18]. Past work on temporal control of the delivery of these growth factors for bone regeneration has been previously described in several thorough reviews [17, 18, 59, 75, 76].
3.2 Genetic material
Delivery of genetic material provides a potential alternative to delivery of growth factors; nucleic acids, including DNA and RNA, can induce changes in gene expression at the transcriptional or post-transcriptional levels. Since there is now a strong understanding of the processes of bone development and repair, there exist known candidate genes that can be used to stimulate bone regeneration or inhibit antagonistic pathways [77]. Genetic material affecting these processes has been studied extensively in 2D cell culture experiments and incorporated into 3D biomaterial scaffolds [78-80].
DNA can encode the same growth factors described in the preceding section. Targeted cells can take up the delivered DNA and then express proteins that may aid in healing a defect. Modifying gene expression eliminates some concerns associated with delivering high concentrations of recombinant human growth factors: the cost and risk of unwanted physiological reactions are decreased because large quantities of expensive proteins are not required, cells continue to produce the growth factor so there is no concern of loss of bioactivity over time, and post-translational modifications are performed by host cells reducing the risk of an immune response to the proteins [79].
DNA that is intended to encode for new protein production must first enter the cell and then reach the nucleus. This can be accomplished using viral or non-viral approaches [81]. As a whole, viral vectors are known for their high transduction efficiency but also potential antigenicity. Since they do not require carriers for their uptake, viral vectors encoding BMP-2 have been injected directly into bone defects [82] or adsorbed onto the surface of polymer scaffolds implanted into bone defects [83] and shown to improve bone healing. Viral vectors differ in their size, cytotoxicity, whether or not they require dividing cells and whether they lead to integration of their cargo into host cell DNA. A thorough review summarizes the advantages and disadvantages of viral vectors that have been used to carry genes for bone regeneration [84]. Once the bone regeneration process is complete, it is usually undesirable for the genes of interest to have permanently integrated into the host genome, as occurs with retroviral and lentiviral vectors [81, 85]. As a result, though they can result in an immune response, recombinant adenoviruses have been the most frequently used viral vectors in bone engineering, as they can be cleared from the body instead of integrating into the genome [79]. Non-viral delivery systems can address some of the drawbacks of viral delivery: they show decreased immunogenicity, and improved safety due to transient effects on gene expression [86]. However, the key challenge of non-viral delivery is that plasmid DNA (pDNA) is a large and negatively charged macromolecule with limited ability to penetrate the negatively charged cell membrane on its own [87]. To overcome this issue, pDNA is typically complexed with cationic lipids or polymers into nanoparticles. These carriers can protect the pDNA from enzymes such as DNAses, and facilitate endocytosis so the pDNA can enter the cell and achieve gene expression [88]. Though much early work utilized polyethyleneimene (PEI) [89] or cationic lipids [90] to complex with DNA to promote entry into the cell, researchers today are developing other synthetic polymers that can be used as non-viral gene carriers to avoid potential cytotoxicity, and are additionally functionalized to improve targeting to the cell population of interest [88]. An alternative to DNA sequences that must enter the nucleus and be transcribed, antisense oligonucleotides are short, single strands of DNA that can pair with complementary mRNA and inhibit its translation [91]. Target sequences have been identified to regulate diverse and clinically relevant functions such as multipotent hematopoietic progenitor cell proliferation [92] and osteoclast bone resorption [93, 94]. Several excellent reviews summarize work on DNA transfection for bone tissue engineering, elaborating upon target genes, transfection modes, in vivo applications, and safety concerns [79, 81, 86, 95]. Controlled release of DNA from many different biomaterial scaffolds has also been demonstrated [96, 97]. Importantly, these systems protect the DNA until it is released, permitting delivery of the DNA, with carrier if needed, to the cell for subsequent uptake, transport to the nucleus and resulting biological effects.
In addition to DNA delivery, genetic material in the form of RNA can also be delivered from biomaterials. While most DNA is introduced to cells to increase the expression of a target gene, new discoveries in the field of RNA interference (RNAi), non-coding RNA sequences which lead to targeted degradation or impaired translation of select mRNA sequences, hold great promise for silencing gene expression at the post-transcriptional level [98, 99]. RNAi involves short interfering RNA (siRNA), short double stranded RNA sequences of which one strand can perfectly base pair with a specific complementary mRNA sequence and induce its degradation, or microRNA (miRNA), similar single stranded sequences which have incomplete base pairing with their target mRNA sequences, allowing them to affect a number of similar mRNAs instead of only one specific sequence [100]. As the field of biology enhances our knowledge of pathways antagonistic to osteogenesis, this technology allows the blocking of relevant genes as a way to enhance osteogenesis. Here, the host genome is not changed, adding to the safety of RNAi. siRNA has been used to silence noggin, a BMP-2 antagonist, to induce ectopic bone formation in mice [101], to knock down chordin, another BMP-2 antagonist, to enhance osteogenic differentiation of hMSCs [102], and to knock down the receptor activator of nuclear factor κB (RANK) to inhibit bone resorption [103]. A number of miRNAs have also been shown to play a role in bone development [104], osteogenic differentiation [105] and vascularization [106]. A variety of systems have been developed for localized presentation of interfering RNA molecules [80, 107], including sequences that stimulate osteogenesis [108]. As more siRNA and miRNA targets for osteogenesis are identified, spatiotemporal control of interfering RNA delivery may be a useful tool to help recapitulate the process of bone development.
3.3 Drugs and small molecules
There are many drugs and small molecules that may be valuable for bone regeneration by serving antibiotic, anti-inflammatory or osteotrophic roles. Antibiotics are used to control infections at a surgical site – in the case of medical devices, bacterial infections pose a significant risk of increased pain, medical costs, and likelihood of device failure [109]. As a result, there is much interest in controlling antibiotic presentation from medical devices [109, 110]. Similarly, controlled antibiotic release from biomaterials may also be used to avoid infections in bone tissue engineering strategies. A number of systems, mostly comprised of ceramic composites, have been designed to present antibiotic agents, including gentamicin, tetracycline, vancomycin and silver, from materials often used for bone tissue engineering [111]. Antibiotic delivery is also used clinically in bone repair: the Masquelet technique releases antibiotics to prevent infection at the surgical site while a vascularized membrane, a pseudo-periosteum, grows around it; 4-12 weeks later, the synthetic spacer is removed and replaced with autografted bone tissue, which is supported biologically by the induced vascularized membrane [112, 113]. Implanting a biomaterial system in the body causes local inflammation, motivating the use of anti-inflammatory drugs to minimize the immune response around the implanted scaffold [114]. These drugs can be glucocorticoids, most typically dexamethasone [115], or non-steroids, including ibuprofen [116]. Localizing both antibiotics and anti-inflammatories to the implant site avoids side effects associated with systemic delivery (e.g., oral or intravenous administration). This review will not focus on delivery of these agents because control over their spatial presentation may be less likely to affect osteogenesis.
Osteogenic drugs have also been delivered from tissue engineering scaffolds with favorable outcomes. Bisphosphonates, which are widely used in the treatment of osteoporosis because they prevent bone resorption, have been released with a degree of control from biomaterial scaffolds, showing concentration-dependent inhibition of osteoclast activity [117, 118]. While these results are limited to in vitro studies, this approach may hold promise especially for repairing bone in patients with a bone disease causing increased bone resorption. Fluvastatin and simvastatin, members of the statin family, have been found to induce bone formation [119-121]. Their release from biomaterial scaffolds was shown to promote osteogenic differentiation of hMSCs [122] and MC3T3 mouse preosteoblast cells [123], and regeneration of nasal bone defects in rabbits [124]. Lastly, parathyroid hormone (PTH) has also been shown to enhance bone formation [125]. With PTH, delivery control is especially important, as continuous exposure can result in bone resorption, but pulsatile, intermittent administration can lead to enhanced bone formation [126, 127]. For this reason, temporal control may enhance the effectiveness of PTH as an osteoinductive agent in bone tissue engineering.
4. Strategies for temporal control over bioactive factor delivery
A wide variety of biomaterial delivery systems have been developed for temporal control of bioactive factor presentation, and many of these systems can be exploited for spatial control as well [128]. For this reason, this review will first summarize techniques for varying the release kinetics of bioactive factors. Most strategies for presentation of bioactive factors from scaffolds include physical entrapment of the factor in the biomaterial; if the factor is free to move through the material then diffusion governs release, otherwise scaffold degradation is the rate limiting step. If a free biomolecule has affinity for the biomaterial, its diffusion out of the scaffold is slowed, leading to more sustained presentation. Alternately, the factor can be covalently tethered to the material, which localizes it to the scaffold until the material degrades or the bond is broken. Lastly, a system can be designed such that an external stimulus triggers the release of the biomolecule. In all cases, the biomolecule carrier system must protect the bioactivity of the bioactive factor while also delivering it at appropriate concentrations over a desired time frame. These factors are functions of the bioactive factor of interest.
Diffusion-based release of a bioactive factor physically entrapped in a biomaterial is the simplest approach, but typically achieves the least control over the timing and location of delivery. The bioactive factor is loaded into the bulk of a biomaterial scaffold, usually by mixing it into a solution before it solidifies or gels or by rehydrating a lyophilized scaffold with solution containing the bioactive factor. These biomaterial scaffolds can then protect the loaded bioactive factor from enzymes in the body; in this way its bioactivity is preserved until it is released to cells [128]. The release kinetics are a function of the ability of the molecule to diffuse out of the scaffold, which is affected by interactions between the scaffold and the biomolecule, as well as the scaffold pore size, architecture and degradation, which changes the pore structure and swelling over time. In purely diffusion-based systems, release profiles are often characterized by an initial burst: free molecules of interest are quickly driven outside of the scaffold by a steep concentration gradient [129]. While this may be desirable in the case of certain molecules, some tissue engineering strategies may require more sustained presentation of the bioactive factors [20]. Additionally, a burst may necessitate higher initial loading because a potentially large fraction of the available biomolecules will be released during the burst [129]; high initial local concentrations may also have adverse effects. When degradation governs delivery, usually by hydrolysis or activity of cell-secreted enzymes, release profiles depend on the scaffold degradation kinetics. These kinetics can be a function of a number of factors, including the molecular weight, concentration and hydrophobicity of the base polymer, the degree of crosslinking and swelling, pH changes due to degradation products, applied mechanical stress/strain and the mode of degradation [130]. While hydrolytic degradation occurs at similar rates in different areas of the body, enzymatic degradation depends on the local concentrations of enzymes, which are often a function of local cellular activity, and release profiles will vary depending on the tissue microenvironment [131].
Many material systems slow diffusion by various intermolecular interactions, permitting more sustained release over days, weeks, or even months compared to diffusion alone. These methods rely on affinity interactions, noncovalent binding that can result from associations between molecules of opposite charge, hydrogen bonding, van der Waals forces or hydrophobic interactions between bioactive factors and the biomaterial to similarly slow their diffusion out of the scaffold [128]. An example of how affinity interactions are used to delay diffusion takes advantage of the net electrostatic charge on some growth factors. BMP-2, TGF-β1, FGF-2 and VEGF, which all have been explored for bone tissue regeneration, carry a net positive charge at physiological pH [132]. These growth factors will thus form polyionic complexes with negatively charged biomaterial matrices such as some gelatins. These electrostatic interactions will slow diffusion, and can serve as the basis of controlled delivery systems [133]. Similarly, DNA itself has a negative charge at physiological pH, but as mentioned previously, it is often complexed with cationic polymers to yield particles of net positive charge [134], which may be exploited to slow the release of DNA from a charged biomaterial matrix . RNA molecules also exhibit negative charge, and electrostatic interactions have been harnessed to achieve localized and controlled release from a biomaterial for sustained gene knockdown for two weeks [135]. Another commonly exploited affinity interaction is growth factor delivery binding to heparin or its derivatives [136]. BMP-2, TGF-β1, FGF-2 and VEGF all exhibit heparin affinity [137-139], and exploiting these interactions can yield more delayed release systems for bone tissue engineering.
Covalent immobilization of a bioactive factor to a biomaterial allows for long-term presentation by delaying diffusion until the scaffold degrades or the covalent bond is broken; a number of these systems have been developed for tissue regeneration [140]. BMP-2 has been covalently coupled to materials such as glass coverslips or slides for in vitro studies, or biomaterial scaffolds including poly(lactic-co-glycolic acid) (PLGA), chitosan, type I collagen, and polycaprolactone (PCL), all with the goal of bone repair [140]. Such presentation may be relevant to tissue engineering because some growth factors in the in vivo environment are sequestered in the ECM by affinity interactions and act without being taken up by cells [141]. For example, tethered BMP-2 has been shown to have increased bioactivity compared to the same amount of free BMP-2: it is not internalized and instead can continue to activate its receptor [142, 143]. Especially relevant for spatial patterning, these coupling reactions can be photo-initiated: the growth factor is first functionalized with a photoreactive group, such as a phenyl azide or acrylate group, and then bound to a biomaterial in the presence of ultraviolet (UV) light, which can be spatially restricted [140].
Finally, stimuli-responsive growth factor delivery systems allow for the creation of dynamic microenvironments with on-demand release. Here, the bioactive factor is released in response to a cell-mediated or externally applied physical or biochemical trigger [144]. For example, matrix metalloproteinase (MMP)-degradable linkages in the backbone of hydrogels can be broken down by cell-secreted MMPs to release BMP-2 [145]. Additional work has been pursued using stimuli ranging from magnetic fields [146], mechanical loading [147] and ultrasound waves [148] to release various bioactive factors. These stimuli-responsive tools are amenable to spatial patterning of growth factor release when the stimuli can be applied to specific regions of a biomaterial.
5. Spatially controlled delivery technologies
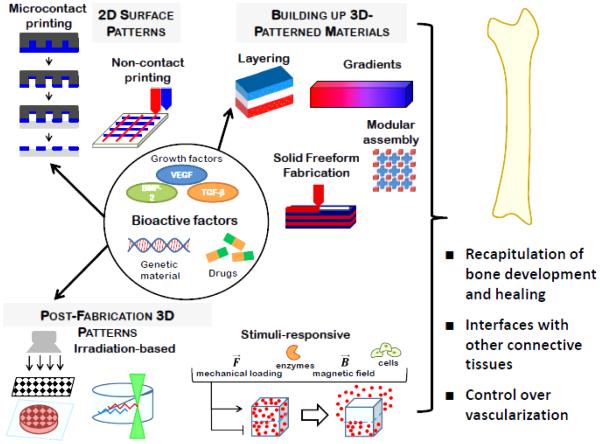
Given the role local presentation of signals may have on the formation of complex tissues, a wide variety of technologies have been engineered to regulate the spatial organization of bioactive factors, and many of these have been applied for bone regeneration. These technologies, illustrated in Figure 1, vary in their complexity and degree of control they allow. This section describes the scientific basis behind each approach, highlighting their use in bone tissue engineering. A summary of the approaches that have been used to drive local osteogenesis by spatially controlling the presentation of bioactive factors is presented in Table 1. Many additional patterning techniques that have not yet been applied to bone regeneration, but have this potential, are also described throughout this section, and summarized in Table 2.
Figure 1.
Schematic illustrating technologies to spatially control bioactive factor presentation.
Table 1.
Examples of spatially controlled patterning of biomolecules for osteogenic applications
| Pattern Creation Technique |
Scaffold Material |
Patterned Molecule(s) |
Cells | Model | Outcome(s) | Ref. |
|---|---|---|---|---|---|---|
| Noncontact printing: Inkjet |
Fibrin | FGF-2 | Human MG- 63 cells |
In vitro | Proliferation dependent on FGF-2 concentration |
[174] |
| Fibrin | BMP-2, BMP-2/ FGF-2 |
Mouse C2C12 cells |
In vitro | Localized osteogenic and tenogenic differentiation |
[176, 177] | |
| Decellularized skin allograft |
BMP-2 | Mouse C2C12 cells |
In vitro | Localized osteogenic and myogenic differentiation |
[179] | |
| BMP-2/ SDF-1/ TGF-β1 |
None | Mouse calvarial defect |
Localized in vivo bone formation |
[179, 180] | ||
| Osteochondral layered scaffolds |
OPF/gelatin microparticles |
TGF-β1 or TGF-β3 |
Rabbit MSCs |
In vitro | Localized osteogenic and chondrogenic differentiation of rabbit MSCs |
[194, 195] |
| PLGA microspheres in segmented polyurethane |
TGF-β1 or BMP-2 |
None | Rabbit knee osteochondral defect |
Formation of new hyaline- like cartilage tissue |
[196] | |
| Gelatin sponges |
Platelet rich plasma, BMP-2, β-TCP |
Horse MSCs | Horse talus osteochondral defect |
Visible defect repair | [198] | |
| Chitosan/ gelatin/ hydroxyapatite |
Plasmid DNA for TGF-β1 and BMP-2 |
Rabbit MSCs |
Rabbit knee osteochondral defect |
Support of both cartilage and subchondral bone formation |
[199] | |
| Cell aggregates with gelatin/ hydroxyapatite microparticles |
TGF-β1 and BMP-2 |
Human MSCs |
In vitro | Localized osteogenic and chondrogenic differentiation |
[200] | |
| Gradient formation |
PLGA and silk microspheres in alginate |
BMP-2 and IGF-1 |
Human MSCs |
In vitro | Gradient of osteogenic and chondrogenic differentiation |
[213] |
| Sintered PLGA microspheres |
BMP-2 and TGF-β1 |
Human MSCs | In vitro | Localized osteogenic and chondrogenic differentiation |
[214] | |
| BMP-2 and TGF-β1 |
None | Rabbit knee osteochondral defect |
Defect filled with bony ingrowth and overlying cartilage layer |
[215] | ||
| Alginate | BMP-2 and TGF-β1 |
Human MSCs |
In vitro | Gradient of osteogenic and chondrogenic differentiation |
[206] | |
| PCL nanofiber mesh |
Insulin and β- glycerophosp hate |
Human ASCs |
In vitro | Localized osteogenic and chondrogenic differentiation |
[216] | |
| Collagen and PLL |
DNA encoding Runx2 |
Rat fibroblasts |
In vitro | Mineralization dependent on DNA gradient |
[217] |
Table 2.
Biomaterial scaffolds with spatially patterned presentation of biomolecules for applications not specifically focused on osteogenesis
| Patterned Molecule(s) |
Surface or 3D Pattern |
Pattern Creation Technique |
Scaffold Material | Ref. | |
|---|---|---|---|---|---|
| Inert markers |
Fluorescent dye | 3D | Multiphoton excitation |
Agarose | [285, 286] |
| Vacuum molding | Collagen/ chitosan |
[245] | |||
| Lock-and-key assembly |
PEGDA | [248] | |||
| Subunit micromanipulation |
[235, 244] | ||||
| 3D printing | [268] | ||||
| Polyvinyl alcohol | Surface | Microcontact printing |
Human lens capsule |
[156] | |
| Cell adhesion molecules |
RGD-containing peptides |
Surface | UV laser light | PEGDA | [186] |
| PEG on silicon wafers |
[190] | ||||
| 3D | UV light projection through a photomask |
PEGDA | [277-279] | ||
| Agarose | [280] | ||||
| Alginate | [282] | ||||
| Multiphoton excitation |
PEGDA | [289-291] | |||
| Proteins | VEGF | Surface | UV light projection through a photomask |
PEGDA | [188] |
| PDGF and FGF- 2 |
[187] | ||||
| VEGF and FGF- 2 |
Surface | Electron beam | PEG on silicon wafers |
[189] | |
| VEGF/anti-VEGF antibody |
3D | Layering | Porous PLGA | [203] | |
| VEGF | 3D | 3D printing channels |
Hydroxyapatite | [257] | |
| FGF-2 | 3D | Stereolithography | PEGDA / heparan | [268] | |
| Gradient | PEGDA | [212] | |||
| Multiphoton excitation |
Agarose | [287] | |||
| Sonic hedgehog and CNTF |
3D | Multiphoton excitation |
Agarose | [288] | |
| Genetic material |
Biotinylated DNA | Surface | Electron beam | PEG-coated glass | [191] |
| Plasmid DNA for GFP |
Surface | Noncontact printing |
Collagen | [181] | |
| Cells in monolayer on tissue culture plastic |
[184] | ||||
| siRNA | 3D | UV light projection through photomask |
Dextran | Alsberg lab unpublished data |
|
| Activation of heat-sensitive luciferase gene switch |
3D | Localized ultrasound application |
Fibrin | [304] | |
5.1 Generating patterns of bioactive factors on scaffold surfaces
There is a great deal of interest in biomaterial surfaces, both as cell culture tools that allow the investigation of basic science questions, and to regulate seeded cell behavior or that of host cells that come in contact with the surface shortly upon implantation for enhancing tissue regeneration. Spatial patterning of bioactive factors on these surfaces has been extensively explored using a variety of innovative technologies, many of which have exciting potential for bone tissue engineering.
5.1.1 Microcontact printing
Lithographic techniques developed by the microelectronics industry for manufacturing integrated circuits and printed circuit boards have been adapted by bioengineers to create micro- and nano-patterned biomaterials. Biocompatible soft lithography can be used to engineer elastomeric stamps and molds with a minimum feature size on the order of tens of nanometers [149]. One technique that has been especially useful for controlling bioactive factor presentation for tissue engineering is microcontact printing. Developed by the Whitesides group, the procedure employs a polydimethylsiloxane (PDMS) stamp made using standard photolithography techniques [150].The stamp is coated by immersion in “ink,” a solution containing the biomolecule of interest, and then direct contact transfers the biomolecule from raised features of the stamp onto a substrate [151]. Some of the first work implementing microcontact printing to study spatial control of cell behavior used printed islands of fibronectin, a cell-adhesive ECM molecule, onto a non-adhesive hard substrate; these studies showed that cell spreading could be limited by controlling the size of the adhesive islands, and were integral to understanding how cell shape controls cell behavior [152, 153]. Microcontact printing was also used to print fibronectin onto substrates coated with poly(N-isopropylacrylamide) [154, 155], a thermoresponsive material that cells can grow on at 37°C, but that undergoes a lower critical solution temperature phase transition when cooled. Decreasing the temperature caused the cells that grew on areas coated in fibronectin to be released as sheets with controlled geometry. In a particularly clinically relevant example, polyvinyl alcohol, a biocompatible polymer that inhibits cell growth and attachment, was printed onto human lens capsule tissue for retinal transplantation in a hexagonal grid micropattern. The organization of retinal or iris pigment epithelial cells was controlled when seeded on the patterned lenses: the cells maintained a globular, epithelioid shape on patterned substrates, as compared to spindle-shaped cells on unpatterned substrates, better mimicking the orientation and shape lost in age-related macular degeneration [156].
While the controlled presentation of ECM signals is valuable, these tools can be applied for printing materials that present other bioactive factors specifically relevant to bone tissue engineering in a spatially regulated way. For example, microcontact printing can stamp solutions containing growth factors, genetic material and/or small molecule therapeutics, or a biomaterial macromer solution containing one or more of these factors, either free or covalently bound, for immediate release or more sustained presentation. In addition, several different stamps can be used to pattern more than one factor onto a single substrate, and backfilling (i.e. modifying the unstamped regions with a polymer like polyethylene glycol (PEG), which does not have intrinsic bioactivity) to create a neutral base [157]. Microcontact printing has been applied to create DNA microarrays [158, 159], to stamp specific proteins or gradients of proteins like bovine serum albumin [160, 161], to stamp controlled patterns of antibodies, which may then bind growth factors of interest [162], to stamp avidin patterns which can then bind biotinylated proteins [163] and to use antibodies on a stamp to select for specific proteins of interest in a solution before stamping them onto a substrate [164-166]. These systems permit examination of the role of these 2D biomolecule patterns in inducing local cell behaviors, including those relevant to osteogenesis.
5.1.2 Non-contact printing
Commercial printer technology has also been embraced by the biomaterials community to achieve high resolution spatial control over substrate surface properties to guide cell behavior. The most common of these, inkjet printing, is a non-contact technique which uses thermal, piezoelectric or magnetic triggers to release ink droplets of volumes ranging from 10 to 150 pL from a nozzle whose position can be carefully controlled in space [167]. If ink is replaced with a solution of biological molecules, the same method can be used to control their spatial presentation. Early work in this field focused on printing proteins onto solid substrates such as glass or tissue culture plastic to control cell adhesion and morphology. For example, researchers modified a commercially available Canon inkjet printer, loading the ethanol-sterilized cartridges with collagen solutions, and printing defined shapes from a document created in Microsoft Office onto glass slides. Such a straightforward approach using off-the-shelf components achieved collagen patterns with 350 μm resolution that localized where smooth muscle cells attached to the substrate [168]. Similar results were seen using laminin patterns generated by inkjet printing to control neuronal adhesion [169]. Inkjet printing was also used for multiple materials: first a uniform non-adhesive PEG background layer was printed onto a slide, and then a second layer of islands of a cell adhesive collagen/poly-D-lysine mixture was printed on top of the PEG. Neurons grown on these substrates adhered only to the collagen/poly-D-lysine, maintaining the patterns after weeks in culture [170]. Multiple printed layers could also be patterned to provide a more complex signaling environment. Growth factors have also been printed using this young technology. IGF-1 and FGF-2 modified with photoreactive phenyl azido groups were loaded into the different cartridges of a Canon printer and deposited onto polystyrene or silicone substrates; the resolution of the printer allowed for creation of 16 different growth factor combinations and concentrations on individual substrates that fit in a standard 24-well culture plate. After printing, the substrates were irradiated with UV light, covalently immobilizing the growth factors on the surfaces, and creating growth factor arrays that were used to study myogenic differentiation of C2C12 cells [171].
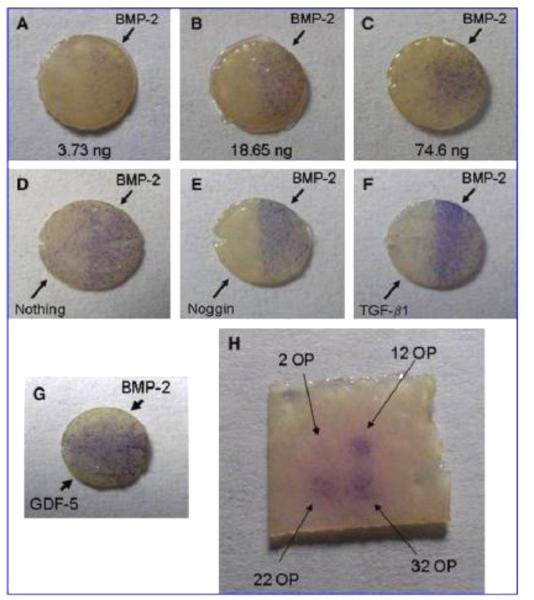
Researchers have since applied inkjet printing for spatial control over the delivery of a variety of growth factors to progenitor and stem cells. By 2005, spatial resolution under 100 nm was possible [172], and inkjet printing was used to pattern FGF-2 onto fibrin hydrogels, relying on affinity between the fibrin and FGF-2 to immobilize the growth factor [173]. When a gradient of FGF-2 concentration and discrete islands of the growth factor were printed, higher amounts of FGF-2 promoted proliferation of human MG-63 "preosteoblastic" osteosarcoma cells seeded on the hydrogel surface [174], locally increasing the number of cells present capable of forming new bone tissue. Printed growth factors can also be used to induce localized stem cell differentiation. For example, on polyacrylamide gel areas with printed FGF-2, neural stem cells were maintained in an undifferentiated state, but on areas printed with fetal bovine serum they differentiated down the smooth muscle cell lineage [175]. In another system relevant to bone repair, mouse muscle-derived stem cells seeded onto fibrin substrates with printed BMP-2 and cultured in myogenic medium underwent osteogenic differentiation in the BMP-2 containing regions, and myogenic differentiation elsewhere [176]. The approach was extended by patterning multiple growth factors (i.e., BMP-2 and FGF-2) with the goal of locally guiding cell differentiation down 3 separate lineages. Muscle-derived stem cells responded as described above, undergoing osteogenic differentiation in response to BMP-2 and myogenic differentiation in the absence of growth factor. In addition, tenocyte markers were upregulated in response to areas patterned with FGF-2 [177]. Such instructive biomaterials may be useful for engineering tendon interfaces to bone and muscle. This growth factor printing technique does not require a substrate with smooth topography: recently, growth factor printing has been performed on a matrix of aligned sub-micron scale polystyrene fibers [178], allowing control of cell alignment in response to the organization of the fibers in addition to growth factor presentation. Additionally, BMP-2 maintained its activity when printed onto microporous scaffolds made from acellular dermis, and led to improved bone healing in mouse calvarial defects in regions of printed BMP-2 compared to regions without growth factor (Figure 2) [179]. Further, co-printing SDF-1 with the BMP-2 augmented bone formation both in vitro and in vivo [180].
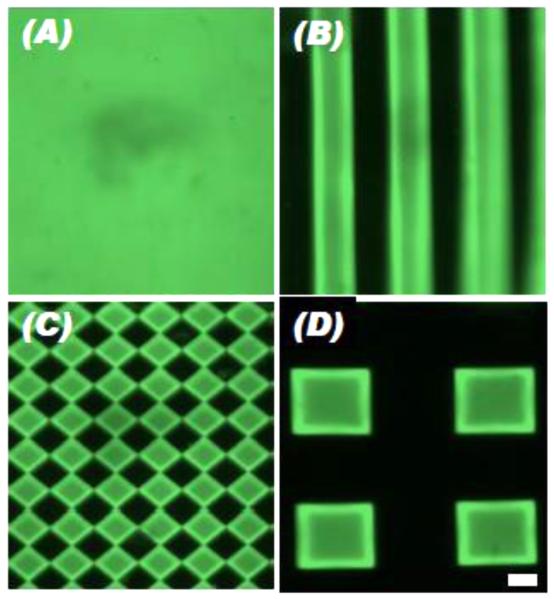
Figure 2.
ALP staining (blue) of C2C12 cells on 5 mm decellularized skin discs printed with bioactive factors resulting from (A-C) varying amounts of BMP-2 printed on the right halves of the scaffolds, (D-F) BMP-2 printed uniformly on the scaffolds with inhibitors printed on the left halves, (G) GDF-5 printed on the left halves, BMP-2 on the right halves, as well as (H) on a square piece with increasing number of BMP-2 overprints (OP). Adapted, with permission, from Cooper, et al. [179]. Copyright Mary Ann Liebert, Inc. 2010.
Another promising application of inkjet printing on 2D substrates is the delivery of genetic material. As a proof of concept, endothelial cells were mixed with naked plasmid DNA encoding green fluorescent protein (GFP), and this solution was printed onto collagen hydrogels. After printing, cells exhibited >90% viability, and >10% transfection efficiency, which was similar to the transfection efficiency obtained when cells on tissue culture plastic were treated with the commercially available Lipofectamine reagent [181]. While transfection efficiency may need to be increased to make this approach clinically applicable, the idea might be translated to deliver any genetic material that would influence cell behaviors such as differentiation or vascular network formation.
Other methods of non-contact printing have been developed, including those that allow for printing not only on dry surfaces but also on surfaces that are submerged in aqueous solutions, which is especially beneficial because they allow printing onto cell-laden materials which must typically be immersed in media during culture. Printing on wet surfaces is accomplished using a polymeric aqueous two-phase system: the surface to be printed on is covered with a PEG solution, and the molecules to be printed are loaded in a dextran solution, which has higher density than the PEG; because the two are immiscible and have low interfacial energy, dispensing the dextran solution near the substrate surface with a pipet or microarray pins can generate micron-scale patterns that are stable over time. With this system, researchers were able to deliver droplets containing GFP plasmid DNA with Lipofectamine in a spatially controlled manner onto cells cultured in monolayer leading to localized GFP expression [182]. The PEG/dextran system was also used to print mouse embryonic stem cells (ESCs) onto a layer of supporting stromal cells to create stem cell colonies of varying sizes [183]. Notably, the addition of media required for cell culture does not wash away the transfection patterns or cell colonies in either of these systems. A dextran/collagen solution could be similarly patterned and gelled in an aqueous PEG environment on top of a layer of living cells, indicating that this biphasic approach could be used to print and pattern polymer solutions [184]. The capacity to pattern gene transfection, cells and biomaterials demonstrates the versatility of this technology. The aforementioned 2D printing tools are promising for monolayer in vitro studies to better understand cellular responses to osteogenic signals, both as tools for high throughput screening and for examining the effects of their spatial presentation. In addition, a patterned coating of bioactive signals on biomaterial constructs can provide localized cues to cells seeded on the scaffold surface or to adjacent host cells to drive bone regenerative processes.
5.1.3. Two-dimensional irradiation-based patterning
Bioactive factors can also be immobilized on the surface of a biomaterial scaffold in controlled regions using UV light and photomasks. This can be very simply applied to create localized regions of photocrosslinked hydrogels, and if a bioactive factor is included in the prepolymer solution, it is effectively patterned with the biomaterial. An interesting application of this approach used a base layer of crosslinked PCL/gelatin nanofibers created using electrospinning, and applied a very thin layer of a solution of PEGDA containing BMP-2 onto this layer. The PEGDA was crosslinked through a photomask, uncrosslinked mononmer was removed, and a solution of FGF-2 was applied and allowed to adsorb to the nanofibers in areas not covered by the PEGDA. As a result, the FGF-2 diffused off of the nanofibers within several days, and in the photocrosslinked regions the BMP-2 entrapped in the hydrogel was released over three weeks. When hMSCs were seeded on the nanofibers, those cultured on scaffolds that released both FGF-2 and BMP-2 showed greater osteogenic differentiation than those cultured on scaffolds releasing either growth factor alone [185].
Chemistries that have been developed to crosslink biomolecules with UV light-reactive moieties to biomaterials are another attractive method for spatial control. For example, peptide attachment to PEG-diacrylate (PEGDA) hydrogels was achieved when the amine groups of RGDS adhesion ligands were covalently coupled to PEG-acrylate, and a solution of the modified peptide covering the surface of PEGDA hydrogels was then irradiated with UV light that was restricted in space by a photomask. In regions exposed to UV light, the acrylate-PEG-RGDS was covalently attached to the hydrogel surface. These RGDS patterns with ~10 μm resolution were shown to affect capillary morphogenesis by endothelial cells [186]. These chemistries are often tested with peptides containing the RGD adhesion peptide sequence, but can be used to control the presentation of other biomolecules. A similar approach was used to couple PDGF and FGF-2 to the surface of PEGDA hydrogels. Acryloyl-PEG-PDGF and acryloyl- PEG-FGF-2 were synthesized using acryloyl-PEG-succinimidyl carbonate, and solutions of these functionalized growth factors were applied to crosslinked PEGDA hydrogels before exposure to UV light through a photomask. Immobilization of these growth factors along with RGDS, led to increased endothelial cell tubule length compared to cells cultured on hydrogels modified with RGDS alone [187]. For patterned VEGF coupling, the growth factor was again PEGylated for crosslinking into PEGDA, but the immobilization was done with laser scanning lithography, using a confocal microscope to focus the laser onto regions of the hydrogel, leading to spatially controlled VEGF presentation [188]. In such a system, the laser parameters, including the power, scan time, and number of scanning iterations, are easily controlled to vary pattern density, and because photomasks are not used, a large number of patterns can easily be created without the need to fabricate new masks.
Electron beam irradiation has been used to attach biomolecules to surfaces with nanoscale resolution. The high energy of an electron beam can form free radicals that initiate crosslinking reactions. For example, an electron beam crosslinked a pattern of styrene-sulfonate-containing PEG-based macromers onto silicon wafers with 100 nm resolution; the resulting substrate could then be incubated with the growth factors VEGF and FGF-2, which adhered to the patterned regions due to the heparin-mimicking properties of the styrene-sulfonate [189]. When PEG-aminooxy was crosslinked in a pattern on the wafers with an electron beam, ketone-functionalized GRGDSPG peptides in solution adhered to the patterned regions via oxime bond formation, causing the material to be cell adhesive and support the growth of human umbilical vein endothelial cells (hUVECs) [190]. Interestingly, electron beam irradiation of a PEG-coated glass substrate created surface patterns of carbon nanodeposits by the phenomenon of electron beam induced deposition: the high energy of the electron beam caused decomposition of organic residues in the atmosphere, which accumulated on the surface as carbon nanodeposits, and to which proteins in solution adhered in concentrations that correlated to the electron beam intensity. Its precision led the technique to be called “painting with biomolecules” [191]. Protein adhesion to the deposits was confirmed with IgG, ferritin, avidin and streptavidin, and biotinylated DNA was shown to have affinity for regions of bound avidin [191]. While nanoscale patterning allows for fine spatial resolution over growth factor presentation, the high energy associated with electron beams may degrade polymeric biomaterials. Despite this limitation, the approach may find strong utility modifying biomaterials such as ceramics and metals with coatings of bioactive factors at high resolution.
5.2. Building up patterned 3D scaffolds
While 2D biomaterial surface modifications are useful, native in vivo signal presentation to cells during development and healing is often tightly regulated and occurs at defined locations in 3D space. For this reason, when advancing beyond material coatings and in vitro monolayer culture tools, it is important to engineer systems where cells can be exposed to 3D microenvironments of patterned bioactive factors. These systems can be used for in vitro studies of cell behavior in an environment more representative of in vivo conditions because it enables cell interactions with the surrounding matrix in all directions. They can also be used as tissue engineering scaffolds: being three dimensional, they can serve the initial space filling and mechanical functions required of scaffolds for tissue regeneration while providing non-uniform instructive signals to cells. Creation of controlled patterns of bioactive factor presentation in scaffolds can be achieved via building up layer-by-layer, mixing prepolymer solutions to create gradients, or assembling from individual subunits. The approaches described in this section are the tools that have been exploited most directly for spatial control of osteogenic molecules and applied for bone tissue engineering.
5.2.1. Layered Scaffolds
The most straightforward method to producing a spatially patterned material is to connect two materials to one another, each delivering a different signal. This method is often used in efforts to generate interface tissues, such as the cartilage-bone transition zone. Such an approach to regenerating osteochondral interfaces was reported as early as 1997, when bilayer scaffolds made of a dense type I collagen layer for the bony side and a porous layer seeded with chondrocytes for the cartilage side were developed and tested showing promising results [192]. The osteochondral interface is an especially appealing target for spatially controlled growth factor delivery, as much work has been done characterizing both the potential of growth factors in the TGF-β superfamily to drive chondrogenesis [193], and that of the BMP subfamily of growth factors to drive osteogenesis [34].
Several groups have leveraged the inductive behavior of these growth factors to create scaffolds with a chondrogenic layer attached to an osteogenic layer. For example, Mikos and colleagues have developed a system based on oligo(polyethylene glycol) fumarate (OPF) with gelatin microparticles to release several different growth factors. Here, the charged nature of gelatin leads to electrostatic interactions with the growth factors, which are charged at physiological pH, delaying their release [132]. The inclusion of rabbit MSCs in OPF hydrogel constructs, with a pro-chondrogenic layer containing either TGF-β1 or TGF-β3 loaded gelatin microspheres, showed that the system could be used for spatial control over cell differentiation in vitro; cells in the growth factor-containing layer expressed chondrogenic markers, while cells in the layer with no growth factor expressed alkaline phosphatase, an osteogenic marker [194, 195]. Similar experiments examined bilayer scaffolds of porous polylactic-co-glycolic acid (PLGA) and segmented polyurethane, with either BMP-2 or TGF-β1 loaded in PLGA microspheres included in the polyurethane layer [196]. PLGA microspheres are a common growth factor delivery vehicle for which release is a function of the microsphere hydrolytic degradation rate and growth factor diffusion [197]. When implanted into a rabbit osteochondral defect with the PLGA-only side of the scaffold in the subchondral bone, and the growth factor-laden polyurethane side lining up with the cartilage, these scaffolds showed promising repair of both the cartilage and underlying bone [196].
As an extension of this idea, recent work has used both osteogenic and chondrogenic growth factors layered in two different scaffold regions to enhance osteogenesis in one layer and chondrogenesis in the other. For example, a bilayer scaffold system used BMP-2 and platelet rich plasma, a growth factor source containing both TGF-β1 and PDGF, for osteochondral defect repair. The system consisted of horse MSCs, both undifferentiated and pre-cultured in chondrogenic media, in bilayer scaffolds in which both layers were made up of gelatin sponges. The chondrogenic layer was loaded with platelet rich plasma, undifferentiated equine bone marrow-derived MSCs, and the MSCs that had been chondrogenically differentiated in vitro. The osteogenic layer contained β-tricalcium phosphate (TCP), as well as BMP-2 and undifferentiated MSCs [198]. These constructs were shown to repair osteochondral defects in the talus of horses [198]. Bilayer osteochondral scaffolds have also been explored for targeted gene delivery. In one study, composite scaffolds comprised of a chitosan-gelatin layer loaded with plasmid DNA for TGF-β1, and a chitosan-gelatin-hydroxyapatite layer mixed with plasmid DNA for BMP-2 were seeded with rabbit MSCs. The tissue constructs led to upregulation of the growth factors that the plasmids encoded, indicating that the gene delivery led to the desired protein expression. More importantly, regional MSC differentiation was observed, and the constructs supported both cartilage and subchondral bone formation in a rabbit knee osteochondral defect [199]. Lastly, it was demonstrated that biphasic high-density hMSC constructs made with incorporated gelatin microspheres releasing TGF-β1 in one layer and mineral-coated hydroxyapatite microspheres releasing BMP-2 in the other layer could drive regional specific hMSC osteogenic or chondrogenic differentiation [200].
Layering techniques are also used in driving vascularization in defined areas, which is of critical importance for bone repair. For instance, the Mooney group has used bilayer made from PLGA microspheres loaded with PDGF and pressed together, sometimes with free VEGF, into discs using gas foaming/particulate leaching, and then stacked. The result was scaffolds with layers of the different growth factors. Growth factors remained confined in the regions they were loaded, and maintained bioactivity: the layers delivering first VEGF and then PDGF led to development of more mature vasculature in a mouse ischemic hindlimb model [201]. Multilayer materials can also allow for improved biomimicry in recapitulating in vivo development, where stimulatory and inhibitory biomolecules are present in spatially restricted areas [202]. This principle was applied in a system of porous PLGA discs either left empty, loaded with VEGF as a proangiogenic molecule, or loaded with anti-VEGF antibody, which is antiangiogenic. The scaffolds consisted of three layers in different combinations, including blank/VEGF/blank or anti-VEGF/VEGF/anti-VEGF. Only the latter composition led to angiogenesis that was spatially restricted to the region where the VEGF was delivered, and formation of stable vasculature in a mouse hindlimb ischemia model [203]. These layering approaches, which allow for discrete regions of bioactive factor presentation, can be a simple tool for evaluating the benefits of separating biochemical signals as opposed to uniformly mixing various bioactive factors throughout a scaffold.
5.2.2. Gradient formation
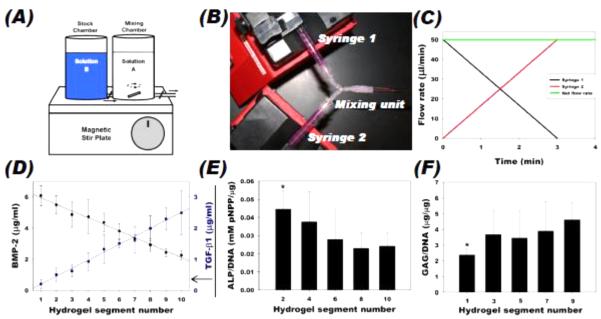
While work with bilayer scaffolds has made some progress in recreating osteochondral interfaces, in vivo, biointerfaces are not discrete layers but instead are established by gradients of mechanical and biochemical cues, driving the formation of tissues with graded properties and composition as described in section 2.2. A number of tools have been developed for creating gradients of bioactive factors, and these are often used for regeneration of the graded osteochondral interface. Gradient making equipment is commercially available; the device most often sold as a “gradient maker” consists of two vertical chambers into which prepolymer solution is poured; one side contains the molecule to be patterned in the desired gradient, and the other does not (or contains a different factor) [204]. A valve connects the two chambers, and when open, allows the material in the first chamber to flow into the second, where they are mixed, usually using a magnetic stir plate (Figure 3A, [205]). The second chamber has an outlet, where the mixture is collected and polymerized. Another system involves two syringe pumps that pump at different rates into a common outlet, which also allows for control of the biomolecule concentration profile [206]. Microfluidic mixing devices have also been used to create gradients of soluble factors. Two inlet ports are each loaded with a different prepolymer solution with or without desired bioactive factors. These ports connect to microchannels that join and split repeatedly, mixing the solutions at each juncture, and ultimately lead to an increased number of output ports that then each contain varying amounts of the two input solutions. Each successive output microchannel contains increasing or decreasing concentrations of the bioactive factors of interest, which can then be combined to form a continuous gradient. Depending on the prepolymer used, the gradient can be crosslinked into place by a variety of mechanisms such as UV light [207]. The same approach can be used for the perfusion of media to cells in culture, constantly presenting the gradient of soluble factors [208].
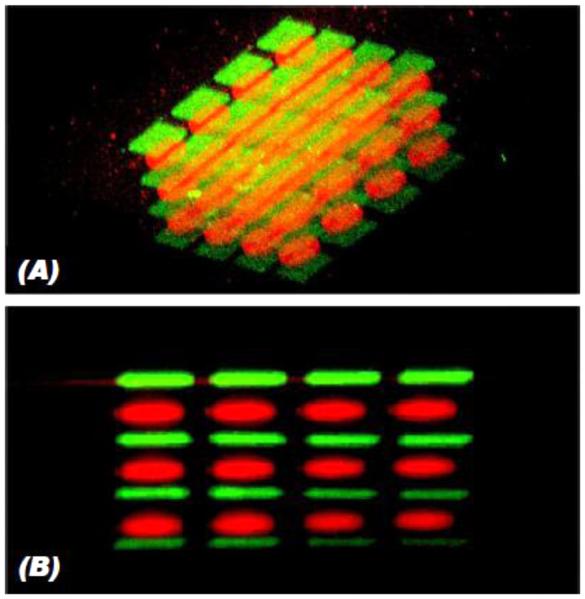
Figure 3.
Examples of bioactive factor gradient formation. (A) Schematic of a commercially available gradient maker design. (B) Dual syringe pump system used for gradient fabrication in alginate/heparin hydrogels, where (C) the flow rate from each syringe is controlled over time to create (D) measurable BMP-2 and TGF-β1 linear gradients in opposite directions. Encapsulated hMSCs expressed increased (E) osteogenic and (F) chondrogenic differentiation markers on the side of the gradient with increased BMP-2 and TGF-β1, respectively. (A) Adapted, with permission, from Chatterjee, et al. [205]. Copyright Bentham Science Publishers 2011. (B-F) Adapted, with permission, from Jeon, et al. [206]. Copyright John Wiley and Sons 2013.
Growth factor gradients are commonly explored [209], as these are present in vivo during healing and development and have been shown in vitro to direct a wide range of cell behaviors along the gradient ranging from neurite outgrowth [210] to branching morphogenesis [211] to stem cell differentiation [208]. For example, a gradient of covalently attached FGF-2 was formed in photocrosslinkable PEGDA hydrogels using a commercially available gradient maker; the FGF-2 was conjugated to a PEG derivative to allow for its photocrosslinking into the bulk hydrogel. The resulting hydrogels increased proliferation and directed migration of smooth muscle cells in the direction of increasing FGF-2 [212]. For osteochondral applications, several systems rely on two opposing gradients: in one direction an increasing gradient of an osteogenic growth factor (usually BMP-2), and a decreasing gradient of a chondrogenic growth factor (usually TGF-β1 or IGF-1) in the other direction, to drive the formation of a transition between bone and cartilage. For example, a commercially available gradient maker was used to make alginate scaffolds with gradients of BMP-2-loaded silk microspheres and IGF-1-loaded silk or PLGA microspheres. The growth factors showed localization and controlled release over time, and led to a gradient of osteogenic and chondrogenic differentiation of encapsulated hMSCs [213]. Alternatively, a syringe pump system was used to create opposing linear gradients using PLGA microspheres loaded with BMP-2 or TGF-β1 that were sintered together after gradient formation to form a solid scaffold. Enhanced hMSC osteogenic and chondrogenic differentiation were observed on the sides of the gradient with increased BMP-2 and TGF-β1, respectively [214]. When the BMP-2-loaded microspheres also contained hydroxyapatite, and a similar process was used for gradient formation, these constructs improved repair of osteochondral defects in rabbit knees with bone ingrowth into the scaffold and an overlying cartilage-like layer [215]. In another hydrogel-based approach, opposing BMP-2 and TGF-β1 gradients were formed using syringe pumps in an alginate/heparin system with encapsulated hMSCs (Figure 3 B-F) [206]. The heparin modification slowed growth factor diffusion due to affinity interactions, inducing regional osteogenesis and chondrogenesis. Other molecules have been used in osteochondral gradients as well: a PCL nanofibrous mesh with gradients of insulin, which stimulates chondrogenic differentiation, and β-glycerophosphate, for promoting mineralization, was shown to result in localized chondrogenesis and formation of mineralized tissue by human adipose-derived stem cells (hASCs) [216].
Local presentation of genetic material can also be controlled via gradients. One of the first such examples reported was collagen scaffolds soaked in a solution containing a positively charged polymer, poly(L-lysine) (PLL). The scaffolds were dipped into the PLL solution at a controlled rate; more PLL was adsorbed onto the end that was in solution for longer, creating a gradient of positive charge on the collagen surfaces. The slopes of these gradients were a function of the dipping speed. Then, the constructs were soaked in a solution containing retroviral DNA encoding Runt-related transcription factor 2 (Runx2), an osteogenic transcription factor, which was noncovalently immobilized in amounts dependent on the local amount of adsorbed PLL. When primary rat fibroblasts were seeded into the matrices and cultured in osteogenic growth medium, a gradient of cellular Runx2 expression was evident, along with a gradient of mineral deposition and construct stiffness, indicating that the cells were expressing an osteogenic phenotype that spatially correlated with the amount of retroviral DNA presented [217]. Further, gradients of siRNA were engineered into photocrosslinkable dextran hydrogels. Using the dual syringe pump mixing system described earlier, a gradient of siRNA against GFP was created, UV-crosslinked into place, and drove differential gene silencing in encapsulated cells along the hydrogel gradient that persisted over time [218]. These approaches may be extended to study gradients of different DNA or RNA sequences to enhance osteogenesis.
5.2.3. Modular assembly
A number of research groups are exploring an approach using modular tissue engineering, where base units, often high cell density aggregates or hydrogel particles with either seeded or encapsulated cells, are formed and assembled into more complex tissues [219]. A key benefit of this approach is the ability to make subunits with different biomaterial compositions, cell types and/or bioactive factors, and arranging them to form a heterogeneous engineered composite that can mimic the spatial variation in native tissue. The different properties of the individual modules allow them to serve different functional roles in the assembled construct. Because one subunit type can be vasculogenic, the approach is frequently used to improve vascularization in engineered constructs [220, 221] including for vascularized bone [222].
Techniques ranging from simple mixing to more complex photopatterning have been developed to make modules in various defined shapes and sizes. Spherical particles can be made relatively easily using a water-in-oil emulsion for water-soluble polymers such as gelatin, and oil-in-water emulsions for water-insoluble polymers such as PLGA, where microsphere size is controlled by variables such as temperature, the amount of solvent used and the speed at which the emulsions are stirred [223]. In ionically crosslinkable polymers, the macromer solution can be dropped into a bath containing dissolved ions, for example divalent cations for an alginate solution, causing it to crosslink in the form of spheres [224]. This method allows for size control by, for example, varying the size of the nozzle or the rate at which the droplets of macromer solution are ejected, and cell encapsulation, as the ionic crosslinking is often gentle enough to maintain cell viability [225]. Microspheres made by both emulsions or ionic crosslinking can be loaded with bioactive factors, either by directly mixing in an aqueous solution of the bioactive factors during synthesis or rehydrating lyophilized hydrogel microspheres with the solution [223, 226]. For the case of high cell density aggregates, cell-cell adhesion interactions are the mechanism that forms the individual modules. Small spherical aggregates can easily be made by hanging drop culture [227], or larger aggregates can be made by culturing cells in a non-adhesive container such as wells of a V-bottom plate, where cell-cell interactions lead to formation of cell clusters, which can be enhanced by centrifuging the plates to force cell aggregation [228]. Biomaterial microparticles of varying size and composition can also be included in the aggregates [229, 230].
Molding techniques allow for flexibility in the shape and size of the individual modules. Molds containing many replicates of micron-scale patterns can easily be made from polymers such as PDMS using approaches including soft lithography. These molds can be rendered nonadhesive by plasma cleaning, and can be used to control the geometry of cell aggregates [231-233]. Thermo-gelling hydrogels, including collagen, Matrigel, and agarose are easily crosslinked in these molds: the molds are loaded with a solution of hydrogel precursor containing the desired cells, and then incubated at 37°C to allow for crosslinking. The hydrogels are then removed by shaking the gels free from the mold and have been shown to maintain high cell viability [234]. Molds can also be used with photopolymerizable hydrogels using the same process but crosslinking with UV light, again with high cell viability [235]. Photomasks that restrict the location of UV light can be used with photopolymerizable hydrogels to eliminate the need for molds. If the light is applied through a photomask to a layer of uncrosslinked polymer solution, potentially containing cells, it can isolate regions of crosslinking creating geometrically defined shapes [236]. Simply rinsing off the uncrosslinked solution leads to a solution of microgels with controlled 3D shapes [237]. While these reports delivered only cells from the individual hydrogels, other signals, including bioactive molecules such as DNA or growth factors, could be localized to specific modules using these techniques. Techniques exist for controlling placement of different cell types within microgels, such as one cell type encapsulated inside of the microgels and another cell type (usually endothelial cells) seeded on their surface [238]. Combined with existing techniques to layer different growth factors on microparticle surfaces [239], such pursuits could be extended to spatially regulate placement of different bioactive factors in or on microparticles.
The simplest method to assemble these constructs into macrotissues is direct mixing of the subunits, which requires no additional equipment, and allows for relatively uniform distribution of a desired bioactive factor throughout the engineered construct. The total amount of bioactive factor loaded and its release kinetics are all variables that can be controlled to drive desired biological effects [229]. The mixing of the modules can be performed in another mold, to form a specific, desired geometry [238, 240]. In the case of high cell-cell adhesion interactions, the cells can grow into a macrotissue held together first by cell adhesion to neighboring cells, and later strengthened by interactions between cells and the ECM they secrete [241]. This mixing is not limited to only spheroid-shaped aggregates; high cell-density rings added to one large well assembled into a multiluminal structure of randomly overlapping rings [232]. For photocrosslinkable polymers, a secondary UV exposure can covalently bind individual microgels to each other. These molds can have complex geometries (e.g., a tube, solid sphere, hollow hemisphere), and can be filled with a variety of different subunit types. For example, PEGDA microgels were assembled into macroconstructs around PDMS templates. In this technique, a PEGDA solution fills the gaps between the microgels, leading the method to be called “micro-masonry,” where the microgels are the bricks and the PEGDA solution is the mortar [242].
Physically manipulating each independent module into a desired position allows for increased control over their placement, but comes at the expense of increased complexity since modules must be individually positioned. For this reason, the method is well suited for constructs made of a small number of subunits. For example, high cell density hMSC rings were formed in custom designed molds, and placed onto rods where 3-6 rings were assembled into tubes to create cartilaginous constructs with potential for tracheal tissue replacement. Incorporation of TGF-β-delivering microspheres enhanced their chondrogenesis [243]. On a smaller size scale, a micromanipulator can be used to move microgels into desired locations. Such an approach is slow, but has been used to make a checkerboard pattern of microgels containing cells stained red and green, demonstrating it can be applied to provide precise spatial control over module position [235]. This principle extends to 3D with the use of microrobots made of magnetic particles in polyurethane and actuated by electromagnets to generate nanonewton forces that can manipulate hydrogel modules in space. The microrobots first build the base level of the desired structure, and then the structure can be built up layer by layer with ramps [244]. All of the aforementioned approaches could be extended to spatially segregating modules containing different bioactive factors.
Intermediate approaches also exist, allowing some guidance in the macrotissue assembly, but without requiring direct manipulation of each individual subunit. For example, vacuum molding is a technique that builds upon molds and direct mixing to achieve pattern formation. The pattern to be molded is cut out of a thin PDMS layer and placed on a porous membrane. Then a solution containing individual microgels or aggregates is poured into the patterned PDMS mold and, a vacuum is applied through the filter. When examined with collagen-chitosan microparticles, the vacuum enhanced microparticle aggregation and removed excess liquid, causing improved packing. The process was also done in steps, where another type of microparticle was applied in a second vacuum application to backfill the space remaining after the construct was removed from the mold, or mixtures of two or more microparticle types were used in each step [245]. These collagen-chitosan microparticles have been shown to induce osteogenic differentiation of hMSCs in response to exogenous media supplements [246], suggesting that the microparticles used in directed assembly systems have potential utility for bone tissue engineering. In an alternate approach, magnetic microgels can be made that respond to externally applied magnetic fields. Micromolded PEGDA or methacrylated gelatin (GelMA) hydrogels containing magnetic nanoparticles were shown to maintain good cell viability and form 3D patterns of fluorescently stained microgels such as layered spheroids. Layers of the hydrogels could be collected on the tip of a magnetic pin, stabilized by filling layers of PEG, which serve a similar role to the mortar in the micromasonry approach described earlier [247].
Interface-directed assembly is another approach to controlling the aggregation of microgels. When microgels are deposited onto the surface of a hydrophobic liquid such as carbon tetrachloride, perfluorodecalin or mineral oil, they float and aggregate due to surface tension and hydrophobicity [237, 248]. While this is a random process, changing the hydrogel shapes can guide them to assemble in a directed manner: lock and key shaped hydrogels fit together in one configuration, and aggregate in that pattern on the liquid surface. A second crosslinking step holds this macroconstruct in place (Figure 4) [248]. This approach can be used to make multilayer constructs by stacking the individual microgel monolayers and crosslinking them into place. For photopolymerizable hydrogel stacks thicker than one centimeter, the maximal penetration depth of UV light in clear hydrogels [249], repeat cycles of UV exposure and the resulting free radical formation can lead to cell death, which will likely restrict this technique to just a few layers. To enhance transport in thick scaffolds and provide space for cell proliferation and ECM deposition, porosity can be induced in these stacked constructs through the use of sacrificial microgels, such as alginate, which can be broken down by calcium chelators with minimal effect on the viability of nearby cells [250].
Figure 4.
Example of modular assembly of microgels. Schematics representing assembly of (A) hexagonal microgels and (D) lock and key shaped microgels, with (B, E) phase contrast and (C, F) fluorescence photomicrographs of centimeter-scale hydrogel constructs with fluorescently labeled encapsulated fibroblasts. Scale bars: 100 μm. Adapted, with permission, from Zamanian et al. [248]. Copyright John Wiley and Sons 2010.
5.2.4. Solid freeform fabrication
To recreate 3D microenvironments both for in vitro studies of cell behavior and tissue engineering, a number of 2D biomolecule printing approaches have been expanded into the third dimension. This is possible due to the advent of additive manufacturing technologies and other mold-less techniques, generally called solid free-form fabrication (SFF). Much work using these technologies focuses on making tissue engineering scaffolds with customized patient-specific geometries but also with highly defined 3D architectures. Importantly, many SFF technologies are mild enough to allow for biomolecule incorporation without causing damage due to high temperatures or toxic solvents, and can control the spatial presentation of these signals [251]. The SFF strategies particularly amenable to delivering osteogenic factors with 3D spatial control fall into the broad categories of 3D printing, stereolithography and fused filament fabrication.
3D printing uses a similar premise to the 2D non-contact printing described earlier, where liquid material is deposited in precisely controlled locations, but in this case the liquid is a binder deposited onto a layer of powder that becomes solid only in the areas treated with the binder. Either the binder-containing cartridge or substrate being printed on can move in the z-plane, allowing the material to be built up in layers and made into a 3D structure. Some biocompatible polymers and ceramics that have been extensively studied for bone tissue engineering can be printed in this way [252, 253]. In one early demonstration of this method, researchers printed a binder solution onto a layer of powdered PCL or PEG, causing the particles to bind and form a solid construct. Notably, microdroplets of dye were interspersed into the constructs at designated locations, demonstrating the utility of this approach to pattern soluble molecules [254]. A key drawback of 3D printing tools, however, is the organic solvents used in some binder solutions, which may damage bioactive factors and limit viable cell encapsulation. To address this problem, aqueous binders have been developed, such as one made with cornstarch, gelatin and dextran in water. However, a scaffold made from such a binder is water soluble, and must be modified for use in an aqueous environment [255]. Another method to use 3D printing to control bioactive factor delivery is to print a designated structure, and then load biomolecules by, for example adsorbing them onto the scaffold surface. This has been demonstrated for the delivery of VEGF as well as the antibiotics tetracycline and vancomycin from TCP scaffolds made by 3D printing ceramic powders using phosphoric acid as the binder solution. After fabrication and heating to set the printed structure, scaffolds for antibiotic delivery were soaked in drug solution for loading, and release kinetics depended on the affinity between the ceramic and the drug [256]. For VEGF presentation, one macroscale Y-shaped channel within each scaffold was printed and loaded with a VEGF solution. As the scaffold dried, the growth factor was adsorbed onto the surface of the ceramic and its bioactivity in vivo was maintained, as the vascular tissue infiltrated the channel during peritoneal implantation in mice [257]. For delivery of combinations of drugs or growth factors, this approach can be implemented with multiple materials with varying affinities in the same scaffold to control spatiotemporal release.
Selective laser sintering is a technique related to 3D printing, but uses a laser instead of a printed solution to crosslink each powder layer. This method is used most often with synthetic polymers, but has also been applied with ceramic/polymer composites and hydroxyapatite alone [258]. While this technique has not been used extensively for bioactive factor delivery, it has been applied to build enclosed crosslinked PCL capsules with methylene blue, a model drug, in their interior. Additional concentric rings of crosslinked PCL around the interior capsules acted as barriers to diffusion, controlling release rate and limiting the initial burst. Later work showed that proteins loaded into microspheres in the powder phase can be protected during the sinter step; bovine serum albumin (BSA) immobilized in calcium phosphate/poly(hydroxybutyrate-co-hydroxyvalerate) microspheres in a scaffold of the same material demonstrated an initial in vitro burst release, but then sustained delivery for four weeks [259].
Stereolithography is also an additive, layer by layer technique: a photopolymerizable liquid macromer solution is exposed to laser or UV light through a unique photomask for each layer, which cures it to form a solid layer with defined geometry. A z-axis controller moves the scaffold in steps of 25-100 μm to expose the next layer for polymerization [260]. The technique is amenable to applications in tissue engineering, as a number of materials used for cell encapsulation and bioactive factor delivery have been “printed” using stereolithography. These include polylactide, which supported preosteoblast proliferation [261], poly(propylene fumarate) (PPF) [262], and PEGDA, which allowed for hydrogel polymerization with encapsulated cells [263, 264]. Particularly relevant for bone tissue engineering, osteoconductive ceramic particles mixed into a solution of a commercial photocrosslinkable monomer solution, Diacryl 101, at up to 53% volume/volume prior to crosslinking; produced a shear thinning suspension favorable for casting and printing using stereolithography [265]. To achieve spatial control of more than one biochemical signal within a scaffold, multiple polymer solutions have be used, but this requires sequential polymerization, crosslinking one solution at a time with rinsing steps in between to remove uncrosslinked macromer solution [266]. For example, two separate solutions of PEGDA and either FITC or Cy-5, two fluorescent dyes, have been printed, each restricted to their 3D patterned regions of the hydrogel after crosslinking [267]. Two solutions of PEGDA, each containing different fluorescently labeled latex microparticles, were also patterned using stereolithography [268], suggesting that other microparticles with known bioactive factor release profiles could be similarly controlled in space. Additionally, polymers with growth factor affinity have been used to permit spatial control over the rate of growth factor release in constructs formed using stereolithography. For example, PEGDA and heparan-modified PEGDA were patterned, and FGF-2 in the solution was retained longer in the regions of heparan-PEGDA [268]. Lastly, cells were patterned in a PEGDA scaffold built up in this way. Each thin layer of PEGDA/acryloyl-PEG-RGDS/cell solution to be crosslinked was deposited onto the surface of an already crosslinked scaffold layer before the new layer's polymerization step; one rinse step at the end removed all unreacted cell-monomer solution [269]. While this study examined layering different cell types, NIH/3T3 cells labeled to fluoresce either green or red, it could also be applied to layer different bioactive factors.
Multiphoton excitation allows for more precise stereolithography because it uses two lasers: at their intersection, the energy is twice that of any point along either individual laser’s path. The excitation levels achieved by these lasers are in the range needed for many UV-reactive chemistries; titanium-sapphire lasers are available with frequencies near 780 nm, and two of these will excite at 390 nm [270], which is in the frequency range used in the light-based chemistries described previously. Only at the intersection point of the two lasers is the energy high enough for crosslinking [271]. The technology was developed for fluorescence microscopy to diminish photobleaching and improve spatial image resolution. When used in stereolithography, a scanner moves the lasers in 3D space to target desired points in a sample. For example, two-photon excitation was used to build up patterned matrices of photocrosslinked BSA or fibrinogen, and the BSA scaffold was shown to release fluorescently labeled dextrans [272].
Fused filament fabrication (FFF), or fused deposition modeling, is a technique based on building a scaffold from individual synthetic polymer fibers extruded from a nozzle, much like that on an inkjet cartridge. While it has been used with some success to form scaffolds with controlled architecture, usually comprised of PCL and applied for bone tissue engineering, FFF has not yet been used to control the spatial presentation of bioactive factors [258]. Bioplotting, where hydrogel fibers are made and assembled under physiological conditions, is a more biologically friendly version of FFF, and has been used with great success for encapsulation of cells [273]. For example, cells in water-soluble polymers such as gelatin or agar can be printed, allowing for fabrication of cell-laden tissue engineering scaffolds with highly controlled microarchitectures [274, 275]. Of particular relevance to bone tissue engineering, hMSCs encapsulated in alginate were printed using this technique, maintaining not only cell viability, but more importantly osteogenic differentiation capacity. Bioplotted scaffolds containing two different groups of cells with different fluorescent labels were fabricated, showing that the technique can pattern more than one type of cell or signal [276].
5.3 Controlling spatial presentation of bioactive factors after scaffold fabrication
As an alternative to building-up approaches, one can also regulate placement and release of bioactive factors after a scaffold is formed. Scaffolds made under harsh conditions, including high temperatures and some solvents, can later be patterned in a gentler environment that minimizes damage to biologics. While initially demonstrated using photomasks to project 2D patterns into 3D materials, more advanced technologies such as multiphoton excitation can generate patterns with sophisticated, challenging geometries in 3D, such as disconnected features and shapes with overhangs (e.g., stalactites). Alternatively, application of external stimuli to responsive materials allows for dynamic patterns that can evolve throughout the regenerative processes.
5.3.1 2D pattern projections
A simple 2D photomask and UV light source can lead to controlled light exposure through a biomaterial, projecting the photomask pattern into 3D. For example, the Anseth research group used thiol-ene click chemistry to couple the amino acid sequence RGDSC to a preformed PEG-based hydrogel. Multiarm PEG-tetraazide macromers and diacetylene functionalized polypeptides formed the hydrogel by copper catalyzed cycloaddition. The polypeptide present throughout the hydrogels also contained lysine residues modified with free alloxycarbonyl groups that could react with the cysteines of the RGDSC adhesion ligands in the presence of UV light. Spatial control was achieved using a photomask to restrict the light application to specific regions, forming a pattern that was uniformly projected in the z-direction throughout the 270 μm thick hydrogels [277]. Hydrogels were washed in fresh media after patterning to remove any unreacted peptide in areas not exposed to UV light, and the addition reaction, dependent on light dosage as well as photoinitiator concentration, gave more control than a simple binary distribution of regions with a single peptide density and regions without peptide [278]. Later, thiol-ene chemistry was used to couple CRGDS adhesion ligands to spatially defined regions in 1 mm thick PEG hydrogels with encapsulated hMSCs. In this work, a solution of norbornine-modified PEG, a polypeptide crosslinker with thiol-containing cysteine residues on each end, and hMSCs was photopolymerized, after which a solution of CRGDS and photoinitiator was allowed to diffuse into the hydrogel and a second UV exposure was applied through a photomask. After a wash step to remove unreacted adhesion ligands, cells maintained high viability and showed increased spreading in regions of coupled CRGDS [279]. These chemistries can be used to spatially control the coupling of other biomolecules with thiol reactive groups, such as thiolated DNA or cysteine-containing peptides or proteins, specifically relevant for bone tissue engineering. In another example, photoresponsive agarose hydrogels were modified throughout with 2-nitro-benzyl-protected cysteines, which were activated by a focused UV laser to create free thiol groups in 3D channels. Then, these reactive moieties were used to tether maleimide-coupled GRGDS to material within these channels. This platform was shown to guide neurite outgrowth [280]. Other coupling reactions that could be implemented with UV irradiation to achieve spatial control over bioactive factor presentation use acrylated peptides in methacrylated alginate hydrogels [281]. For example, with alginate solutions containing acrylated GGGGRGDSP peptide and crosslinked throughout with calcium ions, UV light applied through a photomask created spatially controlled regions of covalently coupled adhesion ligand that could direct MC3T3 preosteoblast cell adhesion and proliferation [282.]. The PEGylated peptides and growth factors in PEGDA hydrogels as described earlier [186-188] could be similarly patterned. Affinity interactions can also be used to create 2D patterns in 3D hydrogels. This approach has been applied to direct presentation of nucleic acids such as siRNA within modified PEG hydrogels (Alsberg laboratory unpublished data, Figure 5). Crosslinking methacrylated heparin into PEGDA [283] or methacrylated alginate [284] hydrogels can also potentially be spatially controlled with photomasks, leading to affinity interactions that will lead to growth factor binding in controlled areas of the biomaterial.
Figure 5.
FITC-labeled siRNA retained (A) uniformly in PEG hydrogels, as well as (B-D) in specific regions controlled by UV exposure through a photomask. Scale bar = 100 μm. Alsberg laboratory unpublished data.
5.3.2. Multiphoton excitation
Multiphoton excitation has been used improve the spatial resolution of stereolithography, and has also been applied for patterning signals in precise locations within preformed 3D hydrogels. One of the first reports of this this new technology to create instructive biomaterials used a standard two-photon microscopy setup, including a commercially available microscope stage and lasers, to create 3D micropatterns of biomolecules in a coumarin-modified agarose hydrogel. A sulfide containing 6-bromo-7-hydroxycoumarin-amine, a custom-synthesized modified version of a commercially available multiphoton-labile protecting group, was conjugated to agarose. In regions exposed to two-photon irradiation, thiols are uncaged from the coumarin-based protecting groups, leaving them available to react with free biomolecules with maleimide moieties present in the hydrogel. Of note, the reactive thiols are stable over time, and can be “written” with a two-photon pattern and then immersed into a solution containing the thiol-reactive compound, which will diffuse into the hydrogel, react in the patterned regions and then diffuse out of other regions during a wash step (Figure 6) [285]. Other chemistries were also developed, for example, using aminocoumarins that are two-photon labile and creates reactive primary amines in areas of light exposure [286]. This light-based deprotection to create reactive thiols was used with two-photon excitation to bind FGF-2 with tight spatial control via disulfide bonds to agarose hydrogels [287]. Taking advantage of more than one specific chemical interaction between scaffolds and biomolecules allows for simultaneous spatial control over multiple factors in one system. For example, streptavidin and barnase, both maleimide-functionalized, were individually patterned into agarose gels by two separate rounds of two-photon irradiation. Then, taking advantage of the affinity complexes that form between streptavidin and biotin, and barnase and barstar, the gels were soaked in a solution containing two different factors, one coupled to biotin and one coupled to barstar, which were then immobilized only on the regions to which they have affinity. When barstar-sonic hedgehog and biotin-ciliary neurotrophic factor, two stem cell differentiation factors, were patterned into discrete squares, circles and channels, they directed neural progenitor cell gene expression and migration [288]. These methods could easily be applied for bone growth factors or other osteogenic molecules.
Figure 6.
Multiphoton patterning in 3D agarose hydrogels showing (A) oblique and (B) side views of a 4 × 4 × 4 array of squares (60 μm per side) of maleimide-conjugated green fluorescent dye (AF488-Mal), and a 4 × 4 × 3 array of circles (50 μ diameter) of maleimide-conjugated red fluorescent dye (AF546-Mal) created in a second multiphoton irradiation step. Adapted, with permission, from Wosnick et al. [285]. Copyright American Chemical Society 2008.
The light-sensitive chemistries described for 2D patterning can also be used with two-photon irradiation for 3D control. For example, acryloyl-PEG-RGDS was allowed to diffuse into crosslinked PEGDA hydrogels, and then conjugated to the PEG network by the two-photon excitation-driven reaction to yield 3D spatial control over RGDS patterns that were shown to guide cell migration [289, 290]. The method was also shown to work with multiple different peptides; each one was soaked into the hydrogels, crosslinked into desired regions, and then washed out before the process was repeated with another peptide. Additionally, the thiol-ene chemistry described earlier was confined to regions of 3D space using two-photon excitation, and used to attach fluorescently tagged proteins and RGD-containing peptides to these designated regions in PEG-based hydrogels [291]. Notably in all of these systems, encapsulated cells maintained high viability that was unaffected by the patterning. Additionally, while much of the preceding work used peptides with fluorescent tags [292], it could be implemented with other acrylated bioactive factors that enhance osteogenesis.
5.3.3 Stimulus-based delivery
Biomaterial systems have been developed to respond to external stimuli for on-demand biomolecule release, allowing a level of temporal control. This is typically accomplished by harnessing two main processes: either the stimuli cause deformation of the material on a size scale that affects convection and/or diffusion of a free biomolecule, or the stimuli disrupt a chemical bond or affinity interaction that tethers the biomolecule to the material. Many of these stimuli can be controlled in space, providing the potential to control bioactive factor presentation spatially at desired time points. As a first example described previously, exposure to light is easily controlled in 2D using photomasks or in 3D using two photon excitation, making light-dependent reactions an exciting target for this approach; photocleavage of bonds that couple growth factors or other molecules to the biomaterial allows for triggered release [293]. The light-controlled coumarin uncaging of molecules described in section 5.3.1 can be performed in the presence of cells, allowing the light to be a spatiotemporal signal for bioactive factor presentation.
Controlling drug release using pH leverages the ability of some materials to change their ionization state in response to a change in environmental pH, leading to conformational changes and swelling that causes them to release their payload [294]. The decreased pH (<6.5) in ischemic and inflamed tissues, especially tumors, and differences in pH along the digestive tract (i.e. pH = 1.0-3.0 in the stomach and pH = 4.8-8.2 in the small intestine), have motivated the development of systems that release their drug payloads in response to local pH, allowing them to target a desired tissue [295]. For example, a hydrogel network of poly(γ-glutamic acid) interpenetrating with sulfonated poly(γ-glutamic acid) was shown to release FGF-2 in response to exposure to pH=4 and pH=6 solutions, while maintaining growth factor bioactivity [296]. The same research group examined pH-responsive poly(acrylic acid) along with poly(N-isopropylacrylamide) (PNIPAm), a polymer with a lower critical solution temperature of 32° C, to make pH and thermally responsive hydrogels that released a model cationic drug [297]. Recently, chitosan and heparin nanoparticles were shown to release doxorubicin, an anti-cancer drug, with different kinetics under acidic conditions (pH=4.8) compared to neutral pH [298]. pH-stimulated release has strong potential in many applications, but spatial control of this approach has not yet been demonstrated.
Mechanical loading can also be used to deform polymer matrices and induce release of biomolecules. For example, when a physically applied step function compressive loading profile was applied to an alginate hydrogel, a burst of a model drug was released before returning to baseline low release levels within 10 minutes. Notably, the system was then used to deliver VEGF in a subcutaneous mouse model, with mechanical stimulation performed in vivo; the growth factor release led to increased blood vessel density around the implant [147]. This technique also lends itself well to spatial control, as nanoindenter technology is widely available and has excellent 2D resolution. The idea was extended for potential clinical use in patient-controlled drug delivery, showing that a drug can be released from a β-cyclodextrin/alginate hydrogel in response to mechanical compressions simulating a patient-controlled squeezing of a device [299]. Micelles, which are well designed for hydrophobic drug delivery, also change conformation in response to mechanical loading, which can trigger release of their payload. Block copolymer micelles of poly(n-butyl acrylate) and poly(acrylic acid) loaded with pyrene as a model drug were used to crosslink polyacrylamide into a hydrogel, and then shown to release the drug in direct response to periodic physically applied strain [300]. An innovative variation to this approach is to use a magnetic field to generate compressive strain, avoiding direct contact with the materials. This was first demonstrated with BSA released from an ethylene-vinyl acetate copolymer (EVAc) matrix, with a single 10 mg magnetized sphere in each hydrogel; an applied magnetic field pulled the magnet through the hydrogel against a flat surface, causing localized compression that led to a 5-10 fold increase in BSA release compared to the release without an applied stimulus [301]. The system was later shown to have similar release behavior in vivo as in vitro [302], and then applied to deliver insulin to diabetic rats [303]. To achieve more uniform hydrogel compression, iron oxide nanoparticles coated with Pluronic 127 were later incorporated into alginate hydrogels. This ferrogel was able to release a drug, mitoxantrone, DNA and a growth factor, SDF-1, in discrete bursts in response to the periodic applications of a magnetic field [146].
An especially interesting example of a physical stimulus to induce local osteogenesis used high intensity focused ultrasound to trigger gene activation with a heat-activated gene switch for luciferace, VEGF or BMP-2. Transfected C3H10T½ cells were shown to produce BMP-2 or VEGF in vitro in response to ultrasound-triggered heating of up to 8°C for 5-15 minutes without loss of cell viability, and when the cells were encapsulated in fibrin hydrogels and injected subcutaneously in mice, they showed localized luciferase expression limited to an area of 30 mm2 [304].
Additionally, chemical stimuli can control bioactive factor presentation, either by physically degrading a barrier that was confining a payload, or by causing conformational changes, such as contracting the polymer network as described above. Hydrogels that use this mechanism to respond to glucose by releasing insulin have been investigated for over 30 years because of their particular relevance to treatment of diabetes. For example, a hydrogel containing glucose oxidase, which converts glucose to gluconic acid and thereby decreases local pH, triggers hydrogel swelling and release of loaded insulin [305]. Later, chitosan/dextran sulfate microparticles with an albumin-containing core that degraded in the presence of chitosanase, were used to release the albumin payload. The capsules released minimal protein without the enzyme present, and release rate could be manipulated depending on whether chitosan or dextran sulfate was on the outer layer of the nanoparticles [306]. Proteins with the ability to change between two or more conformations can also be used as a trigger for release systems. One such protein is calmodulin, which has both collapsed and extended states, depending on whether it is bound to a specific set of ligands. Coupling the calmodulin into a PEGDA network created a hydrogel that could expand or collapse in response to trifluoropernazine, a small molecule drug that induces conformational change in calmodulin. This approach was used to release VEGF from PEG microspheres [307] and bulk hydrogels [308] in response to the ligand-induced conformational change. The PEG-calmodulin microspheres were implemented to release multiple growth factors, VEGF and BMP-2, which are especially relevant to bone tissue engineering [309]. Controlled presentation can be accomplished not only by release of a bioactive factor, but by changing it from cell-accessible to cell-inaccessible states, for example depending on the presence of a PEGylated blocking molecule. RGD-containing peptides which included an acidic leucine zipper domain were immobilized on a gold substrate. When a PEGylated basic leucine zipper in solution was added to the surface, it bound to the acidic leucine zipper part of the peptide, shielding the RGD. Addition of excess free acidic leucine zipper in solution led to competitive binding with the blocking PEGylated basic leucine zipper, freeing the RGD sequence for cell binding [310]. One key benefit of these biochemical triggers for controlling presentation is their effects are unique: chitosanase, a leucine zipper sequence, and trifluoroperazine are all not normally produced in the body, and only the designated trigger, which has minimal off-target effects, will cause the bioactive factors to be available to cells. While controlling these triggering molecules in space has not yet been explored, spatiotemporal control may be possible.
An extension of these biochemical triggers uses cells to produce soluble factors that induce release of a drug, genetic materials or growth factor. In this case, cells usually degrade the chemical linkages that tether a bioactive factor to the matrix, or the biomaterial that contains the encapsulated payload. Controlling the location of cells dictates where the factors are released by leveraging cells’ normal secretion of enzymes that break down the ECM, including MMPs. VEGF proteins, which contain a plasmin-cleavable site, were chemically coupled into hydrogels formed by Michael addition polymerization of 4-arm PEG vinyl sulfone with thiol-containing cell-adhesive and MMP-degradable peptides. This approach provides both covalently immobilized VEGF in the matrix, as well as free VEGF that is released in the presence of cell-secreted plasmin and MMPs. These hydrogels led to improved angiogenesis in a subcutaneous rat model compared to soluble VEGF alone or VEGF-bound hydrogels without the degradable peptide sequence [311]. Later, multiarm PEG vinyl sulfone hydrogels crosslinked using a MMP-sensitive peptide sequence and containing cell adhesion ligands were loaded with thymosin β4, a small peptide that enhances vascularization. After the release was demonstrated due to hydrogel degradation in response to exogenously supplemented MMPs, HUVECs were encapsulated and shown to have improved survival and vascular network formation in the peptide releasing hydrogels [312]. MMP-degradable PEG hydrogels have also been used to release dexamethasone, a glucocorticoid with known osteogenic activity, that led to improved hMSC osteogenic differentiation [313], and to release DNA/PEI complexes that maintained their ability to transfect hMSCs [314]. In contrast to the approaches described earlier in this section, which may allow patient- or physician-mediated spatiotemporal control of bioactive factor delivery throughout the course of a regenerative process, localized cell-responsive release is valuable because it preferentially occurs in regions of high matrix turnover and remodeling.
6. Conclusions and future directions
Spatially controlled bioactive factor presentation will continue to play a critical role in bone tissue engineering strategies, and will be coupled with biomaterials that permit tunable release profiles to achieve true spatiotemporal regulation over delivery. Importantly, for this approach to realize its full potential, the field requires a more advanced understanding of native biological signal presentation during bone development and healing. This includes elucidating bioactive factor concentrations and spatial and temporal distributions during these processes, accounting for the effects of microvascular and interstitial flow, with high resolution [315], and their local influence on target cells. Such knowledge could serve as engineering design criteria for the development of bone regeneration systems driven by patterns of bioactive factors.
Many of the patterning technologies described in this review have only been examined for tissue engineering applications within the last 20 years, but rapid progress has been made in enhancing their enhancing biocompatibility and level of control. This is likely to continue, with toxic fabrication conditions in some techniques, especially certain forms of solid free form fabrication, being replaced with gentler processes that may even allow for cell encapsulation. Methods with proven capacity to pattern just one signal will be expanded for patterning two or more, and efforts will be spent to make techniques less time-intensive while at the same time achieving improved spatial resolution. Many proof of principle techniques described here, as of yet only tested with fluorescently labeled model proteins, will be used to pattern growth factors, genetic material or drugs relevant to osteogenesis.
Patterned materials are used extensively as in vitro tools to better understand and screen cell responses to them, but maintaining patterns of bioactive factors in the complex biological environment found in actual bone defects will be a challenge. Immediately after in vivo implantation, adsorbed serum proteins may block activity of biomolecules presented from a biomaterial surface [316]. Similarly, the cells present at the defect site, including both host and transplanted, will secrete extracellular matrix molecules and soluble factors of their own. Such cell responses, either to patterned signal presentation in a biomaterial or independent of it, could potentially enhance the pattern’s effects, or mask or inhibit the patterned signal, resulting in rapid loss of its influence. To maximize the desired role of controlled spatial presentation of bioactive factors for a specific period of time, the pattern must be maintained in the uncontrolled signaling milieu present in a bone defect, and the delivered factors’ interactions with endogenous or cell secreted signals in this environment need to be investigated. Therefore, in vivo testing of the patterned systems will be vitally important, as results may differ substantially from in vitro experiments. Results with some patterns, including growth factors inkjet printed onto scaffold surfaces, show that the instructive nature of the patterned signals can be retained in the in vivo environment [179, 180], supporting the potential translation of other patterning approaches.
As these technologies move towards clinical translation, an important balance needs to be struck between increased control over signal presentation and degree of fabrication complexity. Because multicomponent systems with complicated fabrication procedures may add increased cost to an ultimate therapy, it will be important to identify applications when the potential benefits of patterning of bioactive factors, such as additional spatial control leading to improved therapeutic outcome compared to uniform presentation of the factors of interest, outweigh this drawback. An important step will be animal testing and human clinical trials comparing these systems to FDA-approved BMP delivery systems, which have shown clinical benefit in healing bone defects and spinal fusion, despite their lack of control over release [7, 9, 12]. Technology for spatially controlling growth factor presentation has advanced rapidly, and continued progress in this area will likely have a significant impact on the future clinical success of bone tissue engineering strategies.
Acknowledgements
We apologize that space limitations prevent us from including all of the excellent work that has been done in this rapidly growing area. The authors gratefully acknowledge funding from the National Institutes of Health (R01AR063194, R56DE022376, R21AR061265, R01AR053733), the Department of Defense Congressionally Directed Medical Research Programs (OR110196), the AO Foundation, and a New Scholar in Aging grant from the Ellison Medical Foundation to EA, and a NSF Graduate Research Fellowship to JES.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- [2].Simon SR. Orthopaedic basic science. Amer Academy of Orthopaedic. 1994 [Google Scholar]
- [3].DeCoster TA, Gehlert RJ, Mikola EA, Pirela-Cruz MA. Management of posttraumatic segmental bone defects. The Journal of the American Academy of Orthopaedic Surgeons. 2004;12:28–38. doi: 10.5435/00124635-200401000-00005. [DOI] [PubMed] [Google Scholar]
- [4].Habal MB, Reddi AH. Bone grafts and bone induction substitutes. Clinics in plastic surgery. 1994;21:525–542. [PubMed] [Google Scholar]
- [5].Langer R, Vacanti JP, Vacanti CA, Atala A, Freed LE, Vunjak-Novakovic G. Tissue engineering: biomedical applications. Tissue engineering. 1995;1:151–161. doi: 10.1089/ten.1995.1.151. [DOI] [PubMed] [Google Scholar]
- [6].Nerem RM, Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1:3–13. doi: 10.1089/ten.1995.1.3. [DOI] [PubMed] [Google Scholar]
- [7].Kanakaris NK, Calori GM, Verdonk R, Burssens P, Biase PD, Capanna R, Vangosa LB, Cherubino P, Baldo F, Ristiniemi J, Kontakis G, Giannoudis PV. Application of BMP-7 to tibial non-unions: A 3-year multicenter experience. Injury. 2008;39(Supplement 2):S83–S90. doi: 10.1016/S0020-1383(08)70019-6. [DOI] [PubMed] [Google Scholar]
- [8].Zimmermann G, Wagner C, Schmeckenbecher K, Wentzensen A, Moghaddam A. Treatment of tibial shaft non-unions: bone morphogenetic proteins versus autologous bone graft. Injury. 2009;40(Supplement 3):S50–S53. doi: 10.1016/S0020-1383(09)70012-9. [DOI] [PubMed] [Google Scholar]
- [9].Kanakaris NK, Lasanianos N, Calori GM, Verdonk R, Blokhuis TJ, Cherubino P, De Biase P, Giannoudis PV. Application of bone morphogenetic proteins to femoral non-unions: a 4-year multicentre experience. Injury. 2009;40(Suppl 3):S54–61. doi: 10.1016/S0020-1383(09)70013-0. [DOI] [PubMed] [Google Scholar]
- [10].Cook SD, Dalton JE, Tan EH, Whitecloud TS, Rueger DC. In-Vivo Evaluation of Recombinant Human Osteogenic Protein (Rhop-1) Implants as a Bone-Graft Substitute for Spinal Fusions. Spine. 1994;19:1655–1663. doi: 10.1097/00007632-199408000-00002. [DOI] [PubMed] [Google Scholar]
- [11].Rodgers MA, Brown JV, Heirs MK, Higgins JP, Mannion RJ, Simmonds MC, Stewart LA. Reporting of industry funded study outcome data: comparison of confidential and published data on the safety and effectiveness of rhBMP-2 for spinal fusion. Bmj. 2013;346:f3981. doi: 10.1136/bmj.f3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McKay B, Sandhu HS. Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine. 2002;27:S66–85. doi: 10.1097/00007632-200208151-00014. [DOI] [PubMed] [Google Scholar]
- [13].King GN. The importance of drug delivery to optimize the effects of bone morphogenetic proteins during periodontal regeneration. Current pharmaceutical biotechnology. 2001;2:131–142. doi: 10.2174/1389201013378716. [DOI] [PubMed] [Google Scholar]
- [14].Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31:2813–2819. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- [15].McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2) Journal of spinal disorders & techniques. 2006;19:483–486. doi: 10.1097/01.bsd.0000211231.83716.4b. [DOI] [PubMed] [Google Scholar]
- [16].Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542–547. doi: 10.1097/01.brs.0000201424.27509.72. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- [17].Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Advanced drug delivery reviews. 2012;64:1257–1276. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Advanced drug delivery reviews. 2012;64:1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. Journal of The Royal Society Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Babensee J, McIntire L, Mikos A. Growth Factor Delivery for Tissue Engineering. Pharmaceutical research. 2000;17:497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- [21].Cao L, Mooney DJ. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Advanced drug delivery reviews. 2007;59:1340–1350. doi: 10.1016/j.addr.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Olsen BR, Reginato AM, Wang W. Bone development. Annual review of cell and developmental biology. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- [23].Blair HC, Zaidi M, Schlesinger PH. Mechanisms balancing skeletal matrix synthesis and degradation. The Biochemical journal. 2002;364:329–341. doi: 10.1042/BJ20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arsenault AL, Ottensmeyer FP. Visualization of early intramembranous ossification by electron microscopic and spectroscopic imaging. The Journal of cell biology. 1984;98:911–921. doi: 10.1083/jcb.98.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. The international journal of biochemistry & cell biology. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- [26].Ortega N, Behonick DJ, Werb Z. Matrix remodeling during endochondral ossification. Trends in cell biology. 2004;14:86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. Journal of cellular biochemistry. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- [28].Cho T-J, Gerstenfeld LC, Einhorn TA. Differential Temporal Expression of Members of the Transforming Growth Factor β Superfamily During Murine Fracture Healing. Journal of Bone and Mineral Research. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- [29].Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. Journal of Bone and Mineral Research. 1999;14:1805–1815. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- [30].Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38:S11–S25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- [31].Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- [33].Phillips A. Overview of the fracture healing cascade. Injury. 2005;36:S5–S7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- [34].Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Advanced drug delivery reviews. 2012;64:1257–1276. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lu HH, Jiang J. Tissue Engineering I. Springer; 2006. Interface Tissue Engineeringand the Formulation of Multiple-Tissue Systems; pp. 91–111. [PubMed] [Google Scholar]
- [36].Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Engineering Part B: Reviews. 2009;15:127–141. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Seidi A, Ramalingam M, Elloumi-Hannachi I, Ostrovidov S, Khademhosseini A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta biomaterialia. 2011;7:1441–1451. doi: 10.1016/j.actbio.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [38].Singh M, Berkland C, Detamore MS. Strategies and applications for incorporating physical and chemical signal gradients in tissue engineering. Tissue Engineering Part B: Reviews. 2008;14:341–366. doi: 10.1089/ten.teb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Collin-Osdoby P. Role of vascular endothelial cells in bone biology. Journal of Cellular Biochemistry. 1994;55:304–309. doi: 10.1002/jcb.240550306. [DOI] [PubMed] [Google Scholar]
- [40].Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, Bouillon R, Carmeliet G. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mechanisms of Development. 2002;111:61–73. doi: 10.1016/s0925-4773(01)00601-3. [DOI] [PubMed] [Google Scholar]
- [41].Villars F, Bordenave L, Bareille R, Amedee J. Effect of human endothelial cells on human bone marrow stromal cell phenotype: role of VEGF? Journal of cellular biochemistry. 2000;79:672–685. doi: 10.1002/1097-4644(20001215)79:4<672::aid-jcb150>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- [42].Deckers MM, arperien M, van der Bent C, Yamashita T, Papapoulos SE, Löwik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- [43].Bouletreau PJ, Warren SM, Spector JA, Steinbrech DS, Mehrara BJ, Longaker MT. Factors in the fracture microenvironment induce primary osteoblast angiogenic cytokine production. Plastic and reconstructive surgery. 2002;110:139–148. doi: 10.1097/00006534-200207000-00025. [DOI] [PubMed] [Google Scholar]
- [44].Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE. Recombinant human platelet-derived growth factor: biology and clinical applications. The Journal of Bone & Joint Surgery. 2008;90:48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- [45].Matsubara H, Hogan DE, Morgan EF, Mortlock DP, Einhorn TA, Gerstenfeld LC. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone. 2012;51:168–180. doi: 10.1016/j.bone.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Huang Y-C, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined Angiogenic and Osteogenic Factor Delivery Enhances Bone Marrow Stromal Cell-Driven Bone Regeneration. Journal of Bone and Mineral Research. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- [47].Kempen DHR, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJA. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- [48].Patel ZS, Young S, Tabata Y, Jansen JA, Wong MEK, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kanczler JM, Ginty PJ, White L, Clarke NMP, Howdle SM, Shakesheff KM, Oreffo ROC. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31:1242–1250. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- [50].Cross M, Dexter TM. Growth factors in development, transformation, and tumorigenesis. Cell. 1991;64:271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- [51].Schlessinger J, Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- [52].Penheiter SG, Mitchell H, Garamszegi N, Edens M, Doré JJ, Jr, Leof EB. Internalization-dependent and-independent requirements for transforming growth factor β receptor signaling via the Smad pathway. Molecular and cellular biology. 2002;22:4750–4759. doi: 10.1128/MCB.22.13.4750-4759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gurkan UA, Gargac J, Akkus O. The sequential production profiles of growth factors and their relations to bone volume in ossifying bone marrow explants. Tissue Engineering Part A. 2010;16:2295–2306. doi: 10.1089/ten.TEA.2009.0565. [DOI] [PubMed] [Google Scholar]
- [54].Damon DH, Lobb RR, D'Amore PA, Wagner JA. Heparin potentiates the action of acidic fibroblast growth factor by prolonging its biological half-life. Journal of Cellular Physiology. 1989;138:221–226. doi: 10.1002/jcp.1041380202. [DOI] [PubMed] [Google Scholar]
- [55].Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends in biochemical sciences. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- [56].Prenzel N, Fischer O, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocrine-related cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- [57].Farhadi J, Jaquiery C, Barbero A, Jakob M, Schaeren S, Pierer G, Heberer M, Martin I. Differentiation-dependent up-regulation of BMP-2, TGF-β1, and V GF expression by FGF-2 in human bone marrow stromal cells. Plastic and reconstructive surgery. 2005;116:1379–1386. doi: 10.1097/01.prs.0000182355.67397.5a. [DOI] [PubMed] [Google Scholar]
- [58].Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. The Journal of bone and joint surgery. 2002;84-A:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- [59].Yun YR, Jang JH, Jeon E, Kang W, Lee S, Won JE, Kim HW, Wall I. Administration of growth factors for bone regeneration. Regenerative medicine. 2012;7:369–385. doi: 10.2217/rme.12.1. [DOI] [PubMed] [Google Scholar]
- [60].Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-α promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proceedings of the National Academy of Sciences. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic Protein-1 (Bone Morphogenetic Protein-7) in the Treatment of Tibial Nonunions A Prospective, Randomized Clinical Trial Comparing rhOP-1 with Fresh Bone Autograft*. The Journal of Bone & Joint Surgery. 2001;83:151–S158. [PMC free article] [PubMed] [Google Scholar]
- [62].Guler H-P, Zapf J, Froesch ER. Short-Term Metabolic Effects of Recombinant Human Insulin-like Growth Factor I in Healthy Adults. New England Journal of Medicine. 1987;317:137–140. doi: 10.1056/NEJM198707163170303. [DOI] [PubMed] [Google Scholar]
- [63].Dahabreh Z, Calori G, Kanakaris N, Nikolaou V, Giannoudis P. A cost analysis of treatment of tibial fracture nonunion by bone grafting or bone morphogenetic protein-7. International orthopaedics. 2009;33:1407–1414. doi: 10.1007/s00264-008-0709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Von Einem S, Schwarz E, Rudolph R. A novel TWO-STEP renaturation procedure for efficient production of recombinant BMP-2. Protein expression and purification. 2010;73:65–69. doi: 10.1016/j.pep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- [65].Seol YJ, Park YJ, Lee SC, Kim KH, Lee JY, Kim TI, Lee YM, Ku Y, Rhyu IC, Han SB. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BM )-2. Journal of Biomedical Materials Research art A. 2006;77:599–607. doi: 10.1002/jbm.a.30639. [DOI] [PubMed] [Google Scholar]
- [65].Lee JS, Lee JS, Murphy WL. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta biomaterialia. 2010;6:21–28. doi: 10.1016/j.actbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lin X, Elliot J, Carnes D, Fox W, Pena L, Campion S, Takahashi K, Atkinson B, Zamora P. Augmentation of osseous phenotypes in vivo with a synthetic peptide. Journal of orthopaedic research. 2007;25:531–539. doi: 10.1002/jor.20303. [DOI] [PubMed] [Google Scholar]
- [68].Saito A, Suzuki Y, Ogata S-I, Ohtsuki C, Tanihara M. Prolonged ectopic calcification induced by BMP-2–derived synthetic peptide. Journal of Biomedical Materials Research Part A. 2004;70A:115–121. doi: 10.1002/jbm.a.30071. [DOI] [PubMed] [Google Scholar]
- [69].Lin X, Takahashi K, Campion S, Liu Y, Gustavsen G, Pena L, Zamora P. Synthetic peptide F2A4-K-NS mimics fibroblast growth factor-2 in vitro and is angiogenic in vivo. International journal of molecular medicine. 2006;17:833–840. doi: 10.3892/ijmm.17.5.833. [DOI] [PubMed] [Google Scholar]
- [70].D'Andrea LD, Iaccarino G, Fattorusso R, Sorriento D, Carannante C, Capasso D, Trimarco B, Pedone C. Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14215–14220. doi: 10.1073/pnas.0505047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jeon O, Song SJ, Yang HS, Bhang S-H, Kang S-W, Sung MA, Lee JH, Kim B-S. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochemical and biophysical research communications. 2008;369:774–780. doi: 10.1016/j.bbrc.2008.02.099. [DOI] [PubMed] [Google Scholar]
- [72].Raiche A, Puleo D. In vitro effects of combined and sequential delivery of two bone growth factors. Biomaterials. 2004;25:677–685. doi: 10.1016/s0142-9612(03)00564-7. [DOI] [PubMed] [Google Scholar]
- [73].Shah NJ, Macdonald ML, Beben YM, Padera RF, Samuel RE, Hammond PT. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials. 2011;32:6183–6193. doi: 10.1016/j.biomaterials.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Geuze RE, Theyse LF, Kempen DH, Hazewinkel HA, Kraak HY, Öner FC, Dhert WJ, Alblas J. A differential effect of bone morphogenetic protein-2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large-animal model. Tissue Engineering Part A. 2012;18:2052–2062. doi: 10.1089/ten.tea.2011.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering—part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Engineering Part B: Reviews. 2013;19:308–326. doi: 10.1089/ten.teb.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Santo VE, Gomes ME, Mano JF, Reis RL. Controlled Release Strategies for Bone, Cartilage, and Osteochondral Engineering—Part II: Challenges on the Evolution from Single to Multiple Bioactive Factor Delivery. Tissue Engineering Part B: Reviews. 2013;19:327–352. doi: 10.1089/ten.teb.2012.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Evans CH, Ghivizzani SC, Robbins PD. Orthopedic gene therapy—lost in translation? Journal of Cellular Physiology. 2012;227:416–420. doi: 10.1002/jcp.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bonadio J. Tissue engineering via local gene delivery:: Update and future prospects for enhancing the technology. Advanced drug delivery reviews. 2000;44:185–194. doi: 10.1016/s0169-409x(00)00094-6. [DOI] [PubMed] [Google Scholar]
- [79].Evans C. Gene therapy for the regeneration of bone. Injury. 2011;42:599–604. doi: 10.1016/j.injury.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Krebs MD, Alsberg E. Localized, Targeted, and Sustained siRNA Delivery. Chemistry – A European Journal. 2011;17:3054–3062. doi: 10.1002/chem.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Partridge KA, Oreffo RO. Gene delivery in bone tissue engineering: progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10:295–307. doi: 10.1089/107632704322791934. [DOI] [PubMed] [Google Scholar]
- [82].Baltzer A, Lattermann C, Whalen J, Wooley P, Weiss K, Grimm M, Ghivizzani S, Robbins P, Evans C. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene therapy. 2000;7:734–739. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- [83].Dupont K, Boerckel J, Stevens H, Diab T, Kolambkar Y, Takahata M, Schwarz E, Guldberg R. Synthetic scaffold coating with adeno-associated virus encoding BMP2 to promote endogenous bone repair. Cell Tissue Res. 2012;347:575–588. doi: 10.1007/s00441-011-1197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Evans CH, Ghivizzani SC, Robbins PD. Getting arthritis gene therapy into the clinic. Nature Reviews Rheumatology. 2010;7:244–249. doi: 10.1038/nrrheum.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. The AAPS journal. 2009;11:671–681. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kimelman Bleich N, Kallai I, Lieberman JR, Schwarz EM, Pelled G, Gazit D. Gene therapy approaches to regenerating bone. Advanced drug delivery reviews. 2012;64:1320–1330. doi: 10.1016/j.addr.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Al-Dosari M, Gao X. Nonviral Gene Delivery: Principle, Limitations, and Recent Progress. AAPS J. 2009;11:671–681. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Advanced drug delivery reviews. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [89].Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proceedings of the National Academy of Sciences. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Askari FK, McDonnell WM. Antisense-oligonucleotide therapy. New England Journal of Medicine. 1996;334:316–318. doi: 10.1056/NEJM199602013340508. [DOI] [PubMed] [Google Scholar]
- [92].Hatzfeld J, Li M-L, Brown EL, Sookdeo H, Levesque J-P, O'Toole T, Gurney C, Clark SC, Hatzfeld A. Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor beta 1 or Rb oligonucleotides. The Journal of experimental medicine. 1991;174:925–929. doi: 10.1084/jem.174.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ishikawa T, Kamiyama M, Tani-Ishii N, Suzuki H, Ichikawa Y, Hamaguchi Y, Momiyama N, Shimada H. Inhibition of osteoclast differentiation and bone resorption by cathepsin K antisense oligonucleotides. Molecular carcinogenesis. 2001;32:84–91. doi: 10.1002/mc.1067. [DOI] [PubMed] [Google Scholar]
- [94].Laitala T, Väänänen H. Inhibition of bone resorption in vitro by antisense RNA and DNA molecules targeted against carbonic anhydrase II or two subunits of vacuolar H (+)-ATPase. Journal of Clinical Investigation. 1994;93:2311. doi: 10.1172/JCI117235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kofron MD, Laurencin CT. Bone tissue engineering by gene delivery. Advanced drug delivery reviews. 2006;58:555–576. doi: 10.1016/j.addr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- [96].Storrie H, Mooney DJ. Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering. Advanced drug delivery reviews. 2006;58:500–514. doi: 10.1016/j.addr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [97].Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nature biotechnology. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- [98].Li YL, Quarles LD, Zhou HH, Xiao ZS. RNA interference and its application in bone-related diseases. Biochemical and biophysical research communications. 2007;361:817–821. doi: 10.1016/j.bbrc.2007.07.123. [DOI] [PubMed] [Google Scholar]
- [99].Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA Translation and Stability by microRNAs. Annual Review of Biochemistry. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- [100].Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Current Opinion in Structural Biology. 2005;15:331–341. doi: 10.1016/j.sbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- [101].Manaka T, Suzuki A, Takayama K, Imai Y, Nakamura H, Takaoka K. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials. 2011;32:9642–9648. doi: 10.1016/j.biomaterials.2011.08.026. [DOI] [PubMed] [Google Scholar]
- [102].Kwong FN, Richardson SM, Evans CH. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis research & therapy. 2008;10:R65. doi: 10.1186/ar2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang Y, Grainger D. siRNA Knock-Down of RANK Signaling to Control Osteoclast-Mediated Bone Resorption. Pharmaceutical research. 2010;27:1273–1284. doi: 10.1007/s11095-010-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Bae Y, Yang T, Zeng H-C, Campeau PM, Chen Y, Bertin T, Dawson BC, Munivez E, Tao J, Lee BH. miRNA-34c regulates Notch signaling during bone development. Human Molecular Genetics. 2012;21:2991–3000. doi: 10.1093/hmg/dds129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhang W-B, Zhong W-J, Wang L. A signal-amplification circuit between miR-18 and nt/β-catenin signal promotes human adipose tissue-derived stem cells osteogenic differentiation. Bone. 2014;58:59–66. doi: 10.1016/j.bone.2013.09.015. [DOI] [PubMed] [Google Scholar]
- [106].Caporali A, Emanueli C. MicroRNA regulation in angiogenesis. Vascular Pharmacology. 2011;55:79–86. doi: 10.1016/j.vph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- [107].Krebs MD, Jeon O, Alsberg E. Localized and Sustained Delivery of Silencing RNA from Macroscopic Biopolymer Hydrogels. Journal of the American Chemical Society. 2009;131:9204–9206. doi: 10.1021/ja9037615. [DOI] [PubMed] [Google Scholar]
- [108].Nguyen MK, Jeon O, Krebs MD, Schapira D, Alsberg E. Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials. 2014;35:6278–6286. doi: 10.1016/j.biomaterials.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chemical Society Reviews. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- [110].Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. Journal of Controlled Release. 2008;130:202–215. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- [111].Mouriño V, Boccaccini AR. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. Journal of the Royal Society Interface. 2010;7:209–227. doi: 10.1098/rsif.2009.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wong TM, Lau TW, Li X, Fang C, Yeung K, Leung F. Masquelet Technique for Treatment of Posttraumatic Bone Defects. The Scientific World Journal. 2014;2014:5. doi: 10.1155/2014/710302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Giannoudis PV, Faour O, Goff T, Kanakaris N, Dimitriou R. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury. 2011;42:591–598. doi: 10.1016/j.injury.2011.03.036. [DOI] [PubMed] [Google Scholar]
- [114].Corry D, Moran J. Assessment of acrylic bone cement as a local delivery vehicle for the application of non-steroidal anti-inflammatory drugs. Biomaterials. 1998;19:1295–1301. doi: 10.1016/s0142-9612(98)00012-x. [DOI] [PubMed] [Google Scholar]
- [115].Duarte ARC, Mano JF, Reis RL. Dexamethasone-loaded scaffolds prepared by supercritical-assisted phase inversion. Acta biomaterialia. 2009;5:2054–2062. doi: 10.1016/j.actbio.2009.01.047. [DOI] [PubMed] [Google Scholar]
- [116].Mortera R, Onida B, Fiorilli S, Cauda V, Brovarone CV, Baino F, Vernè E, Garrone E. Synthesis and characterization of MCM-41 spheres inside bioactive glass–ceramic scaffold. Chemical Engineering Journal. 2008;137:54–61. [Google Scholar]
- [117].Faucheux C, Verron E, Soueidan A, Josse S, Arshad M, Janvier P, Pilet P, Bouler J, Bujoli B, Guicheux J. Controlled release of bisphosphonate from a calcium phosphate biomaterial inhibits osteoclastic resorption in vitro. Journal of Biomedical Materials Research Part A. 2009;89:46–56. doi: 10.1002/jbm.a.31989. [DOI] [PubMed] [Google Scholar]
- [118].Nieto A, Balas F, Colilla M, Manzano M, Vallet-Regí M. Functionalization degree of SBA-15 as key factor to modulate sodium alendronate dosage. Microporous and Mesoporous Materials. 2008;116:4–13. [Google Scholar]
- [119].Maeda T, Matsunuma A, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun. 2001;280:874–877. doi: 10.1006/bbrc.2000.4232. [DOI] [PubMed] [Google Scholar]
- [120].Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- [121].Garrett IR, Mundy G. The role of statins as potential targets for bone formation. Arthritis Res. 2002;4:237–240. doi: 10.1186/ar413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Benoit DSW, Nuttelman CR, Collins SD, Anseth KS. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102–6110. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- [123].Park Y, David A, Park K, Lin C-Y, Than K, Lee K, Park J, Jo I, Park K, Yang V. Controlled Release of Simvastatin from In situ Forming Hydrogel Triggers Bone Formation in MC3T3-E1 Cells. AAPS J. 2013;15:367–376. doi: 10.1208/s12248-012-9442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Mukozawa A, Ueki K, Marukawa K, Okabe K, Moroi A, Nakagawa K. Bone healing of critical-sized nasal defects in rabbits by statins in two different carriers. Clinical Oral Implants Research. 2011;22:1327–1335. doi: 10.1111/j.1600-0501.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- [125].Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic Actions of Parathyroid Hormone on Bone*. Endocrine reviews. 1993;14:690–709. doi: 10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- [126].Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M, Hock JM. Anabolic and catabolic bone effects of human parathyroid hormone (1-34) are predicted by duration of hormone exposure. Bone. 2003;33:372–379. doi: 10.1016/s8756-3282(03)00202-3. [DOI] [PubMed] [Google Scholar]
- [127].Poole KE, Reeve J. Parathyroid hormone—a bone anabolic and catabolic agent. Current opinion in pharmacology. 2005;5:612–617. doi: 10.1016/j.coph.2005.07.004. [DOI] [PubMed] [Google Scholar]
- [128].Nguyen MK, Alsberg E. Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Progress in Polymer Science. 2014 doi: 10.1016/j.progpolymsci.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. Journal of Controlled Release. 2001;73:121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- [130].Siepmann J, Göpferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Advanced drug delivery reviews. 2001;48:229–247. doi: 10.1016/s0169-409x(01)00116-8. [DOI] [PubMed] [Google Scholar]
- [131].Williams DF. Mechanisms of biodegradation of implantable polymers. Clinical Materials. 1992;10:9–12. doi: 10.1016/0267-6605(92)90078-8. [DOI] [PubMed] [Google Scholar]
- [132].Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. Journal of Biomaterials Science, Polymer Edition. 2001;12:77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- [133].Tabata Y, Ikada Y. Protein release from gelatin matrices. Advanced drug delivery reviews. 1998;31:287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- [134].Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nature Reviews Drug Discovery. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- [135].Nguyen K, Dang PN, Alsberg E. Functionalized, biodegradable hydrogels for control over sustained and localized siRNA delivery to incorporated and surrounding cells. Acta biomaterialia. 2013;9:4487–4495. doi: 10.1016/j.actbio.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Joung YK, Bae JW, Park KD. Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration. 2008 doi: 10.1517/17425240802431811. [DOI] [PubMed] [Google Scholar]
- [137].Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annual review of biochemistry. 1989;58:575–602. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- [138].Rider C. Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochemical Society Transactions. 2006;34:458. doi: 10.1042/BST0340458. [DOI] [PubMed] [Google Scholar]
- [139].Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- [140].Masters KS. Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromolecular bioscience. 2011;11:1149–1163. doi: 10.1002/mabi.201000505. [DOI] [PubMed] [Google Scholar]
- [141].Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-anic M. Growth factors and cytokines in wound healing. Wound Repair and Regeneration. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- [142].Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. Journal of Biomedical Materials Research Part A. 2004;71A:528–537. doi: 10.1002/jbm.a.30186. [DOI] [PubMed] [Google Scholar]
- [143].Yamachika E, Tsujigiwa H, Shirasu N, Ueno T, Sakata Y, Fukunaga J, Mizukawa N, Yamada M, Sugahara T. Immobilized recombinant human bone morphogenetic protein-enhances the phosphorylation of receptor-activated Smads. Journal of Biomedical Materials Research Part A. 2009;88:599–607. doi: 10.1002/jbm.a.31833. [DOI] [PubMed] [Google Scholar]
- [144].Mano JF. Stimuli-Responsive Polymeric Systems for Biomedical Applications. Advanced Engineering Materials. 2008;10:515–527. [Google Scholar]
- [145].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proceedings of the National Academy of Sciences. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zhao X, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, Mooney DJ. Active scaffolds for on-demand drug and cell delivery. Proceedings of the National Academy of Sciences. 2010 doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- [148].Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nature Reviews Drug Discovery. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- [149].Xia Y, Whitesides GM. Soft Lithography. Angewandte Chemie International Edition. 1998;37:550–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [150].Rogers JA, Nuzzo RG. Recent progress in soft lithography. Materials Today. 2005;8:50–56. [Google Scholar]
- [151].Xia Y, McClelland JJ, Gupta R, Qin D, Zhao X-M, Sohn LL, Celotta RJ, Whitesides GM. Replica molding using polymeric materials: A practical step toward nanomanufacturing. Advanced Materials. 1997;9:147–149. [Google Scholar]
- [152].Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric Control of Cell Life and Death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- [153].Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned Surfaces for Control of Cell Shape, Position, and Function. Biotechnology Progress. 1998;14:356–363. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- [154].Williams C, Tsuda Y, Isenberg BC, Yamato M, Shimizu T, Okano T, Wong JY. Aligned Cell Sheets Grown on Thermo-Responsive Substrates with Microcontact rinted rotein atterns. Advanced Materials. 2009;21:2161–2164. [Google Scholar]
- [155].Elloumi Hannachi I, Itoga K, Kumashiro Y, Kobayashi J, Yamato M, Okano T. Fabrication of transferable micropatterned-co-cultured cell sheets with microcontact printing. Biomaterials. 2009;30:5427–5432. doi: 10.1016/j.biomaterials.2009.06.033. [DOI] [PubMed] [Google Scholar]
- [156].Lee CJ, Huie P, Leng T, et al. MIcrocontact printing on human tissue for retinal cell transplantation. Archives of Ophthalmology. 2002;120:1714–1718. doi: 10.1001/archopht.120.12.1714. [DOI] [PubMed] [Google Scholar]
- [157].Csucs G, Michel R, Lussi JW, Textor M, Danuser G. Microcontact printing of novel co-polymers in combination with proteins for cell-biological applications. Biomaterials. 2003;24:1713–1720. doi: 10.1016/s0142-9612(02)00568-9. [DOI] [PubMed] [Google Scholar]
- [158].Thibault C, Le Berre V, Casimirius S, Trévisiol E, François J, Vieu C. Direct microcontact printing of oligonucleotides for biochip applications. Journal of nanobiotechnology. 2005;3 doi: 10.1186/1477-3155-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Lange SA, Benes V, Kern DP, Hörber JKH, Bernard A. Microcontact Printing of DNA Molecules. Analytical Chemistry. 2004;76:1641–1647. doi: 10.1021/ac035127w. [DOI] [PubMed] [Google Scholar]
- [160].Mayer M, Yang J, Gitlin I, Gracias DH, Whitesides GM. Micropatterned agarose gels for stamping arrays of proteins and gradients of proteins. PROTEOMICS. 2004;4:2366–2376. doi: 10.1002/pmic.200300748. [DOI] [PubMed] [Google Scholar]
- [161].Von Philipsborn AC, Lang S, Bernard A, Loeschinger J, David C, Lehnert D, Bastmeyer M, Bonhoeffer F. Microcontact printing of axon guidance molecules for generation of graded patterns. Nature protocols. 2006;1:1322–1328. doi: 10.1038/nprot.2006.251. [DOI] [PubMed] [Google Scholar]
- [162].Cornish T, Branch DW, Wheeler BC, Campanelli JT. Microcontact printing: a versatile technique for the study of synaptogenic molecules. Molecular and cellular neuroscience. 2002;20:140–153. doi: 10.1006/mcne.2002.1101. [DOI] [PubMed] [Google Scholar]
- [163].Patel N, Bhandari R, Shakesheff KM, Cannizzaro SM, Davies MC, Langer R, Roberts CJ, Tendler SJ, Williams PM. Printing patterns of biospecifically-adsorbed protein. Journal of Biomaterials Science, Polymer Edition. 2000;11:319–331. doi: 10.1163/156856200743724. [DOI] [PubMed] [Google Scholar]
- [164].Bernard A, Fitzli D, Sonderegger P, Delamarche E, Michel B, Bosshard HR, Biebuyck H. Affinity capture of proteins from solution and their dissociation by contact printing. Nature biotechnology. 2001;19:866–869. doi: 10.1038/nbt0901-866. [DOI] [PubMed] [Google Scholar]
- [165].Renault JP, Bernard A, Juncker D, Michel B, Bosshard HR, Delamarche E. Fabricating Microarrays of Functional Proteins Using Affinity Contact Printing. Angewandte Chemie. 2002;114:2426–2429. doi: 10.1002/1521-3773(20020703)41:13<2320::AID-ANIE2320>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [166].Graber DJ, Zieziulewicz TJ, Lawrence DA, Shain W, Turner JN. Antigen binding specificity of antibodies patterned by microcontact printing. Langmuir. 2003;19:5431–5434. [Google Scholar]
- [167].Cui X, Boland T, D'Lima DD, Lotz MK. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent patents on drug delivery & formulation. 2012;6:149–155. doi: 10.2174/187221112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707–3715. doi: 10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- [169].Turcu F, Tratsk-Nitz K, Thanos S, Schuhmann W, Heiduschka P. Ink-jet printing for micropattern generation of laminin for neuronal adhesion. Journal of neuroscience methods. 2003;131:141–148. doi: 10.1016/j.jneumeth.2003.08.001. [DOI] [PubMed] [Google Scholar]
- [170].Sanjana NE, Fuller SB. A fast flexible ink-jet printing method for patterning dissociated neurons in culture. Journal of neuroscience methods. 2004;136:151–163. doi: 10.1016/j.jneumeth.2004.01.011. [DOI] [PubMed] [Google Scholar]
- [171].Watanabe K, Miyazaki T, Matsuda R. Growth factor array fabrication using a color ink jet printer. Zoological science. 2003;20:429–434. doi: 10.2108/zsj.20.429. [DOI] [PubMed] [Google Scholar]
- [172].Sele CW, von Werne T, Friend RH, Sirringhaus H. Lithography-Free, Self-Aligned Inkjet Printing with Sub-Hundred-Nanometer Resolution. Advanced Materials. 2005;17:997–1001. [Google Scholar]
- [173].Campbell PG, Miller ED, Fisher GW, Walker LM, Weiss LE. Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell organization. Biomaterials. 2005;26:6762–6770. doi: 10.1016/j.biomaterials.2005.04.032. [DOI] [PubMed] [Google Scholar]
- [174].Miller ED, Fisher GW, Weiss LE, Walker LM, Campbell PG. Dose-dependent cell growth in response to concentration modulated patterns of FGF-2 printed on fibrin. Biomaterials. 2006;27:2213–2221. doi: 10.1016/j.biomaterials.2005.10.021. [DOI] [PubMed] [Google Scholar]
- [175].Ilkhanizadeh S, Teixeira AI, Hermanson O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials. 2007;28:3936–3943. doi: 10.1016/j.biomaterials.2007.05.018. [DOI] [PubMed] [Google Scholar]
- [176].Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem cells. 2008;26:127–134. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- [177].Ker ED, Chu B, Phillippi JA, Gharaibeh B, Huard J, Weiss LE, Campbell PG. Engineering spatial control of multiple differentiation fates within a stem cell population. Biomaterials. 2011;32:3413–3422. doi: 10.1016/j.biomaterials.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Ker ED, Nain AS, Weiss LE, Wang J, Suhan J, Amon CH, Campbell PG. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials. 2011;32:8097–8107. doi: 10.1016/j.biomaterials.2011.07.025. [DOI] [PubMed] [Google Scholar]
- [179].Cooper GM, Miller ED, Decesare GE, Usas A, Lensie EL, Bykowski MR, Huard J, Weiss LE, Losee JE, Campbell PG. Inkjet-based biopatterning of bone morphogenetic protein-2 to spatially control calvarial bone formation. Tissue engineering. Part A. 2010;16:1749–1759. doi: 10.1089/ten.tea.2009.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Herberg S, Kondrikova G, Periyasamy-Thandavan S, Howie RN, Elsalanty ME, Weiss L, Campbell P, Hill WD, Cray JJ. Inkjet-based biopatterning of SDF-1β augments BM -2-induced repair of critical size calvarial bone defects in mice. Bone. 2014;67:95–103. doi: 10.1016/j.bone.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Xu T, Rohozinski J, Zhao W, Moorefield EC, Atala A, Yoo JJ. Inkjet-mediated gene transfection into living cells combined with targeted delivery. Tissue Engineering Part A. 2008;15:95–101. doi: 10.1089/ten.tea.2008.0095. [DOI] [PubMed] [Google Scholar]
- [182].Tavana H, Jovic A, Mosadegh B, Lee Q, Liu X, Luker K, Luker G, Weiss S, Takayama S. Nanolitre liquid patterning in aqueous environments for spatially defined reagent delivery to mammalian cells. Nature materials. 2009;8:736–741. doi: 10.1038/nmat2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Tavana H, Mosadegh B, Takayama S. Polymeric Aqueous Biphasic Systems for Non-ontact ell Printing on Cells: Engineering Heterocellular Embryonic Stem Cell Niches. Advanced Materials. 2010;22:2628–2631. doi: 10.1002/adma.200904271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Moraes C, Simon AB, Putnam AJ, Takayama S. Aqueous two-phase printing of cell-containing contractile collagen microgels. Biomaterials. 2013;34:9623–9631. doi: 10.1016/j.biomaterials.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Lee HJ, Koh W-G. Hydrogel Micropattern-Incorporated Fibrous Scaffolds Capable of Sequential Growth Factor Delivery for Enhanced Osteogenesis of hMSCs. ACS Applied Materials & Interfaces. 2014;6:9338–9348. doi: 10.1021/am501714k. [DOI] [PubMed] [Google Scholar]
- [186].Moon JJ, Hahn MS, Kim I, Nsiah BA, West JL. Micropatterning of poly (ethylene glycol) diacrylate hydrogels with biomolecules to regulate and guide endothelial morphogenesis. Tissue Engineering Part A. 2008;15:579–585. doi: 10.1089/ten.tea.2008.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Saik JE, Gould DJ, Watkins EM, Dickinson ME, West JL. Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels. Acta biomaterialia. 2011;7:133–143. doi: 10.1016/j.actbio.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Leslie-Barbick JE, Shen C, Chen C, West JL. Micron-scale spatially patterned, covalently immobilized vascular endothelial growth factor on hydrogels accelerates endothelial tubulogenesis and increases cellular angiogenic responses. Tissue Engineering Part A. 2010;17:221–229. doi: 10.1089/ten.tea.2010.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Christman KL, Vázquez-Dorbatt V, Schopf E, Kolodziej CM, Li RC, Broyer RM, Chen Y, Maynard HD. Nanoscale growth factor patterns by immobilization on a heparin-mimicking polymer. Journal of the American Chemical Society. 2008;130:16585–16591. doi: 10.1021/ja803676r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Kolodziej CM, Kim SH, Broyer RM, Saxer SS, Decker CG, Maynard HD. Combination of Integrin-Binding Peptide and Growth Factor Promotes Cell Adhesion on Electron-Beam-Fabricated Patterns. Journal of the American Chemical Society. 2011;134:247–255. doi: 10.1021/ja205524x. [DOI] [PubMed] [Google Scholar]
- [191].Schlapak R, Danzberger J, Haselgrübler T, Hinterdorfer P, Schäffler F, Howorka S. Painting with Biomolecules at the Nanoscale: Biofunctionalization with Tunable Surface Densities. Nano Letters. 2012;12:1983–1989. doi: 10.1021/nl2045414. [DOI] [PubMed] [Google Scholar]
- [192].Frenkel SR, Toolan B, Menche D, Pitman MI, Pachence JM. Chondrocyte transplantation using a collagen bilayer matrix for cartilage repair. The Journal of bone and joint surgery. British volume. 1997;79:831–836. doi: 10.1302/0301-620x.79b5.7278. [DOI] [PubMed] [Google Scholar]
- [193].Madry H, Rey-Rico A, Venkatesan JK, Johnstone B, Cucchiarini M. Transforming Growth Factor Beta-Releasing Scaffolds for Cartilage Tissue Engineering. Tissue Engineering Part B: Reviews. 2013 doi: 10.1089/ten.TEB.2013.0271. [DOI] [PubMed] [Google Scholar]
- [194].Guo X, Liao J, Park H, Saraf A, Raphael RM, Tabata Y, Kasper FK, Mikos AG. Effects of TGF-beta3 and preculture period of osteogenic cells on the chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in a bilayered hydrogel composite. Acta biomaterialia. 2010;6:2920–2931. doi: 10.1016/j.actbio.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Guo X, Park H, Liu G, Liu W, Cao Y, Tabata Y, Kasper FK, Mikos AG. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials. 2009;30:2741–2752. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Reyes R, Delgado A, Solis R, Sanchez E, Hernandez A, Roman JS, Evora C. Cartilage repair by local delivery of transforming growth factor-beta1 or bone morphogenetic protein-2 from a novel, segmented polyurethane/polylactic-co-glycolic bilayered scaffold. Journal of biomedical materials research Part A. 2013 doi: 10.1002/jbma.34769. [DOI] [PubMed] [Google Scholar]
- [197].Johnson O, Jaworowicz W, Cleland J, Bailey L, Charnis M, Duenas E, Wu C, Shepard D, Magil S, Last T, Jones AS, Putney S. The Stabilization and Encapsulation of Human Growth Hormone into Biodegradable Microspheres. Pharmaceutical research. 1997;14:730–735. doi: 10.1023/a:1012142204132. [DOI] [PubMed] [Google Scholar]
- [198].Seo JP, Tanabe T, Tsuzuki N, Haneda S, Yamada K, Furuoka H, Tabata Y, Sasaki N. Effects of bilayer gelatin/beta-tricalcium phosphate sponges loaded with mesenchymal stem cells, chondrocytes, bone morphogenetic protein-2, and platelet rich plasma on osteochondral defects of the talus in horses. Research in veterinary science. 2013;95:1210–1216. doi: 10.1016/j.rvsc.2013.08.016. [DOI] [PubMed] [Google Scholar]
- [199].Chen J, Chen H, Li P, Diao H, Zhu S, Dong L, Wang R, Guo T, Zhao J, Zhang J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials. 2011;32:4793–4805. doi: 10.1016/j.biomaterials.2011.03.041. [DOI] [PubMed] [Google Scholar]
- [200].Solorio LD, Phillips LM, McMillan A, Cheng CW, Dang PN, Samorezov JE, Alsberg E. Spatially organized differentiation of mesenchymal stem cells within biphasic microparticle-incorporated high cell density osteochondral tissues. (Submitted) doi: 10.1002/adhm.201500598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharmaceutical research. 2007;24:258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- [202].Silver J. Inhibitory molecules in development and regeneration. Journal of neurology. 1994;242:S22–S24. doi: 10.1007/BF00939236. [DOI] [PubMed] [Google Scholar]
- [203].Yuen WW, Du NR, Chan CH, Silva EA, Mooney DJ. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17933–17938. doi: 10.1073/pnas.1001192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Hinton RH, Dobrota M. A simple gradient maker for use with zonal rotors. Analytical Biochemistry. 1969;30:99–110. doi: 10.1016/0003-2697(69)90377-7. [DOI] [PubMed] [Google Scholar]
- [205].Chatterjee K, Young MF, Simon CG., Jr Fabricating gradient hydrogel scaffolds for 3D cell culture. Combinatorial chemistry & high throughput screening. 2011;14:227. doi: 10.2174/138620711795222455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Jeon O, Alt DS, Linderman SW, Alsberg E. Biochemical and Physical Signal Gradients in Hydrogels to Control Stem Cell Behavior. Advanced Materials. 2013;25:6366–6372. doi: 10.1002/adma.201302364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Burdick JA, Khademhosseini A, Langer R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir. 2004;20:5153–5156. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]
- [208].Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab on a Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- [209].Sant S, Hancock MJ, Donnelly JP, Iyer D, Khademhosseini A. Biomimetic gradient hydrogels for tissue engineering. The Canadian journal of chemical engineering. 2010;88:899–911. doi: 10.1002/cjce.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Kapur TA, Shoichet MS. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. Journal of Biomedical Materials Research Part A. 2004;68:235–243. doi: 10.1002/jbm.a.10168. [DOI] [PubMed] [Google Scholar]
- [211].Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Science signaling. 2009;2:ra55. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- [213].Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. Journal of Controlled Release. 2009;134:81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Annals of biomedical engineering. 2010;38:2167–2182. doi: 10.1007/s10439-010-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Engineering Part A. 2011;17:2845–2855. doi: 10.1089/ten.tea.2011.0135. [DOI] [PubMed] [Google Scholar]
- [216].Erisken C, Kalyon DM, Wang H, Örnek-Ballanco C, Xu J. Osteochondral tissue formation through adipose-derived stromal cell differentiation on biomimetic polycaprolactone nanofibrous scaffolds with graded insulin and beta-glycerophosphate concentrations. Tissue Engineering Part A. 2011;17:1239–1252. doi: 10.1089/ten.TEA.2009.0693. [DOI] [PubMed] [Google Scholar]
- [217].Phillips JE, Burns KL, Le Doux JM, Guldberg RE, García AJ. Engineering graded tissue interfaces. Proceedings of the National Academy of Sciences. 2008;105:12170–12175. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Hill MC, Nguyen MK, Jeon O, Alsberg E. Spatial Control of Cell Gene Expression by siRNA Gradients in Biodegradable Hydrogels. Submitted. doi: 10.1002/adhm.201400458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Nichol JW, Khademhosseini A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter. 2009;5:1312–1319. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Engineering Part B: Reviews. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Chamberlain MD, Gupta R, Sefton MV. Bone marrow-derived mesenchymal stromal cells enhance chimeric vessel development driven by endothelial cell-coated microtissues. Tissue Engineering Part A. 2011;18:285–294. doi: 10.1089/ten.tea.2011.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Rouwkema J, Westerweel PE, de Boer J, Verhaar MC, van Blitterswijk CA. The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Engineering Part A. 2009;15:2015–2027. doi: 10.1089/ten.tea.2008.0318. [DOI] [PubMed] [Google Scholar]
- [223].Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. International Journal of Pharmaceutics. 2004;282:1–18. doi: 10.1016/j.ijpharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- [224].Chan L, Jin Y, Heng P. Cross-linking mechanisms of calcium and zinc in production of alginate microspheres. International Journal of Pharmaceutics. 2002;242:255–258. doi: 10.1016/s0378-5173(02)00169-2. [DOI] [PubMed] [Google Scholar]
- [225].Sugiura S, Oda T, Izumida Y, Aoyagi Y, Satake M, Ochiai A, Ohkohchi N, Nakajima M. Size control of calcium alginate beads containing living cells using micro-nozzle array. Biomaterials. 2005;26:3327–3331. doi: 10.1016/j.biomaterials.2004.08.029. [DOI] [PubMed] [Google Scholar]
- [226].Satish C, Satish K, Shivakumar H. Hydrogels as controlled drug delivery systems: Synthesis, crosslinking, water and drug transport mechanism. Indian journal of pharmaceutical sciences. 2006;68:133. [Google Scholar]
- [227].Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Freitas S, Merkle HP, Gander B. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. Journal of controlled release. 2005;102:313–332. doi: 10.1016/j.jconrel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- [229].Solorio LD, Dhami CD, Dang PN, Vieregge EL, Alsberg E. Spatiotemporal regulation of chondrogenic differentiation with controlled delivery of transforming growth factor-β1 from gelatin microspheres in mesenchymal stem cell aggregates. Stem cells translational medicine. 2012;1:632–639. doi: 10.5966/sctm.2012-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Solorio LD, S Fu A, Hernández-Irizarry R. Alsberg, chondrogenic differentiation of human mesenchymal stem cell aggregates via controlled release of TGF-β1 from incorporated polymer microspheres. Journal of Biomedical Materials Research Part A. 2010;92:1139–1144. doi: 10.1002/jbm.a.32440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Svoronos AA, Tejavibulya N, Schell JY, Shenoy VB, Morgan JR. Micro-Mold Design Controls the 3D Morphological Evolution of Self-Assembling Multicellular Microtissues. Tissue Engineering Part A. 2013 doi: 10.1089/ten.tea.2013.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Livoti CM, Morgan JR. Self-assembly and tissue fusion of toroid-shaped minimal building units. Tissue Engineering Part A. 2010;16:2051–2061. doi: 10.1089/ten.tea.2009.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Sasaki J-I, Asoh T-A, Matsumoto T, Egusa H, Sohmura T, Alsberg E, Akashi M, Yatani H. Fabrication of three-dimensional cell constructs using temperature-responsive hydrogel. Tissue Engineering Part A. 2010;16:2497–2504. doi: 10.1089/ten.TEA.2009.0523. [DOI] [PubMed] [Google Scholar]
- [234].McGuigan AP, Bruzewicz DA, Glavan A, Butte M, Whitesides GM. Cell encapsulation in sub-mm sized gel modules using replica molding. PLoS One. 2008;3:e2258. doi: 10.1371/journal.pone.0002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Yeh J, Ling Y, Karp JM, Gantz J, Chandawarkar A, Eng G, Blumling J, Iii, Langer R, Khademhosseini A. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27:5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- [236].Koh W-G, Revzin A, Pishko MV. Poly (ethylene glycol) hydrogel microstructures encapsulating living cells. Langmuir. 2002;18:2459–2462. doi: 10.1021/la0115740. [DOI] [PubMed] [Google Scholar]
- [237].Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proceedings of the National Academy of Sciences. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].McGuigan AP, Sefton MV. Design and fabrication of sub-mm-sized modules containing encapsulated cells for modular tissue engineering. Tissue engineering. 2007;13:1069–1078. doi: 10.1089/ten.2006.0253. [DOI] [PubMed] [Google Scholar]
- [239].Yu X, Khalil A, Dang PN, Alsberg E, Murphy WL. Multilayered Inorganic Microparticles for Tunable Dual Growth Factor Delivery. Advanced Functional Materials. 2014 doi: 10.1002/adfm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Matsunaga YT, Morimoto Y, Takeuchi S. Molding Cell Beads for Rapid Construction of Macroscopic 3D Tissue Architecture. Advanced Materials. 2011;23:H90–H94. doi: 10.1002/adma.201004375. [DOI] [PubMed] [Google Scholar]
- [241].Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Current opinion in cell biology. 1997;9:76–85. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- [242].Fernandez JG, Khademhosseini A. Micro-Masonry: onstruction of 3D Structures by Microscale Self-Assembly. Advanced Materials. 2010:538–2541. doi: 10.1002/adma.200903893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Dikina AD, Strobel HA, Lai BP, Rolle MW, Alsberg E. Engineered Cartilaginous Structures for Tracheal Tissue Replacement. In Preparation. 2104 doi: 10.1016/j.biomaterials.2015.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Tasoglu S, Diller E, Guven S, Sitti M, Demirci U. Untethered micro-robotic coding of three-dimensional material composition. Nature communications. 2014;5 doi: 10.1038/ncomms4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Caldwell DJ, Rao RR, Stegemann JP. Assembly of Discrete Collagen–Chitosan Microenvironments into Multiphase Tissue Constructs. Advanced healthcare materials. 2013;2:673–677. doi: 10.1002/adhm.201200346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Wang L, Rao RR, Stegemann JP. Delivery of mesenchymal stem cells in chitosan/collagen microbeads for orthopedic tissue repair. Cells Tissues Organs. 2013;197:333–343. doi: 10.1159/000348359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Xu F, Wu C.a.M., Rengarajan V, Finley TD, Keles HO, Sung Y, Li B, Gurkan UA, Demirci U. Three-Dimensional Magnetic Assembly of Microscale Hydrogels. Advanced Materials. 2011;3:5–4260. doi: 10.1002/adma.201101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Zamanian B, Masaeli M, Nichol JW, Khabiry M, Hancock MJ, Bae H, Khademhosseini A. Interface-Directed Self-Assembly of Cell-Laden Microgels. Small. 2010;6:937–944. doi: 10.1002/smll.200902326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Alvarez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive Intelligent Drug Delivery Systems†. Photochemistry and Photobiology. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- [250].Yanagawa F, Kaji H, Jang YH, Bae H, Yanan D, Fukuda J, Qi H, Khademhosseini A. Directed assembly of cell-laden microgels for building porous three-dimensional tissue constructs. Journal of Biomedical Materials Research Part A. 2011;97:93–102. doi: 10.1002/jbm.a.33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends in Biotechnology. 2004;22:354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- [252].Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta biomaterialia. 2011;7:907–920. doi: 10.1016/j.actbio.2010.09.039. [DOI] [PubMed] [Google Scholar]
- [253].Habibovic P, Gbureck U, Doillon CJ, Bassett DC, van Blitterswijk CA, Barralet JE. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials. 2008;29:944–953. doi: 10.1016/j.biomaterials.2007.10.023. [DOI] [PubMed] [Google Scholar]
- [254].Wu BM, Borland SW, Giordano RA, Cima LG, Sachs EM, Cima MJ. Solid free-form fabrication of drug delivery devices. Journal of Controlled Release. 1996;40:77–87. [Google Scholar]
- [255].Lam CXF, Mo X, Teoh S-H, Hutmacher D. Scaffold development using 3D printing with a starch-based polymer. Materials Science and Engineering: C. 2002;20:49–56. [Google Scholar]
- [256].Gbureck U, Vorndran E, Müller FA, Barralet JE. Low temperature direct 3D printed bioceramics and biocomposites as drug release matrices. Journal of Controlled Release. 2007;122:173–180. doi: 10.1016/j.jconrel.2007.06.022. [DOI] [PubMed] [Google Scholar]
- [257].Gbureck U, Hölzel T, Doillon CJ, Müller FA, Barralet JE. Direct printing of bioceramic implants with spatially localized angiogenic factors. Advanced Materials. 2007;19:795–800. [Google Scholar]
- [258].Sawkins MJ, Shakesheff KM, Bonassar LJ, Kirkham GR. 3D Cell and Scaffold Patterning Strategies in Tissue Engineering. Recent Patents on Biomedical Engineering. 2013;6:3–21. [Google Scholar]
- [259].Duan B, Wang M. Encapsulation and release of biomolecules from Ca–P/PHBV nanocomposite microspheres and three-dimensional scaffolds fabricated by selective laser sintering. Polymer Degradation and Stability. 2010;95:1655–1664. [Google Scholar]
- [260].Jacobs PF. Fundamentals of stereolithography; Proceedings of the Solid Freeform Fabrication Symposium.pp. 196–211. [Google Scholar]
- [261].Melchels FPW, Feijen J, Grijpma DW. A poly(d,l-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials. 2009;30:3801–3809. doi: 10.1016/j.biomaterials.2009.03.055. [DOI] [PubMed] [Google Scholar]
- [262].Lee K-W, Wang S, Fox BC, Ritman EL, Yaszemski MJ, Lu L. Poly(propylene fumarate) Bone Tissue ngineering Scaffold Fabrication Using Stereolithography: ffects of Resin Formulations and aser Parameters. Biomacromolecules. 2007;8:1077–1084. doi: 10.1021/bm060834v. [DOI] [PubMed] [Google Scholar]
- [263].Dhariwala B, Hunt E, Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue engineering. 2004;10:1316–1322. doi: 10.1089/ten.2004.10.1316. [DOI] [PubMed] [Google Scholar]
- [264].Arcaute K, Mann BK, Wicker RB. Stereolithography of three-dimensional bioactive poly (ethylene glycol) constructs with encapsulated cells. Annals of biomedical engineering. 2006;34:1429–1441. doi: 10.1007/s10439-006-9156-y. [DOI] [PubMed] [Google Scholar]
- [265].Hinczewski C, Corbel S, Chartier T. Ceramic suspensions suitable for stereolithography. Journal of the European Ceramic Society. 1998;18:583–590. [Google Scholar]
- [266].Arcaute K, Mann B, Wicker R. Stereolithography of spatially controlled multi-material bioactive poly(ethylene glycol) scaffolds. Acta biomaterialia. 2010;6:1047–1054. doi: 10.1016/j.actbio.2009.08.017. [DOI] [PubMed] [Google Scholar]
- [267].Lu Y, Mapili G, Suhali G, Chen S, Roy K. A digital micro-mirror device-based system for the microfabrication of complex, spatially patterned tissue engineering scaffolds. Journal of Biomedical Materials Research Part A. 2006;77A:396–405. doi: 10.1002/jbm.a.30601. [DOI] [PubMed] [Google Scholar]
- [268].Mapili G, Lu Y, Chen S, Roy K. Laser-layered microfabrication of spatially patterned functionalized tissue-engineering scaffolds. Journal of Biomedical Materials Research art B: Applied Biomaterials. 2005;75:414–424. doi: 10.1002/jbm.b.30325. [DOI] [PubMed] [Google Scholar]
- [269].Chan V, Zorlutuna P, Jeong JH, Kong H, Bashir R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab on a Chip. 2010;10:2062–2070. doi: 10.1039/c004285d. [DOI] [PubMed] [Google Scholar]
- [270].Fischer A, Cremer C, Stelzer E. Fluorescence of coumarins and xanthenes after two-photon absorption with a pulsed titanium—sapphire laser. Applied optics. 1995;34:1989–2003. doi: 10.1364/AO.34.001989. [DOI] [PubMed] [Google Scholar]
- [271].Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- [272].Pitts JD, Campagnola PJ, Epling GA, Goodman SL. Submicron Multiphoton Free-Form Fabrication of roteins and olymers: Studies of Reaction fficiencies and Applications in Sustained Release. Macromolecules. 2000;33:1514–1523. [Google Scholar]
- [273].Wüst S, Müller R, Hofmann S. Controlled positioning of cells in biomaterials—approaches towards 3D tissue printing. Journal of Functional Biomaterials. 2011;2:119–154. doi: 10.3390/jfb2030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. TRENDS in Biotechnology. 2003;21:157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- [275].Landers R, Hübner U, Schmelzeisen R, Mülhaupt R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials. 2002;23:4437–4447. doi: 10.1016/s0142-9612(02)00139-4. [DOI] [PubMed] [Google Scholar]
- [276].Fedorovich NE, De Wijn JR, Verbout AJ, Alblas J, Dhert WJ. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Engineering Part A. 2008;14:127–133. doi: 10.1089/ten.a.2007.0158. [DOI] [PubMed] [Google Scholar]
- [277].Polizzotti BD, Fairbanks BD, Anseth KS. Three-dimensional biochemical patterning of click-based composite hydrogels via thiolene photopolymerization. Biomacromolecules. 2008;9:1084–1087. doi: 10.1021/bm7012636. [DOI] [PubMed] [Google Scholar]
- [278].DeForest CA, Sims EA, Anseth KS. Peptide-functionalized click hydrogels with independently tunable mechanics and chemical functionality for 3D cell culture. Chemistry of materials. 2010;22:4783–4790. doi: 10.1021/cm101391y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene hotopolymerization. Advanced Materials. 2009;1:5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nature materials. 2004;3:249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- [281].Jeon O, Alsberg E. Photofunctionalization of alginate hydrogels to promote adhesion and proliferation of human mesenchymal stem cells. Tissue Engineering Part A. 2013;19:1424–1432. doi: 10.1089/ten.tea.2012.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Samorezov JE, Morlock CM, Alsberg E. Dual ionic and photocrosslinked alginate hydrogels for micropatterned spatial control of material properties and cell behavior. (Submitted) doi: 10.1021/acs.bioconjchem.5b00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Durst CA, Cuchiara MP, Mansfield EG, West JL, Grande-Allen KJ. Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta biomaterialia. 2011;7:2467–2476. doi: 10.1016/j.actbio.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. Journal of Controlled Release. 2011;154:258–266. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Wosnick JH, Shoichet MS. Three-dimensional Chemical Patterning of Transparent Hydrogels. Chemistry of Materials. 2007;20:55–60. [Google Scholar]
- [286].Wylie RG, Shoichet MS. Two-photon micropatterning of amines within an agarose hydrogel. Journal of Materials Chemistry. 2008;18:2716–2721. [Google Scholar]
- [287].Wylie RG, Shoichet MS. Three-Dimensional Spatial Patterning of Proteins in Hydrogels. Biomacromolecules. 2011;12:3789–3796. doi: 10.1021/bm201037j. [DOI] [PubMed] [Google Scholar]
- [288].Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Shoichet MS. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- [289].Hahn MS, Miller JS. West, Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Advanced Materials. 2006;18:2679–2684. [Google Scholar]
- [290].Lee S-H, Moon JJ, West JL. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials. 2008;29:2962–2968. doi: 10.1016/j.biomaterials.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nature materials. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Hoffmann JC, West JL. Three-dimensional photolithographic patterning of multiple bioactive ligands in poly (ethylene glycol) hydrogels. Soft Matter. 2010;6:5056–5063. [Google Scholar]
- [293].Brieke C, Rohrbach F, Gottschal A, Mayer G, Heckel A. Light-Controlled Tools. Angewandte Chemie International Edition. 2012;51:8446–8476. doi: 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]
- [294].Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Advanced drug delivery reviews. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- [295].Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer research. 1989;49:4373–4384. [PubMed] [Google Scholar]
- [296].Matsusaki M, Akashi M. Novel functional biodegradable polymer IV: pH-sensitive controlled release of fibroblast growth factor-2 from a poly (γ-glutamic acid)-sulfonate matrix for tissue engineering. Biomacromolecules. 2005;6:3351–3356. doi: 10.1021/bm050369m. [DOI] [PubMed] [Google Scholar]
- [297].Asoh T.a., Raneko T, Matsusaki M, Akashi M. Rapid and recise Release from Nano-Tracted oly (N-isopropylacrylamide) Hydrogels ontaining inear Poly (acrylic acid) Macromolecular bioscience. 2006;6:959–965. doi: 10.1002/mabi.200600107. [DOI] [PubMed] [Google Scholar]
- [298].Thomas MB, Radhakrishnan K, Gnanadhas DP, Chakravortty D, Raichur AM. Intracellular delivery of doxorubicin encapsulated in novel pH-responsive chitosan/heparin nanocapsules. International journal of nanomedicine. 2012;8:267–273. doi: 10.2147/IJN.S37737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Izawa H, Kawakami K, Sumita M, Tateyama Y, Hill JP, Ariga K. β-Cyclodextrin-crosslinked alginate gel for patient-controlled drug delivery systems: regulation of host–guest interactions with mechanical stimuli. Journal of Materials Chemistry B. 2013;1:2155–2161. doi: 10.1039/c3tb00503h. [DOI] [PubMed] [Google Scholar]
- [300].Xiao L, Zhu J, Londono JD, Pochan DJ, Jia X. Mechano-responsive hydrogels crosslinked by block copolymer micelles. Soft matter. 2012;8:10233–10237. doi: 10.1039/C2SM26566D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Edelman ER, Kost J, Bobeck H, Langer R. Regulation of drug release from polymer matrices by oscillating magnetic fields. Journal of Biomedical Materials Research. 1985;19:67–83. doi: 10.1002/jbm.820190107. [DOI] [PubMed] [Google Scholar]
- [302].Edelman ER, Brown L, Taylor J, Langer R. In vitro and in vivo kinetics of regulated drug release from polymer matrices by oscillating magnetic fields. Journal of Biomedical Materials Research. 1987;21:339–353. doi: 10.1002/jbm.820210307. [DOI] [PubMed] [Google Scholar]
- [303].Kost J, Wolfrum J, Langer R. Magnetically enhanced insulin release in diabetic rats. Journal of Biomedical Materials Research. 1987;21:1367–1373. doi: 10.1002/jbm.820211202. [DOI] [PubMed] [Google Scholar]
- [304].Wilson CG, Martín-Saavedra FM, Padilla F, Fabiilli ML, Zhang M, Baez AM, Bonkowski CJ, Kripfgans OD, Voellmy R, Vilaboa N, Fowlkes BJ, Francheschi RT. Patterning Expression of Regenerative Growth Factors Using High Intensity Focused Ultrasound. Tissue Engineering Part C: Methods. 2014 doi: 10.1089/ten.tec.2013.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Ishihara K, Kobayashi M, Ishimaru N, Shinohara I. Glucose induced permeation control of insulin through a complex membrane consisting of immobilized glucose oxidase and a poly (amine) Polymer journal. 1984;16:625–631. [Google Scholar]
- [306].Itoh Y, Matsusaki M, Kida T, Akashi M. Enzyme-responsive release of encapsulated proteins from biodegradable hollow capsules. Biomacromolecules. 2006;7:2715–2718. doi: 10.1021/bm060289y. [DOI] [PubMed] [Google Scholar]
- [307].King WJ, Pytel NJ, Ng K, Murphy WL. Triggered drug release from dynamic microspheres via a protein conformational change. Macromolecular bioscience. 2010;10:580–584. doi: 10.1002/mabi.200900382. [DOI] [PubMed] [Google Scholar]
- [308].King WJ, Mohammed JS, Murphy WL. Modulating growth factor release from hydrogels via a protein conformational change. Soft Matter. 2009;5:2399–2406. [Google Scholar]
- [309].King WJ, Toepke MW, Murphy WL. Facile formation of dynamic hydrogel microspheres for triggered growth factor delivery. Acta biomaterialia. 2011;7:975–985. doi: 10.1016/j.actbio.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Liu B, Liu Y, Riesberg JJ, Shen W. Dynamic presentation of immobilized ligands regulated through biomolecular recognition. Journal of the American Chemical Society. 2010;132:13630–13632. doi: 10.1021/ja1054669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmökel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. The FASEB journal. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- [312].Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30:4318–4324. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Yang C, Mariner PD, Nahreini JN, Anseth KS. Cell-mediated delivery of glucocorticoids from thiol-ene hydrogels. Journal of Controlled Release. 2012;162:612–618. doi: 10.1016/j.jconrel.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Lei Y, Segura T. DNA delivery from matrix metalloproteinase degradable poly(ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells. Biomaterials. 2009;30:254–265. doi: 10.1016/j.biomaterials.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nature Reviews Molecular Cell Biology. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- [316].Schmidt DR, Waldeck H, Kao WJ. Biological Interactions on Materials Surfaces. Springer; 2009. Protein adsorption to biomaterials; pp. 1–18. [Google Scholar]