Abstract
Evidence of associations between single nutrients and head and neck cancer (HNC) is still more limited and less consistent than that for fruit and vegetables. However, clarification of the protective mechanisms of fruit and vegetables is important to our understanding of HNC etiology.
We investigated the association between vitamin C intake from natural sources and cancer of the oral cavity/pharynx and larynx using individual-level pooled data from ten case-control studies (5959 cases and 12248 controls) participating in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. After harmonization of study-specific exposure information via the residual method, adjusted odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated using unconditional multiple logistic regression models on quintile categories of ’non-alcohol energy-adjusted’ vitamin C intake. In the presence of heterogeneity of the estimated ORs among studies, we derived those estimates from generalized linear mixed models.
Higher intakes of vitamin C were inversely related to oral and pharyngeal (OR=0.54, 95% CI: 0.45–0.65, for the fifth quintile category versus the first one, p for trend<0.001) and laryngeal cancers (OR=0.52, 95% CI: 0.40–0.68, p for trend=0.006), although in the presence of heterogeneity among studies for both sites. Inverse associations were consistently observed for the anatomical subsites of oral and pharyngeal cancer, and across strata of age, sex, education, body mass index, tobacco, and alcohol, for both cancer sites.
The inverse association of vitamin C intake from foods with HNC may reflect a protective effect on these cancers; however, we cannot rule out other explanations.
Keywords: head and neck cancer, INHANCE, laryngeal cancer, oral and pharyngeal cancer, vitamin C
Introduction
Tobacco smoking and alcohol drinking are the major etiologic factors for cancers of the oral cavity, pharynx and larynx (head and neck cancer (HNC)) [1, 2]. Diet has been associated with HNC risk on the basis of international variation, time trends, and epidemiological research [3, 4, 5, 6], with a well-recognized protective role of fruit and vegetables. To various degrees, higher intakes of non-starchy vegetables, foods containing carotenoids, and fruit in general have been reported to be inversely related to HNC risk [7, 8, 5], especially among heavy smokers and/or drinkers. However, the strength of the evidence concerning fruit and vegetables has recently been downgraded to ’probable’ in the 2007 World Cancer Research Fund report for these cancers and in some important cohort studies for most cancers and for overall cancer risk [9, 10, 11]. This indicated the need for further research.
Fruit and vegetables are rich sources of compounds that have anti-carcinogenic properties, including vitamins, minerals, fiber, and phytochemicals in general. Several of these nutrients and bioactive compounds have antioxidant and antiproliferative activities, modulate steroid hormone concentrations and metabolism, and stimulate the immune system and synthesis and methylation of DNA [12, 13]. Among them, vitamin C from natural sources has been investigated since the early 1980’s in epidemiological studies on upper aerodigestive tract cancer (UADTC) and its subsites, with the vast majority of studies finding inverse associations. However, data are still limited for laryngeal cancer [4, 14, 3].
The International Head and Neck Cancer Epidemiology (INHANCE) consortium [15] was established in 2004 to contribute elucidating the etiology of HNC by providing opportunities for pooled analyses of individual-level data on HNC on a large scale. Dietary habits have been previously investigated within the consortium, with results pointing to a possible protective effect of fruit and vegetables overall (total fruit intake, total vegetable intake excluding potatoes), of selected plant food items (green salad, lettuce, fresh tomatoes, citrus fruits, apples and pears, green vegetables and allium vegetables), as well as of ’a priori’ and ’a posteriori’ dietary patterns rich in fruit and vegetables [16, 17]. The specific goals of this analysis were: 1. to describe and account for central tendency and variation in the intakes of vitamin C for the populations under examination; 2. to investigate the association between vitamin C intake and the risks of two HNC outcomes - oral and pharyngeal cancer and laryngeal cancer; 3. to explore whether effect estimates differ by cancer subsites or across subgroups of subjects, with particular attention to nonsmokers/nondrinkers; 4. to explore the potential interaction effect between the intakes of vitamin C and of other selected factors - putatively associated to HNC and to our main exposure (other selected nutrients, total fruit and vegetables, supplemental use of vitamin C) - on the two HNC outcomes of interest.
Material and methods
Design and participants
Within the version 1.5 of the INHANCE consortium pooled data set, ten case-control studies provided information on vitamin C intake derived from natural sources at the individual level [18, 19, 20, 21, 22, 23, 24, 25, 26, 27]. Details on the individual studies, harmonization of questionnaire data and data pooling methods for the consortium have been previously described [16, 17] and are reported in the Online Supplemental Material (Supplemental Table 1). Briefly, three of the selected studies were from Europe [18, 19, 27], six were from the United States [20, 21, 28, 22, 23, 25, 26], and one from Asia [24]. Six were hospital-based and four were population-based investigations. Study-specific questionnaires included a food-frequency questionnaire (FFQ) section to assess each subject’s usual diet during a reference period preceding cancer diagnosis for cases, or interview for controls. The three studies from Europe (Italy Multicenter, Switzerland, Milan (2006–2009)) used the same FFQ. Previously published studies found that the two FFQs from Italy Multicenter-Switzerland-Milan (2006–2009) and Boston studies were reproducible and valid, the two FFQs from US Multicenter and Memorial Sloan Kettering Cancer Center (MSKCC) studies were valid, and the two FFQs from Los Angeles and North Carolina studies were slightly modified from a previously validated FFQ [29, 30, 28, 31, 32]. The brief FFQs administered in the Buffalo and Japan studies were validated for the intakes of selected nutrients, including vitamin C [33, 34, 35]. Overall, the number and wording of FFQ questions were sufficiently detailed to allow for the calculation of intakes of total energy and several other nutrients [36, 37, 38], through study-specific food composition databases [39, 40, 41, 42, 43, 44].
Written informed consent was obtained from study subjects, and the investigations were approved by the relevant institutional review boards.
Selection of subjects
Cases were included if their tumor had been classified as an invasive tumor of oral cavity, oropharynx, hypopharynx, oral cavity or pharynx not otherwise specified, larynx, or HNC unspecified. Subjects with cancers of the salivary glands [International Classification of Diseases for Oncology, 2nd edition (ICD-O-2) codes C07–C08] or of the nasal cavity/ear/paranasal sinuses (ICD-O-2 codes C30–C31) were excluded. The ICD coding used for the classification into subsites was specified in detail previously [45].
Subjects with missing information on natural vitamin C intake (1075 subjects from 6 studies, who showed missing values on all the nutrient variables, probably due to missing information on the entire dietary section of the questionnaire) were removed from the original data. Subjects having an implausible (< 500 or > 5500 kcal) daily non-alcohol energy intake (defined as: total energy intake (kcal) - 100 * number of drinks per day, as 1 drink per day = 100 kcal) (343 subjects) or those having missing values (544 subjects) on non-alcohol energy intake were excluded from the analysis. Cases with missing information on the site of origin of their cancer (22 subjects, of which 21 belonging to the MSKCC study) were also removed.
Thus, the present analyses included a total of 18207 subjects, with 5959 HNC cases and 12248 controls. There was a total of 1385 oral cavity cancer cases, 1653 oropharyngeal and 571 hypopharyngeal cancer cases (2224 pharyngeal cancer cases), 805 unspecified oral cavity/pharynx cases (giving a total of 4414 oral and pharyngeal cancer cases), and 1545 laryngeal cancer cases.
Definition of the exposure variable
We carried out preliminary checks on vitamin C definitions, reference periods of intake and measurement units across studies. We extracted information on vitamin C intake from natural sources, and we consistently expressed these intakes on a daily basis. Results on vitamin C supplementation have already been published [46].
To assess the comparability of daily intakes across studies, we inspected the kernel density estimation plot [47] representing the study-specific empirical distributions of vitamin C intakes. We also compared study-specific summary statistics across studies. As preliminary checks revealed strong differences across studies, we decided to compute ’non-alcohol energy-adjusted’ vitamin C intakes within each study, referring to the residual method [48].
Statistical analysis
Participants were grouped into five categories according to quintiles of ’non-alcohol energy-adjusted’ (adjusted hereafter) vitamin C intakes calculated among both cases and controls.
We estimated the odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) of oral and pharyngeal cancer (including oral, oropharynx, hypopharynx, unspecified oral/pharynx cancer), and laryngeal cancer, separately, for each quintile category using unconditional multiple logistic regression models [49]. Tests for linear trend were computed for all models scoring the quintiles as numbers from 1 to 5. To adjust for potential confounders, the main analysis included the following set of variables in all the models: age, sex, education, race/ethnicity, study center, cigarette smoking status, cigarette intensity, cigarette duration, cigar smoking status, pipe smoking status, alcohol drinking intensity, and the interaction between cigarette intensity and alcohol drinking intensity (see tables for categories used).
We tested for the presence of heterogeneity among studies for the effect of quintile categories of adjusted vitamin C intake by calculating likelihood ratio tests comparing the deviance statistics from the models including versus excluding the interaction terms between quintile categories and study. As the p-value for heterogeneity among studies was less than 0.1, we used a mixed-effects modelling approach [50, 51] and replaced the fixed-effects ORs and CIs with the corresponding mixed-effects ones. We derived those estimates specifying a random intercept-random slope generalized linear mixed model (GLMM) with a logit link function and binomial family. The random-effects terms included were the random intercept and four random slope terms (one for each quintile category included, except for the reference one), all sharing study center as the common grouping factor. No correlations between random effects were allowed. We reported restricted maximum likelihood (REML) estimates, as they provided better estimates of variance components [50].
As a sensitivity analysis, we further investigated the potential role of other factors putatively associated with HNC and vitamin C intake, including several a priori selected micronutrients (monounsaturated fatty acids, polyunsaturated fatty acids, folate, lutein plus xeaxanthin, total carotenoids, betacarotene equivalents, cryptoxanthin, lycopene, vitamin E, fiber) (quintile categories of adjusted nutrient intake), total fruits and total vegetables (categories of intake based on study-specific quartiles among the controls), and supplement use of vitamin C (never/ever). For each factor, we fitted both the additive and the interaction models including the extra adjustment variable and the interaction terms between quintile categories of adjusted vitamin C intake and extra adjustment variable of interest. We tested for the significance of the adjustment variable or of the interaction effect calculating the corresponding likelihood ratio tests. When the interaction term was non significant at the 0.05 level, we reported results from the additive model. Similarly, non-alcohol energy intake was further adjusted for in the models, to check for a potential reduction in the random error due to a strong association between non-alcohol energy intake and the outcomes of interest, independent of nutrient intake.
For oral and pharyngeal cancer, separate analyses were conducted by anatomical subsite [49]. As the p-value for heterogeneity among studies was less than 0.1, we fitted the GLMMs to derive the subsite estimates. For both cancers, stratified analyses were conducted by age, sex, education, geographic region, control source, study period, body mass index at time of interview, tobacco smoking, and alcohol drinking. Heterogeneity across strata was tested by calculating likelihood ratio tests comparing the deviance statistics from the models including versus excluding the interaction terms between quintile categories of vitamin C intake and stratification variable. When the p-value for heterogeneity among studies was less than 0.1 within strata, we reported mixed-effects estimates derived from the corresponding GLMMs. When, for a single stratification variable, fixed- and mixed-effects models were estimated within different strata, likelihood ratio tests for heterogeneity across strata were based on comparable mixed-effects models and therefore we re-fitted one or more mixed-effects models to replace the original fixed-effects ones.
We examined whether the results from the fixed- and mixed-effects logistic regression models and those from the two-stage random-effects model [52] were comparable to each other in terms of the magnitude of the effect. In the two-stage random-effects model, we excluded the MSKCC study, containing fewer than 100 cases of either cancer site. We quantified inconsistencies across studies and their impact on the analysis by using Cochrane’s Q and the I2 statistics [53, 54]. We also conducted an influence analysis, in which each study was excluded one at a time to ensure that overall estimates were not dependent on any specific study.
All statistical tests were two-sided. Calculations were performed using the open-source statistical computing environment R [55], with its libraries ”lme4” [56] and ”nnet” [57], and Stata (Release 11).
Results
Table 1 shows some descriptive statistics on raw values of vitamin C intake across studies and in all the studies combined. Study-specific distributions were all skewed to the right, with median intakes being always smaller than the corresponding mean intakes.
Table 1.
Descriptive statistics on raw values of vitamin C intake (mg/day) across studies and in all the studies combined. International Head and Neck Cancer Epidemiology (INHANCE) consortium.
| Study name | 20% | Median | Mean | 80% |
|---|---|---|---|---|
| Boston | 67.95 | 122.00 | 135.60 | 190.95 |
| Buffalo | 95.93 | 168.90 | 188.00 | 263.44 |
| Italy Multicenter | 82.34 | 128.10 | 141.30 | 189.85 |
| Japan (2001–2005) | 60.26 | 86.60 | 91.45 | 118.65 |
| Los Angeles | 43.78 | 70.53 | 82.51 | 120.72 |
| Milan (2006–2009) | 78.63 | 124.80 | 139.40 | 192.46 |
| MSKCC | 76.08 | 133.30 | 148.40 | 208.34 |
| North Carolina (2002–2006) | 74.95 | 118.90 | 128.80 | 174.62 |
| Switzerland | 61.24 | 102.00 | 133.80 | 190.35 |
| US Multicenter | 77.48 | 145.80 | 166.80 | 231.37 |
| All studies combined | 68.08 | 113.70 | 133.40 | 183.97 |
ABBREVIATIONS: MSKCC: Memorial Sloan Kettering Cancer Center.
Selected characteristics of cases and controls are shown in Table 2, separately for oral and pharyngeal, and for laryngeal cancer cases. Over 70% of cases and controls were white. The Italy Multicenter, US Multicenter, and North Carolina studies contributed the largest proportion of cases of both cancers combined. The US Multicenter provided cases of oral and pharyngeal cancer only. Cases of both cancers were more frequently and heavily exposed to tobacco and alcohol.
Table 2.
Distribution of cases of oral and pharyngeal, and laryngeal cancers and controls according to selected variables. International Head and Neck Cancer Epidemiology (INHANCE) consortium.
| Oral and pharyngeal cases |
(%) | Controls | (%) | Laryngeal cases |
(%) | Controls | (%) | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||
| <40 | 208 | 4.7 | 681 | 5.6 | 26 | 1.7 | 681 | 5.6 |
| 40–44 | 194 | 4.4 | 563 | 4.6 | 45 | 2.9 | 563 | 4.6 |
| 45–49 | 446 | 10.1 | 949 | 7.7 | 123 | 8.0 | 949 | 7.7 |
| 50–54 | 645 | 14.6 | 1731 | 14.1 | 188 | 12.2 | 1731 | 14.1 |
| 55–59 | 816 | 18.5 | 2079 | 17.0 | 271 | 17.5 | 2079 | 17.0 |
| 60–64 | 713 | 16.2 | 2029 | 16.6 | 290 | 18.8 | 2029 | 16.6 |
| 65–69 | 658 | 14.9 | 1931 | 15.8 | 279 | 18.1 | 1931 | 15.8 |
| 70–74 | 474 | 10.7 | 1540 | 12.6 | 227 | 14.7 | 1540 | 12.6 |
| ≥75 | 260 | 5.9 | 743 | 6.1 | 96 | 6.2 | 743 | 6.1 |
| Missing values | 0 | 0.0 | 2 | 0.0 | 0 | 0.0 | 2 | 0.0 |
| Χ2 (p-value) a | 42.0 (<0.001) | 66.5 (<0.001) | ||||||
| Sex | ||||||||
| Female | 1187 | 26.9 | 3541 | 28.9 | 244 | 15.8 | 3541 | 28.9 |
| Male | 3223 | 73.0 | 8702 | 71.0 | 1300 | 84.1 | 8702 | 71.0 |
| Missing values | 4 | 0.1 | 5 | 0.0 | 1 | 0.1 | 5 | 0.0 |
| Χ2 (p-value)a | 6.3 (0.012) | 117.8 (<0.001) | ||||||
| Race | ||||||||
| Black | 387 | 8.8 | 535 | 4.4 | 116 | 7.5 | 535 | 4.4 |
| Others (with Asian) | 463 | 10.5 | 3089 | 25.2 | 101 | 6.5 | 3089 | 25.2 |
| White (with Hispanic) | 3555 | 80.5 | 8596 | 70.2 | 1324 | 85.7 | 8596 | 70.2 |
| Missing values | 9 | 0.2 | 28 | 0.2 | 4 | 0.3 | 28 | 0.2 |
| Χ2 (p-value)a | 491.5 (<0.001) | 281.7 (<0.001) | ||||||
| Study name | ||||||||
| Boston | 313 | 7.1 | 611 | 5.0 | 71 | 4.6 | 611 | 5.0 |
| Buffalo | 396 | 9.0 | 1190 | 9.7 | 168 | 10.9 | 1190 | 9.7 |
| Italy Multicenter | ||||||||
| Milan | 169 | 3.8 | 621 | 5.1 | 24 | 1.6 | 621 | 5.1 |
| Pordenone | 471 | 10.7 | 1528 | 12.5 | 409 | 26.5 | 1528 | 12.5 |
| Latina | 95 | 2.2 | 425 | 3.5 | 0 | 0.0 | 425 | 3.5 |
| Japan (2001–2005) | 407 | 9.2 | 3002 | 24.5 | 86 | 5.6 | 3002 | 24.5 |
| Los Angeles | 246 | 5.6 | 828 | 6.8 | 60 | 3.9 | 828 | 6.8 |
| Milan (2006–2009) | 131 | 3.0 | 691 | 5.6 | 200 | 12.9 | 691 | 5.6 |
| MSKCC | 74 | 1.7 | 123 | 1.0 | 32 | 2.1 | 123 | 1.0 |
| North Carolina (2002–2006) | 687 | 15.6 | 1120 | 9.1 | 374 | 24.2 | 1120 | 9.1 |
| Switzerland | 367 | 8.3 | 877 | 7.2 | 121 | 7.8 | 877 | 7.2 |
| US Multicenter | ||||||||
| Atlanta | 129 | 2.9 | 134 | 1.1 | 0 | 0.0 | 134 | 1.1 |
| New Jersey | 467 | 10.6 | 459 | 3.7 | 0 | 0.0 | 459 | 3.7 |
| Los Angeles | 398 | 9.0 | 501 | 4.1 | 0 | 0.0 | 501 | 4.1 |
| San Francisco | 64 | 1.4 | 138 | 1.1 | 0 | 0.0 | 138 | 1.1 |
| Χ2 (p-value)a | 1121.5 (<0.001) | 1092.0 (<0.001) | ||||||
| Education | ||||||||
| <= Junior high school | 863 | 19.6 | 2723 | 22.2 | 603 | 39.0 | 2723 | 22.2 |
| Some high school | 885 | 20.0 | 1240 | 10.1 | 258 | 16.7 | 1240 | 10.1 |
| High school graduate | 588 | 13.3 | 1267 | 10.3 | 237 | 15.3 | 1267 | 10.3 |
| Technical school, some college | 1174 | 26.6 | 2305 | 18.8 | 214 | 13.9 | 2305 | 18.8 |
| >= college graduate | 491 | 11.1 | 1703 | 13.9 | 145 | 9.4 | 1703 | 13.9 |
| Missing | 413 | 9.4 | 3010 | 24.6 | 88 | 5.7 | 3010 | 24.6 |
| Χ2 (p-value)a | 766.2 (<0.001) | 503.7 (<0.001) | ||||||
| Smoking status | ||||||||
| Never | 806 | 18.3 | 4868 | 39.7 | 90 | 5.8 | 4868 | 39.7 |
| Former | 1387 | 31.4 | 4330 | 35.4 | 707 | 45.8 | 4330 | 35.4 |
| Current | 2210 | 50.1 | 2986 | 24.4 | 735 | 47.6 | 2986 | 24.4 |
| Missing | 11 | 0.2 | 64 | 0.5 | 13 | 0.8 | 64 | 0.5 |
| Χ2 (p-value)a | 1139.6 (<0.001) | 755.3 (<0.001) | ||||||
| Cigarette intensity (cigarettes/day) | ||||||||
| Never smoker | 806 | 18.3 | 4868 | 39.7 | 91 | 5.9 | 4868 | 39.7 |
| 0to<=10 | 471 | 10.7 | 1949 | 15.9 | 149 | 9.6 | 1949 | 15.9 |
| 10to<=20 | 1466 | 33.2 | 3169 | 25.9 | 628 | 40.6 | 3169 | 25.9 |
| >20 | 1633 | 37.0 | 2137 | 17.4 | 661 | 42.8 | 2137 | 17.4 |
| Missing | 38 | 0.9 | 125 | 1.0 | 16 | 1.0 | 125 | 1.0 |
| Χ2 (p-value)a | 1111.2 (<0.001) | 1015.8 (<0.001) | ||||||
| Duration of cigarette smoking (years) | ||||||||
| Never smoker | 806 | 18.3 | 4868 | 39.7 | 91 | 5.9 | 4868 | 39.7 |
| 0to<=20 | 443 | 10.0 | 2166 | 17.7 | 102 | 6.6 | 2166 | 17.7 |
| >20 | 3132 | 71.0 | 5123 | 41.8 | 1343 | 86.9 | 5123 | 41.8 |
| Missing | 33 | 0.7 | 91 | 0.7 | 9 | 0.6 | 91 | 0.7 |
| Χ2 (p-value)a | 1116.8 (<0.001) | 1133.7 (<0.001) | ||||||
| Cigar smoking | ||||||||
| Never cigar user | 3583 | 81.2 | 8545 | 69.8 | 1323 | 85.6 | 8545 | 69.8 |
| Ever smoked >=100 cigars in a lifetime | 394 | 8.9 | 636 | 5.2 | 118 | 7.6 | 636 | 5.2 |
| Missing values | 437 | 9.9 | 3067 | 25.0 | 104 | 6.7 | 3067 | 25.0 |
| Χ2 (p-value)a | 33.7 (0.008) | 2.8 (0.093) | ||||||
| Pipe smoking | ||||||||
| Never pipe user | 3579 | 81.1 | 8327 | 68.0 | 1325 | 85.8 | 8327 | 68.0 |
| Ever smoked>=100 pipes in a lifetime | 399 | 9.0 | 864 | 7.1 | 115 | 7.4 | 864 | 7.1 |
| Missing values | 436 | 9.9 | 3057 | 25.0 | 105 | 6.8 | 3057 | 25.0 |
| Χ2 (p-value)a | 1.2 (0.027) | 2.8 (0.094) | ||||||
| Alcohol consumption (drinks/day) | ||||||||
| Never drinker | 548 | 12.4 | 3156 | 25.8 | 187 | 12.1 | 3156 | 25.8 |
| <1 | 1030 | 23.3 | 4022 | 32.8 | 250 | 16.2 | 4022 | 32.8 |
| >=1to3 | 973 | 22.0 | 2934 | 24.0 | 344 | 22.3 | 2934 | 24.0 |
| >=3to5 | 647 | 14.7 | 1215 | 9.9 | 250 | 16.2 | 1215 | 9.9 |
| >=5 | 1216 | 27.5 | 921 | 7.5 | 514 | 33.3 | 921 | 7.5 |
| Χ2 (p-value)a | 1442.0 (<0.001) | 1155.2 (<0.001) | ||||||
ABBREVIATIONS: MSKCC: Memorial Sloan Kettering Cancer Center.
Missing values were not considered in the calculation of the Chi-square test.
Table 3 gives separate ORs and the corresponding CIs for oral and pharyngeal and laryngeal cancers by quintile category of adjusted vitamin C intake. Mixed-effects estimates replaced the fixed-effects ones in the presence of heterogeneity among studies (p<0.001). Higher intakes of vitamin C were inversely related to oral and pharyngeal cancer, with an OR of 0.54 (95% CI: 0.45–0.65) for the fifth quintile compared to the first one (p-value for trend <0.001). Similarly, the OR for laryngeal cancer was 0.52 (95% CI: 0.40–0.68) for the last quintile category, with a significant p-value for trend (p=0.006). The extra adjustment for non-alcohol energy intake (either in continuum or in quintile categories of intake) did not substantially modify the ORs and the corresponding CIs. Mixed-effects models including quintiles of non-alcohol energy intake provided the following estimates for adjusted quintile categories of vitamin C intake and oral and pharyngeal cancer: OR=0.88 (95% CI: 0.73–1.05), 0.68 (95% CI: 0.58–0.78), 0.63 (95% CI: 0.53–0.74), 0.54 (95% CI: 0.45–0.64), and for laryngeal cancer: OR=0.83 (95% CI: 0.69–1.00), 0.65 (95% CI: 0.55–0.77), 0.60 (95% CI: 0.51–0.72), 0.52 (95% CI: 0.43–0.64), respectively (data not shown).
Table 3.
Odds ratios (ORs)a for oral and pharyngeal, and laryngeal cancers, and corresponding confidence intervals (95%CIs), on non-alcohol energy-adjusted vitamin C intake quintiles. International Head and Neck Cancer Epidemiology (INHANCE) consortium.
| Controls | Oral and pharyngeal cases |
OR (95% CI)b | Pstudiesc | Laryngeal cases |
OR (95% CI)b | Pstudiesc | |
|---|---|---|---|---|---|---|---|
| I Quintiled | 1359 | 995 | 1e | <0.001 | 431 | 1e | <0.001 |
| II Quintiled | 1768 | 892 | 0.86 (0.71–1.03) | 288 | 0.64 (0.46–0.89) | ||
| III Quintiled | 1945 | 730 | 0.66 (0.58–0.77) | 252 | 0.53 (0.39–0.72) | ||
| IV Quintiled | 1956 | 666 | 0.62 (0.53–0.74) | 210 | 0.48 (0.38–0.62) | ||
| V Quintiled | 1968 | 611 | 0.54 (0.45–0.65) | 221 | 0.52 (0.40–0.68) | ||
| pfor trendf | <0.001 | 0.006 |
Estimated from multiple logistic regression models adjusted for age, sex, education, race/ethnicity, study center, cigarette smoking status, cigarette intensity, cigarette duration, cigar smoking status, pipe smoking status, alcohol drinking intensity and the interaction between cigarette intensity and alcohol drinking intensity.
As heterogeneity among studies was detected (p<0.1), we reported the mixed-effects estimates derived from the corresponding generalized linear mixed model.
P for heterogeneity among studies.
The quantile cut-offs were the following ones:−0.779, −0.367, 0.055,and 0.683.
Reference category.
P for linear trend.
In addition, decreasing ORs with higher intakes of vitamin C were observed across strata of anatomical subsite for oral and pharyngeal cancer: OR=0.52 (95% CI: 0.38–0.72) for oral cavity, OR=0.53 (95% CI: 0.44–0.63) for oropharynx and hypopharynx combined, and OR=0.44 (95% CI: 0.30–0.63) for oral cavity or pharynx not otherwise specified, from random-effects models (p-value for heterogeneity among studies <0.001) (Supplemental Table 2). Separate analyses for oropharynx and hypopharynx cancer sites showed consistent results (OR=0.58, 95% CI: 0.48–0.70, for oropharynx, OR=0.52, 95% CI: 0.38, 0.72, for hypopharynx cancer sites, respectively) and, given the limited number of hypopharynx cancer sites, we decided to combine results for these subsites.
Table 4 shows the ORs of oral and pharyngeal cancer for adjusted vitamin C intake in strata of selected variables. No significant heterogeneity was detected for adjusted vitamin C intake across strata, with consistent inverse associations obtained from mixed-effects models for the fourth quintile category onwards for all the examined strata. However, an appreciable heterogeneity among studies emerged for several strata.
Table 4.
Odds ratios (ORs)a,b for oral and pharyngeal cancer and corresponding confidence intervals (95% CIs) on non-alcohol energy-adjusted vitamin C intake quintiles, in strata of selected covariates. International Head and Neck Cancer Epidemiology (INHANCE) consortium.
| II Quintile OR (95% CI) |
III Quintile OR (95% CI) |
IV Quintile OR (95% CI) |
V Quintile OR (95% CI) |
pstudiesc | |
|---|---|---|---|---|---|
| Age (years) | |||||
| <55 | 0.83 (0.65–1.05) | 0.58 (0.45–0.76) | 0.55 (0.41–0.73) | 0.65 (0.47–0.90) | 0.001 |
| ≥ 55 | 0.84 (0.69–1.03) | 0.69 (0.57–0.83) | 0.63 (0.52–0.77) | 0.49 (0.39–0.63) | < 0.001 |
| pstratad | 0.087 | ||||
| Sex | |||||
| Female | 0.88 (0.67–1.16) | 0.59 (0.44–0.79) | 0.59 (0.45–0.78) | 0.54 (0.37–0.79) | < 0.001 |
| Male | 0.84 (0.68–1.04) | 0.69 (0.59–0.81) | 0.63 (0.52–0.76) | 0.53 (0.45–0.63) | < 0.001 |
| pstratad | 0.514 | ||||
| Education | |||||
| ≤high school graduate | 0.84 (0.69–1.02) | 0.65 (0.53–0.81) | 0.64 (0.52–0.80) | 0.52 (0.42–0.66) | 0.002 |
| ≥some college | 0.90 (0.70–1.18) | 0.72 (0.58–0.88) | 0.62 (0.50–0.77) | 0.61 (0.49–0.76) | 0.014 |
| pstratad | 0.578 | ||||
| Geographic regione | |||||
| Europe | 0.93 (0.71–1.22) | 0.57 (0.45–0.73) | 0.56 (0.40–0.77) | 0.46 (0.35–0.61) | 0.085 |
| America | 0.82 (0.65–1.03) | 0.70 (0.59–0.82) | 0.64 (0.54–0.76) | 0.57 (0.45–0.73) | < 0.001 |
| Asia | 0.72 (0.52–1.00) | 0.67 (0.48–0.94) | 0.57 (0.41–0.79) | 0.43 (0.30–0.62) | NE |
| pstratad | 0.573 | ||||
| Body mass index | |||||
| <25 kg/m2 | 0.80 (0.65–0.98) | 0.62 (0.50–0.75) | 0.53 (0.43–0.65) | 0.52 (0.42–0.64) | 0.016 |
| ≥25 kg/m2 | 0.89 (0.70–1.14) | 0.71 (0.59–0.86) | 0.68 (0.54–0.85) | 0.57 (0.45–0.71) | 0.002 |
| pstratad | 0.254 | ||||
| Tobacco consumption | |||||
| Never user | 1.03 (0.67–1.59) | 0.74 (0.51–1.08) | 0.67 (0.49–0.91) | 0.60 (0.40–0.92) | < 0.001 |
| Former user | 0.77 (0.59–0.99) | 0.75 (0.59–0.97) | 0.59 (0.45–0.76) | 0.56 (0.43–0.74) | 0.560 |
| Current user | 0.78 (0.64–0.95) | 0.61 (0.49–0.75) | 0.65 (0.48–0.88) | 0.62 (0.49–0.80) | 0.051 |
| pstratad | 0.887 | ||||
| Alcohol consumptionf | |||||
| Never/light drinker | 0.97 (0.75–1.24) | 0.69 (0.56–0.85) | 0.61 (0.48–0.78) | 0.64 (0.50–0.82) | < 0.001 |
| Moderate drinker | 0.82 (0.62–1.09) | 0.73 (0.59–0.91) | 0.73 (0.58–0.93) | 0.49 (0.37–0.64) | 0.051 |
| Heavy drinker | 0.74 (0.54–1.02) | 0.49 (0.35–0.69) | 0.42 (0.29–0.59) | 0.46 (0.33–0.65) | 0.835 |
| pstratad | 0.107 | ||||
| Tobacco and alcohol consumption combinedf | |||||
| Never/former user -Never/light drinker | 1.08 (0.72–1.61) | 0.78 (0.60–1.02) | 0.64 (0.49–0.83) | 0.69 (0.53–0.89) | 0.001 |
| Current user- Never/light drinker | 0.79 (0.55–1.13) | 0.57 (0.38–0.84) | 0.59 (0.35–1.01) | 0.76 (0.46–1.25) | 0.024 |
| Never/former user -Moderate drinker | 0.62 (0.44–0.89) | 0.66 (0.47–0.93) | 0.62 (0.44–0.87) | 0.41 (0.26–0.65) | 0.055 |
| Current user -Moderate drinker | 0.86 (0.62–1.18) | 0.70 (0.50–0.96) | 0.70 (0.50–0.99) | 0.53 (0.37–0.77) | 0.476 |
| Never/former user - Heavy drinker | 1.02 (0.63–1.65) | 0.64 (0.38–1.09) | 0.42 (0.24–0.74) | 0.49 (0.29–0.83) | 0.694 |
| Current user -Heavy drinker | 0.68 (0.46–1.01) | 0.60 (0.40–0.91) | 0.51 (0.32–0.80) | 0.55 (0.35–0.87) | 0.866 |
| pstratad | 0.307 | ||||
ABBREVIATIONS: MSKCC: Memorial Sloan Kettering Cancer Center; NE: Not estimable.
Estimated from multiple logistic regression models adjusted for age, sex, education, race/ethnicity, study center, cigarette smoking status, cigarette intensity, cigarette duration, cigar smoking status, pipe smoking status, alcohol drinking intensity and the interaction between cigarette intensity and alcohol drinking intensity, when appropriate.
The I Quintile category was considered as the reference one.
P for heterogeneity among studies. When the p-value was less than 0.1 within strata, we reported the mixed-effects estimates derived from the corresponding generalized linear mixed model.
P for heterogeneity across strata. When, for a single stratification variable, fixed- and mixed-effects models were estimated within different strata, likelihood ratio tests for heterogeneity across strata were based on comparable mixed-effects models and therefore we re-fitted one or more mixed-effects models to replace the original fixed-effects ones. We consistently reported the corresponding stratum-specific mixed-effects models instead of the fixed-effects ones.
Europe included Italy Multicenter, Switzerland and Milan (2006–2009) studies. North America included Boston, Buffalo, Los Angeles, MSKCC, North Carolina (2002–2006), and US Multicenter studies. Asia included Japan study only. As Asia included Japan study only, there was no possibility to assess heterogeneity among studies in the Asia stratum.
The never/light drinker category included never drinkers and subjects who drinks less than 1 drink per day; the moderate drinker category included subjects drinking between 1 (included) and 5 drinks per day; the heavy drinker category included subjects drinking 5 drinks per day or more.
Table 5 shows the ORs of laryngeal cancer for adjusted vitamin C intake in strata of selected variables. Similarly to oral and pharyngeal cancer, an appreciable heterogeneity was observed among studies in several strata, but no significant heterogeneity was detected in general for adjusted vitamin C intake across strata. However, there was an appreciable heterogeneity across strata of geographic region, with a significant inverse association in the three European studies only.
Table 5.
Odds ratios (ORs)a,b for laryngeal cancer and corresponding confidence intervals (95% CIs) on non-alcohol energy-adjusted vitamin C intake quintiles, in strata of selected covariates. International Head and Neck Cancer Epidemiology (INHANCE) consortium.
| II Quintile OR (95% CI) |
III Quintile OR (95% CI) |
IV Quintile OR (95% CI) |
V Quintile OR (95% CI) |
pstudiesc | |
|---|---|---|---|---|---|
| Age (years) | |||||
| <55 | 0.63 (0.44–0.92) | 0.44 (0.28–0.70) | 0.39 (0.25–0.61) | 0.48 (0.32–0.71) | 0.281 |
| ≥ 55 | 0.62 (0.43–0.88) | 0.55 (0.39–0.78) | 0.52 (0.41–0.67) | 0.53 (0.39–0.72) | < 0.001 |
| pstratad | 0.729 | ||||
| Sex | |||||
| Female | 0.76 (0.47–1.24) | 0.57 (0.34–0.95) | 0.42 (0.23–0.77) | 0.69 (0.35–1.34) | 0.002 |
| Male | 0.60 (0.41–0.86) | 0.52 (0.39–0.70) | 0.48 (0.39–0.61) | 0.48 (0.38–0.62) | 0.002 |
| pstratad | 0.716 | ||||
| Education | |||||
| ≤high school graduate | 0.56 (0.38–0.82) | 0.47 (0.32–0.70) | 0.53 (0.34–0.82) | 0.48 (0.35–0.67) | < 0.001 |
| ≥some college | 0.78 (0.52–1.17) | 0.57 (0.32–1.02) | 0.53 (0.35–0.80) | 0.58 (0.38–0.90) | 0.119 |
| pstratad | 0.325 | ||||
| Geographic regione | |||||
| Europe | 0.55 (0.33–0.9) | 0.36 (0.24–0.54) | 0.33 (0.24–0.44) | 0.36 (0.21–0.63) | < 0.001 |
| America | 0.80 (0.54–1.20) | 0.91 (0.68–1.22) | 0.76 (0.56–1.02) | 0.77 (0.57–1.04) | 0.459 |
| Asia | 0.82 (0.39–1.75) | 1.54 (0.81–2.92) | 0.50 (0.22–1.14) | 1.35 (0.67–2.69) | NE |
| pstratad | 0.006 | ||||
| Body mass index | |||||
| <25 kg/m2 | 0.71 (0.53–0.97) | 0.52 (0.34–0.79) | 0.38 (0.25–0.60) | 0.47 (0.3–0.74) | 0.005 |
| ≥25 kg/m2 | 0.60 (0.39–0.90) | 0.50 (0.33–0.75) | 0.57 (0.41–0.79) | 0.52 (0.34–0.79) | < 0.001 |
| pstratad | 0.210 | ||||
| Tobacco consumption | |||||
| Never user | 0.45 (0.18–1.08) | 0.38 (0.15–0.98) | 0.60 (0.27–1.34) | 0.68 (0.32–1.46) | 0.185 |
| Former user | 0.58 (0.41–0.82) | 0.64 (0.43–0.96) | 0.43 (0.29–0.62) | 0.66 (0.47–0.92) | 0.144 |
| Current user | 0.55 (0.41–0.73) | 0.59 (0.39–0.90) | 0.49 (0.35–0.68) | 0.55 (0.41–0.72) | < 0.001 |
| pstratad | 0.845 | ||||
| Alcohol consumptionf | |||||
| Never/light drinker | 0.65 (0.39–1.09) | 0.72 (0.45–1.16) | 0.61 (0.43–0.87) | 0.76 (0.54–1.08) | 0.227 |
| Moderate drinker | 0.51 (0.31–0.84) | 0.38 (0.24–0.61) | 0.43 (0.30–0.61) | 0.39 (0.28–0.54) | 0.004 |
| Heavy drinker | 0.55 (0.37–0.81) | 0.43 (0.28–0.65) | 0.35 (0.22–0.54) | 0.38 (0.25–0.59) | 0.831 |
| pstratad | 0.308 | ||||
| Tobacco and alcohol consumption combinedf | |||||
| Never/former user -Never/light drinker | 0.61 (0.34–1.06) | 0.53 (0.28–0.99) | 0.40 (0.23–0.71) | 0.71 (0.44–1.16) | 0.043 |
| Current user- Never/light drinker | 0.58 (0.31–1.09) | 0.73 (0.39–1.38) | 0.62 (0.39–0.99) | 0.79 (0.49–1.28) | 0.100 |
| Never/former user -Moderate drinker | 0.49 (0.28–0.86) | 0.55 (0.27–1.12) | 0.59 (0.34–1.00) | 0.63 (0.38–1.06) | 0.011 |
| Current user -Moderate drinker | 0.47 (0.31–0.72) | 0.41 (0.23–0.73) | 0.40 (0.24–0.66) | 0.42 (0.22–0.84) | <0.001 |
| Never/former user - Heavy drinker | 0.52 (0.29–0.94) | 0.49 (0.27–0.92) | 0.24 (0.11–0.51) | 0.55 (0.29–1.06) | 0.626 |
| Current user -Heavy drinker | 0.57 (0.34–0.95) | 0.70 (0.33–1.46) | 0.47 (0.20–1.08) | 0.48 (0.26–0.90) | 0.024 |
| pstratad | 0.907 | ||||
ABBREVIATIONS: MSKCC: Memorial Sloan Kettering Cancer Center; NE: Not estimable.
Estimated from multiple logistic regression models adjusted for age, sex, education, race/ethnicity, study center, cigarette smoking status, cigarette intensity, cigarette duration, cigar smoking status, pipe smoking status, alcohol drinking intensity and the interaction between cigarette intensity and alcohol drinking intensity, when appropriate.
The I Quintile category was considered as the reference one.
P for heterogeneity among studies. When the p-value was less than 0.1 within strata, we reported the mixed-effects estimates derived from the corresponding generalized linear mixed model.
P for heterogeneity across strata. When, for a single stratification variable, fixed- and mixed-effects models were estimated within different strata, likelihood ratio tests for heterogeneity across strata were based on comparable mixed-effects models and therefore we re-fitted one or more mixed-effects models to replace the original fixed-effects ones. We consistently reported the corresponding stratum-specific mixed-effects models instead of the fixed-effects ones.
Europe included Italy Multicenter, Switzerland and Milan (2006–2009) studies. North America included Boston, Buffalo, Los Angeles, MSKCC, North Carolina (2002–2006), and US Multicenter studies. Asia included Japan study only. As Asia included Japan study only, there was no possibility to assess heterogeneity among studies in the Asia stratum.
The never/light drinker category included never drinkers and subjects who drinks less than 1 drink per day; the moderate drinker category included subjects drinking between 1 (included) and 5 drinks per day; the heavy drinker category included subjects drinking 5 drinks per day or more.
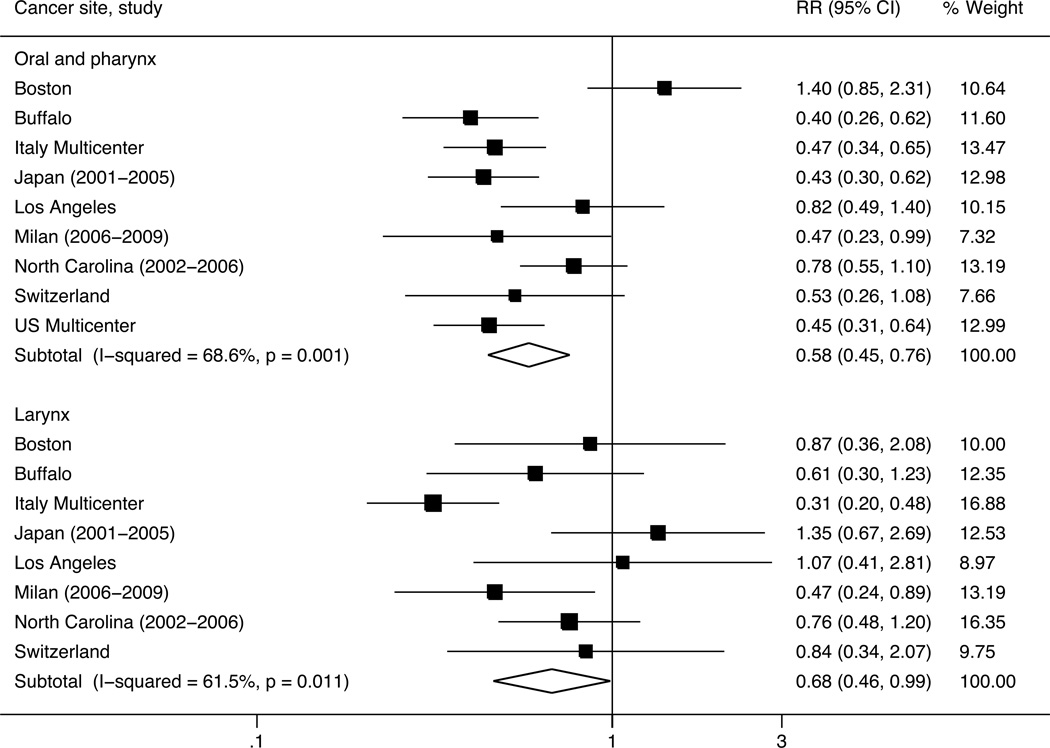
Moreover, results from the fixed- and mixed-effects logistic regression models were comparable to each other in terms of the magnitude of the effect and significance (data not shown). Previous results were also comparable with the results derived from the random-effects meta-analyses [52] comparing each quintile category to the lowest one. Figure 1 shows the forest plots of the pooled and study-specific OR estimates for the associations between the highest versus the lowest quintile of adjusted vitamin C intake and oral and pharyngeal and laryngeal cancers, respectively. For oral and pharyngeal cancer, the pooled OR was 0.58 (95% CI: 0.45–0.76), with corresponding Cochrane’s Q p-value equal to 0.001 and I2 statistic equal to 68.6%. For laryngeal cancer, the pooled OR was 0.68 (95% CI: 0.46–0.99), with corresponding Cochrane’s Q p-value equal to 0.011 and I2 statistic equal to 61.5%. The ORs of oral and pharyngeal cancer were below unity in eight studies (significant in five) and above unity in one study (non significant); the ORs of laryngeal cancer were below unity in six studies (significant in two) and above unity in two studies (non significant). Results from the influence analysis were reassuring, since the exclusion of one study at a time did not materially change the point estimates. For oral and pharyngeal cancer, the point estimates remained significant after the exclusion of any study, whereas, for laryngeal cancer, statistical significance was lost when individually excluding six of the eight studies from the meta-analysis.
Figure 1.
Forest plots of pooled and study-specific odds ratios (ORs) for the associations between the highest versus the lowest quintile categories of non-alcohol energy-adjusted vitamin C intake and oral and pharyngeal, and laryngeal cancers, respectively. International Head and Neck Cancer Epidemiology (INHANCE) consortium.
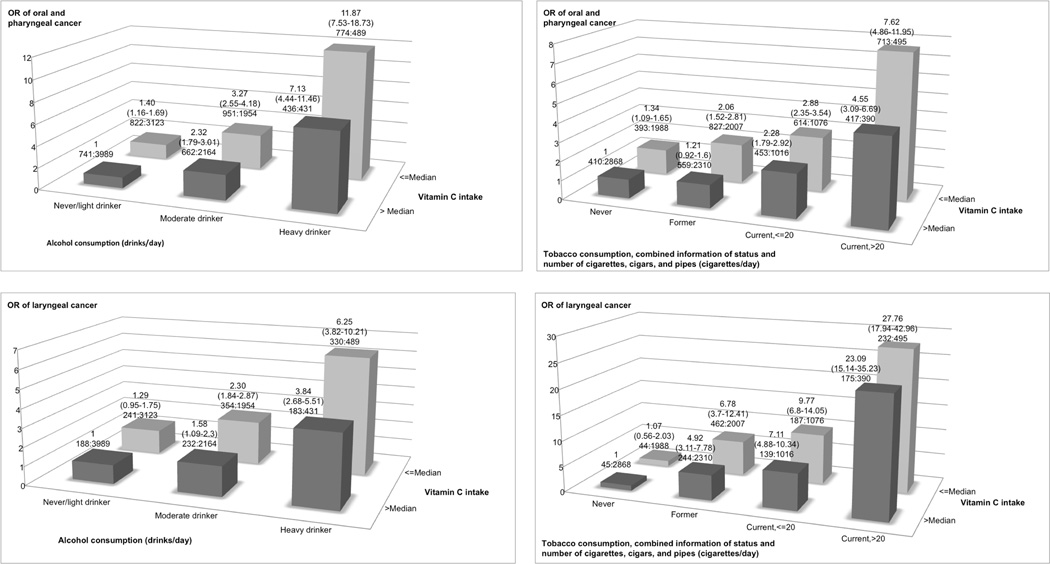
Figure 2 shows the interaction between alcohol or tobacco consumption and adjusted vitamin C intake. For oral and pharyngeal cancer, compared to never and light drinkers (<1 drink/day) in the highest category of vitamin C intake, moderate (>= 1 to < 5 drinks/day) and heavy drinkers (>= 5 drinks/day) in either the low or the high vitamin C intake category had significantly higher ORs, with values ranging approximately from 2 to 12, for drinkers of 5 or more drinks per day in the lowest intake category. Moreover, compared to never smokers in the highest vitamin C intake category, former and current smokers in either the low or the high vitamin C intake category had significantly higher ORs, with values ranging approximately from 2 to 8, for current smokers of more than 20 cigarettes per day in the lowest intake category. Similarly, for laryngeal cancer, moderate and heavy drinkers or former and current smokers in either category of vitamin C intake had a significantly increased OR, with values of about 6 and 28 in the category with the highest exposure to smoking or alcohol and the lowest exposure to vitamin C.
Figure 2.
Odds ratios (ORs)a,b,c of oral and pharyngeal, and laryngeal cancers, and corresponding confidence intervals (95% CIs), according to alcohol or tobacco consumption and ‘non-alcohol energy-adjusted’ vitamin C intake. International Head and Neck Cancer Epidemiology (INHANCE) consortium.
a The odds ratios were derived from mixed-effects logistic regression models adjusted for age, sex, education, race/ethnicity, study center, combined smoking habits of cigarettes, cigars, and pipes, and alcohol drinking, when appropriate.
b The number of cases and controls within each category was indicated below the corresponding odds ratio as: “number of cases : number of controls”.
c The never/light drinker category included never drinkers and subjects who drinks less than 1 drink per day; the moderate drinker category included subjects drinking between 1 (included) and 5 drinks per day; the heavy drinker category included subjects drinking 5 drinks per day or more.
Finally, in the sensitivity analysis including one extra nutrient, the interaction model was selected over the corresponding additive one, from likelihood ratio tests, for fiber intake only, for both cancer sites (p-value=0.04, for oral and pharyngeal cancer, and p-value=0.006, for laryngeal cancer). For the remaining nutrients, the additive model was selected for seven (out of ten) nutrients for oral and pharyngeal cancer, whereas further adjustment for extra nutrients was not significant for laryngeal cancer in any of the fitted models. In either case, the point estimates and the statistical significance were generally in line with the ones from the main analysis. In the model including fiber intake, the ORs of the quintile categories of vitamin C intake were now close to the unity, whereas the ORs of the interaction terms between vitamin C and fiber intakes were generally below the unity, although not always significant, for both cancer sites (data not shown).
In the sensitivity analysis including quartile categories of total fruits or total vegetables, no significant interaction was found between vitamin C and total fruit or total vegetable intakes for either cancer site. The extra adjustment for total fruit or total vegetable intakes was suggested for oral and pharyngeal cancer only, with corresponding ORs for the last quintile category of vitamin C intake given by 0.71 (95% CI: 0.59–0.86) for the model including adjustment by total fruit, and 0.59 (95% CI: 0.50–0.71) for that including adjustment by total vegetable intake (data not shown).
Finally, in the sensitivity analysis including supplemental use of vitamin C, the interaction terms between natural and supplemental use of vitamin C intake were non significant for both cancer sites (p-values equal to 0.74, for oral and pharyngeal cancer, and 0.71 for laryngeal cancer). The extra adjustment for supplemental use of vitamin C intake was significant for oral and pharyngeal cancer only (p-value<0.001), with corresponding ORs still in line with the ones from the main analysis (OR=0.54, 95% CI: 0.41–0.70 for the last quintile category of vitamin C, OR=0.87, 95% CI: 0.77–0.98 for supplemental use of vitamin C) (data not shown).
Discussion
The present analysis shows that, after study-specific adjustment for non-alcohol energy via the residual method, vitamin C intake was inversely and consistently related to oral and pharyngeal, and to laryngeal cancer risk. The identified associations were similar across oral and pharyngeal cancer subsites and in strata of major confounding and risk factors. In particular, these inverse associations were of similar magnitude in never, former, and current smokers, as well as across levels of alcohol drinking. Extra adjustment for potentially related nutrients, supplemental use of vitamin C intake, and non-alcohol energy intake did not materially change the point estimates and the statistical significance of the previous associations. A significant interaction effect with vitamin C intake was found for total fruit consumption and fiber intake, for both cancer sites, and for total vegetable consumption, for laryngeal cancer.
Among possible mechanisms of anti-cancer action, vitamin C has been hypothesized to counteract inflammation and subsequent oxidative damage to DNA, which play a role in the initiation and progression of cancer. Vitamin C may also function as cancer cells killer, due to its pro-oxidant capacity, although the killing of cancer cells is dependent on extracellular H2O2 formation with the ascorbate radical as an intermediate. Moreover, vitamin C may increase collagen synthesis and inhibit hyaluronidase and, on this way, it may prevent cancer spread by increasing extracellular matrix, thus walling in tumors [13, 58]. Finally, this nutrient may act synergistically with other biological antioxidants and radical scavengers in quenching different elements of a radical cascade. This might also justify the strongest evidence in favor of fruit and vegetables, as compared to that on nutrients. Similarly, we cannot exclude that a higher consumption of vitamin C or a more frequent consumption of fruit and vegetables may be a nonspecific indicator of a more affluent and healthy diet [59].
The major strength of our pooled analysis was the availability of a very large series of HNC patients and control subjects, which allowed us to compare vitamin C intake across populations, to examine related overall HNC risk and to explore differences in risks by cancer subsite, geographic region, and alcohol and tobacco consumption.
However, pooled analyses on dietary data pose several challenges. A first issue concerns the type of available dietary data. Nutrients are derived from the questionnaires through country-specific food composition databases. As compared to food-based analyses, this represents an extra step that may be responsible for heterogeneity among studies. Moreover, sources of nutrients may be different across countries. In the present case, a study based on the same FFQ administered in the Italy Multicenter, Milan (2006–2009), and Switzerland studies showed that vitamin C derived from different types of fruit and vegetables, with citrus fruits, kiwi, tomatoes, green salad, apples/pears representing the major sources [60]. In the Japanese 102-item FFQ from which the brief FFQ of the Japan study was derived, vitamin C was supplied by various vegetables and fruits, with spinach, Japanese persimmon, mandarin orange, cabbage, potatoes, but also green tea [61] being relevant sources; however, similar sources ranked in a different way in a rural but otherwise comparable Japanese population, where miso soup was also a very important source of vitamin C [62]. In the Block FFQ used in the US Multicenter, Los Angeles, and MSKCC studies, the main sources of vitamin C were fruit juices, and fruit and vegetables. However, although fruit juices, tomatoes, oranges/tangerines, and potatoes were the leading dietary sources in all socio-demographic subgroups, fortified drinks and southern greens were the major contributors among the young and among the blacks, respectively [63, 64] (for a useful comparison of sources of vitamin C across different dietary questionnaires, see [62]). Keeping this in mind, we observe that, compared to other major nutrients, the validity of vitamin C is more likely to be satisfactory, because it derives from a few major sources, is assessed relatively well by a small number of foods, and these foods are generally consumed all over the world [29, 35]. Moreover, a selection of the top 20 foods contributing most to the total absolute intake accounted for a similar proportion of about 85% of total vitamin C intake from natural sources in the Willett and Block FFQs and in the Western New York FFQ which was used to develop the brief FFQ used in the Buffalo study [33, 64, 65].
A second related issue is the comparability of nutrient intakes across studies. In our scenario, the analysis included only case-control studies, the selected studies were all based on FFQs, the FFQs showed a sufficient level of detail, and some of them were explicitly created to assess consumption of fruit and vegetables and related nutrients [66, 67, 20]. Moreover, we checked for consistency of vitamin C definitions and measurement units, and we excluded the contribution of supplements, whose consumption may represent an extra source of heterogeneity among studies. However, differences existed in the length of the questionnaires and in the wording and design of the questions, and in the food composition databases used to derive nutrient intakes. Moreover, in the Buffalo and Japan studies, diet was queried using brief FFQs, although both the FFQs were specifically designed to provide an assessment of foods providing good sources of vitamins C, and methods for the derivation of the regression weights used to calculate nutrient intakes were carefully examined and provided reassuring results [33, 35].
These differences may have created discrepancies in the study-specific empirical distributions of individual nutrients. To assess the burden of the problem, we carried out preliminary checks using descriptive statistics and a kernel density estimation plot comparing the study-specific empirical distribution of vitamin C intake across studies. We detected systematic differences in the empirical distribution across studies. To partially overcome the problem, we applied the Willett and Stampfer residual method [48] within each study before carrying out the analysis. The extra step of the application of the residual method, instead of the easier and more typical calculation of study-specific quintiles, is driven by the idea that separating out vitamin C from non-alcohol energy intake would allow isolation of the effect of this nutrient from that of total energy intake or energy balance and is suggested when dealing with quantile categories of nutrient intakes, as compared to the standard multivariate approach including both caloric intake and absolute nutrient intakes as terms in the multiple regression model [68]. However, we recognize that this solution may be rough - as between-studies differences are also likely driven by differences in measurement protocols, instruments, study populations, and cultures - and therefore it may not completely solve the issue.
Given the mentioned difficulties in pooling dietary data, together with the different characteristics of the various populations, including variable exposure to alcohol and tobacco, a degree of heterogeneity among studies is to be expected. In our analysis, heterogeneity among studies emerged in the fixed-effects models overall and in several strata of interest, including subsites of oral and pharyngeal cancer. It was confirmed by mixed-effects models results in both GLMM and two-stage method approaches. Our inspection of study-specific findings, influence analyses, and subgroup analyses stratifying by study characteristics pointed to the presence of heterogeneity between European and American studies, especially for laryngeal cancer. However, it is difficult to disentangle the effect of control sources (hospital- versus population-based) from that of geographic region, as the three studies from Europe were all hospital-based and four out of the six American studies were population-based. The apparent heterogeneity cannot, therefore, be attributed beyond reasonable doubt to selection bias and to different types of controls,
In any case, selection bias may be strong in hospital-based case-control studies. since many diseases are related to diet; even population-based designs can be biased due to low or unsatisfactory participation of eligible persons in the source population, although selection bias should be minimized to the extent that participation is not related to the exposure of interest. Recall bias may be another limitation for our pooled analysis, because information about exposure was collected after the onset of HNC. However, diet was not a widely recognized risk factor for HNC, especially in the knowledge of the public at the time of the studies. Therefore, we would expect recall bias to be acceptable during dietary assessment and equally affecting cases and controls. Residual confounding by smoking and alcohol may still be a major issue for these cancers, given the overwhelming role of these risk factors as compared to diet. In the present study, we adjusted for status, intensity, and duration of cigarette smoking, for cigar and pipe use and drinking intensity, as well as for the interaction between cigarette and alcohol intensity. Thus, the residual confounding effect by these factors should have been minimized.
In pooled analyses of binary data, a two-stage method [52] is a simple, valid and practical alternative to a joint model, lending itself to flexibility with respect to differences in design, confounders and data collection across studies. Simulations indicate that, when the individual studies are large, two-stage methods produce nearly unbiased exposure estimates and standard errors of the exposure estimates from a GLMM [69]. However, it is unclear how well the two-stage method would perform if individual studies were smaller, especially when there are a few of them. In the present study, we fitted random-effects models both via GLMMs and the two-stage method, with reassuring results. While the GLMMs estimates are correct by definition, the two-stage method provides an immediate representation of study-specific and pooled risk estimates.
Some of the studies included in the present analysis already contributed to separate original reports on vitamin C intake or provided data for original publications on data partially overlapping with them [70, 67, 71, 72, 73, 24, 27]. Besides them, we are aware of at least fourteen papers that have been reported in the literature to assess the association between vitamin C intake from natural sources and HNC and/or its subsites [5, 4, 3]. Among these, seven provided results on oral and/or pharyngeal cancer [74, 75, 76, 77, 78, 79, 80], four on laryngeal cancer [81, 82, 83, 84], one concerned UADTC and their subsites [85] and two UADTC overall [86, 87]. For oral cavity and/or pharyngeal cancer, significant inverse associations were found in four studies, with [80, 79, 75] or without a linear trend [76]. Some studies showed a weak but non significant protection for higher vitamin C intakes [78, 85, 77], and only one showed an increased but non significant risk [74]. For laryngeal cancer, two studies out of five [81, 84] found an association between low intakes of vitamin C and higher risk of laryngeal cancer, with a significant dose-response relationship, whereas the last three [82, 83, 85] found a weak-to-moderate non significant reduction in risk. For UADTC, in the Iowa Women’s Health cohort, an inverse, but non significant, association was found for vitamin C intake in the original study [86] for cancers of the mouth/pharynx/esophagus combined, but no association was observed in its update for UADTC overall (including also a few cases of cancers of the nasopharynx, larynx, and stomach), after 14 years of follow-up [87]. Moreover, in a study from Uruguay, the overall UADTC risk was significantly lower (p for trend = 0.01) for higher intakes of vitamin C, although the protection was weak and non significant in oral and pharyngeal and laryngeal subsites [85].
Our findings are consistent with evidence from a review on diet and oral and pharyngeal cancer [5], from previous results on food groups from the INHANCE consortium [16] and from the largest European case-control study on diet and UADTC [88], where fruit and vegetables were inversely related to those cancers. They provide extra evidence to integrate with findings from ‘a priori’ and ‘a posteriori’ dietary patterns [16, 17, 89].
In conclusion, clarification of the protective mechanisms of fruit and vegetables is important per se to our understanding of UADTC in general. Although several different factors probably act jointly, our findings suggest that vitamin C intake from foods may protect against cancers of the oral cavity and pharynx, and larynx, respectively.
Supplementary Material
Acknowledgments
MH, CLV, PB and AD designed research; KM, DS, CLV, AO, JZ, DMW, KM, ZFZ, HM, FL, VE, CB, CG, KK, MM, SS, and GP provided single-study databases, commented on manuscript drafts and helped interpret the findings; SCC and YAL prepared the pooled dataset for the analysis; MP provided advice on nutritional issues; VE performed all statistical analyses; VE and FT performed the meta-analysis; VE wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Abbreviations
- CI
confidence interval
- DMV
Department of Motor Vehicles
- FFQ
food-frequency questionnaire
- GLMM
generalized linear mixed model
- HNC
head and neck cancer
- ICD
International Classification of Diseases
- INHANCE
International Head and Neck Cancer Epidemiology
- L
large
- M
medium
- MSKCC
Memorial Sloan Kettering Cancer Center
- NA
not available
- NCI
National Cancer Institute
- NE
Not estimable
- NIH
National Institutes of Health
- OR
odds ratio
- REML
restricted maximum likelihood
- S
small
- UADTC
upper aerodigestive tract cancer.
Footnotes
Conflict of interest: There is no conflict of interest to declare.
Grant sponsor: The INHANCE Pooled Data Project was supported by grants from the National Institutes of Health (NIH), National Cancer Institute, (NCI) R03CA113157 and NIDCR R03DE016611. Individual studies were funded by the following grants: 1. Italy Multicenter study: Italian Association for Research on Cancer (AIRC), Italian League Against Cancer, and Italian Ministry of Research; 2. Swiss study: Swiss League against Cancer and the Swiss Research against Cancer/Oncosuisse [KFS-700 and OCS-1633]; 3. Los Angeles study: NIH [P50CA090388, R01DA011386, R03CA077954, T32CA009142, U01CA096134, R21ES011667] and the Alper Research Program for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center; 4. Boston study: NIH [R01CA078609, R01CA100679]; 5. US multicenter study: The Intramural Program of the NCI, NIH, United States; 6. MSKCC study: NIH [R01CA051845]; 7. Japan study (2001– 2005): Scientific Research grant from the Ministry of Education, Science, Sports, Culture and Technology of Japan (17015052) and grant for the Third-Term Comprehensive 10- Year Strategy for Cancer Control from the Ministry of Health, Labor and Welfare of Japan (H20-002); 8. North Carolina (2002–2006) study: -; 9. Buffalo study: -; 10. Milan study (2006–2009): Italian Association for Research on Cancer (AIRC) and Italian Ministry of Education (PRIN 2009 X8YCBN). Federica Turati was supported by a fellowship from the Italian Foundation for Cancer Research (FIRC).
References
- 1.International Agency for Research on Cancer. IARC Monogr Eval Carcinog Risk Hum. Vol. 83. Lyon, France: IARC; 2004. Tobacco smoke and involuntary smoking. [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Monogr Eval Carcinog Risks Hum. Vol. 96. Lyon, France: IARC; 1988. Alcohol consumption and ethyl carbamate. [PMC free article] [PubMed] [Google Scholar]
- 3.Riboli E, Kaaks R, Esteve J. Nutrition and laryngeal cancer. Cancer Causes Control. 1996;7(1):147–156. doi: 10.1007/BF00115645. [DOI] [PubMed] [Google Scholar]
- 4.Chainani-Wu N. Diet and oral, pharyngeal, and esophageal cancer. Nutr Cancer. 2002;44(2):104–106. doi: 10.1207/S15327914NC4402_01. [DOI] [PubMed] [Google Scholar]
- 5.Lucenteforte E, Garavello W, Bosetti C, La Vecchia C. Dietary factors and oral and pharyngeal cancer risk. Oral Oncol. 2009;45(6):461–467. doi: 10.1016/j.oraloncology.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Meurman JH. Infectious and dietary risk factors of oral cancer. Oral Oncol. 2010;46(6):411–413. doi: 10.1016/j.oraloncology.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 8.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83(5):1126–1134. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 9.Boffetta P, Couto E, Wichmann J, Ferrari P, Trichopoulos D, Bueno-de-Mesquita HB, van Duijnhoven FJ, Büchner FL, Key T, Boeing H, Nöthlings U, Linseisen J, et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2010;102(8):529–537. doi: 10.1093/jnci/djq072. [DOI] [PubMed] [Google Scholar]
- 10.Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96(21):1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 11.George SM, Park Y, Leitzmann MF, Freedman ND, Dowling EC, Reedy J, Schatzkin A, Hollenbeck A, Subar AF. Fruit and vegetable intake and risk of cancer: a prospective cohort study. Am J Clin Nutr. 2009;89(1):347–353. doi: 10.3945/ajcn.2008.26722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu RH. Dietary bioactive compounds and their health implications. J Food Sci. 2013;78(Suppl 1):A18–A25. doi: 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- 13.Grosso G, Bei R, Mistretta A, Marventano S, Calabrese G, Masuelli L, Giganti MG, Modesti A, Galvano F, Gazzolo D. Effects of vitamin C on health: a review of evidence. Front Biosci (Landmark Ed) 2013;18:1017–1019. doi: 10.2741/4160. [DOI] [PubMed] [Google Scholar]
- 14.Block G. Epidemiologic evidence regarding vitamin C and cancer. Am J Clin Nutr. 1991;54(6 Suppl):1310S–1314S. doi: 10.1093/ajcn/54.6.1310s. [DOI] [PubMed] [Google Scholar]
- 15.Conway DI, Hashibe M, Boffetta P, INHANCE consortium. Wunsch-Filho V, Muscat J, La Vecchia C, Winn DM. Enhancing epidemiologic research on head and neck cancer: INHANCE - The international head and neck cancer epidemiology consortium. Oral Oncol. 2009;45(9):743–746. doi: 10.1016/j.oraloncology.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Chuang SC, Jenab M, Heck JE, Bosetti C, Talamini R, Matsuo K, Castellsague X, Franceschi S, Herrero R, Winn DM, La Vecchia C, Morgenstern H, et al. Diet and the risk of head and neck cancer: a pooled analysis in the INHANCE consortium. Cancer Causes Control. 2012;23(1):69–88. doi: 10.1007/s10552-011-9857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edefonti V, Hashibe M, Ambrogi F, Parpinel M, Bravi F, Talamini R, Levi F, Yu G, Morgenstern H, Kelsey K, McClean M, Schantz S, et al. Nutrient-based dietary patterns and the risk of head and neck cancer: a pooled analysis in the International Head and Neck Cancer Epidemiology consortium. Ann Oncol. 2012;23(7):1869–1870. doi: 10.1093/annonc/mdr548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosetti C, Gallus S, Trichopoulou A, Talamini R, Franceschi S, Negri E, La Vecchia C. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1091–1094. [PubMed] [Google Scholar]
- 19.Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S, Monnier P. Food groups and risk of oral and pharyngeal cancer. Int J Cancer. 1998;77(5):705–709. doi: 10.1002/(sici)1097-0215(19980831)77:5<705::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao J, Cao W, Cozen W, Mack TM, Zhang ZF. Polymorphism of Xeroderma Pigmentosum group G and the risk of lung cancer and squamous cell carcinomas of the oropharynx, larynx and esophagus. Int J Cancer. 2006;118(3):714–720. doi: 10.1002/ijc.21413. [DOI] [PubMed] [Google Scholar]
- 21.Peters ES, McClean MD, Liu M, Eisen EA, Mueller N, Kelsey KT. The ADH1C polymorphism modifies the risk of squamous cell carcinoma of the head and neck associated with alcohol and tobacco use. Cancer Epidemiol Biomarkers Prev. 2005;14(2):476–482. doi: 10.1158/1055-9965.EPI-04-0431. [DOI] [PubMed] [Google Scholar]
- 22.Schantz SP, Zhang ZF, Spitz MS, Sun M, Hsu TC. Genetic susceptibility to head and neck cancer: interaction between nutrition and mutagen sensitivity. Laryngoscope. 1997;107(6):765–771. doi: 10.1097/00005537-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF., Jr Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48(11):3282–3287. [PubMed] [Google Scholar]
- 24.Suzuki T, Wakai K, Matsuo K, Hirose K, Ito H, Kuriki K, Sato S, Ueda R, Hasegawa Y, Tajima K. Effect of dietary antioxidants and risk of oral, pharyngeal and laryngeal squamous cell carcinoma according to smoking and drinking habits. Cancer Sci. 2006;97(8):760–767. doi: 10.1111/j.1349-7006.2006.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Divaris K, Olshan AF, Smith J, Bell ME, Weissler MC, Funkhouser WK, Bradshaw PT. Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer Causes Control. 2010;21(4):567–575. doi: 10.1007/s10552-009-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayaprakash V, Rigual NR, Moysich KB, Loree TR, Nasca MA, Menezes RJ, Reid ME. Chemoprevention of head and neck cancer with aspirin: a case-control study. Arch Otolaryngol Head Neck Surg. 2006;132(11):1231–1236. doi: 10.1001/archotol.132.11.1231. [DOI] [PubMed] [Google Scholar]
- 27.Bravi F, Bosetti C, Filomeno M, Levi F, Garavello W, Galimberti S, Negri E, La Vecchia C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br J Cancer. 2013;109(11):2904–2910. doi: 10.1038/bjc.2013.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters ES, Luckett BG, Applebaum KM, Marsit CJ, McClean MD, Kelsey KT. Dairy products, leanness, and head and neck squamous cell carcinoma. Head Neck. 2008;30(9):1193–1195. doi: 10.1002/hed.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decarli A, Franceschi S, Ferraroni M, Gnagnarella P, Parpinel MT, La Vecchia C, Negri E, Salvini S, Falcini F, Giacosa A. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Ann Epidemiol. 1996;6(2):110–118. doi: 10.1016/1047-2797(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi S, Barbone F, Negri E, Decarli A, Ferraroni M, Filiberti R, Giacosa A, Gnagnarella P, Nanni O, Salvini S, La Vecchia C. Reproducibility of an Italian food frequency questionnaire for cancer studies. Results for specific nutrients. Ann Epidemiol. 1995;5(1):69–75. doi: 10.1016/1047-2797(95)92893-d. [DOI] [PubMed] [Google Scholar]
- 31.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1116. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 32.Applied Research Program, National Cancer Institute. Diet History Questionnaire, Version 1.0. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 33.Byers T, Marshall J, Fiedler R, Zielezny M, Graham S. Assessing nutrient intake with an abbreviated dietary interview. Am J Epidemiol. 1985;122(1):41–50. doi: 10.1093/oxfordjournals.aje.a114085. [DOI] [PubMed] [Google Scholar]
- 34.McCann SE, Trevisan M, Priore RL, Muti P, Markovic N, Russell M, Chan AW, Freudenheim JL. Comparability of nutrient estimation by three food frequency questionnaires for use in epidemiological studies. Nutr Cancer. 1999;35(1):4–9. doi: 10.1207/S153279144-9. [DOI] [PubMed] [Google Scholar]
- 35.Tokudome S, Goto C, Imaeda N, Tokudome Y, Ikeda M, Maki S. Development of a data-based short food frequency questionnaire for assessing nutrient intake by middle-aged Japanese. Asian Pac J Cancer Prev. 2004;5(1):40–43. [PubMed] [Google Scholar]
- 36.Public use data tape documentation: Model gram and nutrient composition (computer tape 5702 and 5703) Hyattsville, MD: National Center for Health Statistics; 1982. [Google Scholar]
- 37.HHHQ-DietSys Analysis Software, Version 4.02. Bethesda, MD: National Cancer Institute; 1999. URL http://appliedresearch.cancer.gov/DietSys/materials.html. [Google Scholar]
- 38.Applied Research Program, National Cancer Institute. Diet*Calc Analysis Program, Version 1.4.3. Bethesda, MD: National Cancer Institute; 2005. [Google Scholar]
- 39.Gnagnarella P, Salvini S, Parpinel M. Food composition database for epidemiological studies in Italy. Version 2. 2008 doi: 10.1016/s0304-3835(97)04686-7. URL http://www.bda-ieo.it. [DOI] [PubMed] [Google Scholar]
- 40.Salvini S, Parpinel M, Gnagnarella P, Maisonneuve P, Turrini A. Banca dati di composizione degli alimenti per studi epidemiologici in Italia. Milano, Italy: Istituto Europeo di Oncologia; 1998. [Google Scholar]
- 41.US Department of Agriculture, Agricultural Research Service. Composition of foods, raw, processed, prepared. Agriculture Handbook 8, 1–21 and supplements. 1993 [Google Scholar]
- 42.US Department of Agriculture, Agricultural Research Service. USDA National nutrient database for standard reference, Release 26 and previous versions. Nutrient Data Laboratory Home Page. 2013 URL http://www.ars.usda.gov/ba/bhnrc/ndl.
- 43.Dresser CM. From nutrient data to a data base for a health and nutrition examination survey. Organization, coding and values - real or imputed. Proc Eighth Natl Nutrient Data Base Conference. 1983:92–104. [Google Scholar]
- 44.Standard Tables of Food Composition in Japan, 5th revised version. Tokyo, Japan: Ministry of Finance Printing Bureau; 2000. Resource Council, Science and Technology Agency, the Government of Japan. in Japanese with English translation. [Google Scholar]
- 45.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wünsch-Filho V, Franceschi S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777–779. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Chuang SC, Eluf-Neto J, Menezes A, Matos E, Koifman S, Wünsch-Filho V, Fernandez L, Daudt AW, Curado MP, Winn DM, Franceschi S, et al. Vitamin or mineral supplement intake and the risk of head and neck cancer: pooled analysis in the INHANCE consortium. Int J Cancer. 2012;131(7):1686–1689. doi: 10.1002/ijc.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott DW. Theory, practice and visualization. New York, NY: Wiley; 2005. Multivariate density estimation. [Google Scholar]
- 48.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 49.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York, NY: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 50.Bates DM. lme4: mixed-effects modeling with R. New York, NY: Food and Agriculture Organization of the United Nations; in press, URL http://lme4.R-forge.R-project.org/. [Google Scholar]
- 51.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 52.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–178. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 53.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.R Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2013. R: a language and environment for statistical computing. URL http://www.R-project.org, ISBN 3-900051-07-0. [Google Scholar]
- 56.Bates D, Maechler M, Bolker B. lme4: linear mixed-effects models using S4 classes. 2011 URL http://CRAN.R-project.org/package=lme4, R package version 0.999375-42. [Google Scholar]
- 57.Venables WN, Ripley BD. Modern applied statistics with S. 4th ed. New York, NY: Springer; 2002. URL http://www.stats.ox.ac.uk/pub/MASS4, ISBN 0-387-95457-0. [Google Scholar]
- 58.International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention. Vol. 8. Lyon, France: IARC; 2003. Fruit and Vegetables. [Google Scholar]
- 59.Garavello W, Giordano L, Bosetti C, Talamini R, Negri E, Tavani A, Maisonneuve P, Franceschi S, La Vecchia C. Diet diversity and the risk of oral and pharyngeal cancer. Eur J Nutr. 2008;47(5):280–284. doi: 10.1007/s00394-008-0722-y. [DOI] [PubMed] [Google Scholar]
- 60.Favero A, Salvini S, Russo A, Parpinel M, Negri E, Decarli A, La Vecchia C, Giacosa A, Franceschi S. Sources of macro- and micronutrients in Italian women: results from a food frequency questionnaire for cancer studies. Eur J Cancer Prev. 1997;6(3):277–277. [PubMed] [Google Scholar]
- 61.Imaeda N, Tokudome Y, Ikeda M, Kitagawa I, Fujiwara N, Tokudome S. Foods contributing to absolute intake and variance in intake of selected vitamins, minerals and dietary fiber in middle-aged Japanese. J Nutr Sci Vitaminol (Tokyo) 1999;45(5):519–522. doi: 10.3177/jnsv.45.519. [DOI] [PubMed] [Google Scholar]
- 62.Ogawa K, Tsubono Y, Nishino Y, Watanabe Y, Ohkubo T, Watanabe T, Nakatsuka H, Takahashi N, Kawamura M, Tsuji I, Hisamichi S. Dietary sources of nutrient consumption in a rural Japanese population. J Epidemiol. 2002;12(1):1–8. doi: 10.2188/jea.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Block G, Sorenson A. Vitamin C intake and dietary sources by demographic characteristics. Nutr Cancer. 1987;10(1–2):53–65. doi: 10.1080/01635588709513940. [DOI] [PubMed] [Google Scholar]
- 64.Block G, Dresser CM, Hartman AM, Carroll MD. Nutrient sources in the American diet: quantitative data from the NHANES II survey. I. Vitamins and minerals. Am J Epidemiol. 1985;122(1):13–26. doi: 10.1093/oxfordjournals.aje.a114072. [DOI] [PubMed] [Google Scholar]
- 65.Stryker WS, Salvini S, Stampfer MJ, Sampson L, Colditz GA, Willett WC. Contributions of specific foods to absolute intake and between-person variation of nutrient consumption. J Am Diet Assoc. 1991;91(2):172–178. [PubMed] [Google Scholar]
- 66.Yeh M, Moysich KB, Jayaprakash V, Rodabaugh KJ, Graham S, Brasure JR, McCann SE. Higher intakes of vegetables and vegetable-related nutrients are associated with lower endometrial cancer risks. J Nutr. 2009;139(2):317–322. doi: 10.3945/jn.108.099960. [DOI] [PubMed] [Google Scholar]
- 67.Gridley G, McLaughlin JK, Block G, Blot WJ, Winn DM, Greenberg RS, Schoenberg JB, Preston-Martin S, Austin DF, Fraumeni JF., Jr Diet and oral and pharyngeal cancer among blacks. Nutr Cancer. 1990;14(3–4):219–225. doi: 10.1080/01635589009514096. [DOI] [PubMed] [Google Scholar]
- 68.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 69.Stukel TA, Demidenko E, Dykes J, Karagas MR. Two-stage methods for the analysis of pooled data. Stat Med. 2001;20(14):2115–2120. doi: 10.1002/sim.852. [DOI] [PubMed] [Google Scholar]
- 70.McLaughlin JK, Gridley G, Block G, Winn DM, Preston-Martin S, Schoenberg JB, Greenberg RS, Stemhagen A, Austin DF, Ershow AG, et al. Dietary factors in oral and pharyngeal cancer. J Natl Cancer Inst. 1988;80(15):1237–1243. doi: 10.1093/jnci/80.15.1237. [DOI] [PubMed] [Google Scholar]
- 71.Day GL, Blot WJ, Austin DF, Bernstein L, Greenberg RS, Preston-Martin S, Schoenberg JB, Winn DM, McLaughlin JK, Fraumeni JF., Jr Racial differences in risk of oral and pharyngeal cancer: alcohol, tobacco, and other determinants. J Natl Cancer Inst. 1993;85(6):465–473. doi: 10.1093/jnci/85.6.465. [DOI] [PubMed] [Google Scholar]
- 72.Negri E, Franceschi S, Bosetti C, Levi F, Conti E, Parpinel M, La Vecchia C. Selected micronutrients and oral and pharyngeal cancer. Int J Cancer. 2000;86(1):122–127. doi: 10.1002/(sici)1097-0215(20000401)86:1<122::aid-ijc19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 73.Bidoli E, Bosetti C, La Vecchia C, Levi F, Parpinel M, Talamini R, Negri E, Dal Maso L, Franceschi S. Micronutrients and laryngeal cancer risk in Italy and Switzerland: a case-control study. Cancer Causes Control. 2003;14(5):477–484. doi: 10.1023/a:1024991618398. [DOI] [PubMed] [Google Scholar]
- 74.Zheng W, Blot WJ, Shu XO, Diamond EL, Gao YT, Ji BT, Fraumeni JF., Jr Risk factors for oral and pharyngeal cancer in Shanghai, with emphasis on diet. Cancer Epidemiol Biomarkers Prev. 1992;1(6):441–448. [PubMed] [Google Scholar]
- 75.Kune GA, Kune S, Field B, Kune GA, Kune S, Field B. Oral and pharyngeal cancer, diet, smoking, alcohol, and serum vitamin A and beta-carotene levels: a case-control study in men. Nutr Cancer. 1993;20(1):61–70. doi: 10.1080/01635589309514271. [DOI] [PubMed] [Google Scholar]
- 76.Zheng T, Boyle P, Willett WC, Hu H, Dan J, Evstifeeva TV, Niu S, MacMahon B. A case-control study of oral cancer in Beijing, People’s Republic of China. Associations with nutrient intakes, foods and food groups. Eur J Cancer B Oral Oncol. 1993;29B(1):45–55. doi: 10.1016/0964-1955(93)90010-c. [DOI] [PubMed] [Google Scholar]
- 77.Petridou E, Zavras AI, Lefatzis D, Dessypris N, Laskaris G, Dokianakis G, Segas J, Douglas CW, Diehl SR, Trichopoulos D. The role of diet and specific micronutrients in the etiology of oral carcinoma. Cancer. 2002;94(11):2981–2988. doi: 10.1002/cncr.10560. [DOI] [PubMed] [Google Scholar]
- 78.Marshall JR, Graham S, Haughey BP, Shedd D, O'Shea R, Brasure J, Wilkinson GS, West D. Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. Eur J Cancer B Oral Oncol. 1992;28B(1):9–15. doi: 10.1016/0964-1955(92)90005-l. [DOI] [PubMed] [Google Scholar]
- 79.Rossing MA, Vaughan TL, McKnight B. Diet and pharyngeal cancer. Int J Cancer. 1989;44(4):593–597. doi: 10.1002/ijc.2910440406. [DOI] [PubMed] [Google Scholar]
- 80.Marshall J, Graham S, Mettlin C, Shedd D, Swanson M. Diet in the epidemiology of oral cancer. Nutr Cancer. 1982;3(3):145–149. doi: 10.1080/01635588109513716. [DOI] [PubMed] [Google Scholar]
- 81.Graham S, Mettlin C, Marshall J, Priore R, Rzepka T, Shedd D. Dietary factors in the epidemiology of cancer of the larynx. Am J Epidemiol. 1981;113(6):675–680. doi: 10.1093/oxfordjournals.aje.a113147. [DOI] [PubMed] [Google Scholar]
- 82.Freudenheim JL, Graham S, Byers TE, Marshall JR, Haughey BP, Swanson MK, Wilkinson G. Diet, smoking, and alcohol in cancer of the larynx: a case-control study. Nutr Cancer. 1992;17(1):33–45. doi: 10.1080/01635589209514171. [DOI] [PubMed] [Google Scholar]
- 83.Zheng W, Blot WJ, Shu XO, Gao YT, Ji BT, Ziegler RG, Fraumeni JF., Jr Diet and other risk factors for laryngeal cancer in Shanghai, China. Am J Epidemiol. 1992;136(2):178–181. doi: 10.1093/oxfordjournals.aje.a116484. [DOI] [PubMed] [Google Scholar]
- 84.Esteve J, Riboli E, Pequignot G, Terracini B, Merletti F, Crosignani P, Ascunce N, Zubiri L, Blanchet F, Raymond L, Repetto F, Tuyns AJ. Diet and cancers of the larynx and hypopharynx: the IARC multi-center study in southwestern Europe. Cancer Causes Control. 1996;7(2):240–242. doi: 10.1007/BF00051300. [DOI] [PubMed] [Google Scholar]
- 85.De Stefani E, Ronco A, Mendilaharsu M, Deneo-Pellegrini H. Diet and risk of cancer of the upper aerodigestive tract-II. Nutrients. Oral Oncol. 1999;35(1):22–26. doi: 10.1016/s1368-8375(98)00061-x. [DOI] [PubMed] [Google Scholar]
- 86.Zheng W, Sellers TA, Doyle TJ, Kushi LH, Potter JD, Folsom AR. Retinol, antioxidant vitamins, and cancers of the upper digestive tract in a prospective cohort study of postmenopausal women. Am J Epidemiol. 1995;142(9):955–960. doi: 10.1093/oxfordjournals.aje.a117743. [DOI] [PubMed] [Google Scholar]
- 87.Kasum CM, Jacobs DR, Jr, Nicodemus K, Folsom AR. Dietary risk factors for upper aerodigestive tract cancers. Int J Cancer. 2002;99(2):267–272. doi: 10.1002/ijc.10341. [DOI] [PubMed] [Google Scholar]
- 88.Lagiou P, Talamini R, Samoli E, Lagiou A, Ahrens W, Pohlabeln H, Benhamou S, Bouchardy C, Slamova A, Schejbalova M, Merletti F, Richiardi L, et al. Diet and upper-aerodigestive tract cancer in Europe: the ARCAGE study. Int J Cancer. 2009;124(11):2671–2676. doi: 10.1002/ijc.24246. [DOI] [PubMed] [Google Scholar]
- 89.Bravi F, Edefonti V, Randi G, Ferraroni M, La Vecchia C, Decarli A. Dietary patterns and upper aerodigestive tract cancers: an overview and review. Ann Oncol. 2012;23(12):3024–3029. doi: 10.1093/annonc/mds197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.