Abstract
Elevated Na+ concentration ([Na+]) in the cerebrospinal fluid (CSF) contributes to the development of salt-sensitive hypertension. CSF is formed by the choroid plexus (CP) in cerebral ventricles, and [Na+] in CSF is controlled by transporters in CP. Here, we examined the effect of high salt diet on the expression of urea transporters (UTs) in the CP of Dahl S vs Dahl R rats using real time PCR. High salt intake (8%, for 2 weeks) did not alter the mRNA levels of UT-A (encoded by SLC14A2 gene) in the CP of either Dahl S or Dahl R rats. In contrast, the mRNA levels of UT-B (encoded by SLC14A1 gene) were significantly reduced in the CP of Dahl S rats on high salt diet as compared with Dahl R rats or Dahl S rats on normal salt diet. Reduced UT-B expression was associated with increased [Na+] in the CSF and elevated mean arterial pressure (MAP) in Dahl S rats treated with high salt diet, as measured by radiotelemetry. High salt diet-induced reduction in UT-B protein expression in the CP of Dahl S rats was confirmed by Western blot. Immunohistochemistry using UT-B specific antibodies demonstrated that UT-B protein was expressed on the epithelial cells in the CP. These data indicate that high salt diet induces elevations in CSF [Na+] and in MAP, both of which are associated with reduced UT-B expression in the CP of Dahl S rats, as compared with Dahl R rats. The results suggest that altered UT-B expression in the CP may contribute to an imbalance of water and electrolytes in the CSF of Dahl S rats on high salt diet, thereby leading to alterations in MAP.
INTRODUCTION
Hypertension and its related cardiovascular diseases are the most prevalent cause of death and disability in the world. Epidemiological, migration, intervention, and genetic studies in humans provide very strong evidence of a causal link between high salt intake and hypertension [1]. A positive link has been established between salt intake and elevated blood pressure in 30% and 50% of hypertensive whites and blacks, respectively [2]. Enhanced sympathetic nervous activity plays a major role in the development of salt-induced hypertension both in humans [3] and in genetic animal models, such as Dahl salt-sensitive (Dahl S) rats [4]. Blockade of the neural pathways in the central nervous system (CNS) mediating sympathetic hyperactivity prevent or reverse the hypertension in Dahl S rats [4, 5]. However, the molecular mechanisms in the central nervous system (CNS) underlying the high salt intake-induced sympathoexcitation and hypertension are not yet fully clear.
High salt intake increases sodium concentration [Na+] in the cerebrospinal fluid (CSF) in Dahl S rats and spontaneously hypertensive rats (SHR), whereas CSF sodium shows minimal changes in Dahl salt-resistant (Dahl R) and Wistar-Kyoto (WKY) rats [6, 7]. Elevated CSF Na+ may increase neuronal activity in the CNS, sequentially leading to over-activation of the sympathetic nervous system. This hypothesis is supported by the observation that acute and chronic increases in CSF Na+ by intracerebroventricular (ICV) infusion of hypertonic saline cause sympathetic hyperactivity and hypertension in normotensive Lewis rats as well as in Dahl S rats [8]. CSF is produced by the choroid plexus (CP), a tissue with characteristics similar to kidney, located within the cerebral ventricles. The CSF electrolyte concentration, water secretion, and osmolality balance between CSF and blood are precisely regulated by ion channels, ion exchangers, and transporters located on epithelial cells of the CP. However, transporters that may be altered in the CP of Dahl S rats on high salt diet have not been fully identified.
It is well known that urea transporters (UTs) are expressed in the kidney, and play an important role in water excretion and in the regulation of Na+ concentrating in urine [9]. However, whether UTs are also expressed in the CP and are regulated by high salt diet is unknown. Two genes encode for UTs in mammals: SLC14A1 and SLC14A2, which encode UT-B and UT-A, respectively. Both UT-A and UT-B are expressed in the kidney, where they generate a urea gradient to concentrate urine [10]. UT-B knockout mice have a reduced ability to concentrate urine [11]. UT-B protein is also expressed in erythrocytes as the Kidd (or Jk) antigen, one of the minor blood group antigens. UT-B in erythrocytes also plays an important role in controlling the balance of osmolality across the cytoplasmic membrane, keeping the special shape of erythrocytes [12]. The SLC14A1 gene is located in the chromosome 18, 18q125 region, sitting in a QTL of blood pressure regulatory region in human chromosomes. Thus, the aims of present study were two fold: 1) to determine whether UTs are expressed in the CP; 2) whether their expression is altered by high salt diet in Dahl S versus Dahl R rats.
METHODS
Animals and materials
Adult male Dahl S and Dahl R rats (9 to 10 weeks old) were obtained from Charles River Farms (Wilmington, MA). Rats were housed on a 12:12-hour light/dark cycle in a climate controlled room. Regular rat chow (0.4% Na+) or high salt diet (8% Na+) purchased from Harlan Tekland (Madison, WI) and water were provided ad libitum. All experimental procedures were approved by the North Dakota State University Institutional Animal Care and Use Committee and the Jilin University Institutional Animal Care and Use Committee.
Chronic blood pressure (BP) measurement
Chronic BP was measured with radiotelemetry in free-moving rats, as detailed in our previous publication [13]. After 5-day recovery from telemetry probe implantation surgery, the basal BP and HR were recorded for 3 days. The regular diet was then switched to the high salt diet in 8 Dahl R rats and 8 Dahl S rats; another 8 Dahl R and Dahl S rats were kept on the regular diet. The BP and HR were recorded continuously for 14 days. At the end of the experiment, the brain tissues were harvested and used for assessment of UT expression with Western blots and real-time RT-PCR. The CSF and blood were collected for measurement of [Na+] and [K+] using ion-specific electrodes (Lazar Research Laboratories, Los Angeles, CA).
Western blot analysis of UT-B protein levels
Animals were euthanized with an excessive dose of pentobarbital sodium. Brains were then removed; the choroid plexus and brain tissues were collected; and Western blots were performed as described in our previous publication [14]. The primary antibody (UT-B rabbit polyclonal antibody, Santa Cruz, 1:500) and secondary antibody (goat anti-rabbit IgG horseradish peroxidase-conjugated antibody, Bio-Bad, 1:3,000) were used in current study to detect UT-B protein levels.
Real time PCR measurement of UTs mRNA levels
UT mRNA levels in the choroid plexus of rats were determined by real-time RT-PCR, as described in our previous publication [13]. TaqMan probes specific for rat UT-A and UT-B were purchased from Applied Biosystems Inc (Foster City, CA). Real-time RT-PCR was performed in an Applied Biosystems PRISM 7000 sequence detection system according to the protocol from the manufacturer. Data were normalized to 18S RNA. In each experiment, samples were analyzed in triplicate.
Immunohistochemistry
Immunofluorescence staining of choroid plexus brain sections was performed as described previously [13]. The brain sections containing choroid plexus were incubated with PBS plus 0.5% Tween 20 (PBS-T) containing 5% goat serum. Slices were incubated with primary antibodies (rabbit anti-UT-B 1:500) overnight at 4°C. After being washed with PBS-T, the sections were incubated with secondary antibodies (Alexa Fluor 488 goat anti-rabbit IgG, 1:1000) for 2 hours. The sections were then washed with PBS-T, and examined under a confocal fluorescent microscope (Olympus, Fluoview FV300). The fluorescent images were collected and analyzed with Flow-View software.
Statistical analysis
All data are presented as mean ± SE. Statistical significance was evaluated by 1- or 2-way ANOVA, as appropriate, followed by either a Newman-Keuls or Bonferroni post hoc analysis, where appropriate. Differences were considered significant at P<0.05, and individual probability values are noted in the figure legends.
RESULTS
Effect of high salt diet on MAP and HR
In the first experiment, we confirmed the effect of high salt diet on arterial blood pressure and heart rate in DR and DS rats. Mean arterial pressure (MAP) and heart rate (HR) were measured using radiotelemetry before and after switching to high salt diet. Before dietary treatment, neither MAP nor HR was different between Dahl S and Dahl R rats. However, the MAP and HR were significantly increased by 38±4 mmHg and 49±3 bpm, respectively, by 2-week high salt dietary treatment in Dahl S rats (Figure 1). In Dahl R rats, high dietary salt treatment increased the MAP by only 8±3 mmHg. Together these data demonstrate that the salt-sensitivity of the blood pressure is dramatically enhanced in the Dahl S rats as compared with Dahl R rats.
Figure 1.
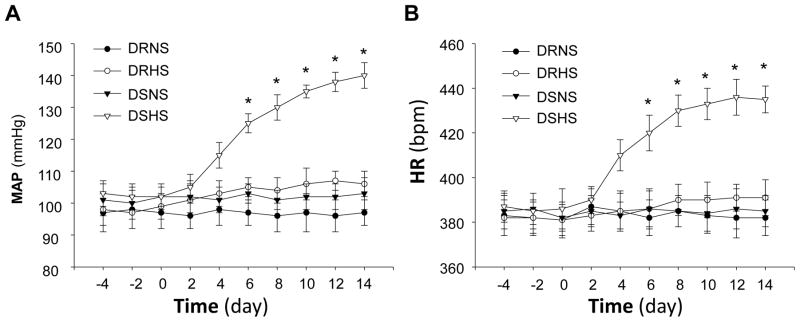
Effect of high salt diet on MAP and HR in Dahl S rats vs. Dahl R rats. The MAP (A) and HR (B) were recorded using radiotelemetry in Dahl S and Dahl R rats before and after switching the diet (at day 0 as indicated in the figure) from regular salt to high salt. The MAP and HR were significantly elevated in Dahl S rats on high salt diet as compared with Dahl S rats on low salt diet or Dahl R rats. Data are mean±SE from 8 rats in each group. *P<0.05 as compared with Dahl R rats on high salt diet.
Effect of high salt diet on [Na+] and [K+] in the CSF and the plasma
We then assessed whether high salt intake alters [Na+] and [K+] in the CSF and in the plasma of Dahl S and Dahl R rats. In both Dahl S and Dahl R rats, sodium levels in the CSF are higher than that in the plasma, suggesting that net sodium excretion from choroid plexus is larger than water excretion. High salt intake treatment for 2 weeks significantly enhanced [Na+] in the CSF of Dahl S rats (Figure 2A). In contrast, the [Na+] in the CSF of Dahl R rats was not significantly altered by the high salt intake, suggesting increased sodium excretion or reduced water excretion into CSF in Dahl S rats as compared with Dahl R rats. However, high salt diet treatment did not significantly alter the [Na+] in plasma of both Dahl S and Dahl R rats. The effects of high salt diet on [K+] in the CSF and in the plasma of Dahl S vs. Dahl R rats are presented in Figure 2B, indicating that the high salt diet treatment did not significantly alter the [K+] in the CSF or in the plasma of both Dahl S and Dahl R rats. In summary, sodium in the CSF is concentrated in the CSF of Dahl S rats after high salt diet treatment.
Figure 2.
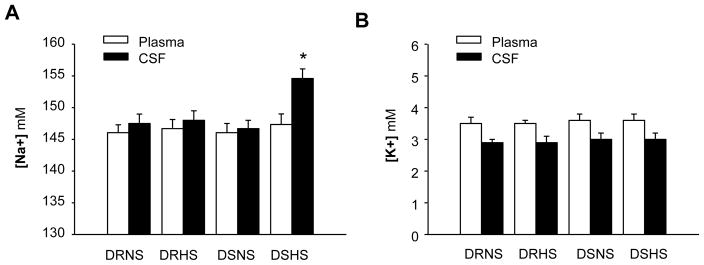
Effect of high salt diet on [Na+] and [K+] in the CSF and the plasma of Dahl S vs. Dahl R rats. A, Bar graphs summarizing the [Na+] in the CSF (filled bars) and the plasma (open bars) of Dahl S (DS) and Dahl R (DR) rats on normal salt diet (NS) and high salt diet (HS) for two weeks. B, Bar graphs summarizing the K+ concentration in the CSF (filled bars) and the plasma (open bars) of Dahl S (DS) and Dahl R (DR) rats on normal salt diet (NS) and high salt diet (HS) for two weeks. The CSF Na+ concentration was significantly elevated in the Dahl S rats on high salt diet. Data are mean±SE from 8 rats in each group. *P<0.05 as compared with Dahl R rats on high salt diet.
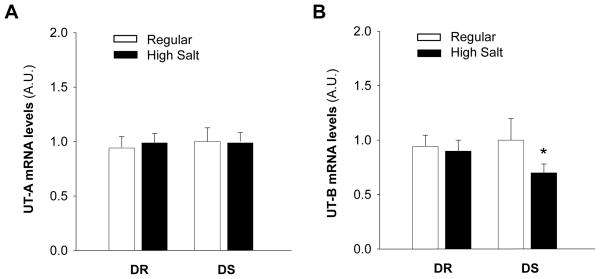
Effect of high salt diet on UT-A and UT-B expression in choroid plexus
It is well known that urea plays a very important role in the regulation of sodium concentration in the urine in the kidney. Since urea is usually transported by specific urea transporters, we next determined the expression of urea transporter A (UT-A) and urea transporter B (UT-B) in the choroid plexus of Dahl S versus Dahl R rats treated with high salt diet. The mRNA levels of UT-A and UT-B were measured in the choroid plexus of Dahl S and Dahl R rats on high salt diet or normal diet using real time RT-PCR. The results are presented in Figure 3, demonstrating that the basal UT-A and UT-B levels are comparable between Dahl S and Dahl R rats. However, the expression of UT-B mRNA was significantly reduced in the choroid plexus of Dahl S rats after two weeks of high salt diet treatment (Fig 3B). In contrast, the UT-A mRNA levels in the choroid plexus were comparable between Dahl S and Dahl R rats on high salt diet or normal diet. Therefore, we focused on the UT-B in choroid plexus in the following several experiments.
Figure 3.
mRNA levels for UTs in choroid plexus of Dahl S vs. Dahl R rats on normal diet and high salt diet. A, Bar graphs summarizing the mRNA levels of UT-A in the choroid plexus of Dahl S (DS) and Dahl R (DR) rats treated with either regular salt or high salt diet for two weeks. B, Bar graphs summarizing the mRNA levels of UT-B in the choroid plexus of Dahl S (DS) and Dahl R (DR) rats treated with either regular salt (RS) or high salt diet (HS) for two weeks. The UT mRNA levels were detected using real-time RT-PCR. Data are mean±SE from 4 repeated experiments and 8 rats in each group. *P<0.05 as compared with Dahl R rats on high salt diet.
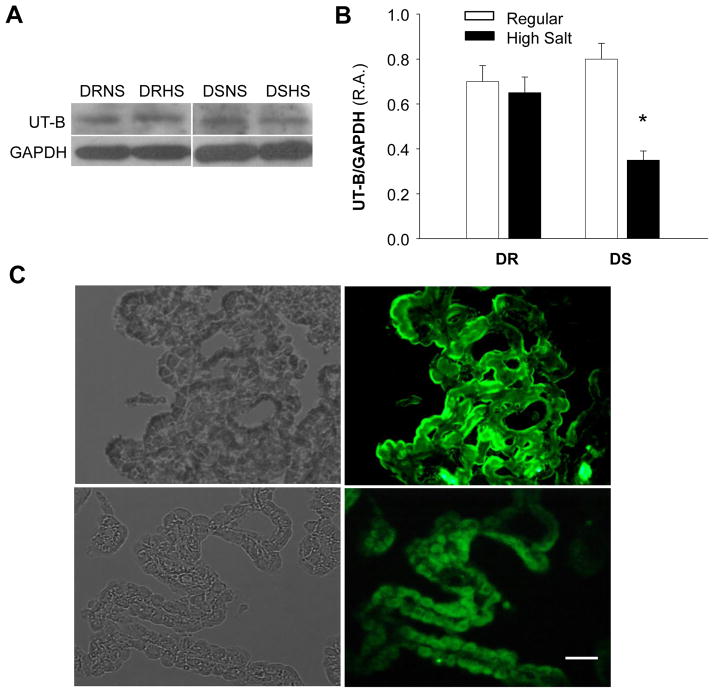
We next determined UT-B protein levels in the choroid plexus of Dahl S and Dahl R rats on high salt or normal diet. The results are presented in Figure 4A and B, indicating that 2-week high salt diet treatment significantly reduced UT-B protein expression in the choroid plexus of Dahl S rats as compared with Dahl R rats. In contrast, high salt diet did not significantly alter UT-B expression in choroid plexus of Dahl R rats. To determine the localization of UT-B protein in the choroid plexus, we performed immunohistochemistry on choroid plexus cross sections using a UT-B specific antibody. The UT-B immunoreactive protein was clearly visualized on the epithelial cell layer of choroid plexus (Fig 4C). The fluorescence density was reduced in the choroid plexus of Dahl S rats treated with high salt diet as compared Dahl R rats with normal diet. In summary, high salt diet significantly reduced UT-B expression in the choroid plexus of Dahl S rats as compared with Dahl R rats.
Figure 4.
UT-B protein expression in the choroid plexus of Dahl S vs. Dahl R rats on regular and high salt diet. A, Representative autoradiograms showing the protein levels of UT-B and GAPDH in the choroid plexus of Dahl S (DS) and Dahl R (DR) treated with either normal salt (NS) or high salt diet (HS) for two weeks. B, Bar graphs showing the protein levels of UT-B in the choroid plexus of Dahl S (DS) and Dahl R (DR) treated with either regular salt or high salt diet for two weeks. Data are normalized using GAPDH. UT-B expression was reduced in the choroid plexus of Dahl S rats on high salt diet. Data are mean±SE from 4 repeated experiments and 8 rats in each group. C, Localization of UT-B protein in the choroid plexus of Dahl S rats on regular diet (upper two panels) and high salt diet (lower two panels). Left two panels showing the choroid plexus in optical phase; and fluorescent micrographs in right two panels showing the same area stained with specific anti-UT-B antibodies. Bar, 100 μm.
DISCUSSION
The current study demonstrates that the expression of UT-B in the choroid plexus is significantly reduced by high salt-diet treatment in salt-sensitive Dahl S rats, suggesting that UT-B may play a key role in the maintenance of sodium and water balance in the CSF during high salt-diet. This conclusion is supported by the following lines of evidence: 1) high salt diet significantly decreased UT-B expression in the CP of Dahl S rats as compared with Dahl R rats; 2) UT-A expression in the CP of Dahl S rats is not altered by high salt diet treatment; 3) UT-B is localized on the epithelial cells of CP, which are responsible for CSF formation; 4) reduced expression of UT-B in the choroid plexus is associated with elevated [Na+] in CSF and increased blood pressure in Dahl S rats after high salt diet treatment.
The results of the present study show that, in rats, UT-B is expressed in the choroid plexus and localized to the epithelial cells. Choroid plexus consists of epithelial cells and fenestrated blood vessels, responsible for the formation of CSF. Ion channels and transporters in the epithelial monolayer cells of choroid plexus regulate the homeostasis of CSF via control of the transportation of electrolytes and water, with a mechanism similar to that in the kidney. In the kidney, UT-B is expressed in the endothelial cells of descending vasa recta [15], where UT-B mediated urea transportation counterregulates the urea concentration gradient in inner medulla, an essential mechanism for the kidney to concentrate urine. Indeed, UT-B-deficient mice have a defect in the ability to form concentrated urine [16]. Thus, we can speculate that UT-B in the choroid plexus may also control water and electrolyte balance between plasma and CSF. Previous studies reveal a CSF level of urea only 0.7–0.8 of that in plasma [17, 18], indicating urea transporters in choroid plexus regulate the urea gradient between plasma and CSF, which may also be important to control water transportation to CSF and electrolyte concentration in the CSF.
An imbalance between electrolyte concentration and water excretion has been observed in hypertensive rats on high salt diet. Data from the present study (Figure 2) demonstrate that the [Na+] in the CSF was significantly increased in Dahl S rats after 2-week high salt diet treatment. This phenomenon has also been observed in spontaneously hypertensive rats [19]. The rise in CSF [Na+] may directly or indirectly stimulate neuronal activity in brain cardiovascular regulatory regions, leading to sympathoexcitation and blood pressure elevation [20. 21]. This hypothesis is supported by studies showing that acute or chronic central ICV infusion of Na+-rich CSF significantly elevate blood pressure, heart rate, and sympathetic nerve activity in both hypertensive [22] and normotensive. Both high Na+ and Cl− are essential for the pressor effect of central ICV infusion [23, 24]. The imbalance of electrolytes and water in the CSF is believed to be involved in the neurogenic mechanism in the development of salt-sensitive hypertension [25]. However, how Na+ concentration in the CSF is elevated in hypertensive rats is not yet fully understood. It has been proposed that epithelial Na+ channels in the choroid plexus are involved in the [Na+] elevation in the CSF [26, 27] in Dahl S and SHR rats on high salt diet. However, the expression of Na+ channels in the choroid plexus is increased in the choroid plexus of Dahl S and SHR rats treated with high salt diet [26, 28]. It is well known that Na+ channels mediate the Na+ influx from CSF into epithelial cells of choroid plexus. Thus, increased Na+ channels in the choroid plexus would reduce [Na+] in the CSF instead of increase [Na+] in the CSF. Our current study examined the expression of another transporter in the choroid plexus, UT-B, and indicates that UT-B expression is significantly reduced in the choroid plexus of Dahl S rats on high salt diet as compared with normotensive rats. Given the high urea concentration in the plasma as compared with CSF [29], it is tempting to consider the possibility that reduced UT-B expression in the choroid plexus induces urea accumulation in the epithelial cells, inducing hyperosmolality, thus, leading to reduced water excretion and concentrated Na+ in the CSF. However, the exact mechanisms still need further investigation in the future.
Another question raised in the current study is how UT-B expression is reduced in the CP of Dahl S rats after high salt diet treatment. In the first possibility, reduced expression of UT-B in the choroid plexus in Dahl S rats could be caused by high [Na+] in the CSF. This mechanism could help epithelial cells of CP to balance the enhanced osmolality caused by increased Na+ in the CSF during high salt diet in Dahl S rats. In another possibility, it has been observed that UT-B expression is significantly reduced by arginine vasopressin (AVP) in kidney [30. 31]. Thus, we can speculate that reduced UT-B expression in the CP may be also caused by elevated AVP levels in Dahl S rats on high salt diet treatment. The results indicate that UT-B in the choroid plexus may play a role in regulating water and electrolyte balance in the CSF and in the development of salt-sensitive hypertension.
Supplementary Material
Highlights.
High salt diet treatment caused hypertension in Dahl salt-sensitive rats
Na+ concentration was elevated in the cerebrospinal fluid by high salt diet
UT-B is expressed in the epithelial cells of choroid plexus
UT-B expression in the choroid plexus was reduced by high salt diet
Acknowledgments
This work was supported by Grants from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH, NS55008), American Heart Association (10GRNT3170012), Natural Science Foundation of China (81000271 and 20110718), Science and Technology Department of Jilin Province, International Collaboration Program (20140414012GH), and Norman Bethune Health Science Center of Jilin University, Young Scholars Program (2013201003).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He FJ, MacGregor GA. Salt, blood pressure and cardiovascular disease. Curr Opin Cardiol. 2007;22:298–305. doi: 10.1097/HCO.0b013e32814f1d8c. [DOI] [PubMed] [Google Scholar]
- 2.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 3.Yatabe MS, Yatabe J, Yoneda M, Watanabe T, Otsuki M, et al. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am J Clin Nutr. 2010;92:77–82. doi: 10.3945/ajcn.2009.29028. [DOI] [PubMed] [Google Scholar]
- 4.Brooks VL, Haywood JR, Johnson AK. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin Exp Pharmacol Physiol. 2005;32:426–32. doi: 10.1111/j.1440-1681.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang BS, White RA, Bi L, Leenen FH. Central infusion of aliskiren prevents sympathetic hyperactivity and hypertension in Dahl salt-sensitive rats on high salt intake. Am J Physiol Regul Integr Comp Physiol. 2012;302:R825–32. doi: 10.1152/ajpregu.00368.2011. [DOI] [PubMed] [Google Scholar]
- 6.Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol. 2004;287:H1160–6. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 7.Simchon S, Manger W, Golanov E, Kamen J, Sommer G, et al. Handling 22NaCl by the blood-brain barrier and kidney: its relevance to salt-induced hypertension in dahl rats. Hypertension. 1999;33:517–23. doi: 10.1161/01.hyp.33.1.517. [DOI] [PubMed] [Google Scholar]
- 8.Huang BS, Ahmad M, Deng AY, Leenen FH. Neuronal responsiveness to central Na+ in 2 congenic strains of Dahl salt-sensitive rats. Hypertension. 2007;49:1315–20. doi: 10.1161/HYPERTENSIONAHA.106.086363. [DOI] [PubMed] [Google Scholar]
- 9.Klein JD, Blount MA, Sands JM. Molecular mechanisms of urea transport in health and disease. Pflugers Arch. 2012;464:561–72. doi: 10.1007/s00424-012-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Chen G, Yang B. Urea transporter physiology studied in knockout mice. Front Physiol. 2012;3:217. doi: 10.3389/fphys.2012.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei T, Zhou L, Layton AT, Zhou H, Zhao X, et al. Role of thin descending limb urea transport in renal urea handling and the urine concentrating mechanism. Am J Physiol Renal Physiol. 2011;301:F1251–9. doi: 10.1152/ajprenal.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmer RT, Klein JD, Bagnasco SM, Doran JJ, Verlander JW. Localization of the urea transporter UT-B protein in human and rat erythrocytes and tissues. Am J Physiol Cell Physiol. 2001;281:C1318–25. doi: 10.1152/ajpcell.2001.281.4.C1318. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Yao F, Raizada MK, O’Rourke ST, Sun C. Apelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ Res. 2009;104:1421–8. doi: 10.1161/CIRCRESAHA.108.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao F, Sumners C, O’Rourke ST, Sun C. Angiotensin II increases GABAB receptor expression in nucleus tractus solitarii of rats. Am J Physiol Heart Circ Physiol. 2008;294:H2712–20. doi: 10.1152/ajpheart.00729.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukaguchi H, Shayakul C, Berger UV, Tokui T, Brown D, et al. Cloning and characterization of the urea transporter UT3: localization in rat kidney and testis. J Clin Invest. 1997;99:1506–15. doi: 10.1172/JCI119313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B, Bankir L, Gillespie A, Epstein CJ, Verkman AS. Urea-selective concentrating defect in transgenic mice lacking urea transporter UT-B. J Biol Chem. 2002;277:10633–7. doi: 10.1074/jbc.M200207200. [DOI] [PubMed] [Google Scholar]
- 17.Johanson CE, Stopa EG, McMillan PN. The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol. 2011;686:101–31. doi: 10.1007/978-1-60761-938-3_4. [DOI] [PubMed] [Google Scholar]
- 18.Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev. 2013;93:1847–92. doi: 10.1152/physrev.00004.2013. [DOI] [PubMed] [Google Scholar]
- 19.Simchon S, Manger W, Golanov E, Kamen J, Sommer G. Handling 22NaCl by the blood-brain barrier and kidney: its relevance to salt-induced hypertension in dahl rats. Hypertension. 1999;33:517–23. doi: 10.1161/01.hyp.33.1.517. [DOI] [PubMed] [Google Scholar]
- 20.Mathai ML, Evered MD, McKinley MJ. Central losartan blocks natriuretic, vasopressin, and pressor responses to central hypertonic NaCl in sheep. Am J Physiol. 1998;275:R548–54. doi: 10.1152/ajpregu.1998.275.2.R548. [DOI] [PubMed] [Google Scholar]
- 21.Miyajima E, Buñag RD. Enhanced sympathetic pressor responses to intracerebrovascularly infused saline in awake salt-loaded rats. Am J Hypertens. 1990;3:117–22. doi: 10.1093/ajh/3.2.117. [DOI] [PubMed] [Google Scholar]
- 22.Gabor A, Leenen FH. Mechanisms mediating sodium-induced pressor responses in the PVN of Dahl rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1338–49. doi: 10.1152/ajpregu.00246.2011. [DOI] [PubMed] [Google Scholar]
- 23.Shah J, Jandhyala BS. Studies on the role(s) of cerebrospinal fluid osmolality and chloride ion in the centrally mediated pressor responses of sodium chloride. Clin Exp Hypertens. 1991;13:297–312. doi: 10.3109/10641969109042064. [DOI] [PubMed] [Google Scholar]
- 24.Ziomber A, Machnik A, Dahlmann A, Dietsch P, Beck FX, et al. Sodium-, potassium-, chloride-, and bicarbonate-related effects on blood pressure and electrolyte homeostasis in deoxycorticosterone acetate-treated rats. Am J Physiol Renal Physiol. 2008;295:F1752–63. doi: 10.1152/ajprenal.00531.2007. [DOI] [PubMed] [Google Scholar]
- 25.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–49. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano M1, Hirooka Y, Matsukawa R, Ito K, Sunagawa K. Mineralocorticoid receptors/epithelial Na(+) channels in the choroid plexus are involved in hypertensive mechanisms in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2013;36:277–84. doi: 10.1038/hr.2012.174. [DOI] [PubMed] [Google Scholar]
- 27.Leenen FH. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta. 2010;1802:1132–9. doi: 10.1016/j.bbadis.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang HW, Amin MS, El-Shahat E, Huang BS, Tuana BS. Effects of central sodium on epithelial sodium channels in rat brain. Am J Physiol Regul Integr Comp Physiol. 2010;299:R222–33. doi: 10.1152/ajpregu.00834.2009. [DOI] [PubMed] [Google Scholar]
- 29.Johanson CE, Woodbury DM. Uptake of [14C] urea by the in vivo choroid plexus-cerebrospinal fluid-brain system: identification of sites of molecular sieving. J Physiol. 1978;275:167–176. doi: 10.1113/jphysiol.1978.sp012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinh-Trang-Tan MM, Lasbennes F, Gane P, Roudier N, Ripoche P, et al. UT-B1 proteins in rat: tissue distribution and regulation by antidiuretic hormone in kidney. Am J Physiol Renal Physiol. 2002;283:F912–22. doi: 10.1152/ajprenal.00359.2001. [DOI] [PubMed] [Google Scholar]
- 31.Combet S, Geffroy N, Berthonaud V, Dick B, Teillet L, et al. Correction of age-related polyuria by dDAVP: molecular analysis of aquaporins and urea transporters. Am J Physiol Renal Physiol. 2003;284:F199–208. doi: 10.1152/ajprenal.00167.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.