Abstract
Background
SFTSV, an emerging tick-borne pathogen that can cause fatal severe fever with thrombocytopenia syndrome (SFTS), was first identified in China in 2009. Limited evidence suggests that SFTSV can be transmitted between humans via blood contact raising concerns over transfusion safety. A study of donor samples from three Chinese blood centers was conducted to investigate the seroprevalence and rate of SFTSV viremia among Chinese blood donors.
Materials and Methods
From April 16 to Oct 31, 2012, 17,208 plasma samples were collected from donors at Xinyang (located in an SFTSV endemic area), Mianyang and Luoyang blood centers. Assessment of anti-SFTSV total antibody was performed on all samples using Enzyme Linked Immunoassay (ELISA). Repeat-reactive samples were tested for SFTSV RNA using reverse transcription (RT) Real-time-PCR assay with Taqman probes. In addition, 9960 of the Xinyang samples were tested in pools of 4 by the same PCR method and each of samples in a reactive pool was tested individually.
Results
Donor seroreactivity rates were: Xinyang, 0.54% (80/14,752); Mianyang, 0.27% (3/1,130); and Luoyang, 0.28% (3/1,326). All seroreactive samples were negative on RT-PCR single-sample testing. Two RT-PCR reactive donor samples were identified, both with estimated viral load <20pfu/ml. The RNA prevalence rate for SFTSV among donors in Xinyang was 0.02%.
Conclusion
This was the first multi-region study of SFTSV sero- and viral-prevalence among Chinese blood donors. Viral prevalence was low and no seroreactive sample was viremic, suggesting limited impact of SFTSV on blood safety in China.
SFTSV, a new bunyavirus that causes Severe Fever with Thrombocytopenia Syndrome (SFTS), was first identified in patients from Xinyang City, Henan Province in 2009, with high initial fatality rates of 12% to 30%1. The epidemic has been expanding from the central to northeast and southeast regions of China2. More recently, similar clinical cases with confirmed SFTSV infection were found in Japan3 and South Korea4, and the Heartland virus, a virus from a phlyogenetic sister group of SFTSV, was detected in the two patients in the United States5, indicating a possible growing epidemic in East Asia and the U.S. The epidemic season in China extends from spring to autumn with most cases occurring from May to July6. Most clinical cases occur in older patients and no specific treatment for SFTS is currently available and1 and 2. Farmers working in hilly areas are believed to be a high-risk population possibly due to occupation related increased risk of exposure to virus carrying ticks2.
The incubation period (time from exposure to clinical disease onset) of SFTSV has been estimated to be one to two weeks, with an average of 9 days, but can be as long as 30 days7,8. Person-to-person transmission caused by blood contact was reported in several studies9–11. A guide for prevention of nosocomial SFTSV infection was promulgated by the Chinese Ministry of Health12. Though transfusion-transmitted infection has not been reported, it is possible that infected donations from asymptomatic donors during the incubation period could transmit this virus to transfusion recipients.
There is virtually no information on the rates of SFTSV seroreactivity or viremia among blood donors; such data are critical to help evaluate SFTSV’s potential impact on blood safety in China. In 2012 a cross-sectional study was launched by the National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) to investigate the seroprevalence and viremia rate of SFTSV in blood donors from three Chinese regions.
Materials and methods
Study sites
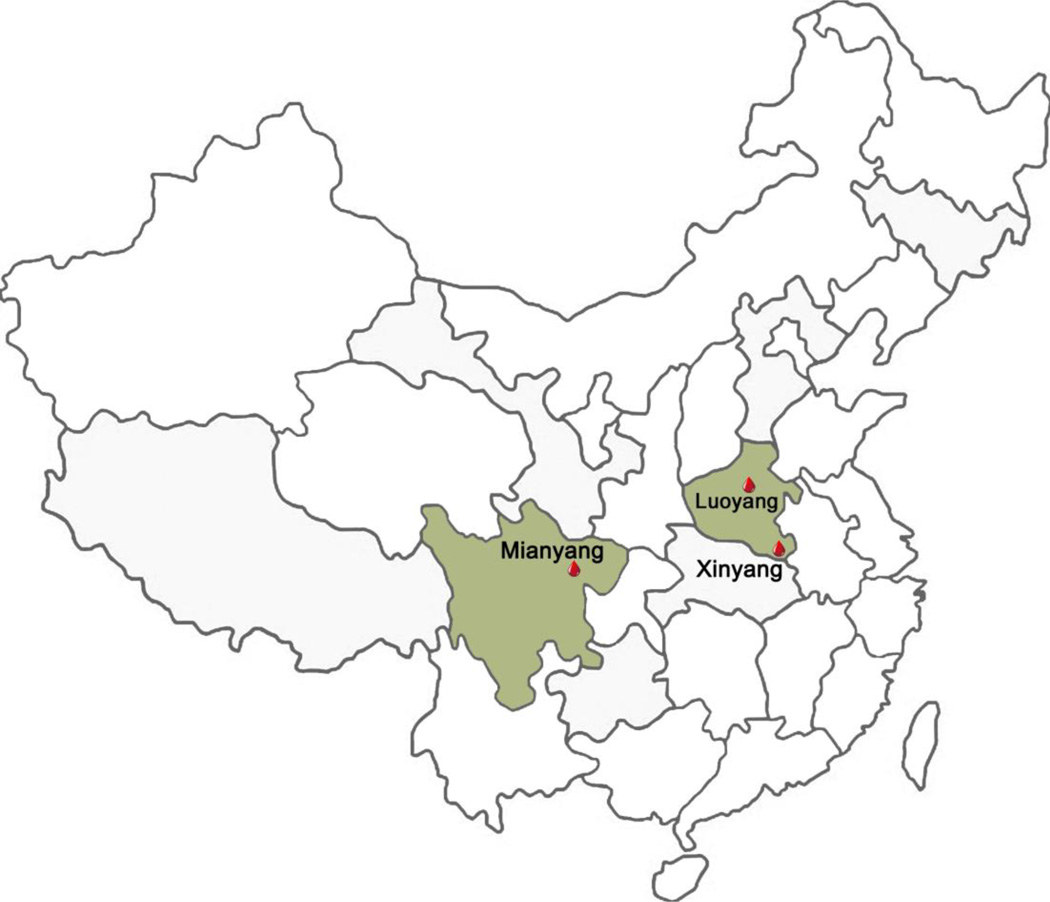
Three blood centers in Xinyang, Mianyang and Luoyang (China) participated in this study. Xinyang Blood Center is in a hilly region in Henan province that has experienced major SFTSV epidemics. Henan was the first Chinese province where cases of SFTSV disease have been reported. Among the 2047 SFTSV cases reported in China in 2011–2012, 48.2% were from Henan Province6 and more than 95% of SFTSV cases in Henan Province reported in 2010 – 2012 came from Xinyang and its surrounding areas13. Luoyang Blood Center is located in a metropolitan region of Henan Province, 220 miles to the north of Xinyang. Mianyang Blood Center, located in Sichuan province, is 750 miles to the southwest of Xinyang, sharing the same hilly terrain (Fig.1). Very few cases of SFTS have been reported in either Mianyang or Luoyang and their surrounding regions.
Fig. 1.
The location of three participating blood centers.
Study populations
Whole blood and apheresis donors who passed the routine pre-donation screening process from April 16 to October 31, 2012 were recruited during the routine donation process. All donors from Xinyang and the first 200 donors in each month from Luoyang and Mianyang were enrolled after obtaining consent to participate in the study. Donors’ demographic characteristics, health history and donation information were recorded in the routine donation database maintained by participating blood centers and transferred to the REDS-III study coordinating center, Research Triangle Institute (RTI) via the Chinese data coordinating center FEi Systems (FEi) through a secure file transfer protocol (FTP) site. Plasma samples were processed from anticoagulated whole blood vacutainer tubes within 24 hours and frozen and stored at −20°C at each blood center before being shipped in batches to the Chinese Institute of Blood Transfusion (IBT) via cold chain transportation.
Fifty plasma samples obtained retrospectively from patients with a clinical diagnosis of SFTS at Xinyang 154 Military Hospital were evaluated for possible use as positive controls. Consent to have their blood samples tested for research purposes was obtained from the patients at the time of collection. Samples were shipped frozen to IBT and stored at −80°C prior to testing. Five ELISA reactive and two RNA positive clinical samples identified by this testing were subsequently used as external controls in the ELISA and PCR testing of blood donor samples.
The study protocol was approved by the Institutional Review Board at all participating institutions including Johns Hopkins University (JHU) on behalf of JHU and FEi; the Institute of Blood Transfusion (IBT) under the Chinese Academy of Medical Sciences (CAMS) on behalf of the three blood centers; and RTI.
Detection of anti-SFTSV total antibody with a commercial ELISA
All samples were tested for anti-SFTSV total antibodies (including IgG and IgM) using an ELISA assay (Xin-Lian-Xin, Inc. Wuxi) in a 96 well format following the manufacturer’s protocol. Selected ELISA reactive clinical samples were used as external controls on the first and last plate during each testing day as an additional quality control measure (see above). The assay is a double-antigen sandwich ELISA that detects SFTSV-specific antibodies that bind to SFTSV recombinant nucleocapsid protein14. Initially reactive samples were retested in duplicate and classified as anti-SFTSV reactive if one or both of the two duplicate repeat assays were reactive (this approach was adopted as there is as yet no commercial confirmatory serologic assay for SFTSV).
Detection of SFTSV RNA with reverse transcriptase (RT)-real-time-PCR assay
Individual sample RT-real-time-PCR testing was conducted on all ELISA positive samples from the three blood centers and on clinical samples from the Xinyang 154 Military Hospital. In addition, about 2/3 of all donations from Xinyang blood center (n=9960, including seronegative and seroreactive samples) collected from May to October were systematically chosen from each month’s collections by selecting every second and third boxes (250 samples per box) out of a total of 60 boxes of stored samples. These plasma samples were tested by real-time reverse-transcriptase -PCR in duplicate in 4-sample mini-pools with 250µL of each sample pooled in a total volume of one mL. A mini-pool was considered positive if either or both of two duplicate assays produced a positive signal at a cycle number (Ct) <45.The four individual samples in each reactive mini-pool were then tested individually in duplicate. Criteria for a positive result at the level of individual testing were more conservative and required that both replicates have Cts<40. The viral load of reactive samples was further determined using quantitative controls that were diluted to create a standard curve. The quantitative controls, which were quantitated in plaque forming units (pfus) were provided by the Centers for Disease Control (Fort Collins, Co, USA). The lower limit for quantitative testing was 20 pfu/mL.
Description of the RT-real-time-PCR assay
The RT and PCR buffers and SFTSV primers were provided by Blood System Research Institute (BSRI, San Francisco, CA, U.S.A.) and optimized for high sensitivity detection of SFTSV virion RNA. SFTSV RNA was extracted using Viral RNA extraction kit (QIAamp, Qiagen, Valencia, CA), per the manufacturer’s instructions. The RNA was eluted in 100µL of water, followed by reverse transcription with RT buffer of 12µL of 10×Solution A + B15, 1.2µL dNTPs (100 mM - no dUTP; Bioline, Germany), 3µLRNase inhibitor (40U/µL; Roche Diagnostics GmbH, Germany), 3µL reverse transcriptase (50 U/µL, Roche Diagnostics GmbH, Germany) and 0.45µL of downstream primer on S segment (100µM, S-R-3)16, at condition of 42°C for 30 min, 100°C for 10 min and cooling to 4°C to synthesis cDNA. Then, 25µL cDNA was added to 51.4µL of PCR mixture consisting of 50 uL Buffer 55 (patented buffer provided by BSRI), 0.5µL dNTPs (100 mM - with dUTP; Bioline, Germany), 0.5µL primers (100µM, S-F-3 and S-R-3), 0.1µL TaqMan probe (100 µM; S-Probe-3) and 0.3µL FastStart Taq (Roche Diagnostics GmbH, Germany). Real-time PCR was performed with conditions of 1 cycle of 95°C for 1 minute followed by 45 cycles of 95°C for 30 seconds and 60°C for 1 minute. Duplicate testing of each sample was performed for individual and mini-pool testing.
Determining the analytic sensitivity of the PCR assay
A test panel for assessing sensitivity was prepared from cultured SFTSV (108.6pfu/mL) received from the Centers for Disease Control (Fort Collins, Co, USA). The virus was diluted serially using plasma. Aliquots of serial dilutions were prepared at concentrations of 10 pfu/mL, 1 pfu/mL, 0.1 pfu/mL and 0.01 pfu/mL and were frozen. These were tested in multiple replicates and interpreted as positive at a (Ct) <45. Based on a logistic regression of the probability of a positive result as a function of log10 concentration for the three non-zero concentrations, 95% of results were expected to be positive at 5.4 pfu/mL, [95% confidence interval (CI): 3.2-8.9 pfu/mL] and 50% were expected to be positive at 0.8 pfu/mL, [95% CI: 0.68–1.04 pfu/mL].
Preparation of PCR controls
To establish the validity of test runs, two clinical samples with low and moderate positive signals by the RT-real-time PCR assay were selected as positive controls, aliquoted, and stored at −80°C. On each plate, two aliquots of each positive control and one negative control were run alongside the donor samples. For runs to be considered valid; the moderate positive control was required to give a positive signal in duplicate. The mean Cts±standard deviation (SD) for the two external controls were 34.9±1.52 (moderate positive control) and 38.9±1.85 (low positive control) over the course of multiple testing runs.
Statistical analysis
Variation in the seroreactivity rates for SFTSV, based on ELISA results, was assessed using a chi square statistics for categorical predictors, such as study site, month, gender or education, and logistic regression for continuous values such as age. Descriptive statistics included means and standard deviations for Cts and exact confidence limits for proportions.
Results
Anti-SFTSV antibody prevalence and seropositive donor profiles
From May to October, 2012, we collected 14,764 donor samples from Xinyang, 1,170 from Mianyang, and 1,350 from Luoyang. Of these 14,752 (Xinyang), 1,130 (Mianyang), and 1,326 (Luoyang) samples were tested for anti-SFTSV antibodies. The exclusion of a small number of samples was due to one of the following: missing data, incomplete donor information, or duplicate samples from the same donor. A total of 139 samples, including 121 samples from Xinyang, 11 from Mianyang, and 7 from Luoyang, were initially reactive on ELISA screening, 86 of which were repeat-reactive and defined as seropositive. The SFTSV seropositive rates among blood donors in these three regions were: Xinyang, 0.54% (80/14,752, 95% CI: 0.42–0.66%); Mianyang, 0.27% (3/1,130, 95%CI: 0.05–0.77%) and Luoyang, 0.28% (3/1326, 95% CI: 0.05–0.78%). These rates were not statistically different (p=0.15). In the epidemic region of Xinyang, there was no month-to-month variation in the seropositive rate. (p=0.22).
Among the 80 seropositive donors from Xinyang, 62.5 %(50/80) were males, most were married (75%, 60/80), the majority had less than high school education (72.5%, 58/80) and 93.75% were younger than 50 years old. The demographic characteristics of the seropositive donors were similar to that of the general donor population in Xinyang. (Table 1). Due to the small number of seropositive donors in Luoyang (n=3) and Mianyang (n=3), we did not perform statistical analysis on the demographics of seropositive donors.
Table.1.
Demographic characteristics of 80 ELISA positive donors from Xinyang
| Positive (ratio, positive/total) |
Negative | Total (samples size:14752) |
||
|---|---|---|---|---|
| Gender(P=0.78; chi square test) | ||||
| Males | 50 (0.53%) | 9,394 | 9,444 | |
| Females | 30 (0.57%) | 5,278 | 5,308 | |
| Marital status(P=0.72; chi square test) | ||||
| Single | 12 (0.44%) | 2,708 | 2,720 | |
| Married | 60 (0.56%) | 10,618 | 10,678 | |
| Missing | 8 (0.59%) | 1,346 | 1,354 | |
| Education(P=0.38; chi square test) | ||||
| Master’s | 2 (1.47%) | 134 | 136 | |
| Bachelor’s | 3 (0.23%) | 1,319 | 1,322 | |
| Associate | 11 (0.44%) | 2,507 | 2,518 | |
| Technician certificate | 5 (0.49%) | 1,019 | 1,024 | |
| High school graduate | 23 (0.55%) | 4,177 | 4,200 | |
| Middle school graduate | 35 (0.65%) | 5,334 | 5,369 | |
| missing | 1 (0.55%) | 182 | 183 | |
| Occupation(P=0.77; chi square test) | ||||
| Student | 4 (0.39%) | 1,025 | 1,029 | |
| Military | 87 | 87 | ||
| Government employee | 3 (0.34%) | 876 | 879 | |
| Health care personnel | 1 (0.64%) | 155 | 156 | |
| Commercial services staff | 15 (0.50% | 3,000 | 3,015 | |
| Factory worker | 3 (0.31%) | 970 | 973 | |
| Farming, fishing, forestry | 4 (0.57%) | 695 | 699 | |
| Working at home | 5 (0.51%) | 981 | 986 | |
| Other | 45 (0.67%) | 6,708 | 6,753 | |
| missing | 175 | 175 | ||
| Age (years old, P=0.84; chi square test) | ||||
| 18–24 | 14 (0.50%) | 2,814 | 2,828 | |
| 25–30 | 11 (0.62%) | 1,766 | 1,777 | |
| 31–40 | 24 (0.56%) | 4,230 | 4,254 | |
| 41–50 | 26 (0.50%) | 5,180 | 5,206 | |
| 51–55 | 5 (0.84%) | 589 | 594 | |
| 56–60 | 93 | 93 | ||
Real-time PCR for SFTSV on seropositive donor samples and seronegative samples from Xinyang
All ELISA positive samples were negative for RNA in individual donation testing. A total of 9,960 Xinyang donor samples (including 56 seropositive and 9904 seronegative samples) were tested in 2,490 pools of four for SFTSV RNA. There were 63 reactive pools of which 51 were reactive in only one of the two duplicate assays and 12 in both duplicates. The Ct for these 63 reactive pools was 40.8±1.9. In contrast, the Cts for the two external positive controls were 34.9±1.52 (reactive on 252 of 252 replicates) and 38.9±1.85 (reactive on 246 of 260 replicates).
When individual samples from the reactive pools were tested in duplicate, only two donor samples were classified as positive (mean Cts of 39.7 and 39.3, respectively) based on our conservative criteria (see methods). The viral load for these two samples was estimated to be lower than 20 pfu/ml. Thus, the overall RNA prevalence rate for SFTSV among donors in Xinyang was 2/9960 or 0.02% (95% CI: >0, 0.07%).
Discussion
In this study, we evaluated nearly 15,000 donations representing more than one third of the number of blood donations collected in 2012 in the SFTSV epidemic region of Xinyang and observed a seropositive rate of 0.54%. In contrast, in Mianyang and Luoyang, two regions with very few reported SFTSV clinical cases, the seroprevalence rates among selected donation samples were 0.27% and 0.28%, respectively. Although the seroprevalence in these two non-epidemic regions was only half of that in the epidemic region of Xinyang, the rates were not statistically different, probably due to the small sample sizes in these two non-epidemic regions.
Higher seroprevalence rates have been reported in other epidemic areas in China when a different ELISA assay (an IgG assay) was used17. Differences in the ELISA assays used and regional SFTSV epidemiological features may have contributed to the differences in rates and illustrate the difficulty in interpreting serologic screening data in the absence of a universally used ELISA kit or a validated confirmatory serologic assay. In this study, we took a relatively conservative approach by defining SFTSV ELISA positive status as being reactive in a screening round of testing as well as reactive on one or duplicate repeat tests. If we had used the same definition used by other Chinese investigators, that is, defining seropositive as ELISA reactive on a single round of testing, our seroprevalence would have been 0.8% in Xinyang, 0.5% in Luoyang, and 1% in Mianyang, comparable to reports based on serosurveys of the healthy general population in other epidemic areas such as Jiangsu Province (0.44%, 11/2510)18and Yiyuan County, Shandong Province (0.8%, n=237)19.
A previous report on 357 laboratory-confirmed SFTS patients in Xinyang 154 Military Hospital from April 2011 to July 2013 indicated more infections in females than males (p=0.011) and a median age of 61 years old among the infected13. A serosurvey in healthy individuals from Jiangsu province did not show a significant difference in age distribution18 but found that farmers were a high-risk population. Most of the donations collected at the Xinyang Blood Center in this study were given by city-dwelling residents with only 4.7% (699/14,752) of donors reporting being a farmer. However, a large proportion of donors (45.8%, 6,753/14752) in Xinyang selected “Other” as their occupation in the donation registration form. Most of them were likely young migrant workers from other areas. Since the age limit of donors prior to 2012 was 18–55 years old, well below the reported median age of SFTSV infected patients, it is not surprising that our data did not detect an association with age.
Out of the 63 reactive pools in our study, most (51/63) were reactive on only one of the two wells in duplicate testing of pools and most reactive results had Cts near the end point of amplification(greater than 40 cycles). Only two seronegative donor samples tested reactive for SFTSV RNA following pool resolution. One possible explanation for pool results not confirming by individual testing is that the Cts in pool testing were at the end point (e.g, high cycle numbers) and were probably due to non-specific amplifications. Another explanation is that some samples had extremely low viral load that would not be consistently detected upon individual donation retesting given our more conservative cycle threshold for determining a positive result. Based on our analytic sensitivity data from dilution panels, the viral loads for the two viremic donor samples were estimated to be lower than 20 pfu/ml, which was near the upper CI (95% CI: 3.2–8.9pfu/ml) for the 95% LOD of the RT PCR assay. We were unable to sequence either of these two samples and it therefore remains possible that the positive PCR results could be due to false positivity.
The two suspected SFTSV RNA positive donors were antibody negative. If these are true positives, this suggests the possibility of transient viremia preceding seroconversion (i.e., window period infection). Given the low viral loads of the suspected viremic donations, it is unclear whether such donations would result in transfusion-transmitted infection. Unfortunately, we were unable to perform any recipient tracing or donor call back studies to further evaluate this possibility.
There is little information on the duration of SFTSV viremia in humans. Cell culture experiments indicate that the exponential growth of SFTSV in primary monocyte-macrophages and Vero cells occurs within 10 hours20. A study in domesticated animals showed that infected dogs had detectable SFTSV RNA on days 8 and 10, but viremia was undetectable on day 1221. Laboratory studies on SFTS among humans reported that SFTSV RNA was detectable at the onset of the illness and for a subsequent 4–7 days; SFTSV antibodies were undetectable when the RT-PCR results were positive22,23. Another study among 986 healthy volunteers in Zhejiang Province also found no viremic persons despite an SFTSV IgG prevalence rate at 7.2%24. Since our study population consisted of healthy donors, viremic individuals with symptoms such as fever should have been excluded at the pre-donation exams. Our ELISA reactive donors were all between the ages of 18 to 55 years old and are presumed to consist of a group of donors who successfully cleared SFTS infection and a group with false positive antibody results. Therefore it is unlikely that any of these donations would result in transfusion transmitted infections.
In conclusion, this was the first investigation of the seroprevalence and rates of viremia of SFTSV among blood donors in China. The low viremia rate in donors from the epidemic Xinyang region suggests limited if any adverse impact of SFTSV on current blood safety. However, the seroprevalence of SFTSV was not insignificant and requires further study. To enable further research it is important that reliable, standardized screening and confirmatory assays automated testing procedures and rigorous testing algorithms are developed. Since the epidemic of SFTSV is still expanding in China and possibly elsewhere in Asia, continued investigations into the routes of SFTSV transmission and rates of SFTSV seropositivity and viremia among donors in epidemic regions are needed. In addition, recipient tracing and look-back studies of recipients of blood from antibody or RNA reactive donors will be important to further evaluate whether this new emerging agent represents a potential threat to blood safety in China as well as globally.
Acknowledgement
The authors acknowledge the Centers for Disease Control (Fort Collins, Co, USA) for providing the cultured SFTSV panel. We also acknowledge Dr. Roger Dodd for his review of the manuscript and Dr. Xutao Deng for his statistical work on the probit analysis. This work was supported by the NHLBI Recipient Epidemiology and Donor Evaluation Study-III (X101222002).
Footnotes
The authors report no conflicts of interest or funding sources.
References
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S, Chai C, Wang C, Amer S, Lv H, He H, Sun J, Lin J. Systematic review of severe fever with thrombocytopenia syndrome:virology, epidemiology, and clinical characteristics. Rev Med Virol. 2014;24:90–102. doi: 10.1002/rmv.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, Senba T, Kaneyuki S, Sakaguchi S, Satoh A, Hosokawa T, Kawabe Y, Kurihara S, Izumikawa K, Kohno S, Azuma T, Suemori K, Yasukawa M, Mizutani T, Omatsu T, Katayama Y, Miyahara M, Ijuin M, Doi K, Okuda M, Umeki K, Saito T, Fukushima K, Nakajima K, Yoshikawa T, Tani H, Fukushi S, Fukuma A, Ogata M, Shimojima M, Nakajima N, Nagata N, Katano H, Fukumoto H, Sato Y, Hasegawa H, Yamagishi T, Oishi K, Kurane I, Morikawa S, Saijo M. The first identification and retrospective study of severe Fever with thrombocytopenia syndrome in Japan. J Infect Dis. 2014;209:816–827. doi: 10.1093/infdis/jit603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013;19:1892–1894. doi: 10.3201/eid1911.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage HM, Godsey MS, Jr, Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding F, Zhang W, Wang L, Hu W, Soares Magalhaes RJ, Sun H, Zhou H, Sha S, Li S, Liu Q, Li Q, Yang W, Huang L, Li C, Yin W. Epidemiologic features of severe fever with thrombocytopenia syndrome in China, 2011–2012. Clin Infect Dis. 2013;56:1682–1683. doi: 10.1093/cid/cit100. [DOI] [PubMed] [Google Scholar]
- 7.Chen HWJ, Liang W. The epidemic survey on first case with novel severe fever with thrombocytopenia bunyavirus in Zhe Jiang province. Journal of International epidemiology and infectious diseases. 2011;38:360. [Google Scholar]
- 8.Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14:763–772. doi: 10.1016/S1473-3099(14)70718-2. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Walker DH, Ren J, Yu XJ. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 2012;12:156–160. doi: 10.1089/vbz.2011.0758. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Wu W, Wang H, Du Y, Liu L, Kang K, Huang X, Ma H, Mu F, Zhang S, Zhao G, Cui N, Zhu BP, You A, Chen H, Liu G, Chen W, Xu B. Human-to-human transmission of severe fever with thrombocytopenia syndrome bunyavirus through contact with infectious blood. J Infect Dis. 2013;207:736–739. doi: 10.1093/infdis/jis748. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Deng B, Zhang J, Cui W, Yao W, Liu P. Person-to-person asymptomatic infection of severe fever with thrombocytopenia syndrome virus through blood contact. Intern Med. 2014;53:903–906. doi: 10.2169/internalmedicine.53.1164. [DOI] [PubMed] [Google Scholar]
- 12.MOH of the people’s republic of China: Guide for severe fever with thrombocytopenia bunyavirus prevention and control. 2010 http://www.moh.gov.cn/mohwsyjbgs/s8348/201010/49272.shtml.
- 13.Cui N, Bao XL, Yang ZD, Lu QB, Hu CY, Wang LY, Wang BJ, Wang HY, Liu K, Yuan C, Fan XJ, Wang Z, Zhang L, Zhang XA, Hu LP, Liu W, Cao WC. Clinical progression and predictors of death in patients with severe fever with thrombocytopenia syndrome in China. J Clin Virol. 2014;59:12–17. doi: 10.1016/j.jcv.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Jiao Y, Zeng X, Guo X, Qi X, Zhang X, Shi Z, Zhou M, Bao C, Zhang W, Xu Y, Wang H. Preparation and evaluation of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. J Clin Microbiol. 2012;50:372–377. doi: 10.1128/JCM.01319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloch EM, Lee TH, Krause PJ, Telford SR, 3rd, Montalvo L, Chafets D, Usmani-Brown S, Lepore TJ, Busch MP. Development of a real-time polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion. 2013;53:2299–2306. doi: 10.1111/trf.12098. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Liang M, Qu J, Jin C, Zhang Q, Li J, Jiang X, Wang Q, Lu J, Gu W, Zhang S, Li C, Wang X, Zhan F, Yao W, Bi Z, Wang S, Li D. Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J Clin Virol. 2012;53:48–53. doi: 10.1016/j.jcv.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 17.Cui F, Cao HX, Wang L, Zhang SF, Ding SJ, Yu XJ, Yu H. Clinical and epidemiological study on severe fever with thrombocytopenia syndrome in Yiyuan County, Shandong Province, China. Am J Trop Med Hyg. 2013;88:510–512. doi: 10.4269/ajtmh.11-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang S, Bao C, Zhou M, Hu J, Tang F, Guo X, Jiao Y, Zhang W, Luo P, Li L, Zhu K, Tan W, Lu Q, Ge H, Chen A. Seroprevalence and risk factors for severe fever with thrombocytopenia syndrome virus infection in Jiangsu Province, China, 2011. Am J Trop Med Hyg. 2014;90:256–259. doi: 10.4269/ajtmh.13-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Zhai S, Wen H, Cui F, Chi Y, Wang L, Xue F, Wang Q, Wang Z, Zhang S, Song Y, Du J, Yu XJ. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg Infect Dis. 2012;18:963–965. doi: 10.3201/eid1806.111345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu B, Qi X, Wu X, Liang M, Li C, Cardona CJ, Xu W, Tang F, Li Z, Wu B, Powell K, Wegner M, Li D, Xing Z. Suppression of the interferon and NF-kappaB responses by severe fever with thrombocytopenia syndrome virus. J Virol. 2012;86:8388–8401. doi: 10.1128/JVI.00612-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu G, Li J, Liang M, Jiang X, Jiang M, Yin H, Wang Z, Li C, Zhang Q, Jin C, Wang X, Ding S, Xing Z, Wang S, Bi Z, Li D. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis. 2013;19:756–763. doi: 10.3201/eid1905.120245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Chai C, Lv H, Lin J, Wang C, Chen E, Zhang Y, Chen Z, Liu S, Gong Z, Jiang J. Epidemiological characteristics of severe fever with thrombocytopenia syndrome in Zhejiang Province, China. Int J Infect Dis. 2014 doi: 10.1016/j.ijid.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Wen HL, Zhao L, Zhai S, Chi Y, Cui F, Wang D, Wang L, Wang Z, Wang Q, Zhang S, Liu Y, Yu H, Yu XJ. Severe fever with thrombocytopenia syndrome, Shandong Province, China, 2011. Emerg Infect Dis. 2014;20:1–5. doi: 10.3201/eid2001.120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang LSJ, Yan J, Lv H, Chai C, Sun Y, Shao Bin, Jiang Jianmin, Chen Zhiping, Zhang Yanjun. Antibodies against severe fever with thrombocytopenia syndrome virus in healthy persons, China, 2013. [Cited on 7/8/2014];Emerg Infect Dis. 2014 Aug; doi: 10.3201/eid2008.131796. http://dx.doi.org/10.3201/eid2008.131796. [DOI] [PMC free article] [PubMed] [Google Scholar]