Abstract
Background
There are no effective treatments that slow the progression of neurodegenerative diseases. A major challenge of treatment in neurodegenerative diseases is appropriate delivery of pharmaceuticals into the cerebrospinal fluid (CSF) of affected individuals. Mesenchymal stromal cells (MSCs – either naïve or modified) are a promising therapy in neurodegenerative diseases and may be delivered directly into the CSF where they can reside for months. In this preclinical study, we evaluated the safety of intrathecal autologous MSCs in a rabbit model.
Methods
Autologous adipose-derived MSCs (or a-CSF) were delivered intrathecally, either with single or repeated injections into the foramen magnum of healthy rabbits, and monitored for 4 and 12 weeks, respectively.
Results
Rabbits tolerated injections well and no definitive MSC-related side effects were observed apart from three rabbits that had delayed death secondary to traumatic foramen magnum puncture. Functional assessments and body weights were equivalent between groups. Gross pathology and histology did not reveal any abnormalities or tumor growth. Complete blood count (CBC) data were normal and there were no differences in CSF IL-6 levels in all groups tested.
Discussion
Our data suggest that intrathecal delivery of autologous MSCs is safe in a rabbit model. Data from this study has supported two successful Investigational New Drug (IND) applications to the FDA, resulting in the initiation of two clinical trials using autologous MSCs in amyotrophic lateral sclerosis and multiple system atrophy.
Keywords: Amyotrophic lateral sclerosis, cell therapy, intrathecal injection, multiple system atrophy, mesenchymal stromal cell, MSC, rabbit, safety study
Introduction
Neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS) and multiple system atrophy cause significant morbidity and mortality and are increasing in incidence due to the aging population. There are no effective therapies that alter the inexorable neuronal loss in these devastating diseases. Autologous mesenchymal stromal cells (MSCs, alternatively referred to as mesenchymal stem cells) shows promise as a therapy in neurodegenerative diseases and may be delivered intrathecally, therefore bypassing the blood-brain barrier. As part of the translation process to treat human disease, regulatory agencies such as the FDA have required animal safety studies as described here that provide insight into the systemic response to intrathecal autologous MSCs.
Proposed mechanisms of action for unmodified MSCs are either growth factor production or modulation of the immune system.1–3 These mechanisms may explain their demonstrated benefit in animal models of ALS 4, multiple system atrophy5 multiple sclerosis 6,7, Parkinson’s disease 8, and stroke 9. Furthermore, MSCs may be modified to produce pharmaceuticals (e.g. growth factors), thus transforming them into excellent candidates for a cell-based drug delivery system that bypasses the blood-brain barrier 10,11.
While most studies of MSCs in the central nervous system have not focused on safety, available reports suggest there are no major side effects. In various animal models, groups have demonstrated that MSCs may safely be injected into the spinal cord and brain of normal or injured animals including mice 12, rats 13, and rhesus monkeys 14. It has been shown that these cells may survive and reside in the cord for up to 3 months after injection, and furthermore can migrate to the site of spinal cord injury 15–17.
Studies of MSCs in human nervous system diseases are limited. Of note, intrathecal injection of autologous bone marrow-derived MSCs demonstrated safety in 25 patients with either ALS or multiple sclerosis 18. A combined intrathecal and intravenous approach with autologous MSCs has provided safety data and initial efficacy in MSA 19. MSCs or other stem cell types have been injected directly into the spinal cord of ALS patients with favorable safety reports 19–21. Overall, these reports provide preliminary evidence of safe applications of MSC delivery into humans.
We have been developing intrathecal delivery of adipose-derived autologous MSCs as an experimental treatment for neurological degenerative diseases, and have active INDs and IRB-approved studies in amyotrophic lateral sclerosis (IND 14788) and multiple system atrophy (IND 15176). As part of the IND process, we had close discussions with the FDA who required demonstration of safety in an animal model. We chose to study rabbits because when compared to rats or mice, rabbits are phylogenetically, anatomically and physiologically more analogous to humans. They are also large enough to monitor and assess physiological changes after injury 22. We now report rabbit adipose provides a source of MSCs that can be isolated, expanded ex vivo, and safely injected back into the subarachnoid space.
Materials and Methods
Animals
All animal experiments were carried out according to the guidelines approved by Mayo Clinic Institutional Animal Care and Use Committee (IACUC). Rabbits were housed according to National Institutes of Health (NIH) and U. S. Department of Agriculture guidelines. All rabbits were held on a 12-hour light-dark cycle on a standard regimen, with food and water ad libitum in conventional housing. Forty-six New Zealand White rabbits (Harlan, Charles River Laboratories, Wilmington, Massachusetts; Harlan Laboratories, Indianapolis, Indiana), both male and female weighing between 2–3kg, aged 3–6 months, were used in this study. The rabbits were kept for 4 (single cell injection group) and 12 weeks (single and repeated cell injection groups), respectively and then euthanized. All rabbits were cared for with availability of experienced veterinarians and researchers.
Primary Culture of Rabbit Mesenchymal Stromal Cells
For adipose tissue harvesting, rabbits were first anesthetized with an intramuscular injection of ketamine (35 mg/kg), xylazine (5 mg/kg) and acepromazine (2.3 mg/kg) 23. Next, the back of animal was shaved and scrubbed with Betadine. Using a #10 scalpel, a 2–3 cm incision through the skin was made in the dorso-medial line of rostro-dorsal region, and a 1X3 cm sample of adipose tissue was excised from the adipose panicles. The skin was sutured with 3.0 vicryl 24.
After harvesting, the adipose tissue was fragmented and cut into small pieces, washed in PBS supplemented with penicillin and streptomycin (1%), and transferred into sterile PBS (Invitrogen). The tissue was washed by centrifugation three times, at 260g for 10 minutes, before being transferred to an open 10cm petri dish in a lamina flow hood. Any discolored tissue and excess vasculature were removed and the tissue diced with a combination of razor blades and scissors. The tissue was re-suspended in 0.075% Collagenase (Worthington Biochemical Corporation) in Hank’s buffer and returned to a 50ml falcon centrifuge tube, and incubated at a 37°C water bath. The tube was inverted every 5 minutes for a period of 45 – 60 minutes, or until the contents of the tube appeared uniformly cloudy. The digested tissue increased to a volume of 50ml with media containing Advanced MEM low glucose (Invitrogen), 5% human platelet lysate (Mayo blood bank), 2 Units per ml Heparin (APP Pharmaceuticals LLC, IL), and 2mM GlutaMAX (Invitrogen), and then centrifuged at 1100g for 10 minutes. The supernatant was removed and the pellet was re-suspended in 10ml ACK (Ammonium-Chloride-Potassium (K)) buffer (Mayo Blood Bank) and incubated at room temperature for 5 –10 minutes before being passed step wise through a 70µm and 40µm filters. A small sample was counted on a hemocytometer in the presence of trypan blue (Sigma) to confirm viability. Cells were plated on either a 75 or 175cm3 tissue culture flask (Falcon) and the media was changed every other day. Flasks of cells were split 1:3 upon reaching 80–90% confluency using 0.25% Trypsin EDTA. At one week, 1× 107 cells were suspended in 200µl of artificial CSF (a-CSF, 119 mM NaCl, 26.2 mM NaHCO3, 2.5 mM KCl, 1 mM NaH2PO4, 1.3 mM MgCl2, 10 mM glucose) for injection. The remaining cell culture was store frozen in FCS (fetal calf serum, Invitrogen) 10% DMSO for future cell injections and characterization 24.
Intrathecal Injection of MSCs
Rabbits were anesthetized as described above, and a 25-gauge needle was aseptically inserted into the cisterna magna between the base of the skull and the first vertebrae 25. 200 µl of CSF was taken and replaced with 200 µl of 1 × 107 autologous MSCs in a-CSF for test groups, or just 200µl of a-CSF for control groups. The animals remained under general anesthesia throughout the procedure and were moved to a recovery area to recover from anesthesia after 30 minutes in the head-down position. The animals were housed and observed for up to 4 or 12 weeks and then euthanized. In human studies, we plan to inject 1–10 × 107 MSCs into the ~125mL total CSF volume in humans. Given that rabbits have ~5mL total CSF volume, the 1 × 107 rabbit MSCs injected correspond to a dose that is 2.5-fold higher relative to the projected maximum human dose.
Study Design
The groups were designed as follows: for the 4-week study: one cisternal injection was performed in either control rabbits (n=7; 200 µl of a-CSF) or experimental rabbits (n=9; 1 × 107 MSC in 200µl a-CSF) and then followed longitudinally for 4 weeks. For the 12-week study, control rabbits (n=10) were injected with 200 µl a-CSF, while single-injection rabbits (n=10) were injected with 200 µl a-CSF containing 1 × 107 MSCs once at the beginning of the study, and repeated-injection rabbits (n=10) were injected with 200 µl a-CSF containing 1 × 107 MSCs three times at week 0 (W0), week 4 (W4) and week 8 (W8).
Post-operative Functional and Behavioral Assessments
Baytril (5mg/kg) and Buprenex (25mg/kg) were given intramuscularly daily for the first week after surgery. Veterinarians and veterinary technicians recorded and maintained regular post-operative monitoring records as per departmental procedure. Animals were observed daily by study staff and any complications or deficits were discussed with Veterinary Medicine. Detailed functional and behavioral assessments (as reported in Results section) were conducted on a daily basis for the first week after surgery/injection, and then on a weekly basis throughout the rest of the study period.
Complete Blood Count (CBC) and IL-6 in CSF
Blood and CSF were taken before surgery (W0), at W2, and W4 for 4-week study groups, while before surgery (W0), at W4, W8 and W12 for 12-week study groups. 500µl blood was drawn from marginal ear vein of the rabbits for hematology tests. Blood samples were analyzed for CBC by using VetScan HM2 hematology system (Abaxis, Union City, CA). 200µl CSF was taken by cisternal puncture 25, which in selected cases was used to measure levels of IL-6 by using Microplate Spectrophotometer (SpectraMax 340PC, Molecular Devices, Sunnyvale, California).
Organ Weight and Histopathological Studies
At the end of the experiment, animals were euthanized with intraperitoneal injection of pentobarbital (65 mg/kg) and transcardially-perfused with 4% paraformaldehyde. All vital organs including heart, liver, spleen, lung, kidneys, brain and spinal cord were dissected, examined, weighed, photographed, fixed and preserved in 10% formalin at 4°C. A photographic image of each organ was taken for all rabbits. Weights were compared between groups as actual weights and percentage of total body weight. Ratios of organ to body weight were calculated for each rabbit. Macroscopic examination was conducted for each organ by 2 researchers. All vital organs, including brain (cortex, midbrain, cerebellum, brainstem) and spinal cord (cervical, thoracic, lumbar and sacral levels) were processed, embedded in paraffin, sectioned to obtain 10 um thick and stained with hematoxylin and eosin (and in some cases Mas-Trichrome) to examine both macro- and microscopic changes 26.
Results
Animal Survival
Of the 54 animals that were enrolled, 46 survived for the duration of the study. In 4W-study groups, 3 out of 19 rabbits died while on study with a mortality of 15.7% that was within our expected mortality rate of 20%. One rabbit died due to anesthesia, and the other two died during the surgical procedures. In 12W-study groups, 5 out of 35 rabbits died while on study with a mortality of 14.3% that was within our expected mortality rate of 20%. Two of the rabbits died during surgical procedures; one at adipose harvesting and one during the first cell injection. These were both thought to be due to anesthesia/airway complications. The remaining three animals were euthanized because of weight loss and failure to thrive at various intervals after surgical injection. These three animals are not included in our analyses, and are discussed in detail below (see Delayed Complication of Cisternal Injection section).
Functional and Behavioral Assessment
Assessments were performed for body weight, rectal temperature, general health condition, wound condition, movement (walking, hopping, and rearing), stimulus response (click, touch approach and pupil response), and pain behaviors (consciousness, chewing, hair pulling). Numerical or scaled data from functional or behavioral studies were analyzed using non-parametric analysis of variance (Kruskal-Wallis) comparing control, single-dose and multidose groups at each time-point and sequentially within groups. Binary data (e.g. presence or absence of hearing response, blink response, etc) were analyzed using a contingency analysis. All functional assessments did not show statistical differences between the experimental and control groups.
Complete Blood Counts (CBC) and IL-6 Levels
Blood studies that were performed were: total white blood cell (WBC), lymphocyte (LYM), monocyte (MON), granulocyte (GRA), lymphocyte percentage (LYM%), monocyte percentage (MON%), granulocyte percentage (GRA%), total red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet (PLT), platelet percentage (PCT), mean platelet volume (MPV), and platelet distribution width (PDW). All blood studies were within normal limits, and there were no statistically significant differences between control and MSC-treated groups.
Four rabbits from each group within the 12W experiment were selected and levels of CSF IL-6 were measured by using Rabbit Interleukin 6 (IL-6) ELISA Kit (Cusabio Biotech Co., LTD). All samples tested were less than the lowest standard (16pg/mL)
Organ and Pathology Studies
Organ weights were compared between groups as actual weights and percentage of total body weight. There were no abnormalities and no significant differences in the body weights and the ratio of organ to body weight between controls and MSC-treated groups (ANOVA p>0.05).
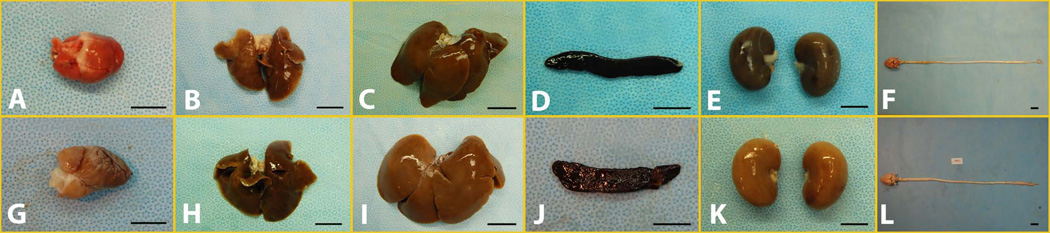
Gross and macroscopic examinations did not show any meaningful difference between the control and MSC-treated groups of 4-week study (Fig 1A–F) and 12-week study (Fig 1G–L). Detailed microscopic examination did not reveal histological deviations in any of the vital organs (including brain and spinal cord) among all groups (Fig 2A–L).
Figure 1.
Gross pictures of major organs, i.e. heart (A), lung (B), liver (C), spleen (D), kidney (E), spinal cord and brain (F), from single injection for 4-week study; and heart (G), lung (H), liver (I), spleen (J), kidney (K), spinal cord and brain (L), from repeated injection for 12-week study. Scale Bar: 2 cm
Figure 2.
Microscopic observation of major organs, i. e. heart (A), lung (B), liver (C), spleen (D), kidney (E), spinal cord and brain (F), from single injection for 4-week study; and heart (G), lung (H), liver (I), spleen (J), kidney (K), spinal cord and brain (L), from repeated injection for 12-week study.
Delayed Complications of Cisternal Injection
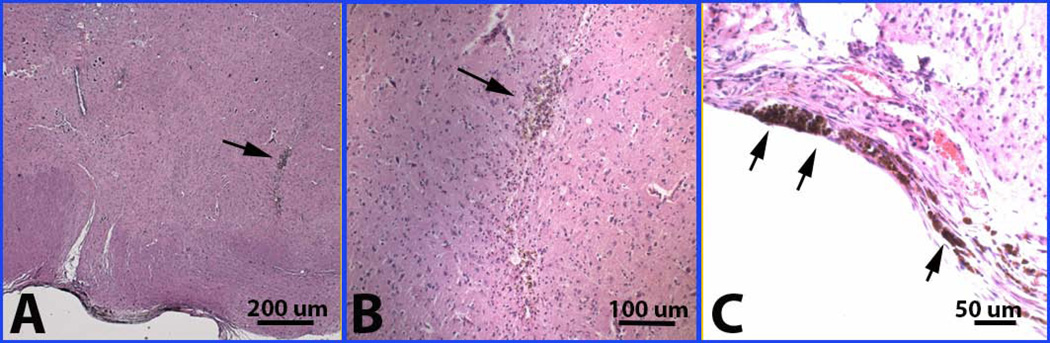
Three animals were euthanized because of weight loss and failure to thrive at various intervals after surgical injection. All major organs, including heart, lungs, kidneys, spleen and liver were normal in the sick rabbits. In the sick rabbits, the main autopsy results of brain and spinal cord examination were: 1) Needle puncture injury: there was a clear-cut antemortem needle puncture with local recent bleeding at the cranio-cervical junction (Fig 3A and 3B). This was felt to represent an inadvertent needle stick at the time of surgical injection; 2) A collection of fibroblast-like cells near encircling the dorsal subdural space adjacent to the needle puncture injury; 3) Bacterial pneumonia - as immediate cause of pre-mortem weight loss and failure to thrive, possibly due to brain stem injury from the needle stick.
Figure 3.
Needle stick as shown with arrow (A) at low power and (B) at higher power, and hemosiderin in meninges (C) in a few animals in both 4-week and 12-week studies.
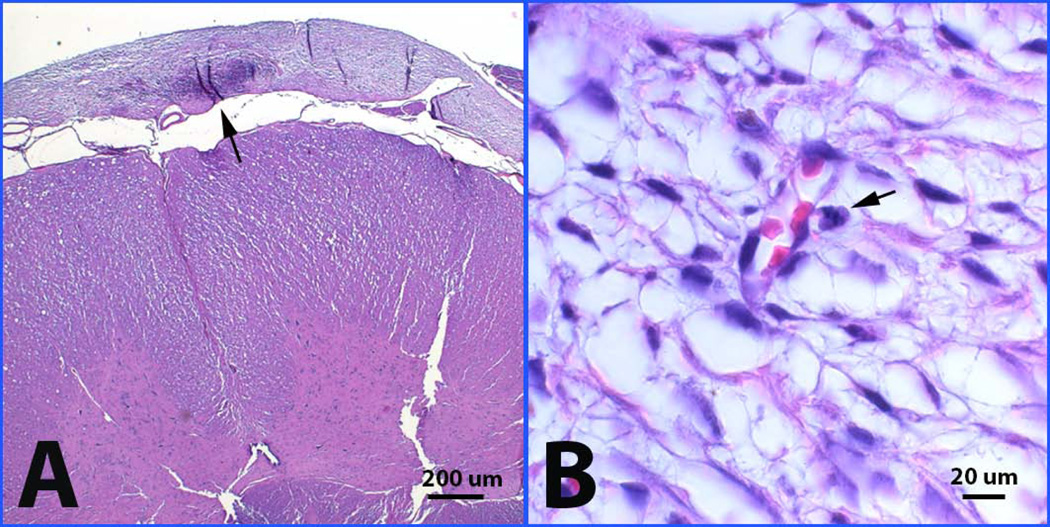
Detailed histopathology of the needle puncture site revealed that the puncture injuries at the upper cervical and cranio-cervical junction level penetrated deep into the spinal cord parenchyma (two thirds of the way through the cord). Hemosiderin-laden macrophages lined the cavity of the needle track and were scattered over the meningeal surfaces of the spinal cord and brain stem. The collection of cells in the subdural space of the puncture site encircled the dorsal aspect of the craniocervical junction. The cells had the appearance of well-differentiated, loosely packed stromal cells or fibroblasts. There were capillaries within the cell collection and signs of recent hemorrhage (red cells interspersed between stromal cells with leaking into the surrounding CSF space) and past hemorrhage (hemosiderin-laden macrophages) (Fig 3C). Abnormal stromal cell accumulation on the edge of the cervical cord was found in two of the cases (Fig 4A). There were no inflammatory infiltrates in the surrounding dural or spinal cord tissue and no evidence of compression or CSF obstruction. Mitotic index of the stromal cells within the cell collection was very low (<2%) with no signs of malignant transformation (Fig 4B). Tissue was stained with human specific cell markers but the level of background staining was too high to allow interpretation. Our final interpretation was that the immediate cause of weight loss and failure to thrive was probably due to hemorrhage within the cell collection with leaking of blood into the CSF, leading to bacterial pneumonia. Furthermore, the cell collection at the site of injury may have represented a MSC collection, as it is known that MSCs migrate to sites of injury.
Figure 4.
Abnormal cell accumulation on the edge of the cervical cord (A) and mitotic change (B) as shown with arrows in one of animals in 12-week study.
Discussion
In our current study we have demonstrated that repeated intrathecal administration of 1 × 107 autologous MSCs into the rabbit cisterna magna is without any demonstrable side effects on multiple tests of general health, function and behavior. We chose this study protocol after close discussion with the FDA in order to mirror our planned human phase I safety studies in ALS and MSA. The amount of MSCs administered into the rabbit was 2.5-fold higher than for our human studies, when estimating equivalent CSF volumes between rabbits and humans. This data was successfully utilized as animal safety data for human INDs at the FDA (INDs 14788 and 15176).
While a concerning risk related to MSCs has been tumor formation, we did not find any evidence of this in our study. MSCs have been reported to have a tumorigenic potential in in vitro and in vivo experiments within long term expanded cell populations 27–29. MSCs were also reported to induce tumor transformation of surrounding cells if loaded into bio-scaffold, possibly inducing a tumor niche 30. This risk will need to be continually monitored in human clinical trials.
There are many unanswered questions regarding MSC therapy in humans. First, it is still unclear what the best route of delivery is: intravenous 18,31, intraarterial 9,32, intrathecal 18,33, intraspinal 20, or a combination of these. To date, studies suggest that each of these routes of administration is safe. Therefore the route of administration will need to be tailored to the specific medical indication. In the case of neurological disease, we argue that intrathecal approach may be most effective. Intrathecal therapy brings along the luxury of bypassing the blood-brain barrier. Furthermore, intrathecal MSC injection offers an important advantage to intraspinal injection in that it reduced the risk of neurological damage. Animal studies have demonstrated that intrathecal injection of stromal cells lead to dispersion throughout the cerebrospinal fluid and the spinal cord surface with some migration into spinal cord lesions 15,34.
Another unresolved issue is how many injections will be necessary to provide a therapeutic benefit. A recent study on stem cells demonstrated the greatest therapeutic benefit in a model of middle cerebral artery occlusion in rats when a one-time injection of high dose of cells was conducted, rather than repeated injection of small dose of cells over several time points 35. However, in a study of clinical and pathological effects of intrathecal injection of MSC-derived neural progenitors in an experimental model of multiple sclerosis, the data suggested that multiple dosing of intrathecally injected MSCs correlated with the enhanced outcome compared to a single dose injection of MSCs 36. Another study demonstrated that single injection of hMSCs was unable to exhibit beneficial effects in superoxide dismutase 1 (SOD1) mice. Repeated injection of hMSCs has reduced weight loss, improved motor performance, reduced motor neuron loss, and more importantly, increased survival in SOD1 transgenic mice 37. Further work clearly is needed in this area.
Despite the obstacles that remain for MSC therapy, a multitude of putative benefits of MSCs continue to be discovered. MSCs are hypo-immune and usually do not elicit allogeneic rejections. Furthermore, MSCs have been shown to possess a wide array of characteristics that make them immunosuppressive. Immunologically, MSCs have been shown to be involved with toll like receptors (TLR), dendritic cells, B and T cells, and T-regulatory cells (Treg) to become immunosuppressive, and thereby greatly minimizing the risks associated with allogenic rejection 38–42. After engraftment, MSCs have shown to direct host cellular machinery towards angiogenesis, neurogenesis, and synaptogenesis. Investigators have also shown injected MSCs enter injured tissues where they alleviate damage, likely due to increased production of growth factors from MSCs and host tissues 43–45.
Pre-clinical MSC studies have begun to transition into human clinical trials 46,47. Ongoing clinical trials and investigations involving MSCs have reached into the realms of ischemic stroke, multiple sclerosis, acute leukemia, graft-versus-host disease, critical limb ischemia, articular cartilage and bone defects, and many others. Recent MSC advances in cardiac repair and bone disorders have shown great promise, but significant obstacles still stand with inconclusive engraftment and lack of standardization of cell characterization 47,48.
There is growing evidence that MSCs may provide therapeutic benefit in neurodegenerative diseases. Several studies have been published suggesting promise in intrathecal injections of MSCs as a possible remedy for ALS 18,49–51. MSC therapy in MSA has also been studied, and there are favorable results from early studies 19. Currently, most human MSC studies use naïve autologous MSCs, but there are developing technologies that modify MSCs to produce growth factors. Using MSCs as a primary vehicle, current clinical trials for MSC injection are showing great promise for treatment of a wide variety of neurodegenerative disorders 47.
In conclusion, intrathecal delivery of autologous MSCs into CSF in rabbits appeared to be safe and did not cause tumor development in rabbits. MSC injection may potentially act as a therapeutic delivery platform for neural growth or protective factors in the treatment for neurodegenerative diseases such as ALS and multiple system atrophy.
Acknowledgement
We thank Jane Meyer for her administrative role in manuscript preparation and Fausto Rodriguez for assistance with neuropathology. This work was supported by a grant from the National Institutes of Health (UL1 TR000135 and K08 169443), the Judith and Jean Pape Adams Charitable Foundation, and the Schmidt, Shannon, and Mayo Foundations.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ANOVA
analysis of variants
- CSF
cerebrospinal fluid
- DMSO
dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- FDA
Food and Drug Administration
- hMSCs
human mesenchymal stem cells
- IACUC
Institutional Animal Care and Use Committee
- IND
investigational new drug
- MEM
minimal essential medium
- MSA
multiple system atrophy
- MSC
mesenchymal stem cell
- PBS
phosphate buffered saline
- TLR
toll-like receptors
Footnotes
Conflicts of Interest:
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to Transfusion.
References
- 1.Rafei M, Campeau PM, Aguilar-Mahecha A, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 2.Prigione I, Benvenuto F, Bocca P, et al. Reciprocal Interactions Between Human Mesenchymal Stem Cells and gamma delta T Cells Or Invariant Natural Killer T Cells. Stem Cells. 2009;27:693–702. doi: 10.1634/stemcells.2008-0687. [DOI] [PubMed] [Google Scholar]
- 3.Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vercelli A, Mereuta OM, Garbossa D, et al. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31:395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Stemberger S, Jamnig A, Stefanova N, et al. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS ONE. 2011;6:e19808. doi: 10.1371/journal.pone.0019808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 7.Gerdoni E, Gallo B, Casazza S, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 8.Park HJ, Lee PH, Bang OY, et al. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem. 2008;107:141–151. doi: 10.1111/j.1471-4159.2008.05589.x. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita T, Kibayashi T, Katayama T, et al. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci Lett. 2011;502:41–45. doi: 10.1016/j.neulet.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Eckert MA, Riazifar H, et al. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cell Int. 2013;2013:435093. doi: 10.1155/2013/435093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono K, Yoshihara K, Suzuki H, et al. Preservation of hematopoietic properties in transplanted bone marrow cells in the brain. J Neurosci Res. 2003;72:503–507. doi: 10.1002/jnr.10588. [DOI] [PubMed] [Google Scholar]
- 13.Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng YB, Liu XG, Liu ZG, et al. Implantation of BM mesenchymal stem cells into injured spinal cord elicits de novo neurogenesis and functional recovery: evidence from a study in rhesus monkeys. Cytotherapy. 2006;8:210–214. doi: 10.1080/14653240600760808. [DOI] [PubMed] [Google Scholar]
- 15.Ohta M, Suzuki Y, Noda T, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Nishida K, Tanaka N, Nakanishi K, et al. Magnetic targeting of bone marrow stromal cells into spinal cord: through cerebrospinal fluid. Neuroreport. 2006;17:1269–1272. doi: 10.1097/01.wnr.0000227993.07799.a2. [DOI] [PubMed] [Google Scholar]
- 17.Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine. 2004;29:1971–1979. doi: 10.1097/01.brs.0000138273.02820.0a. [DOI] [PubMed] [Google Scholar]
- 18.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee PH, Lee JE, Kim HS, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- 20.Mazzini L, Ferrero I, Luparello V, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial. Exp Neurol. 2010;223:229–237. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Glass JD, Boulis NM, Johe K, et al. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- 22.Minguell JJ, Pereira A, Bartholomew P, et al. The intrathecal infusion of mesenchymal stem cells into healthy rabbits is safe and devoid of neurological or clinical complications. J Stem Cell Res Ther. 2011;2 [Google Scholar]
- 23.Santhanam AV, Smith LA, Katusic ZS. Brain-derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke. 2010;41:350–356. doi: 10.1161/STROKEAHA.109.564492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzetti MP, Oliveira IS, Miranda-Ferreira R, et al. Qualitative and quantitative analysis of rabbit's fat mesenchymal stem cells. Acta Cir Bras. 2010;25:24–27. doi: 10.1590/s0102-86502010000100007. [DOI] [PubMed] [Google Scholar]
- 25.Santhanam AV, Smith LA, He T, et al. Endothelial progenitor cells stimulate cerebrovascular production of prostacyclin by paracrine activation of cyclooxygenase-2. Circ Res. 2007;100:1379–1388. doi: 10.1161/01.RES.0000265848.55035.5d. [DOI] [PubMed] [Google Scholar]
- 26.Mamatha SS, Muthukumar SP, Venkateswaran G. Safety evaluation of Mucor rouxii CFR-G15 biomass containing omega-6 fatty acids in rats. Regul Toxicol Pharmacol. 2012;62:183–190. doi: 10.1016/j.yrtph.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Aguilar S, Nye E, Chan J, et al. Murine but Not Human Mesenchymal Stem Cells Generate Osteosarcoma-Like Lesions in the Lung. Stem Cells. 2007;25:1586–1594. doi: 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerlin L, Donnenberg AD, Rubin JP, et al. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 30.Tasso R, Augello A, Carida M, et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009;30:150–157. doi: 10.1093/carcin/bgn234. [DOI] [PubMed] [Google Scholar]
- 31.Osaka M, Honmou O, Murakami T, et al. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343:226–235. doi: 10.1016/j.brainres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Osanai T, Kuroda S, Sugiyama T, et al. Therapeutic effects of intra-arterial delivery of bone marrow stromal cells in traumatic brain injury of rats--in vivo cell tracking study by near-infrared fluorescence imaging. Neurosurgery. 2012;70:435–444. doi: 10.1227/NEU.0b013e318230a795. discussion 444. [DOI] [PubMed] [Google Scholar]
- 33.Kishk NA, Gabr H, Hamdy S, et al. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 2010;24:702–708. doi: 10.1177/1545968310369801. [DOI] [PubMed] [Google Scholar]
- 34.Shi E, Kazui T, Jiang X, et al. Intrathecal injection of bone marrow stromal cells attenuates neurologic injury after spinal cord ischemia. Ann Thorac Surg. 2006;81:2227–2233. doi: 10.1016/j.athoracsur.2005.12.056. discussion 2233–2224. [DOI] [PubMed] [Google Scholar]
- 35.Omori Y, Honmou O, Harada K, et al. Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats. Brain Res. 2008;1236:30–38. doi: 10.1016/j.brainres.2008.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris VK, Yan QJ, Vyshkina T, et al. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J Neurol Sci. 2012;313:167–177. doi: 10.1016/j.jns.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C, Zhou C, Teng JJ, et al. Multiple administrations of human marrow stromal cells through cerebrospinal fluid prolong survival in a transgenic mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2009;11:299–306. doi: 10.1080/14653240902806986. [DOI] [PubMed] [Google Scholar]
- 38.Hass R, Kasper C, Bohm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21:1641–1655. doi: 10.1089/hum.2010.156. [DOI] [PubMed] [Google Scholar]
- 40.English K, Mahon BP. Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem. 2011;112:1963–1968. doi: 10.1002/jcb.23119. [DOI] [PubMed] [Google Scholar]
- 41.Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res Ther. 2010;1:34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bifari F, Pacelli L, Krampera M. Immunological properties of embryonic and adult stem cells. World J Stem Cells. 2010;2:50–60. doi: 10.4252/wjsc.v2.i3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Togel F, Weiss K, Yang Y, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 44.Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 45.Crisostomo PR, Wang Y, Markel TA, et al. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 46.Kim YJ, Park HJ, Lee G, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- 47.Joyce N, Annett G, Wirthlin L, et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trounson A, Thakar RG, Lomax G, et al. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vercelli A, Mereuta OM, Garbossa D, et al. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31:395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Morita E, Watanabe Y, Ishimoto M, et al. A novel cell transplantation protocol and its application to an ALS mouse model. Exp Neurol. 2008;213:431–438. doi: 10.1016/j.expneurol.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Kim H, Kim HY, Choi MR, et al. Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett. 2010;468:190–194. doi: 10.1016/j.neulet.2009.10.074. [DOI] [PubMed] [Google Scholar]