Abstract
Malignant mesothelioma (MM) is a highly invasive and chemoresistant malignancy induced by asbestos fibers. NK4, a HGF antagonist and angiogenesis inhibitor, consists of the N-terminal hairpin domain and four kringle domains of the α-chain of HGF. The therapeutic potential of NK4 has been demonstrated in a variety of tumor types. However, the mechanisms by which NK4 inhibits tumor growth have not been well delineated. In this study, we show that the NK4 adenovirus (Ad-NK4) potently inhibits cell viability, invasiveness, and tumorigenicity of human MM cells. Significantly, we demonstrate for the first time that Ad-NK4 inhibits cancer stem-like cell (CSC) properties as assessed by spheroid formation assay, side population analysis, and flow cytometric sorting of CD24 cells. In addition to inhibiting phosphorylation of Met and AKT, Ad-NK4 markedly suppressed the active form of β-catenin, a key mediator of both Wnt and AKT pathways. We further demonstrate that expression of NK4 suppresses β-catenin nuclear localization and transcriptional activity. Intriguingly, the expression levels of Oct4 and Myc, two critical stem cell factors and downstream targets of β-catenin, were also diminished by Ad-NK4. Furthermore, the strong antitumor effect of NK4 was found to be linked to its ability to inhibit CSCs as revealed by immunohistochemical examination of tumor specimens from a mouse xenograft model of human MM. These findings suggest that NK4 acts as a CSC inhibitor by impeding Met/AKT/β-catenin signaling and holds promise for achieving durable therapeutic responses in MM by constraining the CSC component of these aggressive tumors.
Keywords: malignant mesothelioma, cancer stem-like cells, HGF/Met, NK4, therapy
Hepatocyte growth factor (HGF), also known as scatter factor, was originally identified as a potent hepatocyte mitogen, but subsequent studies revealed that HGF is a multifunctional growth factor that can induce various cellular responses including cell growth, invasion, survival, and morphogenesis. The pleiotropic activities of HGF are mediated through its receptor, a transmembrane tyrosine kinase encoded by the proto-oncogene c-Met. Both HGF and Met are expressed in numerous tissues, although their expression is restricted predominately to cells of mesenchymal and epithelial origin, respectively 1, 2. HGF is synthesized as an inactive single chain precursor molecule that is activated by proteolytic cleavage into disulfide-linked 69-kDa α- and 34-kDa β-chains of the mature form of HGF. The α-chain consists of an N-terminal domain followed by four kringle modules and mediates the binding of HGF to the Met receptor, while the β-chain contains a serine protease-like domain 3.
NK4 is a specific HGF antagonist 4 composed of the N-terminal hairpin domain and four kringle domains of the α-chain of HGF. NK4 exhibits high affinity binding to Met, but does not induce its tyrosine phosphorylation or any biological function. Instead, NK4 competitively inhibits Met activation stimulated by HGF. Surprisingly, NK4 also inhibits angiogenesis induced by basic fibroblast growth factor (bFGF) and vascular endothelial cell growth factor (VEGF) 5, 6, and this antiangiogenic activity does not depend on binding to the Met receptor but rather on its interaction with perlecan, which is a major component of the vascular basement membrane 7. Thus, NK4 has been considered as a bifunctional inhibitor with both HGF antagonist and anti-angiogenesis characteristics. The therapeutic potential of NK4 has been demonstrated in a variety of experimental animal models. NK4 protein administration or NK4 gene therapy inhibited tumor growth, invasion, metastasis, and angiogenesis in various tumor types including cancers of the breast, colon, lung, pancreas, mesothelium, prostate, stomac, and brain8.
Malignant mesothelioma (MM) is a highly aggressive neoplasm arising from mesothelial cells lining the lung, chest wall, heart and abdominal cavity. Exposure to asbestos has been implicated as a major contributory factor in the development of this malignancy. MM is resistant to conventional therapies, with the median survival post-diagnosis ranging from 4 to 12 months 9, 10. We and others have reported that Met is strongly expressed in human MM cell lines 11, and autocrine production of HGF has been found in some MM specimens and MM cell lines 12. HGF stimulation has been shown to increase migration, invasiveness, proliferation, and adhesion in MM cells 13, 14.
Mounting evidence suggests that individual tumors are driven by a small subpopulation of undifferentiated cells dubbed cancer stem-like cells (CSCs) or tumor-initiating cells (T-ICs) 15-17. CSCs are thought to be responsible for cancer initiation and progression and account for resistance to radiotherapy and chemotherapy. Moreover, the resistant CSC population has the potential to re-populate tumors following the killing of the bulk, sensitive tumor cells by standard therapies. Thus, therapeutic modalities aimed at targeting CSCs potentially represent promising approaches for more durable therapeutic responses. Interestingly, recent studies showed that HGF/Met signaling is involved in the maintenance of CSCs in several tumor types and a Met small molecule inhibitor reduced CSC population and suppressed tumor growth of pancreatic cancer18, 19.
In this study, we investigate if the potent antitumor activity of NK4 is attributable to its ability to inhibit CSCs. We show that expression of NK4 via an adenoviral delivery system has pronounced anti-CSC activity on MM cells. NK4 not only suppressed expression levels of several key CSC markers, but also decreased side-population, reduced CD24 positive cells, and inhibited tumor spheroid formation. Furthermore, in a mouse xenograft model of human MM, NK4 adenovirus exhibits strong antitumor activity that was linked to its ability to inhibit cells expressing CSC markers. These results suggest that NK4 acts as a CSC inhibitor through suppressing Met/AKT/β-catenin signaling and provide a novel mechanism for its potent anti-tumor effect.
Materials and Methods
Cell lines
Human MM cell line Meso 10 was established from a surgically explanted primary epithelioid MM as described previously 20, whereas Hmeso was derived from a biphasic MM 21. Neither cell line expressed HGF as assessed by RT-PCR. Cell lines were grown in RPMI 1640 with 10% fetal bovine serum (FBS), supplemented with L-glutamine and penicillin/streptomycin. The adenoviral packaging cell line 293T (ATCC) was cultured in Dulbecco's modified Eagle's medium containing 10% FBS supplemented with 2 mM L-glutamine, 1.0 mM sodium pyruvate, and antibiotics.
Construction of adenoviral vectors
The recombinant NK4 adenovirus was generated by homologous recombination in E. coli using the pAdEasy system 22. Briefly, a NK4 cDNA fragment (encoding HGF residues 1-478) plus a Kozak initiation sequence (GCCACCATG) at its 5′ end and a stop codon (TAG) at its 3′ end was amplified from a plasmid harboring human HGF cDNA (kindly provided by George F. Vande Woude, Van Andel Research Institute, Grand Rapids, MI) and ligated into shuttle vector pShuttle-CMV to create a pShuttle-CMV-NK4 plasmid. After authenticity of the NK4 insert was verified by nucleotide sequencing, E. coli BJ5183 cells were co-transformed with the linearized pShuttle-CMV-NK4 plasmid and the supercoiled adenoviral backbone plasmid pAdEasy-1. Colonies resulting from the transformation were screened for proper recombination by restriction digestion analysis. Then the recombinant NK4 adenoviral plasmid was transfected into HEK293 cells to generate the primary virus stock of recombinant adenovirus AdNK4. The adenovirus was propagated in HEK293 cells and purified by CsCl density gradient ultracentrifugation. The control adenovirus Ad-LacZ, which carries the LacZ gene driven by the CMV promoter, was constructed by using the same protocol described above.
Cell viability assay
The effect of Ad-NK4 on MM cell viability was evaluated by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) reduction conversion assay. Logarithmically-growing Hmeso and Meso 10 cells were seeded in 96-well culture plates and grown in RPMI 1640 medium supplemented with 5% FBS and 10 ng/ml HGF. After overnight incubation, the cells were mock infected or infected with AdLacZ or AdNK4 at a multiplicity of infection (MOI) of 100. After 72 h, 40 μl of MTT at 5 mg/ml was added to each well, and incubation was continued for 2 h. The formazan crystals resulting from the mitochondrial enzymatic activity on the MTT substrate were solubilized with 100 μl of 20% (w/v) SDS, 50% (v/v) N,N-dimethylformamide, pH 4.7 and incubated overnight. Absorbance was measured at 590 nm using a microplate reader. Cell survival was expressed as absorbance relative to that of mock-infected controls.
Cell invasion assay
Cell invasion was measured using 24-well BioCoat Matrigel invasion chambers (Becton Dikinson Labware) and performed essentially as previously described 23, 24. In brief, MM cells were plated in individual culture dishes and mock infected or infected with AdLacZ or AdNK4 as described above. Six hours after infection, cells were trypsinized and suspended in RPMI 1640 containing 1% FBS. Then, 2.5 × 104 cells/well were placed in each upper compartment. The lower compartment contained RPMI 1640 with 10 ng/ml HGF. After incubating for 24 h at 37°C, cells that degraded the Matrigel and migrated through the 8-μm pores of the membrane were fixed with 10% formaldehyde and stained with hematoxylin. The number of invading cells was counted using a microscope, and invasion was estimated as the average number of cells in five fields using a 10x objective.
Side population analysis and CD24 sorting
Hmeso and Meso 10 cells were mock infected or infected with AdLacZ or AdNK4 as described above. Subsequently, cells were suspended in DMEM/ containing 2% FBS at 1×106 cells/ml and stained with Hoechst 33342 dye (5 μg/ml) for 90 min at 37°C with continuous mixing. Following incubation, cells were washed with ice-cold PBS, stained with propidium iodide (1 μg/ml), and maintained at 4°C for flow cytometry analyses and for sorting of side population (SP) fraction using a FACSAria Flow cytometer (Beckton Dickson). The Hoechst dye was excited with an UV laser at 351 to 364 nm, and its fluorescence was measured with a 515-nm side population filter (Hoechst blue) and a 608 EFLP optical filter (Hoechst red). A 540 DSP filter was used to separate the emission wavelengths for flow cytometry analyses. For CD24 analysis, cells were trypsinized, washed, and suspended in PBS containing 2% FBS. After incubation in the dark at 4°C for 30 min with phycoerythrin (PE)-conjugated mouse IgG2b anti human CD24 (BD Pharmingen), cells were washed twice with PBS, followed by FACS analysis using a FACSAria Flow cytometer.
Tumor spheroid formation assay
Tumor spheroid cultures were established as described previously 25. Briefly, single cells were plated in Ultra Low Attachment plates (Corning) in serum-free DMEM-F12 supplemented with 10 ng/mL bFGF, 10 ng/mL EGF, and B27 (all from Invitrogen). In these conditions, cells grew as suspension spherical clusters.
Western blot analysis
MM cells were plated at a density of 5 × 105 cells per well in 6-well tissue culture plates and grown in RPMI 1640/5% FBS. Cells were mock-infected or infected with Ad-LacZ or Ad-NK4 at a MOI of 100. After incubating for 30 h, the cells were treated with HGF (10 ng/ml) for 30 min and solubilized with lysis buffer (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, plus protease and phosphatase inhibitors). The resulting cell lysates were separated by gel electrophoresis, transferred to PVDF membranes, and probed with primary antibodies followed by peroxidase-conjugated secondary antibodies. Protein bands were detected by means of an ECL Western analysis system (Amersham Biosciences, Inc.). An anti-β-gal antibody (Molecular Probes) or anti-HGFα (Santa Cruz) was used to detect adenovirus-mediated expression of LacZ and NK4, respectively. Protein bands were detected by using primary antibodies against Met, phospho-Met, AKT, phospho-AKT, ERK, phosphor-ERK, Nanog, Oct4, Myc (all from Santa Cruz Biotechnology), β-catenin (Cell Signaling Technology), and active β-catenin (anti-ABC, Millipore).
Immunofluorescence staining
For immunofluorescence staining, cells were grown on the surface of cover slides and mock-infected or infected with Ad-LacZ or Ad-NK4 at a MOI of 100. After 30 h, the cells were treated with HGF (10 ng/ml) for 30 min and fixed with 4% paraformaldehyde. After rehydration in PBS, fixed cells were incubated with anti-β-catenin antibody at room temperature for 1 h. FITC-conjugated secondary antibodies were incubated for 30 min at room temperature. The nuclei were stained with DAPI. Cell images were examined and captured with a Nikon Eclipse 80i fluorescent microscope.
Luciferase reporter activity assay
Hmeso and Meso 10 cells were transfected with a TCF/LEF reporter (TOPFlash, Addgene plasmid 12456) or a vector containing mutated TCF/LEF binding sites (FOPFlash, Addgene plasmid 12457) along with renilla luciferase plasmids (Promega) as an internal control. The cells were then infected with Ad-LacZ or Ad-NK4 at a MOI of 100. After 24 hours, cells were lysed directly and luciferase activities were determined by a dual-luciferase reporter assay system (Promega).
Inhibition of tumorigenicity of MM cells by Ad-NK4
Exponentially growing Hmeso cells (5 × 106) were injected peritoneally in 6-week-old nude mice. Four days after implantation, the animals were randomly divided into three groups (6 mice/group) and injected with PBS, Ad-LacZ, or Ad-NK4. For this experiment, 200 μl of PBS or PBS containing Ad-LacZ (1 × 109 pfu) or Ad-NK4 (1 × 109 pfu) was used. The treatment was performed twice a week for a total of five administrations. At the end of week 4, mice were sacrificed by CO2 asphyxiation. The disseminated tumor nodules were collected and weighed. To evaluate the efficacy of Ad-NK4 treatment, the tumor weight in the three groups of mice was compared and analyzed statistically. For immunohistochemical examination, tumor samples were fixed in 10% buffered formalin, embedded in paraffin, and stained with antibodies indicated in the figures. This animal experiment was performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Fox Chase Cancer Center.
Statistical analysis
Data were expressed as means ± SD. Significant differences between groups were determined by analysis of variance and by Student's t test. Differences were considered significant when the P value was less than 0.05.
Results
NK4 suppresses MM cell viability and invasiveness in vitro
We have previously shown that immunohistochemical staining for Met is elevated in human MM specimens compared to that of normal mesothelial cells and that Met was strongly expressed in a panel of nine human MM cell lines analyzed by Western blotting 11. We and others also reported that HGF enhances MM cell viability 12, 26. In the present study, we aimed to evaluate the therapeutic potential of Ad-NK4 in MM. To this end, we first examined the effect of AdNK4 on in vitro cell viability in MM cell lines Hmeso and Meso 10, both of which express high levels of Met protein (Fig. 4A). The cells were grown in the presence of recombinant HGF and mock infected or infected with Ad-LacZ or Ad-NK4 for 3 d. Cell viability was measured by MTT assays. Infection with Ad-LacZ did not show any anti-proliferative effect. In contrast, viability was significantly inhibited by Ad-NK4 in both Hmeso and Meso 10 cells (Fig. 1A).
Figure 4.
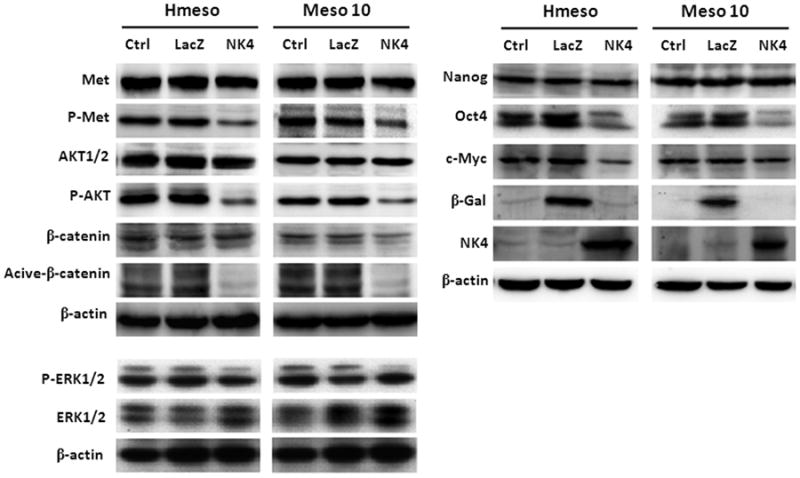
Expression of NK4 inhibits expression of CSC markers. Cells were mock-infected or infected with Ad-LacZ or Ad-NK4. After incubating for 30 hours, the cells were treated with HGF (10 ng/ml) for 30 min followed by immunoblot analysis. Expression of NK4 protein did not have any effect on the level of total Met and AKT proteins, but P-Met and P-AKT were markedly reduced. In contrast, it modestly reduced P-ERK2, but not P-ERK1. Expression of NK4 also inhibited the active form, but not the total protein, of β-catenin. In addition, Ad-NK4 reduced expression of Oct4 in both Hmeso and Meso 10 cells while Myc was suppressed only in Hmeso.
Figure 1.
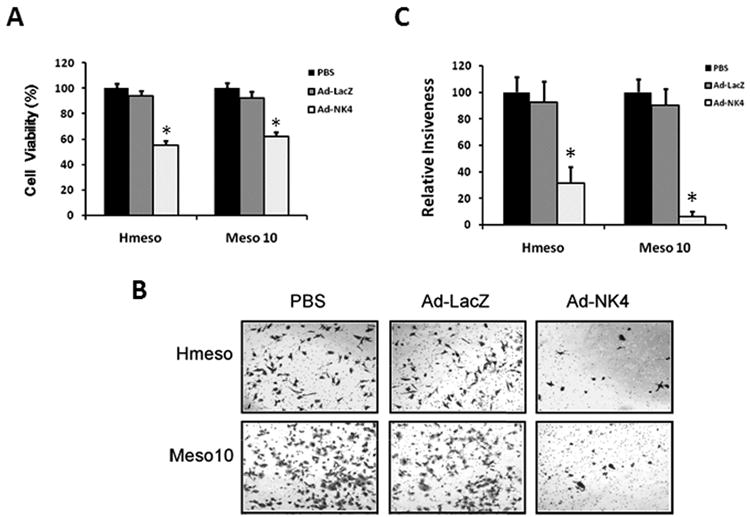
Expression of NK4 inhibits MM cell viability and invasiveness. (A) Inhibition of MM cell viability by Ad-NK4. MM cells Hmeso and Meso 10 were plated at a density of 5 × 103 cells per well in 96-well tissue culture plates and grown in RPMI 1640 medium supplemented with 5% FBS and 10 ng/ml HGF. After overnight incubation, the cells were mock-infected or infected with Ad-LacZ or Ad-NK4. Cells were incubated for another 72 h, followed by MTT assay. Results are expressed as percent cell viability relative to the viability of control mock-infected cells (PBS). Each bar represents the mean value of five replicate wells from a representative experiment (n=3). *, p < 0.01 versus control. (B) Inhibitory effect of Ad-NK4 on invasiveness potential of MM cells in a Matrigel invasion assay. Hmeso and Meso 10 cells were mock-infected (PBS) or infected with Ad-LacZ or Ad-NK4 for 24 h. The following day, cells were seeded in the upper compartment of a Matrigel chamber in serum-free medium and allowed to invade overnight toward 10 ng/ml HGF, used as a chemoattractant, present in the lower compartment. Representative bright-field microscopic fields showing stained cells that invaded through the Matrigel. (C) Quantitative analysis of invasion assays. Ad-NK4 infection markedly decreased the average number of invading cells. Bars represent mean number of counted cells in five different microscope fields ± s.d. *, p < 0.01 compared to mock-infected cells.
Increased cell motility and invasiveness in vitro are thought to correlate with an enhanced malignant and invasive phenotype in vivo 27. Given that MMs are highly invasive tumors 28, 29, we investigated the effects of Ad-NK4 on cell invasiveness in a Matrigel invasion assay. After mock-infection or infection with Ad-LacZ or Ad-NK4, Hmeso and Meso 10 cells were seeded in the upper compartments of the invasion chambers, with HGF present in the lower chambers. Compared to mock-infected cells, the number of invading cells was not affected by infection with Ad-LacZ. However, infection with Ad-NK4 significantly inhibited the invasiveness of both Hmeso and Meso 10 cells (Fig. 1B and C).
NK4 represses side population and CD24 positive cells of MM
Side population (SP) cells are characterized by their high ability to efflux the fluorescent dye Hoechst 33342 from the cytoplasm through ATP-binding cassette transporters such as ABCG2 and MDR1. SP cells account for a small subpopulation of the total cells and exhibit CSC properties in a variety of solid tumors including MM 30. To investigate whether Ad-NK4 could regulate SP cells in MM, Hmeso and Meso 10 cells were subjected to flow cytometric analysis. Untreated Hmeso and Meso 10 cells contained 1.6 % and 0.8 % of Hoechst 33342-dull SP cells, respectively, which were depleted by the ABC transporter inhibitor verapamil (5 μM) in a Hoechst dye exclusion assay. While treatment with Ad-LacZ did not any show obvious effect on SP cells, infection with Ad-NK4 dramatically decreased the percentage SP cells in both cell lines (Fig. 2A).
Figure 2.
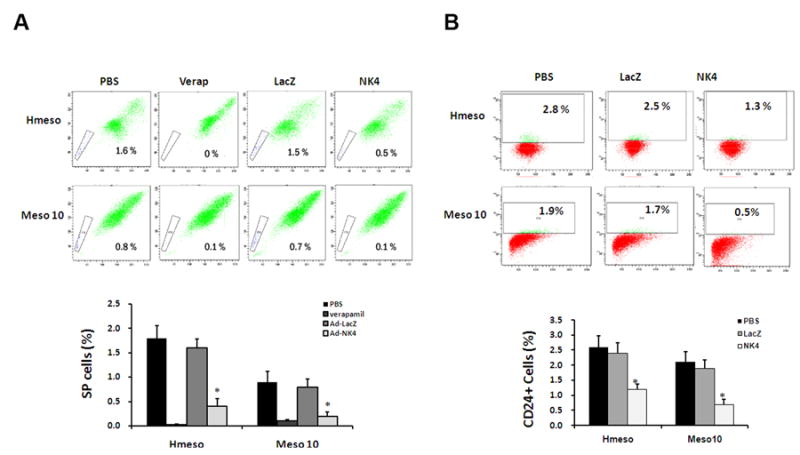
NK4 represses MM side population and CD24+ cells. (A) Hmeso and Meso 10 cells were mock-infected or infected with Ad-LacZ or Ad-NK4 followed by flow cytometric analysis. SP cells were depleted by the ABC transporter inhibitor verapamil and decreased by Ad-NK4, but not by Ad-LacZ. (B) After infection, Hmeso and Meso 10 cells were stained with anti human CD24 antibody and subjected to FACS analysis. Note that infection with Ad-NK4 resulted in a pronounced inhibition of the CD24+ cell population. Bar = mean ± s.d. *, p < 0.01 compared to mock-infected cells.
A recent study has identified CD24 as a helpful marker of CSCs in MM, and its expression has been shown to correlate with the tumorigenic potential of MM cells in animals 30. This finding prompted us to test the effect of Ad-NK4 on CD24 expression in MM cells. As shown in Fig. 2B, uninfected Hmeso and Meso 10 cells contained 2.8% and 1.9% of CD24 positive cells, respectively. Infection with Ad-NK4 significantly reduced the percentage of CD24-positive cells to 1.3-0.6% in the two MM cell lines, whereas Ad-LacZ exhibited no obvious effect on either cell line.
Spheroid formation of MM cells is inhbited by NK4
Unlike in conventional 2D culture system, tumor cells grown on a non-adherent surface form suspended 3D multi-layer spheroids, which are enriched in cancer stem-like cells and known to closely mimic phenotypes of in vivo tumors 25, 31. To determine if NK4 has any effect on tumor spheroid formation, Hmeso and Meso 10 cells were mock infected or infected with Ad-LacZ or Ad-NK4 and cultured in serum-free medium containing bFGF and EGF. After 3 weeks of cultivation, mock infected and Ad-LacZ infected cells formed spheroids. However, the capability of spheroid formation of Ad-NK4 infected cells was severely inhibited in both cell lines (Fig. 3).
Figure 3.
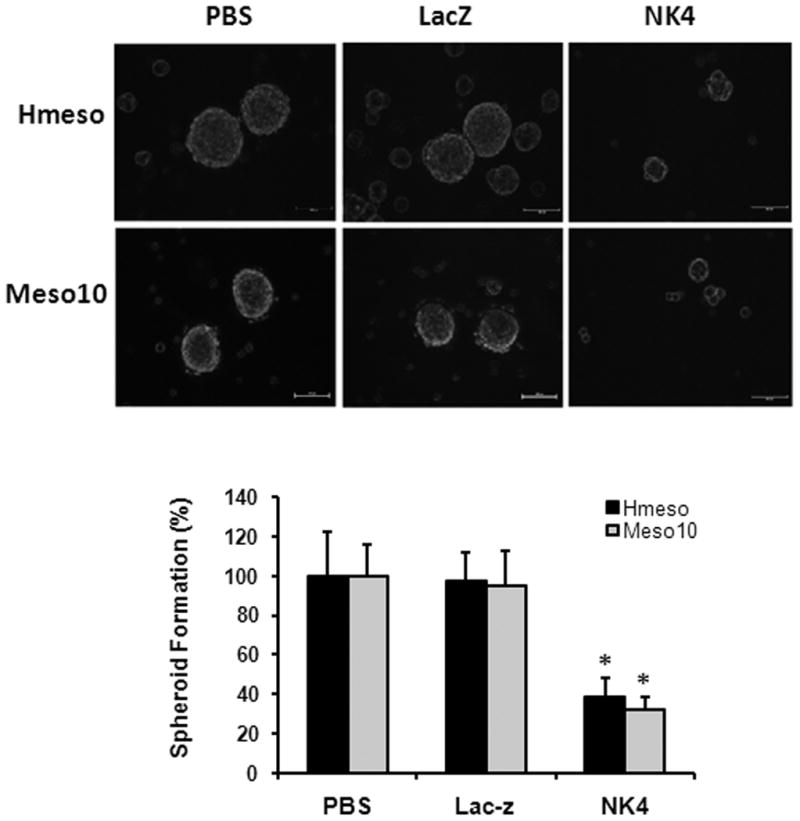
Inhibition of MM spheroid formation by Ad-NK4. Cells were mock-infected, infected with Ad-LacZ or Ad-NK4. After 24 hours, cells were plated on Ultra Low Attachment plates and cultured in spheroid medium containing EGF and bFGF. Infection with Ad-NK4 potently reduced spheroid formation. Bar = mean ± s.d. *, p < 0.01 versus control.
NK4 represses CSC markers in MM cells
To evaluate the potential role of NK4 as an antagonist of CSCs, we first asked if NK4 affects the expression levels of CSC markers. MM cells were mock infected or infected with Ad-LacZ or Ad-NK4 for 30 h. Immunoblot analyses showed that Met protein was highly expressed in both cell lines (Fig. 4A). While infection with either Ad-LacZ or Ad-NK4 had no effect on the level of total Met protein, phosphorylated Met was markedly decreased following infection with Ad-NK4, but not Ad-LacZ, indicating that Met activity was suppressed by NK4. As expected, phosphorylation of AKT, a downstream effector of Met 26, was also reduced after Ad-NK4 infection. However, NK4 only moderately inhibited phosphorylation of ERK2, without any effect on ERK1. Subsequently, we examined if NK4 could inhibit expression of several CSC markers. β-catenin, a key mediator of the Wnt pathway, is essential in sustaining the CSC phenotype in several types of tumors, 32, 33, and its activity is also regulated by AKT/GSK3β signaling 32, 34. Using an antibody specific for the active form of β-catenin, dephosphorylated on Ser37 or Thr41 (anti-ABC, Millipore), we found that β-catenin activity was decreased by Ad-NK4, but not by Ad-LacZ, in both MM cell lines, while total levels of β-catenin were unchanged in Hmeso and mildly reduced in Meso 10 by Ad-NK4. We next examined the effect of Ad-NK4 on the expression of Oct4, Nanog and Myc, which are critical regulators in ES cells and CSCs. Ad-NK4 reduced the expression of Oct4 in both Hmeso and Meso 10, whereas Myc was suppressed only in Hmeso cells. In contrast, expression levels of Nanog were unchanged in both cell lines.
NK4 reduces β-catenin nuclear localization and transcriptional activity
Nuclear localization of β-catenin is a hallmark of Wnt signaling. To confirm the inhibitory effect of Ad-NK4 on β-catenin, we examined subcellular localization of β-catenin in response to Ad-NK4 infection (Fig. 5A). In untreated Hmeso cells, β-catenin was predominantly located at the plasma membrane, with faint staining distributed in the cytoplasm. When cells were treated with HGF, β-catenin was shifted to the nucleus. Treatment with Ad-NK4, but not Ad-LacZ, reversed HGF-induced β-catenin nuclear accumulation. This is consistent with the above immunoblot results showing that Ad-NK4 decreased the levels of dephosphorylated β-catenin, which is transcriptionally active in the nucleus.
Figure 5.
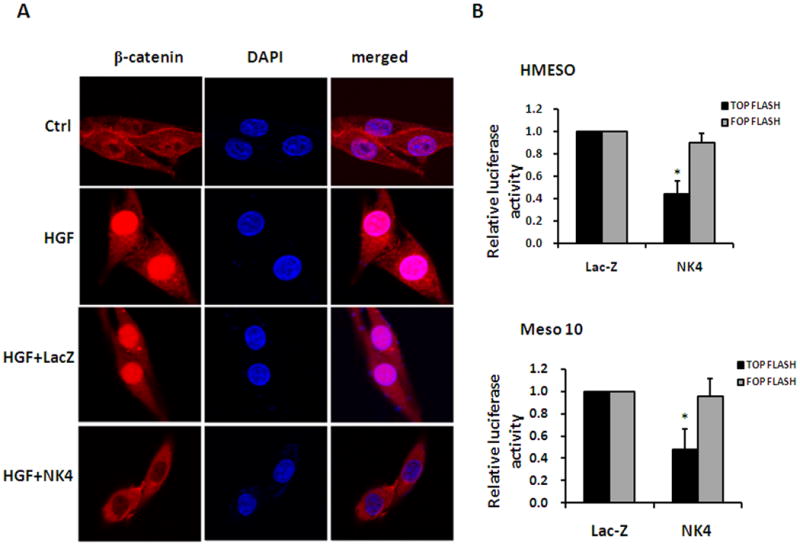
NK4 reduces β-catenin nuclear localization and transcriptional activity. (A) Immunofluorescent staining demonstrating that Ad-NK4 blocks HGF-induced nuclear localization of β-catenin. (B) TOP/FOP luciferase reporter assays performed to determine transcriptional activity of β-catenin. Expression of NK4 inhibited luciferase activity of TOP-Flash but not the negative control vector FOP-Flash in both Hmeso and Meso 10 cell lines (*, p < 0.05).
To validate the effect of NK4 on β-catenin-dependent transcription, we performed TOP/FOP luciferase reporter assay (Fig. 5B). TOP-Flash or FOP-Flash vector that contains wild-type or mutated TCF/LEF binding sites, respectively, was transfected into Hmeso and Meso 10 cells followed by infection with Ad-LacZ or Ad-NK4. As expected, luciferase activity of the control vector FOP-Flash was unchanged in response to expression of either Ad-LacZ or Ad-NK4. In contrast, luciferase activity of TOP-Flash was dramatically inhibited by Ad-NK4 when compared with Ad-LacZ in both cell lines. These results demonstrate that NK4 reduces the nuclear localization of β-catenin and represses its transcriptional activity.
NK4 is more effective than PHA-665752 in suppression of mesothelioma CSCs
To further explore mechanism underlying NK4's anti-CSC activity, we compared effects of NK4 with PHA-665752, which is a selective small molecule inhibitor of Met kinase and exhibits potent antitumor activity in a variety of cancers. We found that both PHA-665752 and NK4 inhibited phosphorylation of Met and suppressed active form of β-catenin in mesothelioma cells. However, NK4 was more effective in suppression of mesothelioma spheroid formation (Fig. 6), suggesting that NK4 targets additional CSC-related molecule(s).
Figure 6. NK4 is more effective than PHA-665752 in suppression of mesothelioma.
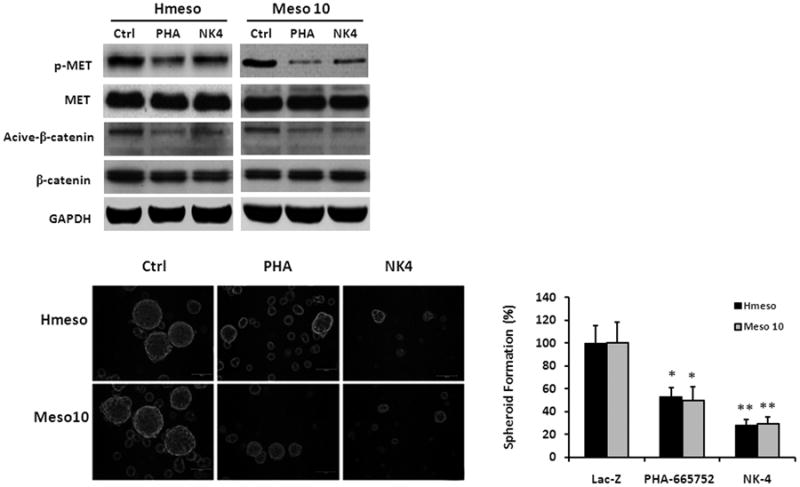
(A) Cells were untreated, treated with PHA-665752 (1 μmol/L), or infected with Ad-NK4. Both PHA-665752 and NK4 inhibited phosphorylation of Met and suppressed active form of β-catenin. (B) NK4 was more effective than PHA-665752 in suppression of mesothelioma spheroid formation. Bars shown represent mean ± s.d. PHA-665752 versus Ad-LacZ (*, p < 0.05); Ad-NK4 versus PHA-665752 (**, p < 0.05).
Ad-NK4 inhibits the tumorigenicity of MM cells
We next examined the anti-tumor efficacy of Ad-NK4 in mice with MM xenografts. The tumorigenic Hmeso cells were injected intraperitoneally in nude mice. Four days after implantation, the animals were injected with PBS, Ad-LacZ, or Ad-NK4 twice a week for a total of five administrations. Compared to PBS, treatment with Ad-LacZ had a mild, statistically insignificant (P>0.05), effect on tumor growth as evaluated based on the average tumor weight per mouse. Treatment with Ad-NK4, however, resulted in a highly significant decrease in tumor growth compared to that with PBS or Ad-LacZ (P<0.01, Fig. 7A). To investigate the potential mechanisms of xenograft tumor growth retardation, immunohistochemical analyses were performed with tumor tissues (Fig. 7B). Activation of Met and AKT, as evaluated by phospho-specific Met and AKT antibodies, was markedly reduced in tumors from mice infected with Ad-NK4 compared to that of mice uninfected or infected with Ad-LacZ. Furthermore, expression of β-catenin and Oct4 was abrogated in Ad-NK4-infected tumors compared to that observed in mock-infected or Ad-LacZ-infected tumors.
Figure 7.
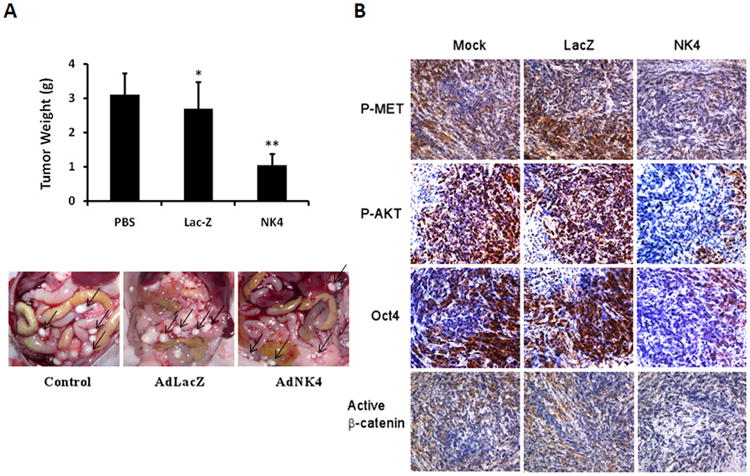
Inhibition of peritoneal MM growth by AdNK4. Intraperitoneal xenografts of human Hmeso cells in nude mice were mock-treated (PBS) or treated with Ad-LacZ or Ad-NK4. The treatment was performed twice a week for a total of five administrations. (A) Weight of individual tumors was determined, and bars shown represent mean weight ± s.d. Ad-LacZ versus PBS (*, p > 0.05). Ad-NK4 versus PBS and Ad-LacZ (**, p < 0.01). Images in lower panel depict tumor growth in representative mice. (B) Immunohistochemical staining of tumor tissues. Phospho-Met, phospho-AKT, Oct4, and active β-catenin were markedly reduced in tumors of mice infected with Ad-NK4 compared to that observed for mice treated with PBS or Ad-LacZ. Photographs were taken at ×400 magnification.
Discussion
NK4 is known to exert its anti-tumor effect by acting as a bifunctional inhibitor, with both HGF antagonist and anti-angiogenesis characteristics. However, the precise mechanisms responsible for this potent anti-tumor effect have required further elucidation. In the present study, we evaluated the therapeutic efficacy of adenoviral-mediated NK4 treatment in MM. Our results show that expression of NK4 substantially reduces Met and AKT activity and inhibits viability, invasiveness, and tumorigenicity of MM cells. These results are consistent with previously reported effects of NK4 observed in MM 35 and other tumor types 8, 36.
Increasing evidence indicates that CSCs initiate and sustain malignancy in a variety of cancers and are responsible for systemic metastases, resistance to chemo- and radiotherapy, and tumor relapses. Recent studies have shown that HGF/Met signaling regulates the invasiveness of tumor-initiating cells in a brain tumor model 37, and activation of Met in prostate cancer cells induced a stem-like phenotype 38. Based on these observations, we investigated whether Ad-NK4 acts against MM CSCs. Our results for the first time demonstrate that Ad-NK4 has anti-CSC activity as assessed by SP analysis, cytometric sorting of CD24 cells, and spheroid formation assay. We also demonstrate that Ad-NK4 suppresses protein expression or activity of several key CSC-related molecules including β-catenin, Oct4, and Myc. β-catenin is a key mediator of Wnt signaling and has been linked to the self-renewal of stem cells and CSCs 39. Other studies have revealed that β-catenin is also responsible for mediating the effect of PTEN/AKT signaling on CSCs 32. Phosphorylation of β-catenin either directly by AKT 32, or indirectly through GSK3β 34, leads to its nuclear translocation and activation. Oct4 and Myc are two critical transcriptional factors that regulate self-renewal and differentiation in embryonic stem cells and are two of the four factors used in reprogramming somatic cells to induced pluripotent stem (iPS) cells 40, 41. Furthermore, Oct4 and Myc are also involved in maintaining CSC properties in a variety of human cancers 42-44.
How did NK4 inhibit the CSC phenotype and molecular markers of such cells? Our previous studies have demonstrated that AKT is phosphorylated and activated by Met signaling and is a major downstream effector of Met in protecting cells from apoptosis 12, 26. Recent work by other investigators and us revealed that AKT is critical in sustaining CSC properties and that this effect is mediated by β-catenin 32, 34. In this study, we have shown that Ad-NK4 not only inhibits activity of Met and AKT, but also of β-catenin, as determined by western blot analyses. We further demonstrated that expression of NK4 markedly suppresses the nuclear localization and transcriptional activity of β-catenin. Intriguingly, the expression levels of Oct4 and Myc were also downregulated by NK4. Since the Myc gene is a transcriptional target of β-catenin 45, suppression of β-catenin activity by Ad-NK4 would be expected to lead to reduced expression of Myc. Our finding of inhibition of Oct4 by NK4 is also consistent with previous observations that expression of Oct4 is regulated by β-catenin. Studies with embryonic stem cells revealed that overexpression of β-catenin prolongs the retention of Oct4 protein 46, 47, whereas knockdown of β-catenin with siRNA decreased Oct4 expression 47. It is noteworthy that Ad-NK4 also significantly reduced the expression of the cell surface marker CD24 (Fig. 2B), which has been reported to be transcriptionally regulated by Wnt/β-catenin signaling 48. In conclusion, our results suggest that NK4 acts as a CSC inhibitor by suppressing Met/AKT/β-catenin signaling and provide a novel mechanism for its potent anti-tumor effect.
Our experiments with PHA-665752 showed that NK4 was more effective than PHA-665752 in suppression of MM CSCs even though both exhibited similar inhibitory activity toward Met and β-catenin in mesothelioma cells, suggesting that NK4 targets addtional CSC-related molecule(s). Previously, Kuba et al. reported that NK4 inhibited bFGF-induced angiogenesis 5, and further study by this group found that NK4 inhibits DNA synthesis induced not only by HGF but also by either bFGF or VEGF 7. A recent study showed that NK4 exhibited potent anti-tumor effects on MM cells 35. NK4, but not anti-HGF antibody, suppressed the proliferation of MM cells in collagen, suggesting that the suppression by NK4 can display effects independent of the HGF-Met pathway 35. Interestingly, the Kringle 1 domain of HGF α-chain alone was able to inhibit EGFR, VEGFR and bFGFR in tumor cells 49, and each of the RTKs is known to be capable of driving CSC phenotypes. Thus the mechanism for NK4's anti-CSC activity may attribute to its ability to mediate multiple effector functions.
Our finding that NK4 acts as a CSC inhibitor has important therapeutic implications for MM. A large body of evidence indicates that MM is a highly invasive and metastatic neoplasm. Despite diverse therapeutic approaches including surgery, radiotherapy and chemotherapy, MM continues to have a very poor prognosis. Conceptually, CSCs are thought to possess the ability to drive cancer resistance and metastasis, and current failure of cancer therapy is attributable to residual CSCs spared by conventional therapies. Thus, the anti-CSC ability of NK4 as demonstrated in this study could make NK4 unique among various agents available to treat this devastating form of cancer.
Novelty and impact.
We demonstrate that NK4, a HGF antagonist and angiogenesis inhibitor, inhibits cancer stem-like cell (CSC) properties and growth of malignant mesothelioma. In addition to inhibiting phosphorylation of Met and AKT, NK4 suppressed the active form of β-catenin, blocked its nuclear translocation, transcriptional activity, and expression of its targets Oct4 and Myc. These findings suggest that NK4 acts as a CSC inhibitor by inhibiting Met/AKT/β-catenin signaling and provide a novel mechanism for NK4's potent anti-tumor effect.
Acknowledgments
This work was supported in part by the Mesothelioma Applied Research Foundation, National Basic Research Program of China, 973 Program No 2010CB529401, and Natural Science Foundation of China grant 81072205 (G. H. Xiao) and NCI grant CA-114047 (B.T. Mossman and J.R. Testa). The investigators declare no conflicts of interest concerning this work.
References
- 1.Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem. 2003;88:408–17. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]
- 2.Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K, Nakamura T. Mechanisms and significance of bifunctional NK4 in cancer treatment. Biochem Biophys Res Commun. 2005;333:316–27. doi: 10.1016/j.bbrc.2005.05.131. [DOI] [PubMed] [Google Scholar]
- 4.Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett. 1997;420:1–6. doi: 10.1016/s0014-5793(97)01475-0. [DOI] [PubMed] [Google Scholar]
- 5.Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000;60:6737–43. [PubMed] [Google Scholar]
- 6.Nakabayashi M, Morishita R, Nakagami H, Kuba K, Matsumoto K, Nakamura T, Tano Y, Kaneda Y. HGF/NK4 inhibited VEGF-induced angiogenesis in in vitro cultured endothelial cells and in vivo rabbit model. Diabetologia. 2003;46:115–23. doi: 10.1007/s00125-002-0954-y. [DOI] [PubMed] [Google Scholar]
- 7.Sakai K, Nakamura T, Matsumoto K. Angioinhibitory action of NK4 involves impaired extracellular assembly of fibronectin mediated by perlecan-NK4 association. J Biol Chem. 2009;284:22491–9. doi: 10.1074/jbc.M109.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura T, Sakai K, Matsumoto K. Anti-cancer approach with NK4: Bivalent action and mechanisms. Anticancer Agents Med Chem. 2010;10:36–46. doi: 10.2174/1871520611009010036. [DOI] [PubMed] [Google Scholar]
- 9.Pass HI, Vogelzang N, Hahn S, Carbone M. Malignant pleural mesothelioma. Curr Probl Cancer. 2004;28:93–174. doi: 10.1016/j.currproblcancer.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Bueno R. Multimodality treatments in the management of malignant pleural mesothelioma: an update. Hematol Oncol Clin North Am. 2005;19:1089–97. vii. doi: 10.1016/j.hoc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Altomare DA, You H, Xiao GH, Ramos-Nino ME, Skele KL, De Rienzo A, Jhanwar SC, Mossman BT, Kane AB, Testa JR. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene. 2005;24:6080–9. doi: 10.1038/sj.onc.1208744. [DOI] [PubMed] [Google Scholar]
- 12.Harvey P, Warn A, Dobbin S, Arakaki N, Daikuhara Y, Jaurand MC, Warn RM. Expression of HGF/SF in mesothelioma cell lines and its effects on cell motility, proliferation and morphology. Br J Cancer. 1998;77:1052–9. doi: 10.1038/bjc.1998.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klominek J, Robert KH, Sundqvist KG. Chemotaxis and haptotaxis of human malignant mesothelioma cells: effects of fibronectin, laminin, type IV collagen, and an autocrine motility factor-like substance. Cancer Res. 1993;53:4376–82. [PubMed] [Google Scholar]
- 14.Harvey P, Clark IM, Jaurand MC, Warn RM, Edwards DR. Hepatocyte growth factor/scatter factor enhances the invasion of mesothelioma cell lines and the expression of matrix metalloproteinases. Br J Cancer. 2000;83:1147–53. doi: 10.1054/bjoc.2000.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 16.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 17.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–2. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Li A, Glas M, Lal B, Ying M, Sang Y, Xia S, Trageser D, Guerrero-Cazares H, Eberhart CG, Quinones-Hinojosa A, Scheffler B, et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Natl Acad Sci U S A. 2011;108:9951–6. doi: 10.1073/pnas.1016912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hage C, Rausch V, Giese N, Giese T, Schonsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013;4:e627. doi: 10.1038/cddis.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taguchi T, Jhanwar SC, Siegfried JM, Keller SM, Testa JR. Recurrent deletions of specific chromosomal sites in 1p, 3p, 6q, and 9p in human malignant mesothelioma. Cancer Res. 1993;53:4349–55. [PubMed] [Google Scholar]
- 21.Reale FR, Griffin TW, Compton JM, Graham S, Townes PL, Bogden A. Characterization of a human malignant mesothelioma cell line (H-MESO-1): a biphasic solid and ascitic tumor model. Cancer Res. 1987;47:3199–205. [PubMed] [Google Scholar]
- 22.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589–93. [PubMed] [Google Scholar]
- 24.Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006 doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- 25.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2001;98:247–52. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 28.Sterman DH, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005;10:266–83. doi: 10.1111/j.1440-1843.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 29.Zervos MD, Bizekis C, Pass HI. Malignant mesothelioma 2008. Curr Opin Pulm Med. 2008;14:303–9. doi: 10.1097/MCP.0b013e328302851d. [DOI] [PubMed] [Google Scholar]
- 30.Ghani FI, Yamazaki H, Iwata S, Okamoto T, Aoe K, Okabe K, Mimura Y, Fujimoto N, Kishimoto T, Yamada T, Xu CW, Morimoto C. Identification of cancer stem cell markers in human malignant mesothelioma cells. Biochem Biophys Res Commun. 2011;404:735–42. doi: 10.1016/j.bbrc.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 31.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 32.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nature Genetics. 2007;39:189–98. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, Huelsken J. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–3. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 34.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y, Sakai K, Ueki J, Xu Q, Nakamura T, Shimada H, Matsumoto K. Inhibition of Met/HGF receptor and angiogenesis by NK4 leads to suppression of tumor growth and migration in malignant pleural mesothelioma. Int J Cancer. 2010;127:1948–57. doi: 10.1002/ijc.25197. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto K, Nakamura T, Sakai K. Hepatocyte growth factor and Met in tumor biology and therapeutic approach with NK4. Proteomics. 2008;8:3360–70. doi: 10.1002/pmic.200800156. [DOI] [PubMed] [Google Scholar]
- 37.Tamase A, Muraguchi T, Naka K, Tanaka S, Kinoshita M, Hoshii T, Ohmura M, Shugo H, Ooshio T, Nakada M, Sawamoto K, Onodera M, et al. Identification of tumor-initiating cells in a highly aggressive brain tumor using promoter activity of nucleostemin. Proc Natl Acad Sci U S A. 2009;106:17163–8. doi: 10.1073/pnas.0905016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Leenders GJ, Sookhlall R, Teubel WJ, de Ridder CM, Reneman S, Sacchetti A, Vissers KJ, van Weerden W, Jenster G. Activation of c-MET induces a stem-like phenotype in human prostate cancer. PLoS One. 2011;6:e26753. doi: 10.1371/journal.pone.0026753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–8. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 42.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–8. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 44.Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27:993–1005. doi: 10.1002/stem.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 46.Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW. beta-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214–27. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam H, Patel S, Wong J, Chu J, Lau A, Li S. Localized decrease of beta-catenin contributes to the differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2008;372:601–6. doi: 10.1016/j.bbrc.2008.05.116. [DOI] [PubMed] [Google Scholar]
- 48.Shulewitz M, Soloviev I, Wu T, Koeppen H, Polakis P, Sakanaka C. Repressor roles for TCF-4 and Sfrp1 in Wnt signaling in breast cancer. Oncogene. 2006;25:4361–9. doi: 10.1038/sj.onc.1209470. [DOI] [PubMed] [Google Scholar]
- 49.Shen Z, Yang ZF, Gao Y, Li JC, Chen HX, Liu CC, Poon RT, Fan ST, Luk JM, Sze KH, Li TP, Gan RB, et al. The kringle 1 domain of hepatocyte growth factor has antiangiogenic and antitumor cell effects on hepatocellular carcinoma. Cancer Res. 2008;68:404–14. doi: 10.1158/0008-5472.CAN-07-2081. [DOI] [PubMed] [Google Scholar]


