Abstract
Previous studies have demonstrated both synergistic and opposing effects of Akt and Mek1/2 in various cell functions and disease states. Furthermore, Akt has been reported to inhibit and activate cRaf/Mek pathway, suggesting that their mutual interaction and cooperation may be cell type, stimuli and/or context specific. While PI3-Kinase/Akt and cRaf/Mek pathways have been implicated in the regulation of extracellular matrix (ECM) remodeling, mutual interactions between these two pathways and their specific contributions to the events leading to ECM synthesis and assembly is not clear. We investigated the specific role of Akt1 and Mek1 in ECM synthesis and assembly by NIH 3T3 fibroblasts and how these effects were reconciled to mediate overall ECM remodeling. Our study identified that cooperation between Akt1 and Mek1 is necessary to mediate ECM synthesis. Whereas Akt1 activation resulted in Mek1 activation as evidenced by increased ERK1/2 phosphorylation, Mek1 inhibition using U0126 or DN-Mek1 resulted in enhanced Akt1 phosphorylation. Interestingly, both Akt1 and Mek1 activities were needed for the synthesis and assembly of ECM. The effect of Akt1 and Mek1 on ECM synthesis was reconciled through activation of p70 S6-Kinase via phosphorylation at T421/S424 and S411, respectively. Furthermore, Akt1 and Mek1 cooperated in mediating ECM assembly via activation of integrin β1. Together, we show for the first time that Akt1 and Mek1 pathways cooperate in the regulation of ECM remodeling by the fibroblasts.
Keywords: Akt1, Mek1, mTOR, p70 S6-Kinase, Extracellular matrix, Integrin
1. Introduction
Extracellular matrix (ECM) is a non-cellular structural network composed of mainly proteins such as fibronectin, collagens, laminins and elastin as well as proteoglycans such as dermatan sulfate and hyaluronan [1]. Fibroblasts, the major source of ECM, also perform their assembly during tissue remodeling [2], and are precursors of pathologic myofibroblasts that mediate tissue fibrosis [3]. ECM provides the necessary scaffold to the surrounding cells to group them together and confer flexibility and strength.
Research from our laboratory has demonstrated the key role of serine-threonine kinase Akt1 (Protein kinase Bα) in ECM remodeling in vitro and in vivo. Our studies in Akt1−/− mice revealed that expression and assembly of collagen matrix in normal skin and during cutaneous wound healing, as well as the expression of laminin in the vascular basement membrane are all impaired in the absence of Akt1 gene [4, 5]. Pharmacological inhibition of Akt or ablation of Akt1 gene resulted in impaired fibronectin matrix assembly via modulation of Rac1 and P21 activated kinase pathway [6, 7]. Furthermore, secretion of fibronectin and collagen types I, II and V was also regulated by Akt1 via mTOR signaling in response to the pro-fibrotic factor transforming growth factor-β (TGFβ) or agents such as bleomycin [8]. Recently, we also demonstrated that, apart from the pro-fibrogenic role of Akt1 in fibroblasts, sustained activation of Akt1 leads to myofibroblast differentiation associated with enhanced expression of alpha-smooth muscle cell actin (αSMA) contractile protein through serum response factor (SRF) and myocardin-mediated pathway [9]. These studies indicated that fine tuning of Akt1 activity is necessary to maintain ECM homeostasis in tissues.
Another pathway activated during a fibrogenic response that has been implicated in ECM remodeling is the cRaf/Mek/ERK1/2 pathway [10]. However, reports on mutual interactions between cRaf/Mek and PI3 kinase/Akt pathways are highly conflicting. An early study done in HEK293 cells indicated that Akt is responsible for the direct phosphorylation of cRafS259 leading to inhibition of Mek/ERK1/2 pathway [11]. Another study showed that Akt can keep cRaf in an inactive as well as an active form depending upon the phosphorylation status of another serine residue (cRafS338) by an unknown kinase that promote its interaction with adaptor protein 14-3-3 [12]. Whereas one study indicated that P21 activated kinase-1 (Pak1) is responsible for the activation of cRaf signaling in mediating oncogenic transformation in Rat-1 fibroblasts [13], our group showed that Akt1 and cRaf pathways can be coupled by phosphorylation of cRafSer259 by Akt1 and cRafSer338 by Pak1 [14]. While many follow-up studies conducted early on have reported mutual activity inhibition and opposing effects of Akt and cRaf pathways in physiology [15, 16], most recent studies by multiple laboratories [17–20], including ours [14, 21] demonstrated a cooperation between Akt and cRaf/Mek signaling in multiple cellular processes in vitro, and physiology and pathology in vivo. These reports suggest that the cross-talk between Akt and cRaf/Mek pathways may be cell, tissue or context dependent and warrants additional research to investigate the role of Akt and cRaf/Mek cooperation in multiple cellular and physiological processes.
In our study, we identified that cooperation between Akt1 and Mek1 pathways is necessary for overall ECM remodeling by the fibroblasts. Whereas Akt1 was primarily responsible for ECM secretion and the role of Mek1 was more prominent in ECM assembly, both Akt1 and Mek1 activities were needed for each of these inter-dependent facets of ECM remodeling. One of the candidates that reconciled the effects of Akt1 and Mek1 pathways in the regulation of ECM remodeling was p70 S6-kinase (p70S6K), which is activated by both Akt1/mTOR and Mek1/ERK1/2 pathways through phosphorylation at specific Threonine421 and Serine424 residues on p70S6K, respectively. On the other end, Akt1 and Mek1 cooperated in activating integrin β1 in mediating ECM assembly. Overall, we report novel insights into the role of p70S6K in harmonizing the Akt1 and Mek1 pathways in the regulation of ECM remodeling.
2. Materials and Methods
2.1. Reagents, cell lines and antibodies
NIH 3T3 fibroblasts were obtained from ATCC (Manassas, VA) and maintained in DMEM (HyClone, Logan, UT) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin in a 5% CO2 incubator at 37°C. Primary antibodies against phospho-AktS473, phospho-MekS217/221, Pan-Mek, phospho-S6 ribosomal protein235/236, phosphop-70S6KT421/S424, phospho-ERK1/242/44, phospho-4EBP37/46 and pan-Akt were purchased from Cell Signaling (Boston, MA). Primary antibodies against phospho-p70S6KSer411 were purchased from Abcam (Cambridge, MA). Bleomycin and primary antibodies against β-actin and fibronectin were purchased from Sigma (St. Louis, MO). Anti-collagen-I was purchased from Rockland (Gilbertsville, PA). All HRP-conjugated secondary antibodies were obtained from Bio-Rad (Hercules, CA) and Alexa-labelled secondary antibodies were purchased from Life Technologies (Carlsbad, CA). TGFβ was purchased from R&D (Minneapolis, MN), whereas LY294002 inhibitor, U0126 inhibitor, and Rapamycin were purchased from EMD Millipore Bioscience (San Diego, CA). PF4708671 inhibitor was obtained from Santa Cruz Biotechnology (Dallas, TX). Adenoviral particles encoding CA-Mek1 and DN-Mek1 were obtained from Cell Biolabs, Inc. (San Diego, CA), whereas retroviral plasmids encoding CA-Akt1 and DN-Akt1 were generated in the laboratory [6].
2.2. Virus-mediated transfections
For adenoviral infection, NIH 3T3 fibroblasts were grown to 75 % confluence in 6-well plates. Next, cells were washed with 1X PBS and 1 ml of medium, supplemented with 10 µg of polybrene, followed by 5×108 PFU/ml of adenoviral particles encoding DN-Mek1 (Mek1-S217A/S221A) or 1×108 PFU/ml of adenoviral particles encoding CA-Mek1 (Mek1-S217E/S221E). After 24 h, medium was replaced and incubated for additional 24 h (~95% transfection efficiency was obtained). For retroviral infection, phoenix cells were transfected with pBabe CA-Akt1 (Myristoylated Akt1) or pBabe DN-Akt1 (Akt1-K179M) plasmids with puromycin resistance. After 48 h, retroviral particles were collected and added to NIH 3T3 Fibroblasts in 1 ml of medium, supplemented with 10 µg of polybrene. After antibiotic selection of stable NIH 3T3 fibroblasts, 100% transfection was confirmed by GFP staining. Primary human foreskin fibroblasts, passages 11–16, were kindly gifted by Dr. Tatiana Byzova, Cleveland Clinic, Cleveland, OH. Human foreskin fibroblasts were transfected by retroviral infection and subjected to puromycin selection, and re-infected by adenoviral particles for coexpression of proteins.
2.3. Analysis of ECM secretion
A modification of previously described protocol was used for the analysis of secreted fibronectin [8]. Fibroblasts, either treated with 5 µM U0126, 5 µM LY294002, 25 nM Rapamycin and/or 10 µM PF4708671 alone or together with cells transfected with: pBabe empty vector, CA-Akt1 vector, DN-Akt1 vector, DN-Mek1 or CA-Mek1 particles, respectively or in combination of CAAkt1 with DN-Mek1 or DN-Akt1 with CA-Mek1, were grown in six-well plates in serum-free medium. Conditioned media were collected after 16 h. Next, cells were lysed using lysis buffer after treatment with control vehicle (DMSO) or TGFβ (100 pM). Fibronectin and collagen type I from the conditioned media was concentrated by TCA precipitation and was re-dissolved in normal saline, and pH adjusted using NaOH solution. Cell lysates and conditioned media were subjected to Western analyses of secreted ECM proteins using specific antibodies.
2.4. Analysis of fibronectin matrix assembly
Fibronectin matrix assembly was analyzed based on the immuno-fluorescence staining of insoluble fibronectin fibrils as described previously [6, 7]. Briefly, NIH 3T3 fibroblasts, stably transfected with pBabe empty vector, CA-Akt1 or DN-Akt1 were co-transfected with Ad-DN-Mek1 Ad-CA-Mek1, particles, Retro-CA-Akt1 co-transfected with Ad-DN-Mek1 and Retro-DN-Akt1 co-transfected with Ad-CA-Mek1 particles, were plated on cell culture chamber slides (Fisher scientific, Pittsburgh, PA) at 90–100 % of confluence and cultured for the next 8 h in serum free medium. Next, cells were fixed with 2% paraformaldehyde in 1 × PBS for 30 min followed by blocking with 5 % BSA for 1 h at room temperature. The fixed and blocked cells were incubated with primary antibody against fibronectin (dilution 1:1000) overnight at 4°C, followed by washing with 1 × PBS (3 × for 15 min each). Next, Alexa Fluor 488-labeled secondary antibodies were added and incubated room temperature for 1 h (dilution 1:1000). Slides were washed and mounted with Vectashield (Vector Laboratories). The images were taken using Zeiss fluorescent microscope (Zeiss Axiovert100M, Carl Zeiss, Germany).
2.5. HUTS-4 Binding Assay
Activation-dependent ligand HUTS-4 antibodies were used to determine the activation status of β1 integrins [6, 22]. To analyze HUTS-4 binding, serum-starved human foreskin fibroblasts were cultured in a 96-well plate (85–95% of confluence) and were incubated with HUTS-4 antibodies (30 µg /ml) for 40 min. Wells were washed with PBS, fixed in 2% paraformaldehyde and further incubated with anti-rabbit Alexa 555 antibodies for another 1 h. Plate was then subjected to quantification of fluorescence in a Synergy HT multi-plate reader with excitation wavelength at 484/20 nm and emission wavelength at 590/35 nm.
2.6. Cell adhesion and spreading assay
Cell adhesion assays were performed as previously described [6]. NIH 3T3 fibroblasts (1 × 105) were suspended in serum-free medium and were immediately transferred to fibronectin-coated 12-well plates. After incubation at 37 °C for 45 min, the wells were washed three times with 1X PBS. Cells were then fixed with 4% paraformaldehyde and stained with 1% crystal violet solution, and adherent cells were examined microscopically to study the number and diameter of the spreading cells as quantified manually and using Image J software.
2.7. Western analysis
Whole cell lysates were prepared using lysis buffer [50 mM Tris-HCl (pH=7.4), 1% TritonX-100, 150mM NaCl, 1mM EDTA, 2mM Na3VO4, and 1X Complete protease inhibitors (Roche Applied Science, Indianapolis, IN)]. The protein concentration was measured by the Dc protein assay (Bio-Rad, Hercules, CA). Western analyses were performed using standard Laemmli's method as done previously [8]. Densitometry was done using NIH Image J software.
2.8. Statistical Analysis
All the data are presented as means ± SD. To determine significant differences between treatment and control values, we used either one way ANOVA for groups of 3 or more, or the Student’s two-tailed t test for studies including 2 independent groups. The significance was set at 0.05 levels (marked with symbols wherever data are statistically significant).
3. Results
3.1. PI3Kinase/Akt and Mek1/2/ERK1/2 pathways modulate the synthesis and secretion of ECM proteins
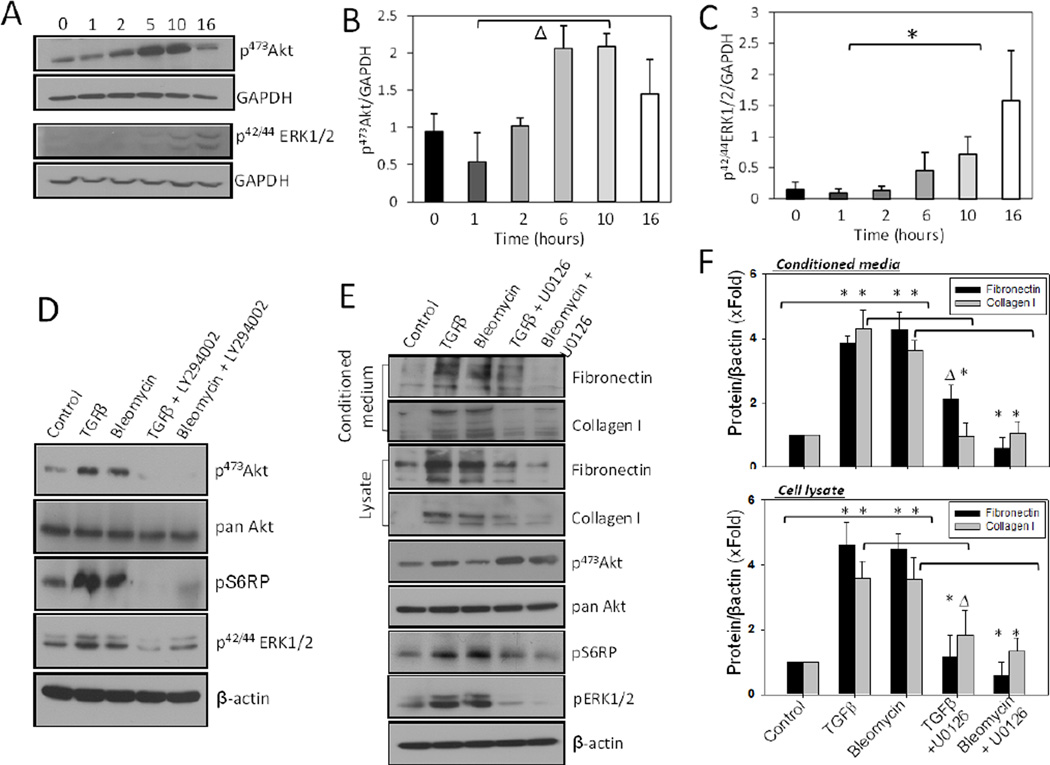
First, we determined the time-course of changes in the phosphorylation of Akt and ERK1/2 in response to 100 pM TGFβ up to16 h (Figure 1A–C). While treatment of NIH 3T3 fibroblasts with 100 pM of TGFβ or 1 mU of bleomycin up to 16 h increased phosphorylation of Akt, ERK1/2 and ribosomal S6 protein (S6RP; substrate of mTOR), co-treatment with 5 µM PI3 kinase inhibitor LY294002 inhibited both TGFβ- and bleomycin-induced phosphorylation of Akt and ERK1/2, suggesting that both Akt and ERK1/2 pathways mediate TGFβ-mediated function downstream of PI3 kinase activation (Figure 1D). Whereas our previous studies have demonstrated the importance of Akt-mTOR pathway in ECM secretion, in our current study, co-treatment with Mek1/2 inhibitor U0126 significantly inhibited the synthesis and secretion of fibronectin and collagen Type I in cell lysates and conditioned media, respectively, associated with a decrease in the phosphorylation of Mek1/2 substrate ERK1/2 (Figures 1E and F). Interestingly, U0126 treatment resulted in increased phosphorylation of Akt, but reduced phosphorylation of S6RP in NIH 3T3 fibroblasts (Figure 1E).
Figure 1. Inhibition of Mek1 and Akt1 decreases synthesis and secretion of ECM proteins.
(A) NIH3T3 fibroblasts were treated with 100 pM of TGFβ and cell lysates prepared at various time intervals were subjected for Western analysis of changes in the phosphorylation of Akt and ERK1/2. (B) Band densitometry analysis of NIH 3T3 cell Western blot images showing changes in the levels of pS473Akt levels at different time points post TGFβ treatment. (C) Band densitometry analysis of NIH 3T3 cell Western blot images showing changes in the levels of p42/44ERK1/2 levels at different time points post TGFβ treatment. (D) NIH3T3 fibroblasts were treated with 100 pM of TGFβ or 1mU of Bleomycin and co-treated with 5 µM of LY294002 inhibitor (PI3 kinase inhibitor) in serum free medium. After 16 hours, cells were lysed using lysis buffer. (E) NIH 3T3 fibroblasts were treated with 100 pM of TGFβ or 1mU Bleomycin and co-treated with 5 µM of U0126 compound (Mek1 inhibitor) in serum free medium. After 16 hours, conditioned media were collected, followed by TCA precipitation, and cells were lysed using lysis buffer. Both, the precipitated proteins from conditioned media and cell lysates were subjected to Western blot analysis to detect fibronectin and collagen I, whereas the cell lysates were additionally analyzed for changes in phosphorylation level in molecules of the Akt-mTORcRaf pathway such as: Akt, S6 ribosomal protein, and ERK1/2. (F) Band densitometry analysis of NIH 3T3 cell lysate and conditioned medium Western analysis indicating changes in the expression of fibronectin and collagen I with TGFβ and bleomycin treatment in the presence and absence of Mek1 inhibitor. Data presented as mean ± SD (*p<0.001; Δp<0.01; n=3).
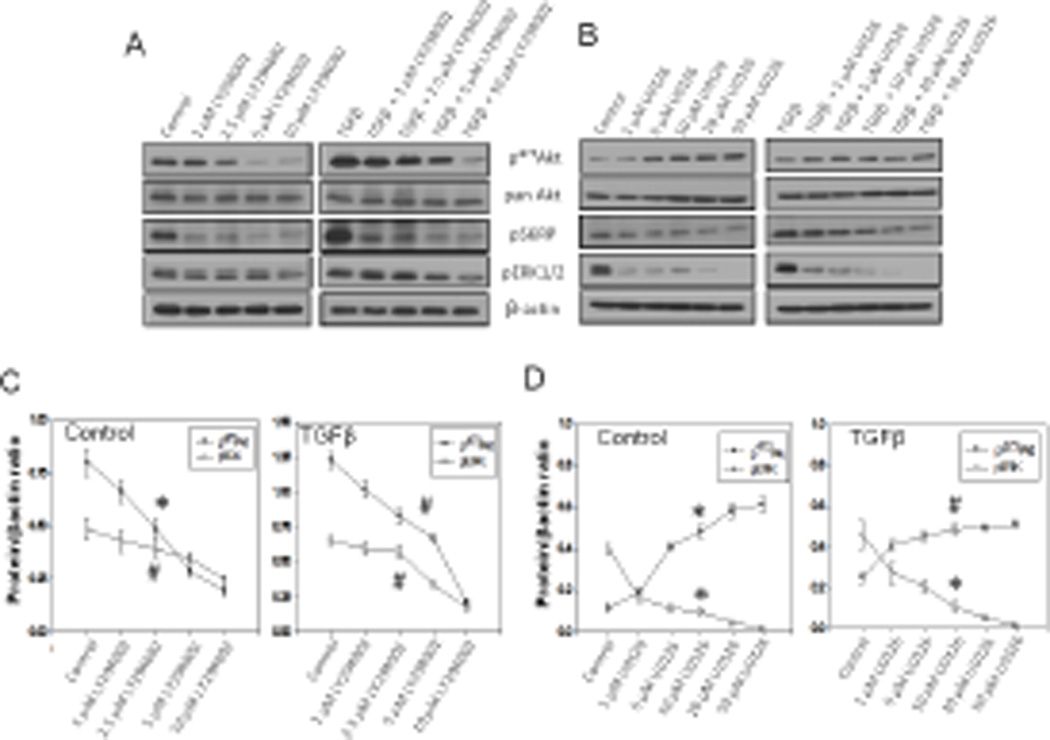
In order to further confirm this observation, we performed a dose-response study of PI3 kinase and Mek inhibitors (LY294002 and U0126, respectively) on Akt, ERK1/2 and mTOR activity modulation. Our study revealed that 1–10 µM range of LY294002 significantly inhibited Akt, S6RP and ERK1/2 phosphorylations in a dose-dependent manner in the presence and absence of TGFβ (Figures 2A and C). In contrast, Mek inhibition by 1–50 µM range of U0126, although reduced ERK1/2 and S6RP phosphorylations, resulted in significantly increased Akt phosphorylation in the presence and absence of TGFβ (Figures 2B and D). These results suggest the potential involvement of Mek-ERK1/2 pathway, in addition to Akt, in the activity regulation of mTOR and synthesis and secretion of ECM proteins.
Figure 2. Mek1 and PI3K inhibition modulates Akt, mTOR and ERK1/2 activities in a dose-dependent manner.
(A) Western blot analysis of cell lysates from NIH 3T3 fibroblasts treated with LY294002 inhibitor at doses: 1, 2.5, 5, and 10 µM for 16 hours, in the presence and absence of 100 pM TGFβ. (B) Western blot analysis of cell lysates from NIH 3T3 fibroblasts treated with U0126 inhibitor at doses: 1, 5, 10, 20, and 50 µM for 16 hours, in the presence and absence of 100 pM TGFβ. All cell lysates were subjected to detect changes in phosphorylation level of: Akt, S6 ribosomal protein and ERK1/2. (C) Graph representing changes in the phosphorylation level of Akt and ERK1/2 after cell treatment with LY294002 inhibitor; the data are presented as mean ± SD (n=3). (D) Graph representing changes in the phosphorylation of Akt and ERK1/2 after cell treatment with U0126 inhibitor. Data presented as mean ± SD (*p<0.001; Δp<0.01; #p<0.05; n=3).
3.2. Both Mek1 and Akt1 are important for ECM protein synthesis in fibroblasts
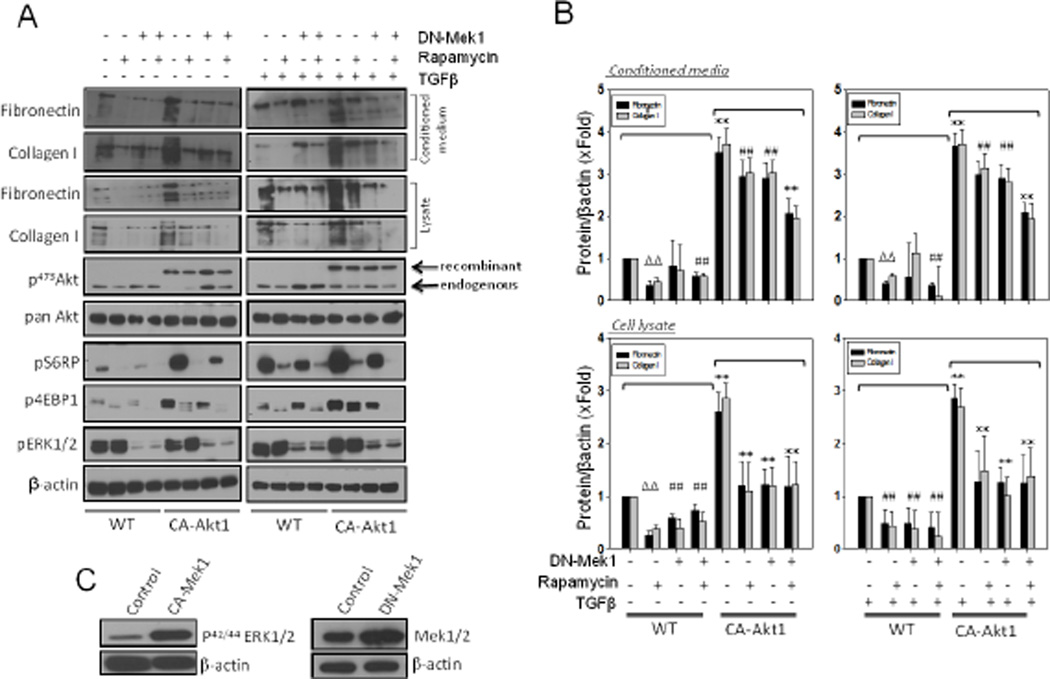
Control and TGFβ-stimulated NIH 3T3 fibroblasts were treated with mTOR inhibitor rapamycin and/or expressed them with DN-Mek1 in the presence and absence of stable expression of CA-Akt1. Effect of rapamycin and Akt1 expression on mTOR activity was determined through the analysis of phosphorylation of mTOR substrates p4EBP1 and pS6RP (Figure 3A). In control and TGFβ-stimulated NIH 3T3 fibroblasts, treatment with rapamycin or expression of DN-Mek1 significantly inhibited expression of fibronectin and collagen type I in cell lysates. Interestingly, although rapamycin treatment reduced the levels of fibronectin and collagen I in the conditioned medium, expression of DN-Mek1 did not exhibit any significant effect on ECM levels in the conditioned medium (Figures 3A and B). Whereas cells containing CA-Akt1 expressed significantly higher levels of ECM (~3 fold) in both the cell lysates and the conditioned medium, this effect was significantly inhibited by co-expression of DN-Mek1 or treatment with rapamycin. Whereas rapamycin and DN-Mek1 had only a modest effect on inhibiting CA-Akt1-mediated ECM expression, a combination of rapamycin treatment and DN-Mek1 expression exhibited a significant additive effect in inhibiting CA-Akt1-induced fibronectin and collagen I expression (Figures 3A and B). Increased levels of p42/44 ERK in cells expressing CA-Mek1 and increased expression of Mek1 in cells expressing DN-Mek1, compared to vector control cells is shown in Figure 3C. Interestingly, although DN-Mek1 expression significantly reduced the expression of ECM proteins in fibroblasts, no significant change in either fibronectin or collagen I levels were observed in the conditioned media in the absence or presence of TGFβ. Another important finding from our experiment was that expression of DN-Mek1 in fibroblasts, although reduced phosphorylation of ERK1/2, it resulted in increased Akt phosphorylation in control and TGFβ treated cells (Figure 3A). As observed in the case of U0126 treatment, expression of DNMek1 resulted in partial inhibition of S6RP phosphorylation despite Akt activation. This effect of DN-Mek1 was also observed in CA-Akt1 expressing fibroblasts (Figure 3A), thus suggesting the role of Mek1 in the activation of mTOR signaling and subsequent regulation of ECM remodeling.
Figure 3. Both Mek1 and Akt1 are important for ECM protein synthesis in fibroblasts.
(A) NIH 3T3 fibroblasts were viral transfected with either CA-Akt1 and/or DN-Mek1 and/or co-treated with 25 µM of Rapamycin (mTOR inhibitor) in serum free medium with or without TGFβ stimulation. After 16 hours, conditioned media were collected, followed by TCA precipitation and cells were lysed using lysis buffer. Precipitated proteins from conditioned media were subjected to Western blot analysis to detect fibronectin and collagen type I, whereas cell lysates were analyzed for changes in the phosphorylation level of the molecules of AktmTOR-cRaf signaling pathways such as: Akt, S6 ribosomal protein, 4EBP1 and ERK1/2. (B) Graph showing changes in the synthesis and secretion of fibronectin and collagen I in cell lysates and conditioned media from cells being either transfected with Ad-DN-Mek1 and/or treated with Rapamycin inhibitor. (C) Western blot images of NIH 3T3 cell lysates transfected with Ad-CA-Mek1 and Ad-DN-Mek1 showing expression of viral expressed proteins. Data presented as mean ± SD (*p<0.001; Δp<0.01; #p<0.05; n=3).
3.3. Akt1 and Mek1 differentially regulate ECM secretion and assembly
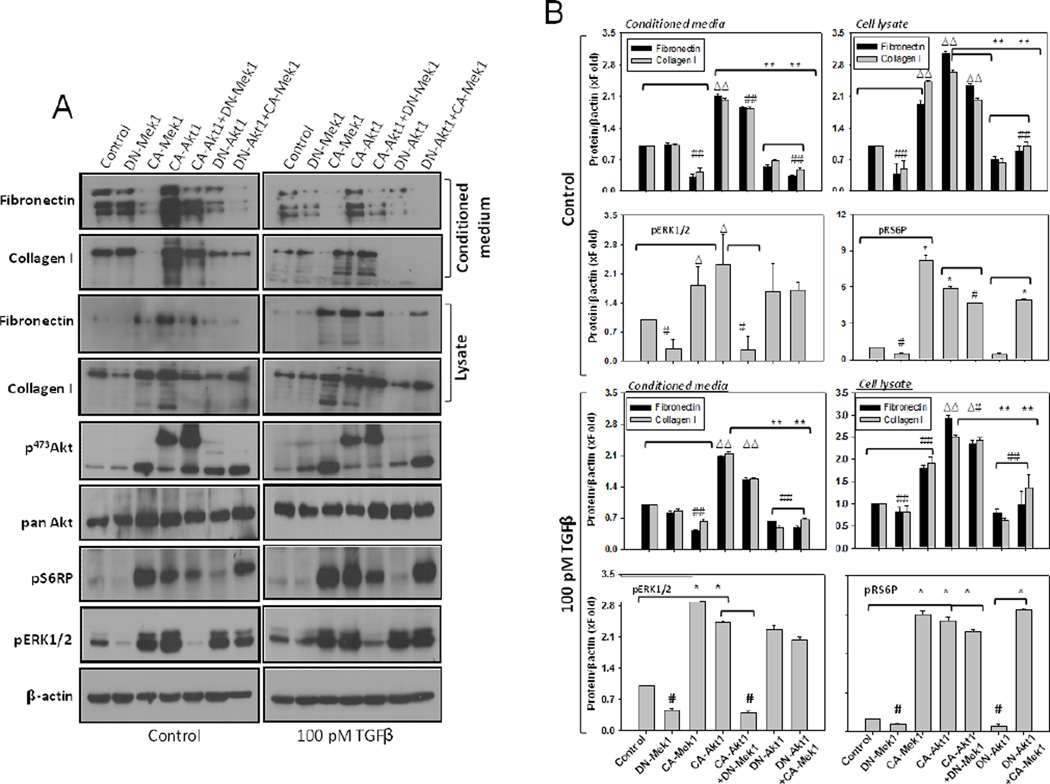
In our analysis, whereas DN-Mek1 expression in fibroblasts resulted in reduced ECM levels in the cell lysates, significant difference was not observed in the conditioned media collected from DN-Mek1 expressing fibroblasts, compared to control (Figures 4A and B). In contrast, whereas CA-Mek1 expression resulted in reduced ECM levels in the conditioned medium, a significant increase in both fibronectin and collagen I was observed in the cell lysate, compared to control (Figures 4A and B). Similar results were observed even in the presence of TGFβ stimulus (Figures 4A and B). Together, these data suggested specific roles for Akt1 and Mek1 in fibroblasts in mediating ECM secretion and assembly, respectively.
Figure 4. Modulation of Akt1 and Mek1 activities, in turn, modulates secretion of ECM proteins by the fibroblasts.
(A) Western blot analysis of cell lysates and conditioned media from NIH 3T3 fibroblasts transfected with CA-Akt1, DN-Akt1, CA-Mek1 and DN-Mek1, respectively or in combination of CA-Akt1 with DN-Mek1 and DN-Akt1 with CA-Mek1, followed by treatment with DMSO (control vehicle; left panel) or 100 pM TGFβ (right panel) in serum free medium. (B) Bar graphs showing densitometry analysis of cell lysates and conditioned media for fibronectin and collagen I, as well as phosphorylated Akt, S6 ribosomal protein, and ERK1/2, normalized to βactin, followed by treatment with DMSO (control vehicle; above) or 100 pM TGFβ (below) in serum free medium.. Data presented as mean ± SD (*p<0.001; Δp<0.01; #p<0.05; n=3).
3.4. Cooperation between Akt1 and Mek1 pathway is necessary for overall ECM remodeling
We next expressed NIH 3T3 fibroblasts with DN-Akt1, DN-Mek1, CA-Akt1 and CA-Mek1 alone and in various combinations. Surprisingly, expression of DN-Mek1 and CA-Mek1 both resulted in increased phosphorylation of Akt (Figures 4A and B). In contrast, the effect of Akt1 on ECM levels in the cell lysate and the conditioned media was as expected. Whereas DN-Akt1 expression in fibroblasts resulted in significant reduction in the levels of ECM proteins in both cell lysates and the conditioned media, expression of CA-Akt1 resulted in increased ECM production. Although DN-Mek1 resulted in increased Akt1 phosphorylation in NIH 3T3 fibroblasts, expression of CA-Akt1 significantly increased ERK1/2 phosphorylation, which was reduced in DN-Akt1 fibroblasts (Figures 4A). Interestingly, whereas both CA-Mek1 and CAAkt1 resulted in increased phosphorylation of mTOR substrate S6RP and inactive isoforms of Akt1 and Mek1 inhibiting S6RP phosphorylation, effect of DN-Akt1 expression on S6RP was reversed with co-expression of CA-Mek1. In contrast, increased phosphorylation of S6RP with CA-Akt1 expression was blunted upon co-expression of DN-Mek1. This suggested that Mek1-mediated effect on ECM remodeling is mediated through its ability to modulate S6RP phosphorylation independent of Akt1 and mTOR pathways. Also, Akt1-mediated effect on ECM assembly, but not secretion, may be due to the ability of Akt1 to activate Mek1 pathway.
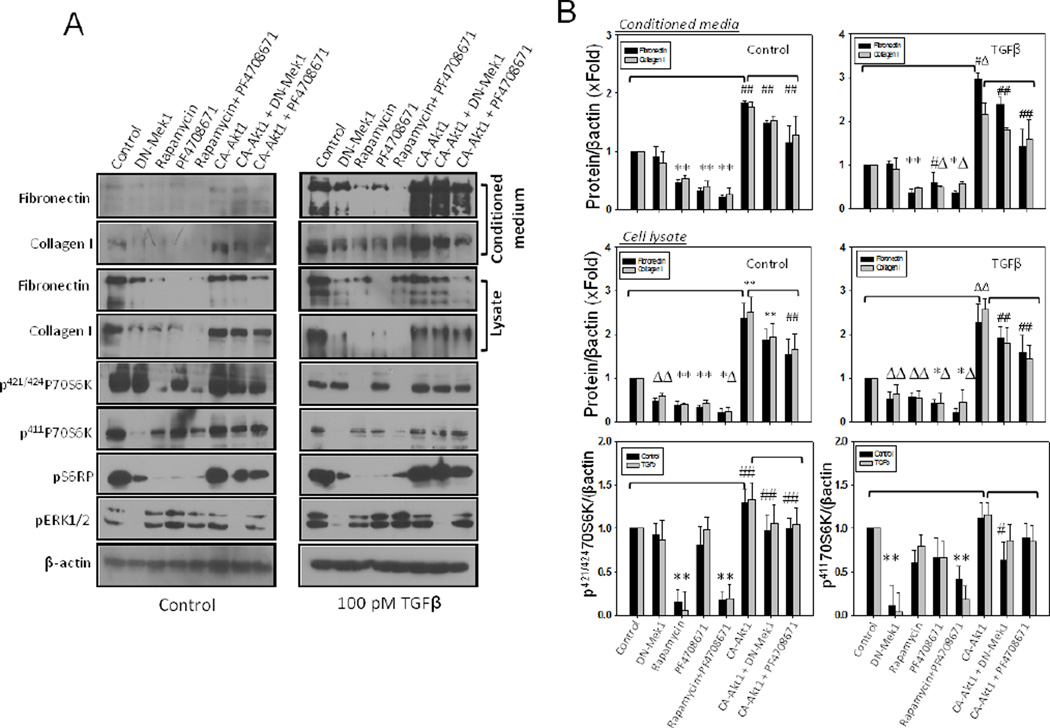
3.5. p70 S6-kinase (p70S6K) reconciles Akt1 and Mek1 pathways in the regulation of ECM secretion
Next, we examined if Mek1 is able to activate p70S6K via a mechanism different from the Akt1-mTOR pathway. We specifically looked into changes in the phosphorylation of Serine 411 residue on p70S6K, which has also been shown to be involved in its activation [25]. Our analysis showed that while DN-Mek1 had no effect on pT421/S424p70S6K levels, it significantly reduced pS411p70S6K levels, which in turn, correlated with the phosphorylation of S6RP phosphorylation (Figure 5A). As expected, treatment with p70S6K inhibitor, PF4708671, inhibited the expression levels of collagen I and fibronectin in both cell lysates and conditioned media in the presence or absence of TGFβ. This effect was at par with mTOR inhibition with rapamycin (Figures 5A and B). Increased ECM secretion as a result of CA-Akt1 expression in fibroblasts, was partially inhibited by treatment with PF4708671, thus suggesting that p70S6K is a connecting link between Akt1-mTOR and Mek1 pathways in the regulation of ECM secretion.
Figure 5. p70S6 kinase synergizes Akt1 and Mek1-mediated synthesis and secretion of ECM proteins by the fibroblasts.
(A) Western blot images of cell lysates and conditioned media from either NIH 3T3 fibroblasts expressing either CA-Akt1 or DN-Mek1 and/or co-treated with Rapamycin (mTOR inhibitor) or PF4708671 (p70S6 Kinase inhibitor), followed by treatment with DMSO or 100 pM TGFβ in serum free medium. (B) Bar graphs showing densitometry analysis of cell lysates and conditioned media for the synthesized and secreted fibronectin and collagen I expressing either CA-Akt1 or DN-Mek1 and/or co-treated with Rapamycin or PF4708671, followed by treatment with DMSO or 100 pM TGFβ in serum free medium. Data presented as mean ± SD (*p<0.001; Δp<0.01; #p<0.05; n=3).
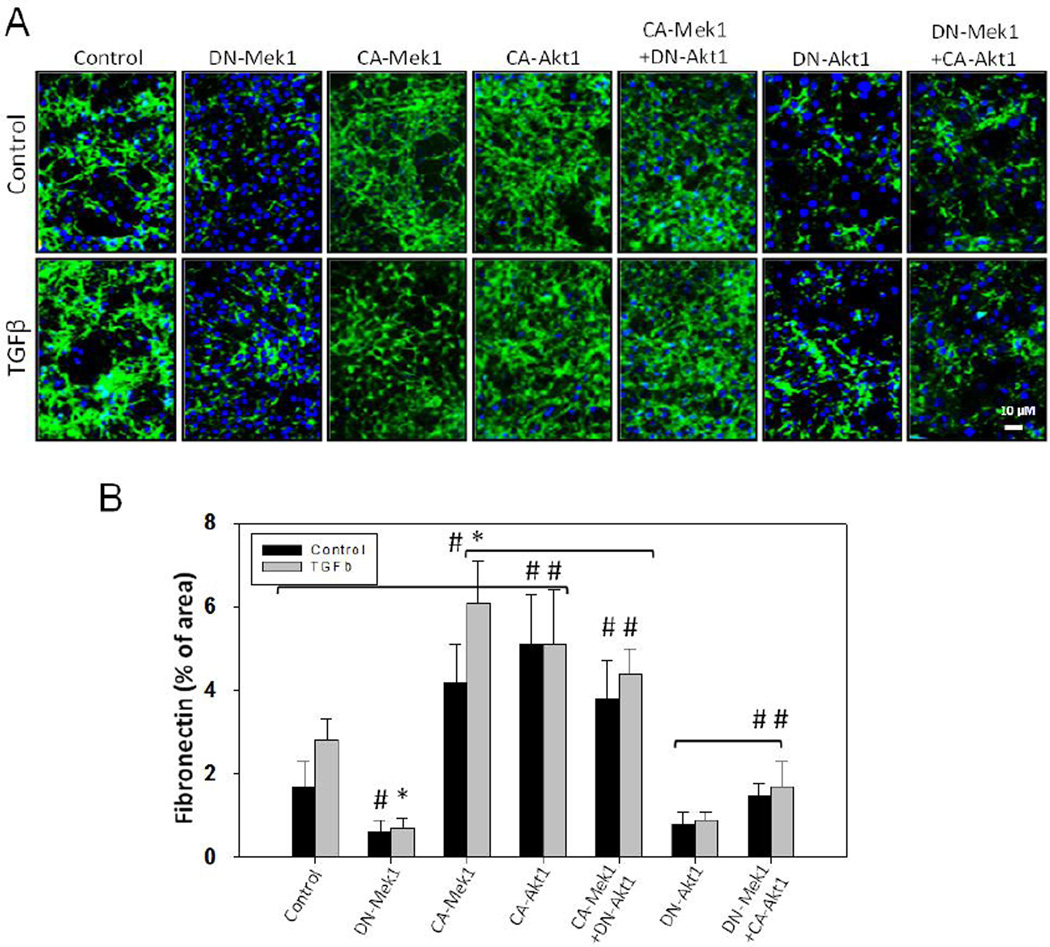
3.6. Akt1 and Mek1 cooperate in mediating fibronectin matrix assembly
We next performed fibronectin matrix assembly assay in NIH 3T3 fibroblasts expressing DNMek1, CA-Mek1, DN-Akt1, CA-Akt1 as well as CA-Mek1 in combination with DN-Akt1, and DN-Mek1 in combination with CA-Akt1. We observed that expression of CA-Akt1 significantly enhanced fibronectin matrix assembly; an effect that is impaired with DN-Akt1 expression (Figure 6A and B). Similarly, whereas expression of CA-Mek1 enhanced fibronectin matrix assembly, expression of DN-Mek1 significantly impaired the process (Figure 6A and B). Interestingly, while a modest inhibition of fibronectin matrix assembly was observed in cells coexpressing CA-Mek1 and DN-Akt1 as compared to CA-Mek1 expressing controls, only a partial rescue in fibronectin matrix assembly was observed in cells co-expressing DN-Mek1 and CAAkt1 (Figure 6A and B), suggesting that Mek1 activity is necessary for the overall fibronectin matrix remodeling.
Figure 6. Akt1/Mek1 axis signaling regulates overall fibronectin matrix assembly by the fibroblasts.
(A) Representative fluorescent pictures of NIH3T3 fibroblasts expressing either CAAkt1, DN-Akt1, CA-Mek1, DN-Mek1 or a combination of CA-Akt1 with DN-Mek1 or DNAkt1 with CA-Mek1 treated with 100pM TGFβ (or DMSO) in serum free medium. After 8 hours, cellular layers were immunostained for fibronectin and probed with Alexa 488. (B) Bar graph showing NIH-ImageJ based quantitative analysis of assembled fibronectin by the fibroblast layers expressing either CA-Akt1, DN-Akt1, CA-Mek1, DN-Mek1, or a combination of CA-Mek1 with DN-Akt1 or DN-Mek1 with CA-Akt1 treated with 100pM TGFβ (or DMSO) in serum free medium. Data presented as mean ± SD (*p<0.001; #p<0.05; n=3).
3.7. Akt1 and Mek1 cooperation mediated integrin activation
We next determined if Akt1-Mek1-p70S6K signaling is involved in the regulation of integrin β1 function in fibroblasts. Our results indicated that fibroblasts expressing either CA-Akt1 or CA-Mek1 resulted in enhanced adhesion on to fibronectin, a ligand for integrin β1 (Figure 7A and B). Interestingly, an additive effect of CA-Mek1 over the effect of CA-Akt1 on integrin activation was not observed in cells co-expressing CA-Akt1 and CA-Mek1. Next, we subjected human foreskin fibroblasts transfected with various Akt1 and Mek1 plasmids, either alone or in combinations, and in the presence and absence of p70S6K inhibitor, to determine their effect on HUTS-4 binding, a highly specific assay for integrin β1 activation. In our results, whereas cells expressing DN-Akt1 or DN-Mek1 exhibited impaired HUTS-4 binding, cells expressing CA-Akt1 or CA-Mek1 exhibited enhanced HUTS-4 binding compared to vector control cells (Figure 7C). Treatment of cells co-expressing CA-Mek1 and CA-Akt1 with p70S6K inhibitor PF4708671 significantly inhibited cell adhesion, spreading and HUTS-4 binding indicating the role of p70S6K in the process (Figure 7A–C).
Figure 7. Akt1/Mek1 axis signaling regulates integrin activation in fibroblasts.
(A) Representative bright field images of NIH3T3 fibroblasts expressing either CA-Akt1 or a combination of CA-Akt1 with CA-Mek1 in the presence and absence of p70S6 kinase inhibitor PF4708671 in serum free medium showing the number of cells attached to plated fibronectin in 6-well plates. (B) Bar graph showing NIH-ImageJ based quantitative analysis of number of cells adhered to fibronectin with the expressions as above. (C) Bar graph showing HUTS-4 binding fluorescence analysis of human foreskin fibroblasts expressing either DN-Akt1, CA-Akt1, DN-Mek1, CA-Mek1 or a combination of CA-Akt1 with CA-Mek1 in the presence and absence of p70S6 kinase inhibitor PF4708671 in serum free medium. (D) Schematic representation of how p70S6 Kinase harmonizes Akt1 and Mek1 pathways in extracellular matrix remodeling. Data presented as mean ± SD (*p<0.001; Δp<0.01; #p<0.05; n=4).
Collectively, our results clearly demonstrated that Akt1 and Mek1 synergize their effects in mediating ECM secretion and assembly, and that activation of p70S6K via phosphorylation at T421/S424 and S411 by Akt1 and Mek1/ERK1/2, respectively, is at least in part responsible for their cooperation in mediating ECM secretion and assembly (Figure 7D).
4. Discussion
Fibronectin and collagens, the major elements of the ECM, interact with integrins to form the meshwork that determines the structure and function of tissues such as skin, brain, lung, and heart [26]. Synthesis, secretion and assembly of ECM proteins are the crucial steps in the process of ECM remodeling. We [4, 6–8] and others [27, 28] have demonstrated the integral role of Akt1/mTOR signaling in ECM secretion. Apart from the Akt1/mTOR pathway, cRaf/Mek1/2 signaling has also been implicated in the regulation of ECM remodeling, particularly in the assembly of ECM proteins [10]. However, reports on the mutual interactions and activity modulation between Akt and cRaf/Mek1/2 pathways in various in vitro and in vivo settings have been conflicting [11],[12]. Thus, further investigation is necessary to determine the net effect of individual and/or combined activity modulation of Akt and Mek1/2 pathways in fibroblast will have on ECM remodeling. In the current study, we demonstrated for the first time that Akt1 synergizes with Mek1 in the regulation of ECM secretion and assembly by the fibroblasts in vitro, and this cooperation is mediated through p70 S6K and integrin β1.
Akt was first reported to inhibit cRaf activity through direct phosphorylation at S259 leading to subsequent inhibition of Mek1/2/ERK1/2 pathway in HEK293 cells [11]. Follow-up studies from various laboratories confirmed that cRaf and Akt pathways mutually inhibit their activities in response to certain growth factors and often elicit opposite effects under physiological and pathological [15, 16, 29]. These reports questioned if at all any cooperation between Akt and Mek pathways is possible in physiology or pathology or whether the cooperation or mutual inhibition of these pathways is cell or context dependent. But, an elegant study from Joseph Avruch laboratory identified the sequence of events involved in the activity modulation of cRaf [12]. This study, which provided insights on the complex nature of cRaf activity regulation by Ras, Akt and other unknown kinases, also revealed that Akt can both inhibit and activate cRaf signaling depending on the status of other signaling pathways. Accordingly, although cRafS259 phosphorylation by Akt inhibited cRaf activity, phosphorylation of cRafS338 by an unknown kinase promoting its interaction with adaptor protein 14-3-3 prevailed over the inhibitory effect of Akt, and was necessary for the stable activation of cRaf. The kinase responsible for the phosphorylation of cRafS338 was later identified to be Pak1 [30].
Numerous studies demonstrated the cooperation between Akt and cRaf/Mek signaling in various cellular, physiological and pathological processes including adaptive angiogenesis and cancer [17–20]. We showed that phosphorylation of cRafS338 by Pak1 in Rat-1 fibroblasts activates cRaf and is necessary for the coupling of Akt1 and cRaf pathways in mediating oncogenic transformation [14, 31]. We also showed that combined inhibition of Akt and Mek pathways had significantly higher effect on the inhibition of prostate cancer cell motility, proliferation and colony formation as compared to monotherapy [21]. These findings suggest that mutual activity modulation and cooperation between Akt and Mek pathways may be cell, tissue or context specific. Recently, cooperation between Akt and Mek pathways have also been reported in colorectal [32], rhabdomyosarcoma [33] and many other cancers [34, 35]. While most of these studies are focused on cancers where different signaling pathways are already deregulated, details regarding mutual activity regulation between Akt1 and Mek1 in many physiological processes are only emerging.
Whereas increased ERK1/2 activity in the mouse endothelial cells is achieved through down regulation of PI3 kinase activity or by suppressing Akt1 activity [29], same study indicated activation of ERK1/2 in a zebra fish model promoting arteriogenesis via inhibition of PI3 Kinase/Akt pathway. In contrast, a recent study in a tumor model implicated the role of ERK-2 in the re-activation of Akt pathway [36]. In another study, PI3 Kinase/Akt and Mek/ERK1/2 pathways did not influence each other’s activity, but co-targeting them inhibited uveal melanoma cell proliferation [37]. Interestingly, our study revealed that an increase or decrease in Akt1 activity, both results in enhanced ERK1/2 phosphorylation. Similarly, a decrease in Mek1/2 activity via pharmacological (U0126) or genetic approaches or increase in Mek1 activity, both resulted in a significant increase in Akt phosphorylation. While the mechanisms leading to this interesting cross-talk between Akt1 and Mek1 is not clear from our current study, our data however demonstrate that both these signaling pathways are activated together in response to TGFβ and cooperate in the modulation of ECM remodeling.
It is not clear how the effects of Akt1 and Mek1/2 cooperation are reconciled in mediating ECM remodeling. From our past studies, we know that Akt1 utilizes mTOR for the synthesis of ECM proteins [8] and activates integrin β1 [6] in mediating assembly of ECM proteins. Hence, we sought to determine if Mek-ERK1/2 pathway can cooperate with Akt1 in activating mTOR and integrin β1 signaling in mediating ECM secretion and assembly, respectively. While inhibition of mTOR with rapamycin had no effect on ERK1/2 phosphorylation, genetic and pharmacological inhibition of Mek1 reduced phosphorylation of S6RP, one of the secondary substrates of mTOR, but not 4EBP1, a direct substrate of mTOR. This indicated that Mek1, although did not influence mTOR activity, may be responsible for the activation of a downstream substrate of mTOR, thus cooperating with Akt1 in regulating ECM remodeling.
Since S6RP is phosphorylated by p70S6K, an mTOR substrate, we next determined if Akt1 and Mek1 have independent ways to activate this kinase. A major known mechanism for the activation of p70S6K is by its phosphorylation at residues T421/S424 by mTOR [24]. In addition to this, phosphorylation of another residue implied in p70S6K activation of S411 [38], and the kinase that is responsible for this phosphorylation is yet to be identified. Nevertheless, there is a report that cRaf/Mek pathway may be responsible for the phosphorylation of S411 on p70S6K [25]. Our study showed that whereas DN-Mek1 inhibition resulted in a decrease in S411 p70S6K phosphorylation, expression of CA-Mek1 resulted in increased S411 p70S6K phosphorylation. Furthermore, specific inhibition of p70S6K resulted in reduced expression of fibronectin and collagen I by the fibroblasts. These results demonstrated that Akt1 and Mek1 reconciled their effects on ECM secretion through activation of p70S6K.
Another interesting observation from our study is that, although CA-Mek1 expression in fibroblasts resulted in increased fibronectin and collagen I expression, their levels in the conditioned medium was significantly lesser. This usually happens when there is increased integrin activation subsequently leading to increased conversion of soluble ECM proteins into insoluble fibers attached to the cells [39, 40]. Our study revealed that while activation of both Akt1 and Mek1 resulted in increased fibronectin matrix assembly, and HUTS-4 binding, activation of Mek1 in DN-Akt1 expressing fibroblasts significantly rescued the defects in ECM assembly. Since integrin β1 is necessary for fibronectin matrix assembly [6] via involving Rac1/P21 activated kinase-1 (Pak1) pathway [7], our studies reveal the importance of Mek1, in addition to Akt1, in the process. Our findings support an important observation from another group on the role of p70S6K in the activation of Rac1 and cdc42 in cytoskeletal remodeling and integrin activation [41]. Overall, we demonstrate the cooperation between Akt1 and Mek1 in the regulation of ECM secretion and assembly through activation of p70S6K and integrin β1, respectively. We suggest that co-targeting Akt1 and Mek1 for ECM-related diseases may be more effective as compared to monotherapy. However, validation of the results from our in vitro experiments in vivo using suitable animal models will be necessary to strengthen this conclusion.
HIGHLIGHTS.
The cooperation between Akt1 and Mek1 in extracellular matrix remodeling is unclear
The synergism between Akt1 and Mek1 mediates extracellular matrix remodeling
p70 S6-Kinase couples Akt1 and Mek1 pathways in fibroblasts
Acknowledgements
Funds were provided by the American Heart Association – Scientist Development Grant (0830326N) and in part by the National Institutes of Health grant (R01 HL103952), University of Georgia Research Foundation, Wilson Pharmacy Foundation and UGA College of Pharmacy through intramural grants to PRS. MA was supported by the American Heart Association pre-doctoral fellowship (13PRE17100070). This material is the result of work supported with resources and the use of facilities at the Charlie Norwood VAMC, Augusta, GA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have declared that no conflicts of interest exist.
References
- 1.Mecham RP. Overview of extracellular matrix. In: Bonifacino Juan S, et al., editors. Current protocols in cell biology / editorial board. Unit 10 11. Chapter 10. 2012. [DOI] [PubMed] [Google Scholar]
- 2.Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? American journal of physiology. Gastrointestinal and liver physiology. 2011;301:G950–G955. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramann R, DiRocco DP, Humphreys BD. Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. The Journal of pathology. 2013;231:273–289. doi: 10.1002/path.4253. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nature medicine. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somanath PR, Chen J, Byzova TV. Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis. 2008;11:277–288. doi: 10.1007/s10456-008-9111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somanath PR, Kandel ES, Hay N, Byzova TV. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. The Journal of biological chemistry. 2007;282:22964–22976. doi: 10.1074/jbc.M700241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somanath PR, Byzova TV. 14-3-3beta-Rac1-p21 activated kinase signaling regulates Akt1-mediated cytoskeletal organization, lamellipodia formation and fibronectin matrix assembly. Journal of cellular physiology. 2009;218:394–404. doi: 10.1002/jcp.21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goc A, Choudhary M, Byzova TV, Somanath PR. TGFbeta- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin (mTOR) Journal of cellular physiology. 2011;226:3004–3013. doi: 10.1002/jcp.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdalla M, Goc A, Segar L, Somanath PR. Akt1 mediates alpha-smooth muscle actin expression and myofibroblast differentiation via myocardin and serum response factor. The Journal of biological chemistry. 2013;288:33483–33493. doi: 10.1074/jbc.M113.504290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winters BS, Raj BK, Robinson EE, Foty RA, Corbett SA. Three-dimensional culture regulates Raf-1 expression to modulate fibronectin matrix assembly. Molecular biology of the cell. 2006;17:3386–3396. doi: 10.1091/mbc.E05-09-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 12.Tzivion G, Luo Z, Avruch J. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature. 1998;394:88–92. doi: 10.1038/27938. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Chen Z, Ambrose D, Liu J, Gibbs JB, Chernoff J, Field J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Molecular and cellular biology. 1997;17:4454–4464. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somanath PR, Vijai J, Kichina JV, Byzova T, Kandel ES. The role of PAK-1 in activation of MAP kinase cascade and oncogenic transformation by Akt. Oncogene. 2009;28:2365–2369. doi: 10.1038/onc.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha D, Bannergee S, Schwartz JH, Lieberthal W, Levine JS. Inhibition of ligand-independent ERK1/2 activity in kidney proximal tubular cells deprived of soluble survival factors up-regulates Akt and prevents apoptosis. The Journal of biological chemistry. 2004;279:10962–10972. doi: 10.1074/jbc.M312048200. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Bar-Eli M, Meloche S, Brodt P. Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. The Journal of biological chemistry. 2004;279:19683–19690. doi: 10.1074/jbc.M313145200. [DOI] [PubMed] [Google Scholar]
- 17.Zugasti O, Rul W, Roux P, Peyssonnaux C, Eychene A, Franke TF, Fort P, Hibner U. Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt. Molecular and cellular biology. 2001;21:6706–6717. doi: 10.1128/MCB.21.19.6706-6717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo M, Sakurai H, Ueno Y, Ohtani O, Saiki I. Activation of MEK/ERK and PI3K/Akt pathways by fibronectin requires integrin alphav-mediated ADAM activity in hepatocellular carcinoma: a novel functional target for gefitinib. Cancer science. 2006;97:155–162. doi: 10.1111/j.1349-7006.2006.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor JR, Lehmann BD, Chappell WH, Abrams SL, Steelman LS, McCubrey JA. Cooperative effects of Akt-1 and Raf-1 on the induction of cellular senescence in doxorubicin or tamoxifen treated breast cancer cells. Oncotarget. 2011;2:610–626. doi: 10.18632/oncotarget.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebi H, Costa C, Faber AC, Nishtala M, Kotani H, Juric D, Della Pelle P, Song Y, Yano S, Mino-Kenudson M, Benes CH, Engelman JA. PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P-Rex1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:21124–21129. doi: 10.1073/pnas.1314124110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goc A, Al-Husein B, Kochuparambil ST, Liu J, Heston WW, Somanath PR. PI3 kinase integrates Akt and MAP kinase signaling pathways in the regulation of prostate cancer. International journal of oncology. 2011;38:267–277. [PubMed] [Google Scholar]
- 22.Goc A, Liu J, Byzova TV, Somanath PR. Akt1 mediates prostate cancer cell microinvasion and chemotaxis to metastatic stimuli via integrin beta(3) affinity modulation. British journal of cancer. 2012;107:713–723. doi: 10.1038/bjc.2012.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. The international journal of biochemistry & cell biology. 2011;43:47–59. doi: 10.1016/j.biocel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 24.An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Iqbal IG, Winblad B, Pei JJ. Upregulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer's disease. The American journal of pathology. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, Marumo F, Hirata Y, Inagami T. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. The Journal of biological chemistry. 1999;274:36843–36851. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 26.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Current opinion in cell biology. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Lee Y, Seo JE, Cho KH, Chung JH. Caveolin-1 increases basal and TGF-beta1-induced expression of type I procollagen through PI-3 kinase/Akt/mTOR pathway in human dermal fibroblasts. Cellular signalling. 2008;20:1313–1319. doi: 10.1016/j.cellsig.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Yang T, Liang Y, Lin Q, Liu J, Luo F, Li X, Zhou H, Zhuang S, Zhang H. miR-29 mediates TGFbeta1-induced extracellular matrix synthesis through activation of PI3K-AKT pathway in human lung fibroblasts. Journal of cellular biochemistry. 2013;114:1336–1342. doi: 10.1002/jcb.24474. [DOI] [PubMed] [Google Scholar]
- 29.Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, Mulligan- Kehoe MJ, Byzova TV, Peterson RT, Simons M. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. The Journal of clinical investigation. 2010;120:1217–1228. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. The Journal of biological chemistry. 2005;280:24698–24705. doi: 10.1074/jbc.M413374200. [DOI] [PubMed] [Google Scholar]
- 31.Kichina JV, Goc A, Al-Husein B, Somanath PR, Kandel ES. PAK1 as a therapeutic target. Expert opinion on therapeutic targets. 2010;14:703–725. doi: 10.1517/14728222.2010.492779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Q, Cai W, Zheng Y, Evers BM, She QB. ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene. 2013 doi: 10.1038/onc.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renshaw J, Taylor KR, Bishop R, Valenti M, De Haven Brandon A, Gowan S, Eccles SA, Ruddle RR, Johnson LD, Raynaud FI, Selfe JL, Thway K, Pietsch T, Pearson AD, Shipley J. Dual blockade of the PI3K/AKT/mTOR (AZD8055) and RAS/MEK/ERK (AZD6244) pathways synergistically inhibits rhabdomyosarcoma cell growth in vitro and in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5940–5951. doi: 10.1158/1078-0432.CCR-13-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S, Malaponte G, Mazzarino MC, Fagone P, Nicoletti F, Basecke J, Mijatovic S, Maksimovic-Ivanic D, Milella M, Tafuri A, Chiarini F, Evangelisti C, Cocco L, Martelli AM. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget. 2012;3:1068–1111. doi: 10.18632/oncotarget.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer treatment reviews. 2013;39:935–946. doi: 10.1016/j.ctrv.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Toulany M, Minjgee M, Saki M, Holler M, Meier F, Eicheler W, Rodemann HP. ERK2- dependent reactivation of Akt mediates the limited response of tumor cells with constitutive K-RAS activity to PI3K inhibition. Cancer biology & therapy. 2013;15 doi: 10.4161/cbt.27311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babchia N, Calipel A, Mouriaux F, Faussat AM, Mascarelli F. The PI3K/Akt and mTOR/P70S6K signaling pathways in human uveal melanoma cells: interaction with B-Raf/ERK. Investigative ophthalmology & visual science. 2010;51:421–429. doi: 10.1167/iovs.09-3974. [DOI] [PubMed] [Google Scholar]
- 38.Papst PJ, Sugiyama H, Nagasawa M, Lucas JJ, Maller JL, Terada N. Cdc2-cyclin B phosphorylates p70 S6 kinase on Ser411 at mitosis. The Journal of biological chemistry. 1998;273:15077–15084. doi: 10.1074/jbc.273.24.15077. [DOI] [PubMed] [Google Scholar]
- 39.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Current opinion in cell biology. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annual review of cell and developmental biology. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou MM, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]