Abstract
Importance
Typical cognitive aging may be defined as age associated changes in cognitive performance in individuals who remain free of dementia. Ideally the full adult age spectrum should be included to assess brain imaging findings associated with typical aging.
Objective
To compare age, sex and Apolipoprotein E (APOE ε4) effects on memory, brain structure (adjusted hippocampal volume, HVa) and amyloid PET in cognitively normal individuals aged 30 to 95 years old.
Design, Setting, and Participants
Cross sectional observational study (Marc 2006 to October 2014) at an academic medical center. We studied 1246 cognitively normal subjects; 1209 participants aged 50–95 years old enrolled in a population-based study of cognitive aging and 37 self-selected volunteers aged 30–49.
Main Outcomes and Measures
Memory, HVa, and amyloid PET
Results
Overall, memory worsened from age 30 years through the 90s. HVa worsened gradually from 30 years to the mid-60s and more steeply beyond that age. The median amyloid PET was low until age 70 years and increased thereafter. Memory was worse in men than women overall (p<0.001) and more specifically beyond age 40 years. HVa was lower in men than women overall (p<0.001) and more specifically beyond age 60 years. There was no sex difference in amyloid PET at any age. Within each sex, memory performance and HVa were not different by APOE ε4 at any age. From age 70 years onward APOE ε4 carriers had significantly greater median amyloid PET load than noncarriers. However the ages at which 10% of the population were amyloid PET positive were 57 years for APOE ε4 carriers and 64 years for non-carriers.
Conclusions and Relevance
Male sex is associated with worse memory and HVa among cognitively normal individuals while APOE ε4 is not. In contrast, APOE ε4 is associated with greater amyloid PET values (from age 70 years onward) while sex is not. Worsening memory and HVa occur at earlier ages than abnormal amyloid PET. Therefore, neuropathological processes other than β-amyloidosis must underlie declines in brain structure and memory function in middle age. Our findings are consistent with a model of late-onset Alzheimer’s disease in which β-amyloidosis arises in later life on a background of preexisting structural and cognitive decline that is associated with aging and not with β-amyloid deposits.
Keywords: Cognitive Aging, Amyloid Imaging, Alzheimer Disease, Memory Performance, Brain Atrophy
Introduction
Typical cognitive aging may be defined as age-associated changes in cognitive performance in individuals who remain free of dementia. Interrelationships among biomarkers of β-amyloid, neurodegeneration, and cognitive performance have been the focus of much recent literature. However studies that include all of these variables have focused predominantly on elderly individuals, typically included few, if any, individuals younger than 60 years, and tended to be composed of selected volunteers rather than population-based samples1–3. We measured memory performance, hippocampal volume, and β-amyloidosis as a function of age using cross-sectional data from a large sample of cognitively normal individuals 30–95 years old. Individuals were grouped by sex and Apolipoprotein E (APOE ε4) status. The present study differs from a recent publication4 in which our group examined neither memory performance, nor individuals younger than 50 years and in which our independent variables were not continuous measures. Differentiating features of the present study compared with other multimodality imaging studies in aging are (1) inclusion of the full adult age spectrum, 30–90 years, (2) the population-based nature of 97.0% of our participants, (3), our transformation of the imaging and cognitive measures to a common scale to facilitate comparison across different modalities, and (4) the large sample size. Our objectives were to compare age, sex and APOE ε4 effects on memory performance, hippocampal volume, and amyloid positron emission tomography (PET) across the adult life span.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Mayo Clinic and Olmsted Medical Center (Rochester, Minnesota) institutional review boards. Written informed consent was obtained from all participants.
Subject methods
We studied 1246 cognitively normal individuals from two different cohorts. The largest group (n=1209) was 50 to 95 years old and comprised participants enrolled in the Mayo Clinic Study of Aging (MCSA). The MCSA is a population-based study of cognitive aging among Olmsted County, MN, residents 5. The Olmsted County population is enumerated in the eligible age strata. From this enumeration, we select individuals for recruitment using an age- and sex-stratified random sampling strategy. These individuals were then invited to participate. The second group (n=37) was 30 to 49 years old, equally stratified by 5-year age-groups and sex (referred to as young normal). These individuals were self-selected volunteers and were not population-based. The study dates were March 2006 to October 2014.
All subjects in this study were judged to have no cognitive impairment according to published criteria 5. All 1246 individuals (MCSA and young normals) underwent identical PET, MRI and memory testing protocols which included the Auditory Verbal Learning Test (AVLT). The sum of trials 1 through 5 plus the immediate and delayed recall trials (possible total score of 105) was the learning and memory performance measure (referred to as memory) used in our analyses.
Imaging Methods
Amyloid PET imaging was performed with 11C Pittsburgh Compound B (PIB).6 Standardized uptake value ratios (SUVR) were formed from the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, posterior cingulate, and precuneus regions-of-interest normalized to the whole cerebellum.7, 8 MRI was performed at 3T and hippocampal volume was measured with available software (FreeSurfer, version 5.3.0; https://surfer.nmr.mgh.havard.edu/). Total intracranial volume (TIV) was measured using an in-house method.4
Statistical Methods
Some subjects were enrolled in the MCSA prior to availability of amyloid PET and received prior cognitive testing. To eliminate confounding due to the well-established learning effect on serial AVLT performance in cognitively normal individuals, we created a partial residual that adjusted for education and the number of times a subject had taken the AVLT prior to baseline which for this study was the date of the imaging studies. This adjusted AVLT measure can be interpreted as the difference, in number of words correctly recalled, from the expected number for a person given his or her education and number of previous exposures to the test. To adjust hippocampal volume for total intracranial volume (TIV), we fit a regression model among the 133 individuals aged 30 to 59 years old of hippocampal volume versus TIV. The adjusted hippocampal volume (HVa) was defined as the residual from this model 8 and can be interpreted as the difference (in cubic centimeters) compared to the expected hippocampal volume given a person’s head size.
Memory performance, HVa, and amyloid PET levels are reported in modality-specific native units and also in “centiloid-like” units (scale 0 to 100).9 This process is similar to scaling biomarkers from normal to maximum abnormal levels (as described by Jack et al10). To create reference points for scaling, we defined 0 (normal) for the scaled units as the 95th percentile for memory and HVa and the 5th percentile for amyloid PET among the young normal study participants aged 30 to 49 years old. We defined 100 (abnormal) for the scaled units as the 5th percentile for HVa and the 95th percentile for amyloid PET among a group of 42 individuals with moderately demented Alzheimer’s disease (AD) (Clinical Dementia Rating, 1–3). We defined 100 (abnormal) for memory based on the 5th percentile among a larger group of 382 individuals with moderately demented AD (Clinical Dementia Rating, 1–3) who underwent memory testing but not necessarily MRI and PET. These individuals with AD were participants in the MCSA or Mayo Alzheimer’s Disease Research Center and had undergone the same battery of evaluations as our study participants. An individual’s memory, HVa, or amyloid PET in native units was scaled linearly to centiloid-like units (Figure 1S).
We used quantile regression to estimate median (rather than mean) memory, HVa, and amyloid PET versus age by sex and APOE ε4 status. Quantile regression is particularly appropriate for modeling amyloid PET because its distribution is highly skewed and not conditionally normal even after log or other parametric transformations. For each response variable, we fit a single model that included age, sex, and APOE ε4 status along with all two-way interactions. To allow for nonlinear associations with age, we modeled age with restricted cubic splines using knots at ages 50, 75, and 80 years11. As recommended by Harrel,11 we prespecified the knot locations based on the distribution of ages in our data set and to serve as reference points to support a broad class of flexible nonlinear curves.
We used the percentile bootstrap based on 5000 replicates to report 95% CIs for the median memory, HVa, or amyloid PET as a function of age and to report 95% CIs for differences in medians between two measures or between two groups. We base inferences on whether 95% CIs for differences include the null value of zero.
We also report the p-values for a general sex effect for each outcome from a four degree of freedom Wald test which tests the additive and interaction terms involving sex. Similarly, we report the p-values for a general APOE ε4 effect for each outcome.
We assessed the influence of individuals younger than 50 years on model fit and our conclusions. This assessment was performed with a sensitivity analysis limited to individuals aged 50 years or older.
In a secondary analysis, we fit a logistic regression model with age and APOE ε4 genotype to predict the probability of abnormal amyloid PET and used the estimates from this model to identify the age at which the probability reached 10% for both APOE ε4 carriers and noncarriers. To be consistent with our group’s recent publications4, 12, 13, we defined abnormal as a SUVR of 1.4 or greater. Sex was not included in the model because it was not significantly associated with the probability of abnormal amyloid PET.
Results
Demographic features, imaging and memory performance data by age group are found in the Table. There was no significant difference in age by sex but APOE ε4 carriers were on average one year younger than noncarriers (median, 71 vs 72 years; p=0.04). The proportion of APOE ε4 carriers did not differ by sex. Educational level was not different by APOE ε4 status, but men were slightly more educated than women (median, 16 vs 14 years of education; p < 0.001). We show the young normal volunteers separately from the MCSA participants, who are grouped into 15-year age strata to illustrate the effects of advancing age (Table and Fig 1S).
Table.
Characteristics of All Participantsa
| Young Normal | MCSA | ||||
|---|---|---|---|---|---|
| Characteristic | Overall (n = 1246) | 30–49 y (n = 37) | 50–64 y (n = 320) | 65–79 y (n = 628) | 80–95 y (n = 261) |
| Age, y | 72 (63 to 78) [30 to 95] | 39 (34 to 44) [30 to 49] | 60 (55 to 62) [50 to 64] | 73 (69 to 76) [65 to 79] | 83 (82 to 86) [80 to 95] |
| Male sex, No. (%) | 655 (52.6) | 18 (48.6) | 155 (48.4) | 327 (52.1) | 155 (59.4) |
| APOE ε4+, No. (%) | 340 (27.3) | 15 (40.5) | 91 (28.4) | 176 (28.0) | 58 (22.2) |
| Educational level, y | 14 (12 to 16) [8 to 20] | 16 (14 to 16) [12 to 20] | 16 (13 to 16) [9 to 20] | 14 (12 to 16) [8 to 20] | 14 (12 to 16) [8 to 20] |
| AVLT sum of trials | 61 (50 to 73) [20 to 105] | 78 (65 to 86) [50 to 99] | 69 (59 to 78) [34 to 100] | 60 (49 to 71) [24 to 105] | 51 (41 to 63) [20 to 103] |
| Adjusted AVLT sum of trials | −16 (−28 to −4) [−66 to 25] | 5 (−9 to 10) [−26 to 23] | −6 (−17 to 3) [−40 to 22] | −17 (−27 to −6) [−66 to 25] | −30 (−39 to −19) [−65 to 24] |
| Amyloid PET SUVR | 1.33 (1.27 to 1.44) [1.10 to 3.03] | 1.21 (1.19 to 1.23) [1.11 to 1.48] | 1.27 (1.23 to 1.31) [1.10 to 2.24] | 1.35 (1.30 to 1.45) [1.13 to 3.03] | 1.44 (1.33 to 1.86) [1.18 to 2.96] |
| HV, cm3 | 7.5 (6.9 to 8.2) [3.9 to 11.6] | 8.6 (7.9 to 9.2) [6.8 to 11.6] | 8.2 (7.6 to 8.8) [5.8 to 10.5] | 7.5 (6.9 to 8.1) [3.9 to 10.1] | 6.8 (6.2 to 7.2) [3.9 to 8.7] |
| HVa, cm3 | −0.90 (−1.61 to −0.22) [−4.41 to 2.44] | 0.18 (−0.33 to 0.41) [−0.91 to 2.44] | −0.15 (−0.59 to 0.30) [−3.02 to 2.04] | −0.99 (−1.60 to −0.47) [−3.80 to 1.23] | −1.76 (−2.41 to −1.32) [−4.41 to 0.48] |
| Range of study dates | Mar 2006 to Oct 2014 | Jun 2012 to Jan 2013 | Mar 2012 to Oct 2014 | Mar 2006 to Oct 2014 | May 2006 to Apr 2014 |
Abbreviations: AVLT, Auditory Verbal Learning Test; HV, hippocampal volume; HVa, adjusted HV; MCSA, Mayo Clinic Study of Aging; PET, positron emission tomography; SUVR, standardized uptake value ratio.
Unless otherwise indicated, data are given as the median (interquartile range) [range].
Associations of memory, HVa and amyloid PET versus age by sex and APOE ε4 are illustrated in Figs 1, 2 and 2S and are interpreted descriptively. Plots comparing differences in outcomes among APOE ε4 carriers versus noncarriers within men and within women isolate the effect of APOE ε4 within sex (Fig 3). Plots comparing differences in outcomes among men versus women within carriers and within noncarriers isolate the effect of sex within APOE ε4 genotype (Fig 3).
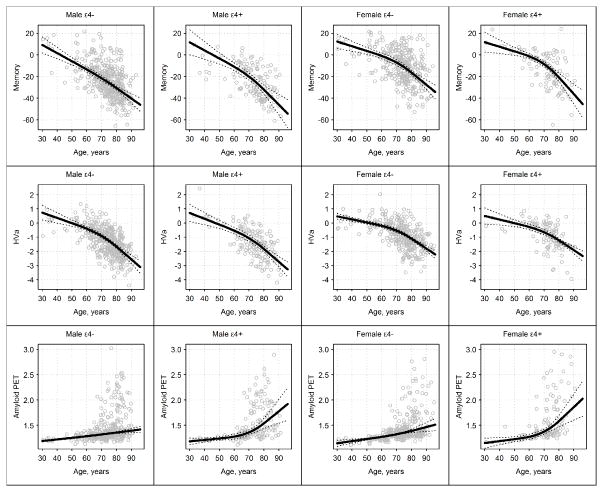
Figure 1.
Memory, Adjusted Hippocampal Volume (HVa), and Amyloid Positron Emisison Tomography (PET) in Modality-Specific Units by Age, With Participants Categorized Into 4 Groups by Sex and APOE ε4 Genotype (Carriers vs Noncarriers). Solid lines represent estimated median regression lines, while dotted lines represent 95% bootstrap CIs. Knots were placed at ages 50, 75, and 80 years.
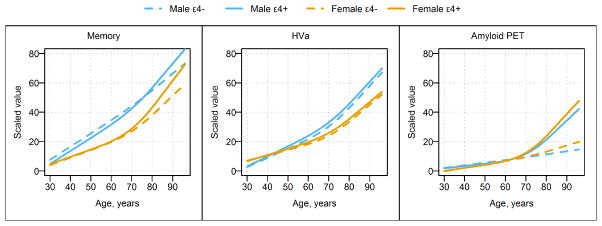
Figure 2.
Estimated Median Regression Lines in Scaled Units vs Age for All 4 Demographic Groups, With Separate Panels for Memory, Adjusted Hippocampal Volume (HVa), and Amyloid Positron Emission Tomography (PET). Knots were placed at ages 50, 75, and 80 years. Blue lines represent relationships in men, and orange represent relationships in women. Solid lines represent APOE ε4 carriers, and dashed lines APOE ε4 noncarriers.
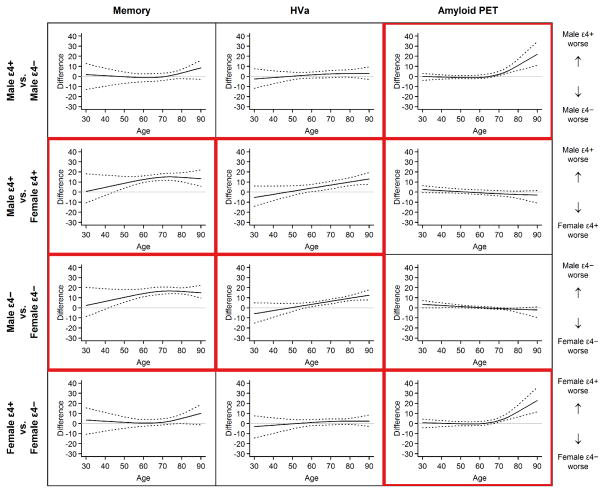
Figure 3.
Plots of Groupwise Differences in Scaled Units for Memory, Adjusted Hippocampal Volume (HVa), and Amyloid Positron Emission Tomography (PET). Comparisons are shown for differences among APOE ε4 carriers vss noncarriers within sex and for male vs female within APOE ε4 genotype. The solid line in each plot represents the estimated difference in medians, while the dotted lines represent 95% bootstrap CIs for this difference. A horizontal line at 0 (ie, no difference) is shown for reference. Plots in which significant groupwise differences were found are outlined in red. This red outlining illustrates a pattern showing differences in memory and HVa were due to sex not APOE ε4, while differences in amyloid PET were due to APOE ε4 and not sex.
These difference plots (Fig 3) illustrate approximate ages at which significant differences were present in the outcomes by sex and APOE ε4 status. That is, when the 95% bootstrap confidence interval (CI) around the median is above 0, we interpret this result as a significant difference in the outcomes by the group of interest at those ages. Therefore, plots in Figs 1, 2 and 2S are interpreted qualitatively while plots in Fig 3 are interpreted statistically.
Age, sex and APOE ε4 group effects on memory
In all four groups, median memory performance worsened from age 30 through the 90s (Figs 1, 2 and 2S) with a steeper decline after age 70 years in male APOE ε4 carriers and in women. Memory was worse in men than women overall (p<0.001) and more specifically beyond age 40 years (Fig 3). There was no difference in memory by APOE ε4 status (p=0.24, Fig 3); however, carriers trended toward worse memory beyond age 80 years (Fig 3). Individual values within each group follow a Gaussian distribution around the median for age (Figs 1 and 2S).
Age, sex and APOE ε4 group effects on HVa
In all four groups, HVa worsened gradually from age 30 years to the mid-60s and more steeply beyond that age (Figs 1, 2 and 2S). HVa was lower in men than in women overall (p<0.001) and more specifically beyond age 60 years (Fig 3). Within each sex, HVa was not different by APOE ε4 status (p=0.15, Fig 3). Individual values within each group follow a Gaussian distribution around the median for age (Figs 1 and 2S).
Age sex and APOE ε4 group effects on amyloid PET
Unlike memory or HVa, the distribution of amyloid SUVR by age is highly skewed above age 65 years (Figs 1 and 2S). Amyloid PET was different by APOE ε4 status (p<0.001). The median amyloid PET value has a slight upward trend from age 30 years through the 90s among APOE ε4 noncarriers. In APOE ε4 carriers there is a slight upward trend until age 70 years and then a steeper increase in the median after that (Figs 1, 2 and 2S). While the median amyloid PET was greater in APOE ε4 carriers compared to noncarriers over age 70 years (Fig 3), the ages at which 10% of the population were classified as amyloid PET positive were 57 (95% CI, 53–59 years) for APOE ε4 carriers and 64 years (95% CI, 62–66 years) for noncarriers (Fig 4). Sex differences in amyloid PET were not significant (p=0.25); however, women trended toward greater β-amyloid beyond age 70 years (Fig 3).
Figure 4.
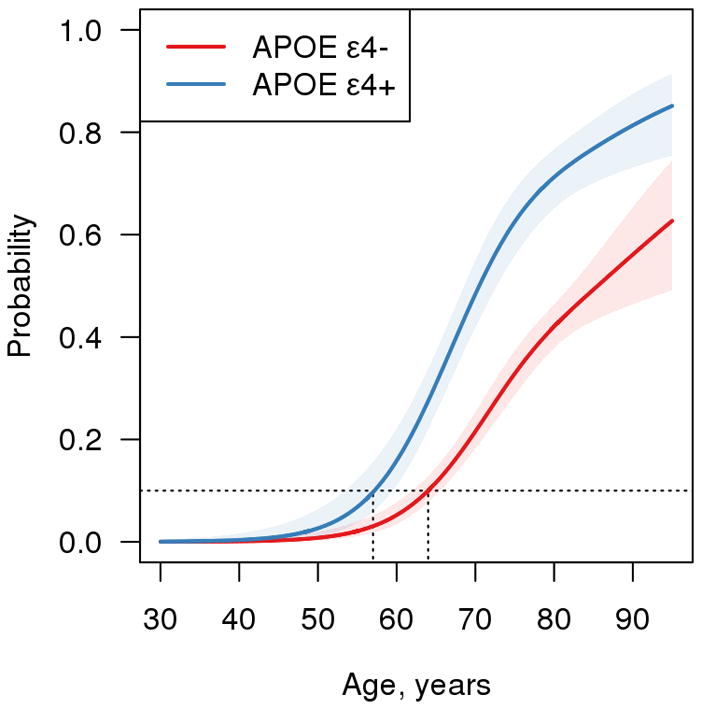
Probability of Amyloid Positron Emission Tomography (PET) Positivity vs Age by APOE ε4 Genotype. The data are estimated from a logistic model. Amyloid PET positivity was defined as a SUVR of 1.4 or greater. The estimated age at which 10% (the dashed horizontal line) of the population is positive is 57 years (95% CI, 53–59 years) for APOE ε4 carriers and 64 years (95% CI, 62–66 years) for APOE ε4 noncarriers.
Comparisons between memory, HVa and amyloid PET versus age within group
Both memory and HVa were more abnormal than amyloid PET beyond age 30 to 40 years in all four groups (Figs 3S and 4S).
Discussion
Our major findings are that the median amyloid PET is greater in cognitively normal APOE ε4 carriers compared to noncarriers older than age 70 years and the age at which 10% of the carriers are classified as amyloid PET positive is 7 years year younger compared with noncarriers. Male sex is associated with worse memory and HVa among cognitively normal individuals, while APOE ε4 is not. Declining memory performance and HVa occur at earlier ages than abnormal amyloid PET.
The estimated age at which 10% of our APOE ε4 carriers were amyloid PET positive was 57 years compared with 64 years for noncarriers. These ages depend on the cut-point used for amyloid PET positivity as well as the threshold chosen for the proportion who are positive.
However, we wanted to make a concrete statement about the age at which abnormal amyloid PET first appears in the population and operationalized this finding as the age when the frequency of abnormality in the population reaches 10%. This is more robust than reporting the age when an abnormal scan first occurs in a single individual, which is very sensitive to outliers. In addition, our data show that from the age of 70 years onward, APOE ε4 carriers had significantly greater median amyloid PET than noncarriers. These results are consistent with the well-established link between APOE ε4 and increased risk of β-amyloidosis.2, 3, 14, 15, 16, 17 In turn, β-amyloidosis increases the risk of cognitive impairment and dementia 1, 18–24.
Overall age-dependent trends in our data are largely consistent with prior studies that show progressive declines in memory 25, 26 and brain volumes 27, 28 with age. Recognition that AD pathology, particularly amyloid plaques, can exist in situ for over a decade or longer without producing overt cognitive symptoms 1, 29–31 has raised the idea that subclinical declines in brain structure and cognitive function in middle age are often due to underlying β-amyloid deposition. However, we found that memory and HVa worsen continuously from age 30 years onward and that these trends are established before obviously abnormal amyloid PET values appear in the population (Figs 1, 2 and 2S). Memory and HVa values are symmetrically distributed around the population age median which implies that declines in bran structure and memory are a fundamental characteristic of typical aging. In contrast amyloid PET values are skewed above age 65 years (Figs 1 and 2S) such that some individuals accumulate high amyloid loads, while many survive to old age without developing significant β-amyloidosis. The differing distributions of memory and HVa vs amyloid PET around population medians with age imply that declining memory and HVa must have some mechanistic independence from β-amyloid accumulation. Also, direct comparisons of memory, HVa and amyloid PET within each group (Figs 3S and 4S) show that memory and HVa were consistently more abnormal than amyloid PET beyond age 30 to 40 years. We acknowledge that amyloid PET measures only fibrillar amyloid deposits and therefore potential effects of soluble β-amyloid cannot be assessed. Given this caveat, our data are nonetheless consistent with the concept that age-related degenerative processes affecting brain structure and cognitive function that are unrelated to fibrillar β-amyloid deposition 8, 13, 32–37 exist from at least age 30 years onward and are characteristic of typical aging. Reasonable candidates for non-AD processes associated with structural and functional decline in middle age are cerebro-vascular disease and its risk factors, including primary age-related tauopathy, 38, 39 brain aging in the absence of any specific pathophysiological process,36, 40 or combinations of these. Our data are consistent with models of late-onset AD in which β-amyloidosis, which defines preclinical AD,41 typically arises in later life on a background of pre-existing age-related cognitive and structural decline 12, 13, 34, 38, 42–46.
With regard to sex effects, we found that men perform worse than women on memory beginning in their 40s, as has been shown previously.47 We also found that HVa was smaller in men than women beyond age 60 years. This sex effect on memory and HVa was likely not due to sex differences in age or APOE ε4 because differences in age by sex or by APOE ε4 were small (median, approximately 1 year different) and because there were no differences in APOE ε4 prevalence by sex. Men were slightly more educated than women (median, 16 vs 14 years of education). However, if anything, this factor would tend to enhance memory performance in men compared with women, which is opposite from what we found. Moreover, we adjusted memory for educational level and practice effects. This detrimental effect of male sex on memory and HVa must also be independent from β-amyloid deposition because (1) we found no sex differences in amyloid PET at any age (Fig 3) and (2) sex differences were present in memory (beginning at age 40 years) well before abnormal amyloid PET first appeared in the population (Figs 1, 3, 4 and 2S). These sex differences in memory and HVa could be developmental,48 a hormonal protective effect,49 or attributable to a greater prevalence of adverse life style related exposures (eg, vascular risk factors) in men.27
Perhaps the most controversial findings from this study come from comparing the associations between sex vs APOE ε4 on age-dependent trends in memory and HVa. Some prior studies are consistent with our finding of no association between APOE ε4 and hippocampal volume in cognitively normal individuals.50, 51 However, other studies have indicated that, among cognitively normal individuals without β-amyloid deposition, APOE ε4 carriers have hypo-metabolism in AD-like regions,52 abnormal functional connectivity,53, 54 worse cognitive performance,55 and smaller regional brain volumes.56, 57 Such findings have been taken as evidence that APOE ε4 exerts harmful effects throughout life on brain structure and function that are independent from its role in promoting β-amyloid deposition.58, 59 In contrast, we found that, while male sex was associated with smaller hippocampal volume and worse memory, APOE ε4 carriers within each sex did not have worse memory or HVa than noncarriers at any age.60 Had we examined other imaging measures (eg, fluorodeoxyglucose F18 PET or functional MRI) or perhaps other cognitive indexes, the findings might have been different. Nonetheless, our results paint a different picture than is presented in much of the recent imaging literature, which has focused great attention on the effects of APOE ε4 but little on the effect of sex on brain structure and function.
Our study has limitations. While all individuals 50 years or older in our sample were derived from an epidemiologically defined cohort, the non-population based nature of those 30 to 49 years old and the small sample size in this age range are acknowledged limitations. However, when obvious inflection points exist in the plots of memory, HVa, and amyloid PET vs age, they occur well within the age range of 50 years to the 90s of the MCSA cohort (Figs 1, 2, 2S and 3S), and not at the age junction of the young normal group and the MCSA cohort. In addition, a sensitivity analysis indicated that plots (among those 50 years or older) of memory, HVa, and amyloid PET vs age did not change when individuals younger than 50 years were excluded. This finding indicates that the imbalance in numbers of individuals aged 50 years or older vs younger than 50 years did not unduly influence conclusions. Another limitation is that cross-sectional studies tend to confound age effects with birth cohort effects.61 However, cohort effects are unavoidable when examining age-dependent trends covering a range of 60 years. Within-individual longitudinal data typically found in studies with intensive multimodality imaging (1–5 years) will not ameliorate birth cohort effects when the research questions of interest are age trends spanning 60 years. A final methodological point concerns interpretation of results given that all participants were cognitively normal. Individuals who remain cognitively normal into old age represent a subset of those who were members of their birth cohort at younger ages.
Despite these limitations, we believe that this study of typical aging reveals interesting sex and APOE ε4 effects on age-related trends in brain structure, function and β-amyloidosis. To date, these effects have not been widely appreciated. Our findings are consistent with a model of late-onset AD in with β-amyloidosis arises in later life on a background of preexisting structural and cognitive decline that is associated with aging and not with β-amyloid deposits.
Supplementary Material
Memory, HVa, and Amyloid PET in Scaled Units by Age With Subjects Broken Into Four Groups by Sex and APOE ε4 Genotype (APOE ε4 Carrier vs Noncarrier). Solid lines represent the estimated median regression line while dotted lines indicate 95% bootstrap confidence intervals. Knots were placed at 50, 75, and 80 years of age. Values near 0 indicate normal values and values near 100 indicate most abnormal values.
Estimated Median Regression Line for Memory, HVa, and Amyloid PET in Scaled Units vs Age by Sex and APOE ε4 Genotype (APOE ε4 Carrier vs Noncarrier). Knots were placed at 50, 75, and 80 years of age. Orange lines represent the relationship between memory and age for each demographic group, blue lines represent relationship for HVa, and green lines represent relationship for amyloid PET.
Plots of the Difference in Medians for Memory vs HVa (Row 1), Memory vs Amyloid PET (Row 2), and HVa vs Amyloid PET (Row 3) for Ages 30 to 90 years by Demographic Group (Columns) in Scaled Units. The solid line in each plot indicates the estimated difference in medians while the dotted lines indicate 95% bootstrap confidence intervals for this difference. A horizontal line at 0, i.e., no difference, is shown for reference.
Acknowledgments
Study Funding: NIH (R01-AG011378, RO1 AG041851, U01-AG06786)
Footnotes
Disclosures
This study was sponsored by National Institute on Aging (R01-AG011378, RO1 AG041851, U01-AG06786); the Alexander Family Professorship of Alzheimer’s Disease Research.
Dr. Jack has provided consulting services for Eli Lily. He receives research funding from the NIH (R01-AG011378, U01-AG024904, RO1 AG041851, R01 AG37551, R01AG043392, U01-AG06786), and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation.
Ms. Wiste reports no disclosures.
Mr. Weigand reports no disclosures.
Dr. Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH.
Dr. Vemuri reports no disclosures.
Dr. Mielke reports no disclosures.
Dr. Lowe is a consultant for Bayer Schering Pharma and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the NIH (NIA, NCI), the Elsie and Marvin Dekelboum Family Foundation, the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia & Lymphoma Society.
Mr. Senjem reports no disclosures.
Dr. Gunter reports no disclosures.
Dr. Machulda reports no disclosures.
Mr. Gregg reports no disclosures.
Dr. Pankratz has been funded by the NIH (R01AG040042, U01AG06786, Mayo Clinic Alzheimer’s Disease Research Center/Core C P50AG16574/Core C, and R01AG32990).
Dr. Rocca serves on the editorial boards for Revista de Neurologia (in Spanish), Clinical Epidemiology & Risk Management, Maturitas, Menopause, and Journal of Chinese Clinical Medicine; and receives research support from the NIH (R01 AG034676 (PI), U01 AG006786 (Co-I), P50 AG04417 (Co-I), and K12 HD 065987 (PI)).
Dr. Petersen serves on scientific advisory boards for Elan Pharmaceuticals, Wyeth Pharmaceuticals, and GE Healthcare; receives royalties from publishing Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH (P50-AG16574 (PI) and U01-AG06786 (PI), R01-AG11378 (Co-I), and U01–24904 (Co-I)).
Author Contributions
Dr. Jack (Jack.Clifford@mayo.edu) conceptualization of the study, analysis and interpretation of data, drafting and revising manuscript
Ms. Wiste (Wiste.Heather@mayo.edu) conceptualization of the study, analysis and interpretation of data, drafting and revising manuscript, statistical analysis
Mr. Weigand (Weigand.Stephen@mayo.edu) conceptualization of the study, analysis and interpretation of data, drafting and revising manuscript, statistical analysis
Dr. Knopman (Knopman@mayo.edu) drafting and revising manuscript
Dr. Vemuri (Vemuri.Prashanthi@mayo.edu) drafting and revising manuscript
Dr. Mielke (Mielke.Michelle@mayo.edu) drafting and revising manuscript
Dr. Lowe (VLowe@mayo.edu) drafting and revising manuscript
Mr. Senjem (Senjem.Matthew1@mayo.edu) drafting and revising manuscript, technical support
Dr. Gunter (Gunter.Jeffrey@mayo.edu) drafting and revising manuscript, technical support
Dr. Machulda (Machulda.mary@mayo.edu) drafting and revising manuscript
Mr. Gregg (Gregg.Brian@mayo.edu) technical support
Dr. Pankratz (Pankratz.Vernon@mayo.edu) drafting and revising manuscript, statistical analysis
Dr. Rocca (rocca@mayo.edu) drafting and revising manuscript
Dr. Petersen (Peter8@mayo.edu) drafting and revising manuscript
References
- 1.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigue KM, Kennedy KM, Devous MD, Sr, et al. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78(6):387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson SC, Christian BT, Okonkwo OC, et al. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiol Aging. 2014;35(3):576–584. doi: 10.1016/j.neurobiolaging.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. The Lancet Neurology. 2014;13(10):997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 7.Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26(2):600–608. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to NIA-AA crtiteria for preclinical Alzheimer’s disease. Ann Neurol. 2012;71(6):765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klunk WE, Koeppe RA, Price JC, et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimer’s & dementia. 2015;11(1):1–15. doi: 10.1016/j.jalz.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 12.Jack CR, Jr, Wiste HJ, Knopman DS, et al. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82(18):1605–1612. doi: 10.1212/WNL.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology. 2013;81(20):1732–1740. doi: 10.1212/01.wnl.0000435556.21319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleisher AS, Chen K, Liu X, et al. Apolipoprotein E epsilon4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging. 2013;34(1):1–12. doi: 10.1016/j.neurobiolaging.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Mormino EC, Brandel MG, Madison CM, et al. Not quite PIB-positive, not quite PIB-negative: Slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2012;59(2):1152–1160. doi: 10.1016/j.neuroimage.2011.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vemuri P, Wiste HJ, Weigand SD, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67(3):308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80(14):1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dore V, Villemagne VL, Bourgeat P, et al. Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 2013;70(7):903–911. doi: 10.1001/jamaneurol.2013.1062. [DOI] [PubMed] [Google Scholar]
- 20.Landau SM, Mintun MA, Joshi AD, et al. Amyloid deposition, hypometabolism, and longitutdinal cognitive decline. Ann Neurol. 2012;72(4):578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowe CC, Bourgeat P, Ellis KA, et al. Predicting Alzheimer disease with beta-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol. 2013;74(6):905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- 22.Lim YY, Pietrzak RH, Bourgeat P, et al. Relationships Between Performance on the Cogstate Brief Battery, Neurodegeneration, and Abeta Accumulation in Cognitively Normal Older Adults and Adults with MCI. Arch Clin Neuropsychol. 2015;30(1):49–58. doi: 10.1093/arclin/acu068. [DOI] [PubMed] [Google Scholar]
- 23.Chetelat G, Villemagne VL, Pike KE, et al. Relationship between memory performance and beta-amyloid deposition at different stages of Alzheimer’s disease. Neuro-degenerative diseases. 2012;10(1–4):141–144. doi: 10.1159/000334295. [DOI] [PubMed] [Google Scholar]
- 24.Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71(8):961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. Rey’s verbal learning test: normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. Journal of the International Neuropsychological Society : JINS. 2005;11(3):290–302. doi: 10.1017/S1355617705050344. [DOI] [PubMed] [Google Scholar]
- 26.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 27.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 29.Benzinger TL, Blazey T, Jack CR, Jr, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013;110(47):E4502–4509. doi: 10.1073/pnas.1317918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jack CR, Jr, Wiste HJ, Lesnick TG, et al. Brain beta-amyloid load approaches a plateau. Neurology. 2013;80(10):890–896. doi: 10.1212/WNL.0b013e3182840bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack CR, Jr, Vemuri P, Wiste HJ, et al. Evidence for Ordering of Alzheimer Disease Biomarkers. Arch Neurol. 2011;68(12):1526–1535. doi: 10.1001/archneurol.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jagust W. Apolipoprotein E, neurodegeneration, and Alzheimer disease. JAMA Neurol. 2013;70(3):299–300. doi: 10.1001/jamaneurol.2013.726. [DOI] [PubMed] [Google Scholar]
- 33.Chetelat G. Alzheimer disease: Abeta-independent processes-rethinking preclinical AD. Nat Rev Neurol. 2013;9(3):123–124. doi: 10.1038/nrneurol.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer’s disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. J Neurosci. 2013;33(13):5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. Brain changes in older adults at very low risk for Alzheimer’s disease. J Neurosci. 2013;33(19):8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nebes RD, Snitz BE, Cohen AD, et al. Cognitive aging in persons with minimal amyloid-beta and white matter hyperintensities. Neuropsychologia. 2013;51(11):2202–2209. doi: 10.1016/j.neuropsychologia.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 39.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. doi: 10.1007/s00401-014-1349-0. Accepted in 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77(2):219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Assocation workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duyckaerts C, Hauw JJ. Prevalence, incidence and duration of Braak’s stages in the general population: can we know? Neurobiol Aging. 1997;18(4):362–369. doi: 10.1016/s0197-4580(97)00047-x. discussion 389–392. [DOI] [PubMed] [Google Scholar]
- 43.Delacourte A, Sergeant N, Wattez A, et al. Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp Gerontol. 2002;37(10–11):1291–1296. doi: 10.1016/s0531-5565(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 44.Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer’s Disease. Neuron. 2013;80(6):1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musiek ES, Holtzman DM. Origins of Alzheimer’s disease: reconciling cerebrospinal fluid biomarker and neuropathology data regarding the temporal sequence of amyloid-beta and tau involvement. Current opinion in neurology. 2012;25(6):715–720. doi: 10.1097/WCO.0b013e32835a30f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Storandt M, Head D, Fagan AM, Holtzman DM, Morris JC. Toward a multifactorial model of Alzheimer disease. Neurobiol Aging. 2012;33(10):2262–2271. doi: 10.1016/j.neurobiolaging.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerhan JR, Folsom AR, Mortimer JA, et al. Correlates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Gerontology. 1998;44(2):95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 48.Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310(5749):819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- 49.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 50.Jack CR, Jr, Petersen RC, Xu YC, et al. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann Neurol. 1998;43(3):303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Protas HD, Chen K, Langbaum JB, et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 2013;70(3):320–325. doi: 10.1001/2013.jamaneurol.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheline YI, Morris JC, Snyder AZ, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw P, Lerch JP, Pruessner JC, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6(6):494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- 57.Alexander GE, Bergfield KL, Chen K, et al. Gray matter network associated with risk for Alzheimer’s disease in young to middle-aged adults. Neurobiol Aging. 2012;33(12):2723–2732. doi: 10.1016/j.neurobiolaging.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jagust WJ, Landau SM. Apolipoprotein E, Not Fibrillar beta-Amyloid, Reduces Cerebral Glucose Metabolism in Normal Aging. J Neurosci. 2012;32(50):18227–18233. doi: 10.1523/JNEUROSCI.3266-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf AB, Valla J, Bu G, et al. Apolipoprotein E as a beta-amyloid-independent factor in alzheimer’s disease. Alzheimers Res Ther. 2013;5(5):38. doi: 10.1186/alzrt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim YY, Ellis KA, Pietrzak RH, et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79(16):1645–1652. doi: 10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]
- 61.Arking RA. Biology of Aging: Observations and principles. 2. Sunderland, MA: Sinauer Associates; 1998. Measuring age-related change in individuals; pp. 61–105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Memory, HVa, and Amyloid PET in Scaled Units by Age With Subjects Broken Into Four Groups by Sex and APOE ε4 Genotype (APOE ε4 Carrier vs Noncarrier). Solid lines represent the estimated median regression line while dotted lines indicate 95% bootstrap confidence intervals. Knots were placed at 50, 75, and 80 years of age. Values near 0 indicate normal values and values near 100 indicate most abnormal values.
Estimated Median Regression Line for Memory, HVa, and Amyloid PET in Scaled Units vs Age by Sex and APOE ε4 Genotype (APOE ε4 Carrier vs Noncarrier). Knots were placed at 50, 75, and 80 years of age. Orange lines represent the relationship between memory and age for each demographic group, blue lines represent relationship for HVa, and green lines represent relationship for amyloid PET.
Plots of the Difference in Medians for Memory vs HVa (Row 1), Memory vs Amyloid PET (Row 2), and HVa vs Amyloid PET (Row 3) for Ages 30 to 90 years by Demographic Group (Columns) in Scaled Units. The solid line in each plot indicates the estimated difference in medians while the dotted lines indicate 95% bootstrap confidence intervals for this difference. A horizontal line at 0, i.e., no difference, is shown for reference.