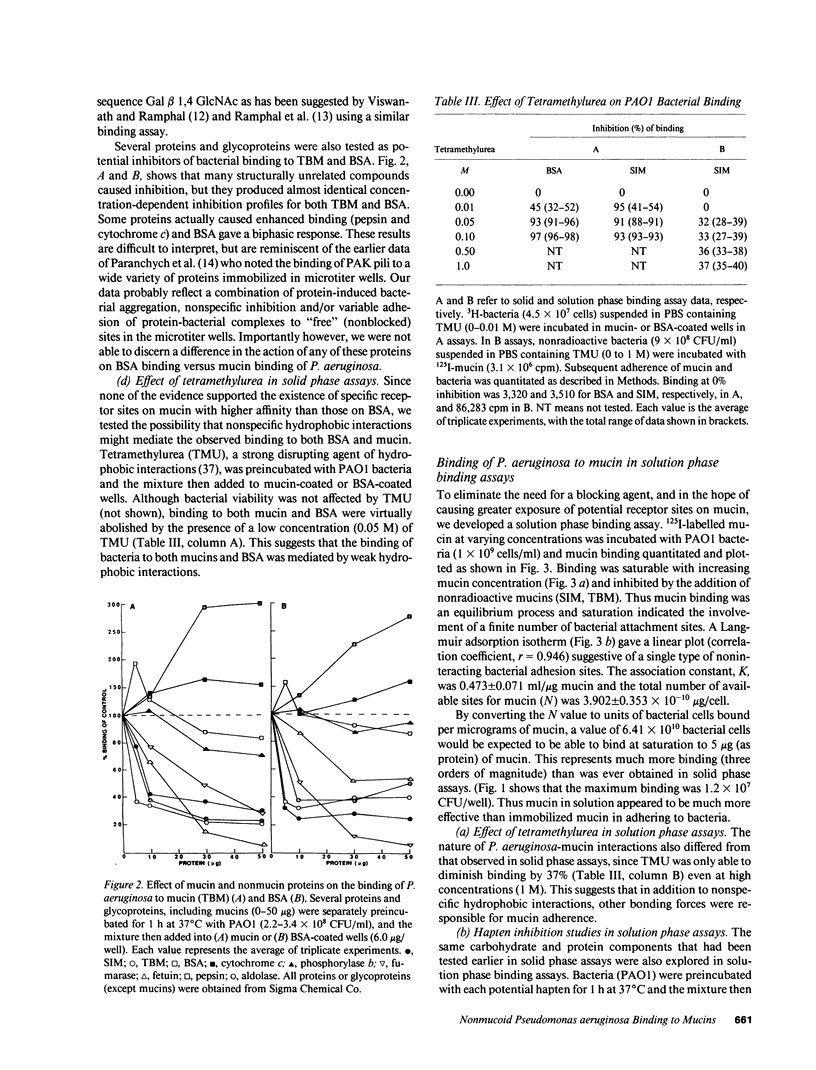
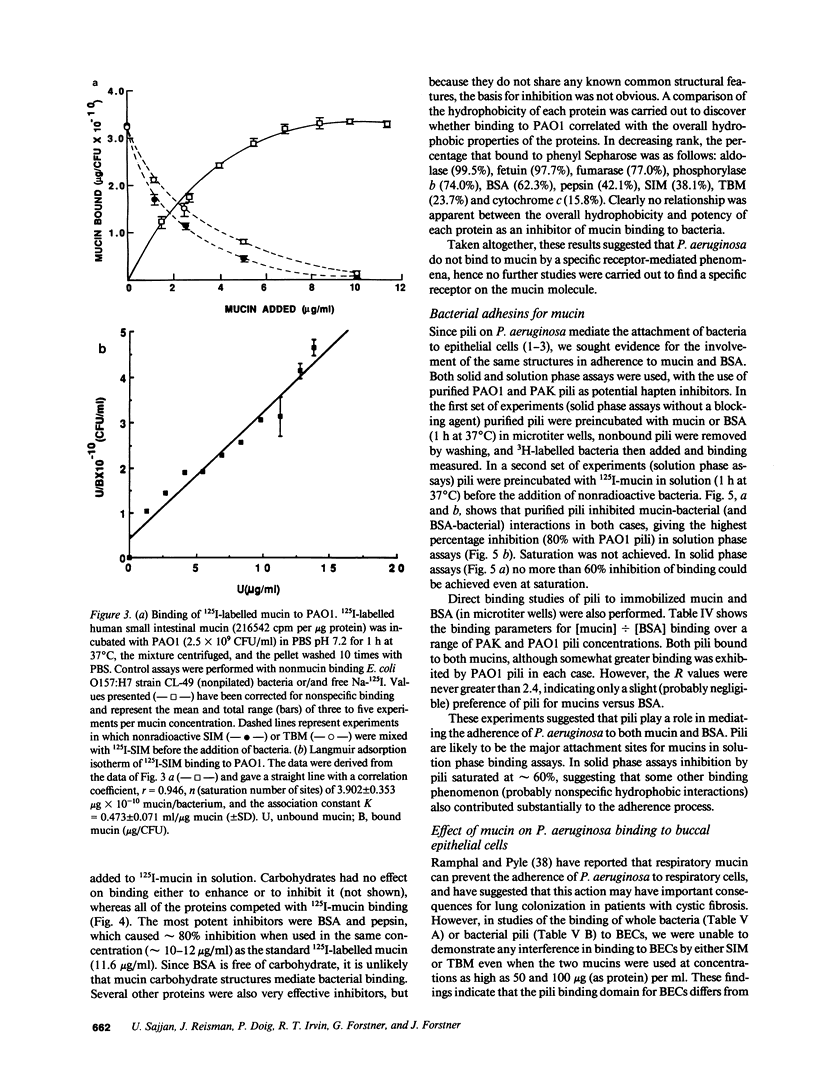
Abstract
Lung infections due to Pseudomonas aeruginosa and Pseudomonas cepacia are common in patients with cystic fibrosis. Initial colonization is due to nonmucoid P. aeruginosa, while later mucoid variants emerge and are associated with chronic infection. P. cepacia colonization tends to be more prevalent in older patients. The present study was conducted to discover whether highly purified mucins (from cystic fibrosis sputum and control intestinal secretions) exhibited specific binding of nonmucoid P. aeruginosa. In vitro solid phase microtiter binding assays (with or without a blocking agent) as well as solution phase assays were conducted. Bacteria bound to both mucins via bacterial pili, but no differences in binding capacity were noted between the mucins. Unlike P. cepacia (described in the accompanying manuscript) there was also no preferential binding of P. aeruginosa to mucins versus bovine serum albumin, casein, gelatin, or a host of structurally unrelated proteins and glycoproteins. Carbohydrate hapten inhibition studies did not suggest the existence of specific mucin carbohydrate receptors for P. aeruginosa. In solid phase assays a low concentration (0.05 M) of tetramethylurea abolished P. aeruginosa bacterial binding to both mucins as well as to BSA, whereas in solution phase assays mucin binding to bacteria was not completely disrupted by tetramethylurea. Specific monoclonal antipilus antibodies did not inhibit binding to a greater extent than did Fab fragments of normal mouse IgG. Binding of strains PAO1 and PAK (and isolated PAK pili) to buccal epithelial cells was not influenced by the presence of mucin in binding assay mixtures. Our findings do not support the widely held notion that specific mucin receptors are responsible for the attachment of P. aeruginosa pili, nor do they support the idea that there is a competitive interference by mucins of bacterial binding to respiratory cells. In patients with cystic fibrosis, it would seem unlikely therefore that initial colonization of the lungs by P. aeruginosa is due to a 'selective tropism' of these bacteria for respiratory mucin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N., Hansson G. C., Leffler H., Riise G., Svanborg-Edén C. Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect Immun. 1990 Jul;58(7):2361–2366. doi: 10.1128/iai.58.7.2361-2366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore R. S., Christie C. D., Smith G. J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989 Dec;140(6):1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Kaufman B., Khorrami A., Enriquez J. I., Manna B. Fibronectin: source of mannose in a highly purified respiratory mucin. Inflammation. 1988 Oct;12(5):433–446. doi: 10.1007/BF00919437. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Pitt T. L. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J Gen Virol. 1974 Jul;24(1):1–15. doi: 10.1099/0022-1317-24-1-1. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee K., Petri W. A., Jr, Innes D. J., Ravdin J. I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Paranchych W., Sastry P. A., Irvin R. T. Human buccal epithelial cell receptors of Pseudomonas aeruginosa: identification of glycoproteins with pilus binding activity. Can J Microbiol. 1989 Dec;35(12):1141–1145. doi: 10.1139/m89-189. [DOI] [PubMed] [Google Scholar]
- Doig P., Sastry P. A., Hodges R. S., Lee K. K., Paranchych W., Irvin R. T. Inhibition of pilus-mediated adhesion of Pseudomonas aeruginosa to human buccal epithelial cells by monoclonal antibodies directed against pili. Infect Immun. 1990 Jan;58(1):124–130. doi: 10.1128/iai.58.1.124-130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Todd T., Sastry P. A., Lee K. K., Hodges R. S., Paranchych W., Irvin R. T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988 Jun;56(6):1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Garber N., Sharon N., Shohet D., Lam J. S., Doyle R. J. Contribution of hydrophobicity to hemagglutination reactions of Pseudomonas aeruginosa. Infect Immun. 1985 Oct;50(1):336–337. doi: 10.1128/iai.50.1.336-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M. R., Townsend R. R., Lee Y. C. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem. 1988 Apr;170(1):54–62. doi: 10.1016/0003-2697(88)90089-9. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachran D. W., Irvin R. T. Adhesion of Pseudomonas aeruginosa to human buccal epithelial cells: evidence for two classes of receptors. Can J Microbiol. 1985 Jun;31(6):563–569. doi: 10.1139/m85-105. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Tredgett M. W., Sheehan J. K., Thornton D. J., Notman D., Govan J. R. Mucinophilic and chemotactic properties of Pseudomonas aeruginosa in relation to pulmonary colonization in cystic fibrosis. Infect Immun. 1990 Jun;58(6):1489–1495. doi: 10.1128/iai.58.6.1489-1495.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani N., Zaidi T. S., Gigstad J. E., Jungalwala F. B., Barza M., Baum J. Binding of Pseudomonas aeruginosa to neutral glycosphingolipids of rabbit corneal epithelium. Infect Immun. 1990 Jan;58(1):114–118. doi: 10.1128/iai.58.1.114-118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Frost L. S. The physiology and biochemistry of pili. Adv Microb Physiol. 1988;29:53–114. doi: 10.1016/s0065-2911(08)60346-x. [DOI] [PubMed] [Google Scholar]
- Paranchych W., Sastry P. A., Drake D., Pearlstone J. R., Smillie L. B. Pseudomonas pili. Studies on antigenic determinants and mammalian cell receptors. Antibiot Chemother (1971) 1985;36:49–57. [PubMed] [Google Scholar]
- Paranchych W., Sastry P. A., Frost L. S., Carpenter M., Armstrong G. D., Watts T. H. Biochemical studies on pili isolated from Pseudomonas aeruginosa strain PAO. Can J Microbiol. 1979 Oct;25(10):1175–1181. doi: 10.1139/m79-182. [DOI] [PubMed] [Google Scholar]
- Pasloske B. L., Sastry P. A., Finlay B. B., Paranchych W. Two unusual pilin sequences from different isolates of Pseudomonas aeruginosa. J Bacteriol. 1988 Aug;170(8):3738–3741. doi: 10.1128/jb.170.8.3738-3741.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt T. L. Biology of Pseudomonas aeruginosa in relation to pulmonary infection in cystic fibrosis. J R Soc Med. 1986;79 (Suppl 12):13–18. [PMC free article] [PubMed] [Google Scholar]
- Plotkowski M. C., Beck G., Tournier J. M., Bernardo-Filho M., Marques E. A., Puchelle E. Adherence of Pseudomonas aeruginosa to respiratory epithelium and the effect of leucocyte elastase. J Med Microbiol. 1989 Dec;30(4):285–293. doi: 10.1099/00222615-30-4-285. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Guay C., Pier G. B. Pseudomonas aeruginosa adhesins for tracheobronchial mucin. Infect Immun. 1987 Mar;55(3):600–603. doi: 10.1128/iai.55.3.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Houdret N., Koo L., Lamblin G., Roussel P. Differences in adhesion of Pseudomonas aeruginosa to mucin glycopeptides from sputa of patients with cystic fibrosis and chronic bronchitis. Infect Immun. 1989 Oct;57(10):3066–3071. doi: 10.1128/iai.57.10.3066-3071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa in the lower respiratory tract. Infect Immun. 1983 Jul;41(1):339–344. doi: 10.1128/iai.41.1.339-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Vishwanath S. Why is Pseudomonas the colonizer and why does it persist? Infection. 1987 Jul-Aug;15(4):281–287. doi: 10.1007/BF01644139. [DOI] [PubMed] [Google Scholar]
- Rosengren J., Pählman S., Glad M., Hjertén S. Hydrophobic interaction chromatography on non-charged Sepharose derivatives. Binding of a model protein, related to ionic strength, hydrophobicity of the substituent, and degree of substitution (determined by NMR). Biochim Biophys Acta. 1975 Nov 18;412(1):51–61. [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Characteristics of binding of Escherichia coli serotype O157:H7 strain CL-49 to purified intestinal mucin. Infect Immun. 1990 Apr;58(4):860–867. doi: 10.1128/iai.58.4.860-867.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany A., Slomiany B. L., Witas H., Aono M., Newman L. J. Isolation of fatty acids covalently bound to the gastric mucus glycoprotein of normal and cystic fibrosis patients. Biochem Biophys Res Commun. 1983 May 31;113(1):286–293. doi: 10.1016/0006-291x(83)90464-3. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sorensen K., Brodbeck U. Assessment of coating-efficiency in ELISA plates by direct protein determination. J Immunol Methods. 1986 Dec 24;95(2):291–293. doi: 10.1016/0022-1759(86)90419-9. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985 May;48(2):331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A. H., van der Mei H. C., Slot J. W. Relationship of cell surface morphology and composition of Streptococcus salivarius K+ to adherence and hydrophobicity. Infect Immun. 1987 Feb;55(2):438–445. doi: 10.1128/iai.55.2.438-445.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley A. W., Forstner J. F., Forstner G. G. Structure of intestinal-mucus glycoprotein from human post-mortem or surgical tissue: inferences from correlation analyses of sugar and sulfate composition of individual mucins. Carbohydr Res. 1983 Apr 16;115:151–163. doi: 10.1016/0008-6215(83)88143-9. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



