Abstract
Polycystic ovary syndrome (PCOS) is characterized by insulin resistance, elevated circulating leukocytes, and hypothesized to have higher adipose tissue inflammation. Aerobic exercise reduces circulating leukocytes and improves insulin sensitivity in obese individuals, but the effect of exercise on inflammation in PCOS is not known. We investigated circulating leukocytes, insulin sensitivity by euglycemic-hyperinsulinemic clamp, serum pro- and anti-inflammatory markers (hsCRP, TNF-α, total and high molecular weight adiponectin), and abdominal subcutaneous adipose tissue (SAT) gene expression of proinflammatory markers in 8 PCOS women and 8 obese control females matched for BMI. Additionally, in a prospective study, the 8 women with PCOS underwent a 16-week aerobic exercise regimen with the same measures performed post-intervention. Compared to controls, white blood cell counts (WBC) were 30% higher (p = 0.04) and circulating total adiponectin levels were 150% lower (p = 0.03) in women with PCOS at baseline/pre-exercise conditions. SAT gene expression of macrophage migration inhibitory factor (MIF, p < 0.01) and interleukin-6 (IL-6, p < 0.05) were also lower in women with PCOS. In response to 16 weeks of aerobic exercise, insulin sensitivity improved (p < 0.01) and WBC counts decreased (p = 0.02). The exercise-induced change in WBC and circulating neutrophils correlated inversely with changes in glucose disposal rate (r= -0.73, p=0.03; and r= -0.82, p=0.01, respectively). Aerobic exercise reduced serum leptin (p < 0.05) after 4 weeks, trended to reduce the ratio of leptin-to-high molecular weight adiponectin (p < 0.1) by the 8th week, and significantly increased serum dehydroepiandrosterone sulfate (DHEA-S, p < 0.001) after 16 weeks. In conclusion, women with PCOS have higher circulating leukocytes compared to controls, which can be reversed by aerobic exercise and is associated with improvements in insulin sensitivity.
Keywords: Inflammation, Polycystic Ovary Syndrome, Insulin Resistance, Adipose, Obese
Introduction
Polycystic Ovary Syndrome (PCOS) is a complex endocrine disorder affecting 5-10% of women at reproductive age [1-3]. In addition to menstrual irregularity, hyperandrogenemia, and polycystic ovarian morphology, PCOS is highly associated with abdominal obesity and insulin resistance [4-6]. Chronic, low-grade inflammation, a condition associated with obesity [7] and type 2 diabetes [8, 9], is a risk factor for cardiovascular disease [10, 11]. Recent evidence suggests that low-grade inflammation may contribute to the metabolic dysregulation associated with PCOS [12].
Although the exact sources of low-grade inflammation associated with insulin resistance and obesity are still debated, adipose tissue inflammation is a likely culprit [13-16]. Adipose tissue from women with PCOS as well as pre-adipocytes treated with testosterone have shown to have reduced lipolytic function [17, 18]. Adipose tissue dysfunction, here defined as reduced lipolytic function, is also seen in insulin resistance [19]. Adipose tissue inflammation occurs as a result of monocyte and neutrophil infiltration into adipose tissue to “clean up” dysfunctional and dying adipocytes [20-22]. Macrophage accumulation coupled with dysfunctional adipocytes produces pro-inflammatory cytokines and chemokines that spill into the circulation and thus contribute to systemic, chronic, low-grade inflammation [23-25].
Aerobic exercise has been shown to reduce inflammation [26-29]. However, very few studies have focused on how exercise affects adipose tissue inflammation [30]. Prior studies in women with PCOS have shown that there are indeed improvements in insulin resistance with exercise [31, 32]. Importantly though, no study to date has examined the role of exercise in adipose specific inflammation in women with PCOS. We have previously shown that menstrual function, insulin resistance, aerobic capacity, and, importantly, adipose tissue lipolytic function were all improved after 16-weeks of moderate intensity aerobic exercise in women with PCOS [33, 34]. Improvements in inflammation with aerobic exercise in PCOS women could potentially mediate such improvements in insulin sensitivity. The objectives of this study were: 1) compare markers of systemic and adipose-specific inflammation in 8 women with PCOS and 8 BMI-matched controls; and 2) investigate the effects of 16-weeks of aerobic exercise on adipose and systemic inflammation in the 8 women with PCOS. We hypothesized that women with PCOS would have higher systemic inflammation, which would be attenuated following 16-weeks of exercise.
Materials and Methods
Subjects, Experimental design, and Exercise Protocol
As previously reported [33, 34], 8 women with PCOS were matched with women without signs of abnormal menses or hyperandrogenemia. The diagnosis of PCOS was assessed by the Rotterdam criteria [35, 36]. Women with PCOS had to possess two of the following criteria: confirmation by medical history of menstrual irregularity (oligo- or amenorrhea), presence of more than 10 ovarian follicles 2-9mm in diameter as assessed by MRI, or either clinical (hirsutism score) or serum measures of androgen excess (elevated free androgen index, FAI). Other causes of oligomenorrhea (hyperprolactinemia, congenital adrenal hyperplasia, Cushing's syndrome, hyperthyroidism) were excluded by medical history. All women in our PCOS group possessed more than 10 ovarian follicles measured 2-9mm in diameter, and all had irregular menses. Women in the control group were excluded from participation in our study for exercise training, use of contraceptive medications, and menstrual cycle irregularity together with androgen excess. All women in our control group had FAI values below 3.6 as defined as a cut-off value for FAI in the assessment of PCOS according to Hahn et al [37]. Additionally, all women in our control group reported regular menstruation, thus confirming that they did not have PCOS based on the Rotterdam guidelines. Following an overnight fast, blood and subcutaneous adipose tissue samples were collected, body composition was assessed by dual x-ray absorptiometry (DXA, QDR 4500A; Hologics, Bedford, MA) and insulin sensitivity was determined by a hyperinsulinemic-euglycemic clamp (120-minutes at 80mU/min/m2). Aerobic capacity (VO2max) was measured during a graded treadmill test (TrueMax 2400; ParvoMedics, Salt Lake City, UT). After baseline testing, women with PCOS underwent 16 weeks of aerobic exercise at the Health and Fitness Center of the Pennington Biomedical Research Center with all exercise sessions performed on a treadmill at 55% VO2max five times per week under supervision. Details of the exercise protocol are provided elsewhere [33]. Exercise was performed initially to achieve an exercise energy expenditure of 4% of the participants’ estimated energy requirement during the first 4 weeks, then incrementally increased to 6% for weeks 5-8, 8% for weeks 9-12, and finally 10% for weeks 13-16. A fasted blood sample was collected every 4 weeks throughout the exercise program. The study was approved by the Institutional Review Board of Pennington Biomedical Research Center and participants provided informed written consent before participating (Clinicaltrials.gov registration NCT01150539).
Biochemical Assays
High sensitive C-reactive protein (hsCRP) and dehydroepiandrosterone sulfate (DHEAS) were determined by chemiluminescent immunoassay (Immulite 2000™, Siemens Healthcare Diagnostics, Deerfield, IL); Tumor necrosis factor-alpha (TNF-α) by immunoassay (Luminex 100™, Luminex Corp. Austin, TX); leptin and adiponectin (total and high molecular weight) by radioimmunoassay (Linco Research Inc., St Charles, MO); serum lipids by an enzymatic assay on a Beckman Coulter DXC 600 (Beckman Coulter, Brea, CA); and complete blood counts using a Beckman Coulter DxH 800 using the Coulter principle (Beckman Coulter, Brea, CA).
Hyperinsulinemic-euglycemic Clamp
Insulin sensitivity was measured as previously described using a hyperinsulinemic euglycemic clamp. [33]. After catheter placement, a primed infusion of insulin (80 mU/min/m2) was initiated for 120 min with plasma glucose levels measured every 5 min; at this insulin infusion level, all splanchnic glucose output was assumed to be blocked. Exogenous 20% glucose was infused at a variable rate to maintain plasma glucose concentration at 90 mg/dL. Glucose disposal rate (GDR) was adjusted for kilograms of fat free mass (FFM) + 17.7, denoted as estimated mean body size (EMBS) [38].
Subcutaneous Adipose Tissue Biopsy for Real Time PCR for Gene Expression
Subcutaneous adipose tissue was obtained from the abdomen with a 5-mm needle using the Bergstrom technique after local anesthesia (5 mL 1:1 mixture of 0.5% bupivicaine and 2% lidocaine). Adipose tissue was immediately snap frozen in liquid nitrogen and stored at −80°C until analysis. Total RNA was extracted from approximately 100 mg of adipose tissue using miRNEasy Kits (Qiagen, Valencia, CA). RNA extracts were converted into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Gene expression was carried out using Real Time-PCR with TaqMan gene expression assays embedded on microfluidic cards on the ABI 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Gene expression assays were performed for the following genes: plasminogen activator inhibitor 1 (PAI-1, Hs01126603_m1), macrophage migration inhibitory factor (MIF, Hs00236988_g1), interleukin 6 (IL-6, Hs00985639_m1), cluster of differentiation 68 (CD68, Hs00154355_m1), monocyte chemotactic protein 1 (MCP1, Hs00234140_m1), tumor necrosis factor, alpha (TNFa, Hs00174128_m1), adiponectin (AdipoQ, Hs00605917_m1), and cyclophilin B (PPIB, Hs00168719_m1). Relative gene expression was assessed using Ct normalized to Cyclophilin B (PPIB) gene expression.
Statistical Analysis
All analyses were performed using GraphPad Prism Software, version 5.0 (GraphPad Software, La Jolla, CA). The Mann-Whitney test was used for cross-sectional comparisons between PCOS and Obese Control women when data was not normally distributed, and an Independent Samples t-test was used when the data was normally distributed (Table 1 and Figure 2). Paired t-tests were used when data was normally distributed and Wilcoxon signed ranked tests were used with data was not normally distributed when comparing measured between pre- and post-exercise variables in the women with PCOS when data was only collected for those two time points (Table 2). A one-way, repeated measures ANOVA along with Dunnett's multiple comparisons post-hoc test were used to evaluated significance in variables that were measured in women with PCOS every 4 weeks during the duration of the exercise intervention (Table 3). Additionally, paired t-tests or Wilcoxon signed ranked paired tests used to compare variables from baseline to changes with exercise intervention were reported in instances when they were found to be significant, and Dunnett's test was not (Table 3). Pearson r correlation coefficients were used to evaluate the significance of relations between changes in insulin sensitivity and changes in circulating leukocytes with exercise in women with PCOS. P < 0.05 was considered statistically significant.
Table 1.
Anthropometric and Metabolic Characteristics between Controls and Women with PCOS.
| Control | PCOS | p value | |
|---|---|---|---|
| Pre-Exercise | |||
| Age (yrs) | 33.9 ± 16.2 | 25.6 ± 3.1 | 0.20 |
| Weight (kg) | 75.3 ± 11.5 | 84.1 ± 16.6 | 0.24 |
| BMI (kg/m2) | 28.1 ± 5.0 | 32.1 ± 5.2 | 0.13 |
| % Fat | 31.3 ± 7.6 | 38.2 ± 5.2 | 0.20 |
| FM (kg) | 23.9 ± 8.6 | 32.8 ± 10.8 | 0.27 |
| FFM (kg) | 51.4 ± 7.5 | 51.4 ± 6.6 | 0.99 |
| VO2max (mL/FFM) | 23.7 ± 6.4 | 27.5 ± 3.7 | 0.27 |
| GDR (mg/min/EMBS) | 7.4 ± 3.9 | 5.1 ± 1.8 | 0.18 |
| HOMA-IR (AU) | 3.5 ± 3.8 | 3.6 ± 2.7 | 0.94 |
| SHGB (nmol/L) | 35.5 ± 18.3 | 30.9 ± 31.7 | 0.73 |
| Testosterone (ng/dL) | 36.9 ± 8.0 | 92.4 ± 41.2 | 0.006 |
| FAI (AU) | 1.2 ± 0.6 | 18.8 ± 14.2 | 0.02 |
| WBC (× 103/μL) | 6.7 ± 2.1 | 8.7 ± 1.4 | 0.04 |
| Neutrophils (× 103/μL) | 3.9 ± 1.7 | 4.2 ± 1.2 | 0.61 |
| hsCRP (mg/L) | 3.8 ± 5.2 | 4.6 ± 6.1 | 0.79 |
| Triglycerides (mg/dL) | 89.3 ± 52.6 | 95 ± 49.8 | 0.82 |
| Total Cholesterol (mg/dL) | 184.9 ± 27.6 | 165.5 ± 40.7 | 0.28 |
| HDL-C (mg/dL) | 63.5 ± 17.6 | 50.6 ± 11.7 | 0.10 |
| LDL-C (mg/dL) | 103.9 ± 18.9 | 95.9 ± 24.1 | 0.48 |
| Cholesterol/HDL | 3.1 ± 0.8 | 3.3 ± 0.4 | 0.48 |
| HDL/LDL | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.26 |
| DHEA-S (μg/dL) | 130.9 ± 49.2 | 183.2 ± 80.3 | 0.13 |
| Total Adiponectin (μg/mL) | 6.7 ± 3.3 | 2.7 ± 1.3 | 0.003 |
Data is represented as mean ± SD. BMI, Body Mass Index; FM, Fat Mass; FFM, Fat Free Mass; GDR, Glucose Disposal Rate; EMBS, Estimated Mean Body Size; SHGB, Sex Hormone Binding Globulin; FAI, Free Androgen Index; WBC, White Blood Cell Count; hsCRP, high sensitivity C-Reactive Protein; HDL-C, High Density Lipoprotein-Cholesterol; LDL-C, Low Density Lipoprotein-Cholesterol; DHEA-S, Dehydroepiandrosterone-Sulfate
Figure 2.
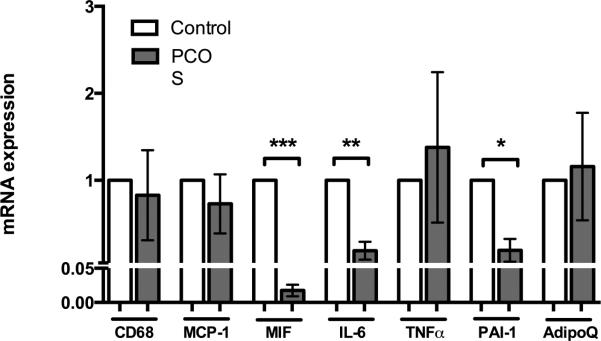
Gene expression data from abdominal subcutaneous adipose tissue between controls compared to PCOS women at baseline before exercise training. All gene expression is expressed as arbitrary units reflecting fold change compared to controls. CD68 – Cluster of Differentiation 68, a marker of macrophages; MCP-1 – Monocyte Chemotactic Protein 1; MIF – Macrophage Migration Inhibitory Factor; IL-6 – Interleukin 6; TNFα – Tumor Necrosis Factor, alpha; PAI-1 – Plasminogen-Activator Inhibitor 1; and AdipoQ - Adiponectin. Graphs represent means ± SEM. *, p < 0.10; **, p <
Table 2.
Anthropometric and Metabolic Characteristics from Pre- and Post-Exercise Intervention in Women with PCOS
| PCOS | p value | ||
|---|---|---|---|
| Pre-Exercise | Post-Exercise | ||
| Age (yrs) | 25.6 ± 3.1 | -- | -- |
| Weight (kg) | 84.1 ± 16.6 | 83.5 ± 18.2 | 0.67 |
| BMI (kg/m2) | 32.1 ± 5.2 | 31.8 ± 5.7 | 0.63 |
| % Fat | 38.2 ± 5.2 | 36.8 ± 6.0 | 0.24 |
| FM (kg) | 32.8 ± 10.8 | 31.6 ± 11.6 | 0.53 |
| FFM (kg) | 51.4 ± 6.6 | 51.9 ± 7.2 | 0.12 |
| VO2max (mL/FFM) | 27.5 ± 3.7 | 31.0 ± 5.0 | 0.007 |
| GDR (mg/min/EMBS) | 5.1 ± 1.8 | 6.6 ± 2.1 | 0.001 |
| HOMA-IR (AU) | 3.6 ± 2.7 | 3.5 ± 1.9 | 0.85 |
| SHGB (nmol/L) | 30.9 ± 31.7 | 28.8 ± 16.4 | 0.79 |
| Testosterone (ng/dL) | 92.4 ± 41.2 | 86.0 ± 47.7 | 0.45 |
| FAI (AU) | 18.8 ± 14.2 | 17.2 ± 15.8 | 0.27 |
Data is represented as mean ± SD. BMI, Body Mass Index; FM, Fat Mass; FFM, Fat Free Mass; GDR, Glucose Disposal Rate; EMBS, Estimated Mean Body Size; SHGB, Sex Hormone Binding Globulin; FAI, Free Androgen Index
Table 3.
Serum TNFa and Adipokines in women with PCOS
| PCOS | ||||||
|---|---|---|---|---|---|---|
| Pre-Exercise | Exercise Duration | p value | ||||
| Week 4 | Week 8 | Week 12 | Week 16 | |||
| WBC (× 103/μL) | 8.7 ± 1.4 | 8.1 ± 1.6 | 8.2 ± 1.4 | 7.6 ± 1.4b | 7.5 ± 1.1b | 0.02 |
| Neutrophils (× 103/μL) | 4.2 ± 1.2 | 4.2 ± 1.3 | 4.8 ± 1.4 | 4.1 ± 1.4 | 4.1 ± 1 | 0.13 |
| hsCRP (mg/L) | 4.6 ± 6.1 | 4.5 ± 4.9 | 3.5 ± 2.8 | 4.4 ± 5.2 | 4.7 ± 4.5 | 0.47 |
| Triglycerides (mg/dL) | 95 ± 49.8 | 77.3 ± 49.4 | 71.7 ± 34.3 | 84.3 ± 44.8 | 76.6 ± 37.4c | 0.16 |
| Total Cholesterol (mg/dL) | 165.5 ± 40.7 | 174 ± 35.7 | 174.7 ± 35.6 | 169.1 ± 36 | 179.8 ± 38.1c | 0.25 |
| HDL-C (mg/dL) | 50.6 ± 11.7 | 57.9 ± 11.3b | 58.8 ± 14.1b | 57.9 ± 13.4b | 59.3 ± 11.4b | < 0.001 |
| LDL-C (mg/dL) | 95.9 ± 24.1 | 100.5 ± 21.8 | 101.6 ± 19.8 | 94.3 ± 23.7 | 105.2 ± 24.7c | 0.20 |
| Cholesterol/HDL | 3.3 ± 0.4 | 3.0 ± 0.5 | 3.0 ± 0.4a | 2.9 ± 0.6b | 3.0 ± 0.4c | 0.07 |
| HDL/LDL | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1b | 0.5 ± 0.1 | 0.05 |
| DHEA-S (μg/dL) | 183.2 ± 80.3 | 232.3 ± 132.4b | 226.2 ± 129.5b | 226.5 ± 120.3b | 219 ± 87.7c | 0.09 |
| Total Adiponectin (μg/mL) | 2.7 ± 1.3 | 2.5 ± 0.6 | 2.6 ± 0.8 | 2.6 ± 1.2 | 2.4 ± 1 | 0.95 |
| TNFa (pg/mL) | 12.8 ± 3.3 | 12.5 ± 4.6 | 10.6 ± 4.4c | 12.1 ± 3.1c | 13.1 ± 4.7 | 0.55 |
| Leptin (μg/mL) | 33.1 ± 11.7 | 24.7 ± 13.6b | 25.5 ± 13.8a | 24.6 ± 13.3b | 26.1 ± 13.9 | 0.07 |
| HMW-Adiponectin (ng/mL) | 28.2 ± 24.4 | 32.2 ± 23.8 | 45.8 ± 47.9 | 38 ± 37 | 33 ± 19.7 | 0.30 |
| Leptin/Total Adiponectin | 10.5 ± 2.4 | 9.3 ± 5.5 | 9.2 ± 6.3 | 9.3 ± 6.7 | 9.6 ± 5.1 | 0.97 |
| Leptin/HMW-Adiponectin | 2.6 ± 2.2 | 0.7 ± 0.3 | 1 ± 1.1 | 0.9 ± 0.8b | 0.7 ± 0.3b | 0.03 |
Data is represented as mean ± SD. The p values presented in the table were obtained from repeated measures one-way ANOVAs. Trends and significance indicated from Dunnett's multiple comparisons tests:
p < 0.10 vs Pre-Exercise PCOS
p < 0.05 vs Pre-Exercise PCOS.
Significance indicated from paired t-tests:
p < 0.05 vs Pre-Exercise PCOS.
WBC, White Blood Cell Count; hsCRP, high sensitivity C-Reactive Protein; HDL-C, High Density Lipoprotein-Cholesterol; LDL-C, Low Density Lipoprotein-Cholesterol; DHEA-S, Dehydroepiandrosterone-Sulfate; TNFa, Tumor Necrotizing Factor alpha; HMW, High Molecular Weight.
Results
Women with PCOS have higher leukocyte inflammation markers, which are reversed by exercise
As shown in Table 1, women with PCOS were adequately matched with controls with no significant differences in body composition, insulin sensitivity (GDR), and aerobic capacity (VO2max); although as expected women with PCOS had higher serum testosterone and free androgen index (FAI). Sixteen weeks of aerobic exercise significantly increased insulin sensitivity and aerobic capacity, but did not alter body composition in women with PCOS (Table 2). Prior to exercise intervention, women with PCOS had a 30% higher white blood cell count (WBC; p = 0.04), but no significant differences in neutrophil counts compared to controls (Table 1). There were no significant difference in high sensitivity C-reactive protein (hsCRP, Table 1) between women with PCOS and controls.
Sixteen weeks of aerobic exercise resulted in declines in WBC at week 12 (WBC, p = 0.04) and at week 16 (WBC, p = 0.01), with no changes in neutrophil counts (Table 3). Changes in circulating WBC and neutrophils with aerobic exercise were inversely associated with changes in glucose disposal rate (p = 0.03, Figure 1A; and p = 0.01, Figure 1B, respectively). There were no significant differences in hsCRP levels (Table 3), but tumor necrosis factor-alpha (TNFα) was significantly lower at week 12 (p = 0.03, Table 3).
Figure 1.
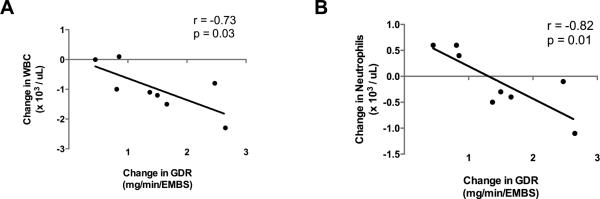
Changes in White Blood Count (WBC) and Neutrophils between baseline and following 16 weeks of exercise inversely correlated to changes in Glucose Disposal Rate (GDR) adjusted to estimated mean body size (EMBS). A) Pearson Correlation between changes in WBC and changes in GDR/EMBS, and B) Pearson Correlation between changes in Neutrophil counts and changes in GDR/EMBS.
Women with PCOS have reduced adipose tissue inflammation, but altered circulating adipokine profiles with exercise
Subcutaneous abdominal adipose tissue revealed lower mRNA expression of macrophage migration inhibitory factor (MIF, p < 0.01, Figure 2), interleukin-6 (IL-6, p < 0.05, Figure 2), and a trend towards decreased plasminogen activator inhibitor-1 (PAI-1, p < 0.10, Figure 2) in women with PCOS compared to controls. There were no differences between PCOS women and controls for the mRNA expression of cluster of differentiation marker 68 (CD68), monocyte chemotactic protein-1 (MCP-1), TNFα, or adiponectin (AdipoQ, Figure 2). However, total serum adiponectin concentrations were three-fold lower in women with PCOS (p = 0.003, Table 1)
Total serum adiponectin was not changed with exercise in women with PCOS (Table 3). Similarly, we did not see significant changes in serum high molecular weight adiponectin (HMW Adiponectin). However, at week 16, 5 out of the 8 participants increased their HMW adiponectin levels with aerobic exercise, and 4 of those 5 increased their HMW adiponectin levels greater than two fold. Leptin levels were found to be significantly lower after 4 weeks (p = 0.04) and at 12 weeks (p = 0.05) of exercise; and tended to be lower at week 8 (p = 0.08, Table 3). The leptin-to-total adiponectin was unchanged with exercise (Table 3) but the ratio of Leptin-to-HMW Adiponectin was significantly lower from baseline levels at week 12 (p = 0.03) and 16 (p = 0.01) of aerobic exercise (Table 3). There was no change with aerobic exercise in abdominal subcutaneous adipose tissue gene expression in women with PCOS for PAI-1, IL-6, MIF, CD68, TNFα, AdipoQ, or MCP-1 (data not shown).
Aerobic exercise improves serum lipids and increases DHEA-S levels in women with PCOS
There were no differences in serum lipids, with the exception of a trend towards higher HDL-C (p = 0.10) in controls, or DHEA-S at baseline (Table 1). Exercise reduced fasting triglyceride concentrations at week 16 (p = 0.02, Table 3). HDL-C levels increased at week 4 (p = 0.002), week 8 (p < 0.003), week 12 (p = 0.002), and at week 16 (p < 0.001), with significant increases in total cholesterol (p = 0.01) and LDL cholesterol (p = 0.02) at week 16 (Table 3). Importantly, the ratio of total cholesterol-to-HDL was found to significantly decrease by week 12,(p = 0.03), while the ratio of HDL-to-LDL cholesterol significantly increased above baseline levels during week 12 (p = 0.01, Table 3). Finally, DHEA-S levels increased significantly at week 4 (p = 0.04) and at the end of 16 weeks of exercise (p = 0.006); and trended towards an increase during week 8 (p = 0.09) and week 12 (p = 0.09, Table 3).
Discussion
In this study, we found that women with PCOS had higher circulating WBC compared with BMI matched female controls. This confirmed the findings in Orio et al [39], whose study additionally found a significant correlation between increased WBC and increased insulin resistance. Similarly, we found that 16 weeks of aerobic exercise reduced WBC counts, and that the changes in total WBC as well as neutrophils correlated inversely with changes in glucose disposal rate. This represents the first study in women with PCOS to demonstrate a relation with exercise between reductions in circulating leukocytes and improvements in insulin sensitivity.
Chronic, low-grade inflammation is a characteristic of many metabolic diseases, including obesity, metabolic syndrome and Type 2 Diabetes [7, 8, 16]. Given the metabolic derangement in women with PCOS, this syndrome has also been hypothesized to be associated with increased levels of low grade inflammation [12]. Though we found differences in WBC, which were reduced by exercise, we did not find differences in other prominent circulating inflammatory factors hsCRP or TNFα between women with PCOS and controls. Additionally, though we found moderate decreases in TNFα at week 8 and week 12, these differences were not carried through to the end of the intervention; and no changes were seen in serum levels of hsCRP. Therefore, we investigated the possibility of adipose tissue and altered adipokine levels to be a potential source of systemic inflammation in women with PCOS.
Dysfunction of the adipose tissue in women with PCOS has been a much studied phenomenon within the recent past (reviewed in [40]). In subcutaneous adipose tissue, women with PCOS had decreased gene expression levels of MIF, IL-6, and a trend towards decreased PAI-1. Similarly, Chazenbalk et al. also found lower IL-6 expression in adipose tissue of PCOS women compared to controls [41]. However, no other differences were noted on the gene expression level between controls and women with PCOS for CD68, MCP-1, TNFα, or adiponectin. In addition, there were no alterations in adipose gene expression with exercise in our study. We next examined circulating adipokine levels since they have been shown to influence systemic inflammation. Adiponectin displays cardio-protective and anti-inflammatory properties [42, 43]. Previous reports on serum adiponectin levels in women with PCOS have been conflicting, with some showing lower levels and others showing no differences [44-46]. Our study showed a three-fold reduction in total adiponectin levels compared to controls but with no difference in the gene expression of adiponectin in the adipose tissue. With aerobic exercise, we saw no changes in serum levels in either total or HMW adiponectin, which is not entirely surprising given previous reports on aerobic exercise and adiponectin levels [47, 48]. On the other side, PCOS is associated with hyperleptinemia [49, 50], to which high leptin levels are associated with inflammation [51]. In our study, exercise significantly reduced leptin levels after 4 weeks and 12 weeks of exercise, and trended towards lower leptin levels at week 8: all of which occurred without reductions in body weight or percent body fat (Table 2). Since differential ratios of leptin and adiponectin have been shown to be elevated in women with PCOS compared to age and weight matched controls [52], we investigated the ratios of leptin-toadiponectin and leptin-to-HMW adiponectin. We found no differences in the ratio of leptin-to-adiponectin, but we did find significant decreases in the ratio of leptin-to-HMW adiponectin during weeks 12 and 16 suggesting an alteration in adipokine profiles going from pro-inflammatory to anti-inflammatory and cardio-protective. Together these findings show that women with PCOS have higher circulating leukocyte concentrations, which is perhaps not directly related to adipose tissue inflammatory recruitment of circulating leukocytes given the adipose tissue gene expression data. Aerobic exercise, though, did appear to alter circulating adipokines without changing the gene expression of adipose tissue inflammatory markers.
Since PCOS is associated with increased cardiovascular disease risk (reviewed in [53]), which is likewise associated with inflammation [54], we examined circulating lipids and other cardiovascular risk markers. With the exception of lower HDL-C in women with PCOS, there were no differences in serum lipids compared to controls. HDL cholesterol has been well documented to relieve inflammation and reduce atherosclerotic plaque in blood vessels, thereby indirectly ameliorating the inflammatory response [55, 56]. With aerobic exercise, there were significant increases in HCL-C from the 4th to the 16th week. Importantly, there were reductions in the ratio of total cholesterol-to-HDL at week 12 of aerobic exercise, as well as increases in the HDL-to-LDL ratio at week 12 of aerobic exercise. DHEA-S has been shown to be cardio-protective and thoroughly studied in aging research due to reductions in DHEA-S levels with increasing age [57, 58]. DHEA-S levels were not different between controls and women with PCOS; however, DHEA-S levels were greatly increased after 16 weeks of exercise. To the best of our knowledge, this is the first time it has been shown that DHEA-S levels increased in PCOS women with aerobic exercise. This, along with increases in HDL-C and altered circulating adipokines, would give powerful evidence that exercise is beneficial for women with PCOS because it would not only potentially relieve inflammation, but also provide additional cardio-protection.
We acknowledge that our study is limited by the fact that our control participants did not perform the exercise regimen along side the women with PCOS. A randomized control trial or a prospective controlled study would have been a stronger study to fully evaluate differences in exercise response to women with PCOS along with weight matched controls. However, given the striking differences between circulating white blood cell counts between controls and pre-exercise levels in women with PCOS, along with of course the diagnostic differences in circulating free androgen index in women with PCOS, we infer that valid conclusions can be made from the cross-sectional component of our study. Furthermore, given the robust changes in both aerobic fitness (VO2max) and insulin sensitivity (GDR) with 16-weeks of aerobic exercise in women with PCOS, we conclude that the prospective study design in the women with PCOS would yield cogent and beneficial results into the nature of exercise mediated reductions in inflammation in women with PCOS.
In conclusion, results from our study showed that 16 weeks of aerobic exercise reduces circulating leukocyte inflammation and improves insulin sensitivity in women with PCOS. Importantly, women with PCOS did not show additional adipose tissue inflammation compared to controls, and exercise did not affect local inflammatory gene expression in adipose tissue. However, aerobic exercise was shown to reduce leptin levels independent of a reduction in fat mass and improve the ratio of leptin-to-HMW adiponectin, thus switching the adipokine profile from being more pro-inflammatory to more anti-inflammatory. Finally, aerobic exercise was shown to improve serum lipids and increase levels of DHEAS, thus improving cardio-protective mechanisms. These results show that regular aerobic exercise that does not induce weight loss may have multiple benefits for improving the pathophysiology of PCOS and thus may be an important treatment option for many patients.
Highlights.
Women with PCOS have higher circulating leukocytes
Aerobic Exercise reduces circulating leukocytes
Exercise related declines in leukocytes relates to insulin sensitivity
Cardio-protective factors (HDL, HMW-Adiponectin, DHEAS) increased with exercise
Acknowledgements
The authors wish to thank Bhavaani Jayaram, Ph.D., Anne Gilmore, Ph.D., and Eric Ravussin Ph.D. for helpful conversations and advice on the manuscript. We also wish to thank Philip Ebenezer Ph.D., Jamie LaGrange and Mindy Gaubert for their technical assistance with adipose tissue RNA isolation, and we wish to thank Richard Carmouche and Susan Newman of the Genomics Core Facility of Pennington Biomedical Research Center. Finally, we wish to sincerely thank all the research volunteers for their participation in this study.
This investigation was supported in part by a grant from the Health and Performance Enhancement Division of Pennington Biomedical Research Center (L.M.R.) and a Nutrition Obesity Research Center (NORC) grant NIH 1P30 DK072476 (E.R.). This work utilized the Genomics Core Facility at Pennington Biomedical Research Center, which is supported in part by COBRE (NIH P20 RR021945). C.S.T. is supported by a NHMRC Early Career Fellowship (#1037275). L.M.R. is supported by NIH grant R00HD060762.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose.
Clinicaltrials.gov registration: NCT01150539
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Boyle JA, et al. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. Med J Aust. 2012;196(1):62–6. doi: 10.5694/mja11.10553. [DOI] [PubMed] [Google Scholar]
- 3.March WA, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 4.Barber TM, et al. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 2006;65(2):137–45. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- 5.Dunaif A, et al. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41(10):1257–66. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- 6.Stepto NK, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–84. doi: 10.1093/humrep/des463. [DOI] [PubMed] [Google Scholar]
- 7.Visser M, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 8.Patino R, et al. Circulating monocytes in patients with diabetes mellitus, arterial disease, and increased CD14 expression. Am J Cardiol. 2000;85(11):1288–91. doi: 10.1016/s0002-9149(00)00757-8. [DOI] [PubMed] [Google Scholar]
- 9.Festa A, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102(1):42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Festa A, et al. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int. 2000;58(4):1703–10. doi: 10.1046/j.1523-1755.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 11.Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. 2011;75(12):2739–48. doi: 10.1253/circj.cj-11-1184. [DOI] [PubMed] [Google Scholar]
- 12.Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95(3):1048–58. e1–2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 14.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 15.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 17.Dicker A, et al. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47(3):420–8. doi: 10.1007/s00125-003-1324-0. [DOI] [PubMed] [Google Scholar]
- 18.Ek I, et al. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab. 1997;82(4):1147–53. doi: 10.1210/jcem.82.4.3899. [DOI] [PubMed] [Google Scholar]
- 19.Reynisdottir S, et al. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J Clin Invest. 1994;93(6):2590–9. doi: 10.1172/JCI117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity- related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talukdar S, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012 doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bullo M, et al. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11(4):525–31. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 24.Weyer C, et al. Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima Indians. Atherosclerosis. 2002;161(1):233–42. doi: 10.1016/s0021-9150(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 25.Juge-Aubry CE, et al. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes. 2003;52(5):1104–10. doi: 10.2337/diabetes.52.5.1104. [DOI] [PubMed] [Google Scholar]
- 26.Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol. 2007;293(3):F914–9. doi: 10.1152/ajprenal.00272.2007. [DOI] [PubMed] [Google Scholar]
- 27.Vieira RP, et al. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am J Respir Crit Care Med. 2007;176(9):871–7. doi: 10.1164/rccm.200610-1567OC. [DOI] [PubMed] [Google Scholar]
- 28.Nunes RB, et al. Physical exercise improves plasmatic levels of IL-10, left ventricular end diastolic pressure, and muscle lipid peroxidation in chronic heart failure rats. J Appl Physiol. 2008;104(6):1641–7. doi: 10.1152/japplphysiol.00062.2008. [DOI] [PubMed] [Google Scholar]
- 29.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 30.Tam CS, et al. Little evidence of systemic and adipose tissue inflammation in overweight individuals(dagger). Front Genet. 2012;3:58. doi: 10.3389/fgene.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison CL, et al. The impact of intensified exercise training on insulin resistance and fitness in overweight and obese women with and without polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012;76(3):351–7. doi: 10.1111/j.1365-2265.2011.04160.x. [DOI] [PubMed] [Google Scholar]
- 32.Hutchison SK, et al. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96(1):E48–56. doi: 10.1210/jc.2010-0828. [DOI] [PubMed] [Google Scholar]
- 33.Moro C, et al. Aerobic exercise training improves atrial natriuretic peptide and catecholamine- mediated lipolysis in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(7):2579–86. doi: 10.1210/jc.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redman LM, Elkind-Hirsch K, Ravussin E. Aerobic exercise in women with polycystic ovary syndrome improves ovarian morphology independent of changes in body composition. Fertil Steril. 2011;95(8):2696–9. doi: 10.1016/j.fertnstert.2011.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Rotterdam E.A.-S.P.c.w.g. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 37.S Hahn WK, Tan S, Mann K, Janssen OE. Prognostic value of free testosterone and free androgen index in detecting the polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114(S 1) [Google Scholar]
- 38.Lillioja S, Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev. 1988;4(5):517–40. doi: 10.1002/dmr.5610040508. [DOI] [PubMed] [Google Scholar]
- 39.Orio F, Jr., et al. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(1):2–5. doi: 10.1210/jc.2004-0628. [DOI] [PubMed] [Google Scholar]
- 40.Villa J, Pratley RE. Adipose tissue dysfunction in polycystic ovary syndrome. Curr Diab Rep. 2011;11(3):179–84. doi: 10.1007/s11892-011-0189-8. [DOI] [PubMed] [Google Scholar]
- 41.Chazenbalk G, et al. Abnormal expression of genes involved in inflammation, lipid metabolism, and Wnt signaling in the adipose tissue of polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97(5):E765–70. doi: 10.1210/jc.2011-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohashi K, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285(9):6153–60. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohashi T, et al. Correlation between circulating adiponectin levels and coronary plaque regression during aggressive lipid-lowering therapy in patients with acute coronary syndrome: subgroup analysis of JAPAN-ACS study. Atherosclerosis. 2010;212(1):237–42. doi: 10.1016/j.atherosclerosis.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Panidis D, et al. Serum adiponectin levels in women with polycystic ovary syndrome. Hum Reprod. 2003;18(9):1790–6. doi: 10.1093/humrep/deg353. [DOI] [PubMed] [Google Scholar]
- 45.Toulis KA, et al. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update. 2009;15(3):297–307. doi: 10.1093/humupd/dmp006. [DOI] [PubMed] [Google Scholar]
- 46.Wickham EP, 3rd, et al. Total and high-molecular weight adiponectin in women with the polycystic ovary syndrome. Metabolism. 2011;60(3):366–72. doi: 10.1016/j.metabol.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hulver MW, et al. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283(4):E861–5. doi: 10.1152/ajpendo.00150.2002. [DOI] [PubMed] [Google Scholar]
- 48.Ando D, et al. Effects of exercise training on circulating high molecular weight adiponectin and adiponectin oligomer composition: a randomized controlled trial. J Atheroscler Thromb. 2009;16(6):733–9. doi: 10.5551/jat.2089. [DOI] [PubMed] [Google Scholar]
- 49.Pusalkar M, et al. Obesity and polycystic ovary syndrome: association with androgens, leptin and its genotypes. Gynecol Endocrinol. 2010;26(12):874–82. doi: 10.3109/09513590.2010.487586. [DOI] [PubMed] [Google Scholar]
- 50.Yildizhan R, et al. Serum retinol-binding protein 4, leptin, and plasma asymmetric dimethylarginine levels in obese and nonobese young women with polycystic ovary syndrome. Fertil Steril. 2011;96(1):246–50. doi: 10.1016/j.fertnstert.2011.04.073. [DOI] [PubMed] [Google Scholar]
- 51.Galletti F, et al. Hyperleptinemia is associated with hypertension, systemic inflammation and insulin resistance in overweight but not in normal weight men. Nutr Metab Cardiovasc Dis. 2012;22(3):300–6. doi: 10.1016/j.numecd.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Golbahar J, et al. Leptin-to-adiponectin, adiponectin-to-leptin ratios, and insulin are specific and sensitive markers associated with polycystic ovary syndrome: a case-control study from Bahrain. Metab Syndr Relat Disord. 2012;10(2):98–102. doi: 10.1089/met.2011.0075. [DOI] [PubMed] [Google Scholar]
- 53.Randeva HS, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33(5):812–41. doi: 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee CD, et al. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001;154(8):758–64. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 55.Navab M, et al. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8(4):222–32. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 56.Barter PJ, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 57.Tagashira H, et al. Distinct cardioprotective effects of 17beta-estradiol and dehydroepiandrosterone on pressure overload-induced hypertrophy in ovariectomized female rats. Menopause. 2011;18(12):1317–26. doi: 10.1097/gme.0b013e31821f915b. [DOI] [PubMed] [Google Scholar]
- 58.Orentreich N, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551–5. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]


