Abstract
Recent advances in cancer stem cell biology have shown that cancer stem–like cells with epithelial–mesenchymal transition (EMT) phenotypes are more aggressive and cause relapse; however absence of a specific marker to isolate these EMT stem-like cells hampers research in this direction. Cell surface markers have been identified for isolating cancer stem-like cells, but none has been identified for isolating cancer stem-like cells with EMT phenotype. Recently, we discovered that Vimentin, an intracellular EMT tumor cell marker, is present on the surface of colon metastatic tumor nodules in the liver. In this study, we examined the potential of targeting cell surface Vimentin (CSV) to isolate stem-like cancer cells with EMT phenotype, by using a specific CSV-binding antibody, 84-1. Using this antibody, we purified the CSV positive, CD133-negative (csVim+CD133−) cell population from primary liver tumor cell suspensions and characterized for stem cell properties. The results of sphere assays and staining for the stem cell markers Sox2 and Oct4A demonstrated that csVim+CD133− cells have stem-like properties similar to csVim−CD133+ population. Our investigation further revealed that the csVim+CD133− cells had EMT phenotypes, as evidenced by the presence of Twist and Slug in the nucleus, the absence of EpCAM on the cell surface and basal level of expression of epithelial marker E-cadherin. The csVimentin negative CD133 positive stem cells do not have any EMT phenotypes. csVim+CD133− cells exhibited more aggressively metastatic in livers than csVim−CD133+ cells. Our findings indicate that csVim+CD133− cells are promising targets for treatment and prevention of metastatic hepatocellular carcinoma.
Keyowrds: EMT, Liver cancer stem cells, HCC, Metastasis
Introduction
Hepatocellular carcinoma (HCC) is the most fatal form of cancer and the third leading cause of cancer related death worldwide 1. In the United States, the number of HCC cases are increasing, and the age-adjusted incidence have doubled compared to previously reported rates 2. The development of liver cancer can be considered as the downstream effect of cirrhosis and fibrosis which occur due to chronic insults or viral infection that progress over decades. Even with aggressive treatment such as liver transplantation, chemoembolization, or surgical resection of a tumor 3, massive recurrence still occurs with HCC patients during their life span 4. Several studies have found an association between recurrence and cancer stem cells, which have shown chemoresistance in different types of tumors5, 6. Characterization of these slow-growing, dormant, and drug-resistant cells may be useful for development of treatments to improve HCC outcomes.
Phenotypic heterogeneity is one of the hallmarks of cancer stem cells. Increasing evidence suggests that liver cancer stem cells (LCSCs) are a highly heterogeneous population expressing different markers such as CD133 7, CD24 4, CD13 8, CD90 9 and EpCAM 10. Our laboratory recently identified the well-known EMT marker Vimentin as a novel cell surface marker while screening metastatic liver nodules of patients with primary colorectal cancer. Previously, our laboratory showed that Vimentin is expressed on the surface of circulating tumor cells having EMT phenotypes 11. On the basis of these observations, we examined the potential of targeting Vimentin to isolate stem-like cancer cells with EMT phenotype, by using a cell surface Vimentin-binding antibody. We isolated from primary liver tumor a pure population of LCSCs that expressed Vimentin on their surface and were CD133 negative (csVim+CD133−). Upon characterization of this novel population, we discovered that csVim+ CD133− cells have stem like properties, differentiation ability and tumorigenic properties similar to csVim−CD133+. However, unlike csVim−CD133+ cells, csVim+CD133− cells had the epithelial–mesenchymal transition (EMT) phenotype and metastasized aggressively.
The study described in this article demonstrates the use of Vimentin for isolating a putative stem cell population that may serve as a novel therapeutic target in efforts reduce the rates of metastasis and relapse in HCC patients.
Materials and Methods
Isolation and Culture of primary tumor cell lines
A tumor-bearing MST−/− mouse was euthanized and tumors were resected from liver. A single cell suspension was prepared as described previously 7, 12. Both csVimentin positive and negative populations were sorted using α-Vimentin using MACS column. Then CD133-positive and negative cells from those two populations were sorted in a similar fashion. csVim+CD133−, csVim−CD133+ and csVim−CD133− cells were cultured with supplements as described previously 7. Liver metastasis samples resected from human colorectal cancer patients were obtained in accordance with a protocol approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center.
Differentiation Assays
Freshly sorted csVim+CD133− cells were cultured in hepatocyte specific condition as described previously 13 and to differentiate into cholangiocytes, we followed this protocol 14. Albumin staining (1:50) was performed in differentiated hepatocytic cells using the protocol as described previously 15.
Western blot
Cell lysis and Western blots with antibodies to E-cadherin or GAPDH were performed as previously described 16. For all samples, total protein was determined by the BCA method (Pierce). Western blots were detected by enhanced chemiluminescence (Cell Signaling technologies).
Subcutaneous inoculation of sorted cells in NSG mice
105 fresh sorted csVim+CD133− or csVim−CD133+ cells were inoculated in 8–10 weeks old NOD scid gamma chain knockout mice (NSG) either subcutaneous injections. Mice were euthanized as in accordance with the guidelines approved by the IACUC.
Immunofluorescent staining of tissue sections
Frozen sections of liver tumors were stained with albumin antibody (1:50 dilution) and a secondary antibody conjugated with Alexa Fluor-488 (1:300 dilution) was used.
Gross metastasis assay
Fifty thousand freshly sorted csVim+CD133− or csVim−CD133+ cells were injected intraosseously into the right legs of NSG mice. Right legs were amputated 2 weeks after post inoculation and the mice were sacrificed 2 weeks after post-amputation. Gross metastases were quantified in each liver. Representative images were taken with a digital camera.
Results
Because the rate of HCC relapse remains very high, we sought to characterize EMT-positive LCSCs, as this cell population is known to promote relapse and drug resistance in other cancers 17. To date, no specific markers have been identified for isolating these EMT-positive LCSCs. By screening for mislocalized surface expression of proteins on primary tumor cells, we identified the well-known EMT marker Vimentin on the surface of cells from metastatic liver nodules from patients with colorectal cancer (Supplementary Fig. 1). We fractionated a novel cell population from primary HCC tumors that we termed csVim+CD133−. These cells were cuboidal and formed colonies. Their purity was confirmed by cell surface staining for Vimentin and CD133 (Supplementary Fig. 2). To characterize stem cell properties of csVim+CD133−, we used csVim−CD133+ LCSCs as CD133 is considered to be the most widely accepted stem cell marker in different tissues. We then assessed the stem-like properties of these cells by Matrigel sphere formation assay and by staining spheres for stem cell-associated markers Sox2 and Oct4A (Fig. 1a and b). To further establish the stem cell phenotype, csVim+CD133− cells were stained for pluripotency markers, such as SSEA4, on their surface (Supplementary Fig. 3). The results indicated that csVim+CD133− cells have a stem-like phenotype.
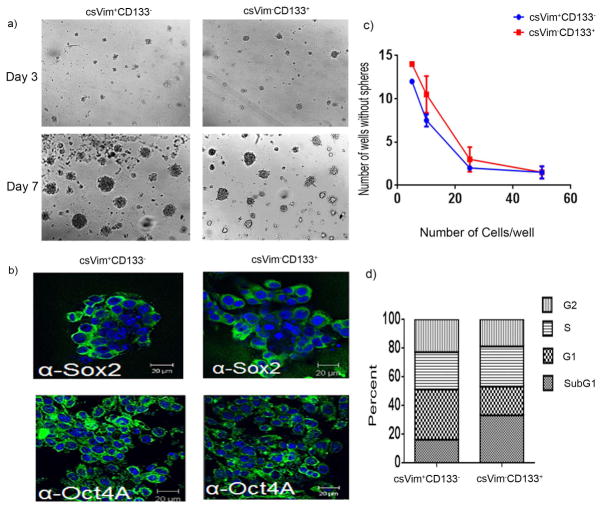
Figure 1. A novel csVim+CD133- population isolated from primary HCC exhibited stem-like properties.
(a) Images of spheres formed in Matrigel by csVim+CD133− and csVim−CD133+ populations on days 3 and 7. (b) Stem cell-associated transcription factors Sox2 and Oct4a were detected using fluorescence microscopy in Matrigel culture. (c) Cells from each population were laid on Matrigel at densities of 5, 10, 25, and 50 cells/well, with 15 replicate samples per density. The numbers of spheres formed at different seeding densities in 15 wells were calculated. (d) Percentages of csVim+CD133− and csVim−CD133+ populations in sub-G1, G1, S, and G2 phases, determined by flow cytometry. Representative data were shown as mean ± SEM.
To determine the purity of stem cells among the csVim+CD133− and csVim−CD133+ subpopulations, we performed limiting dilution assays. The assays revealed that csVim+CD133− cells formed spheres at a rate similar to that for the csVim−CD133+ population at seeding densities from 5 to 50 cells/well (Fig. 1c). Additionally, cell cycle analysis revealed that many more cells were in G1-S-G2 phase in the csVim+CD133− population than in the csVim−CD133+ population (Fig. 1d). Taken together, these data indicated that csVim+CD133− cells have stem-like properties and actively traverse the cell cycle.
Liver stem cells are known to undergo differentiation into hepatocytes and cholangiocytes under suitable culture conditions. To determine their differentiation potential, csVim+CD133− cells were cultured in hepatocyte-specific conditions. We found that csVim+CD133− cells underwent differentiation to hepatocytes, as evidenced by albumin expression and positive periodic acid-Schiff staining, indicating glycogen storage (Fig. 2a–c). These cells also differentiated into cholangiocytes by forming star-shaped structures with branching and lumens (Fig. 2d).
Figure 2. A novel csVim+CD133− subpopulation enriched from primary HCC showed differentiation.
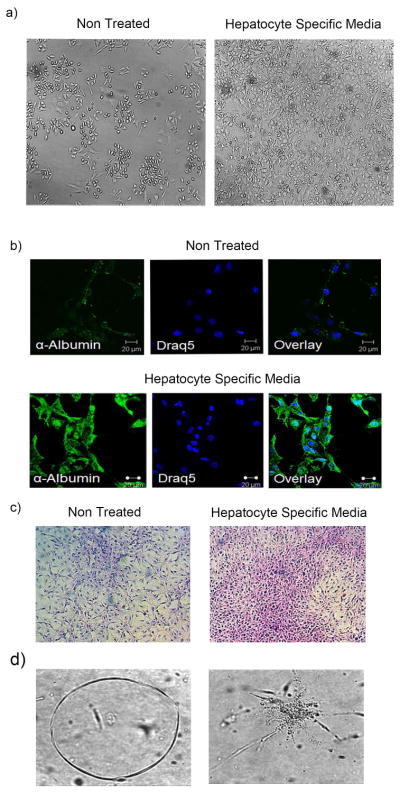
(a) csVim+CD133− cells under normal conditions (nontreated) and in hepatocyte-specific medium. (b) Immunofluorescent confocal microscopy was used to detect albumin expression in csVim+CD133− cells in both nontreated and hepatocyte-specific media. (c) Glycogen storage content of csVim+CD133− cells in nontreated and hepatocyte-specific media, determined by periodic acid-Schiff staining. (d) csVim+CD133− cells formed lumen- and asterisk-shaped structures in cholangiocyte-specific culture conditions.
To determine tumorigenic potential, we evaluated both in vitro and in vivo tumorigenic potentials of csVim+CD133− cells (Supplementary Fig. 4). Experimental studies showed that csVim+CD133− cells are slow growing tumor compared to csVim−CD133+ cells. Also, we tested tumorigenic potential of csVim−CD133− cells, it also showed similar result as csVim+CD133− cells in vivo (Supplementary Fig. 4). Previous studies exhibited that slow-growing HCC tumors have a high recurrence rate and are chemoresistant (10). Thus, the tumors produced by csVim+CD133− cells may have a greater chance of relapse and metastatic potential than the tumors produced by csVim−CD133+ cells.
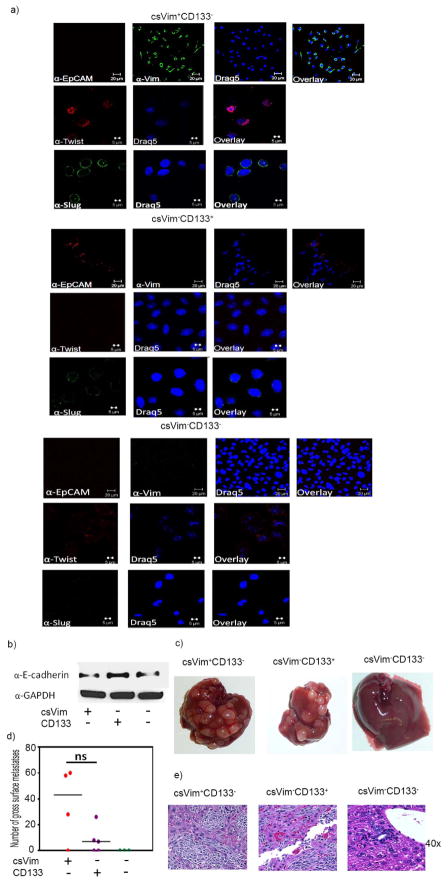
Given that EMT is associated with aggressive tumors and metastasis and that Vimentin is considered an EMT marker, our detection of the EMT marker Twist and Slug in freshly sorted csVim+CD133− cells inside the nucleus was as expected. Interestingly, no expression of the epithelial cell surface marker EpCAM was detected in csVim+CD133− cells (Fig. 3a). In contrast, csVim−CD133+ and csVim−CD133− cells did not express Twist and Slug but did express EpCAM on their surface. Also, western blot analysis of E-cadherin, an epithelial marker confirmed that csVim+CD133− cells are more mesenchymal type compared to csVim−CD133+ cells (Fig. 3b). These expression patterns clearly indicate the EMT nature of csVim+CD133− cells, in contrast to csVim−CD133+ and csVim−CD133− cells. Furthermore, intraosseous inoculation in NSG mice demonstrated that csVim+CD133− cells produced more liver metastases than did the csVim−CD133+ and csVim−CD133− population (Fig. 3c and d). Histological examination of liver tumor tissues showed no gross differences between csVim+CD133− and csVim−CD133+ tumors (Fig. 3d).
Figure 3. csVim+CD133− cells exhibit EMT like phenotypes and metastasize at a higher rate than do csVim−CD133+ and csVim−CD133− cells.
(a) Freshly sorted csVim+CD133−, csVim−CD133+ and csVim−CD133− cells were stained for EpCAM, Twist and Slug. (b) Fifty thousand csVim+CD133− (four mice) or csVim−CD133+ cells (five mice) or csVim−CD133− cells (three mice) were injected intraosseously into the right legs of NSG mice. Two weeks postinoculation, the right legs were amputated. The images are representative of liver metastases observed at week 4 postinoculation. (c) Numbers of gross metastatic liver nodules. P value is 0.0486 (d) Hematoxylin-eosin-stained sections of metastatic liver tumor tissues (original magnification ×400). One-way Anova analyses: *P < .05; **P < .01; ***P< .001.
Our identification of the novel csVim+CD133− cell population sheds new light on EMT-positive LCSCs. An understanding of the biology of this cell population can be used to reduce HCC metastasis.
Discussion
The transition from primary CSCs to EMT-positive CSCs is a significant step in HCC progression 18. Recent studies suggest that cancer stem cells having EMT phenotypes are associated with sensitivity and resistance to chemotherapy in various tumor models 19, 20. Identification of this novel population will greatly facilitate to understand their intrinsic resistance towards neoplastic drugs, dormancy and effective therapeutic strategy. In this study, we isolated from primary HCC a novel cell population, csVim+CD133−, which exhibited stem-like cells properties by forming spheres in Matrigel, expressing Oct4A and Sox2, and differentiating into hepatocytes and cholangiocytes.
The presence of LCSCs in HCC has been proven, and chemotherapeutic and other strategies have been used to reduce LCSCs 4, 8, 9. However, early and late intrahepatic relapses occur frequently 21, 22. Several lines of evidence suggest that EMT-positive cancer stem cells regulate sensitivity and resistance to chemotherapy in various tumor models 17. The present study for the first time identifies the existence of Vimentin on the surface of LCSCs and presents a separation technique to enrich EMT-positive LCSCs directly from primary tumor cells. These csVim+CD133− cells are considered EMT-positive cells because these cells also express low E-cadherin and high nuclear twist, slug two other critical EMT markers beside Vimentin; In agreement with this conclusion, these csVim+CD133− cells have superiority metastatic potential compared to the well-known csVim−CD133+ stem cells in vivo. As Vimentin is a universal EMT-marker in different types of cancer models, its surface expression could be utilized to enrich and characterize EMT positive CSCs from different tissue specific cancers. Further investigation of EMT-positive CSCs may unveil some of the key mechanisms of cancer relapse and to target selected pathways to prevent recurrence in different tissue specific cancers.
Supplementary Material
Novelty of the manuscript.
The discovery of EMT positive liver cancer stem cells using a novel marker that detects cell surface Vimentin.
Impact of the manuscript.
This manuscript describes a novel population of cancer stem cells csVimentin+CD133− that play an active role in tumor relapse and metastasis.
Acknowledgments
Special thanks to Dr. Randy Johnson for providing tumor-bearing MST−/ − mice. Work in the authors’ laboratory was supported by a grant from the National Institutes of Health to Dr. Shulin Li (NIH R01CA120895).
Footnotes
“The authors disclose no potential conflicts of interest.”
References
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Carr BI. Hepatocellular carcinoma: current management and future trends. Gastroenterology. 2004;127:S218–24. doi: 10.1053/j.gastro.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–58. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 6.Chuthapisith S, Eremin J, El-Sheemey M, Eremin O. Breast cancer chemoresistance: emerging importance of cancer stem cells. Surg Oncol. 2010;19:27–32. doi: 10.1016/j.suronc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Rountree CB, Ding W, He L, Stiles B. Expansion of CD133-expressing liver cancer stem cells in liver-specific phosphatase and tensin homolog deleted on chromosome 10-deleted mice. Stem cells. 2009;27:290–9. doi: 10.1634/stemcells.2008-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, Barnard GF, Doki Y, et al. CD13 is a therapeutic target in human liver cancer stem cells. The Journal of clinical investigation. 2010;120:3326–39. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–66. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–24. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satelli A, Mitra A, Cutrera JJ, Devarie M, Xia X, Ingram DR, Dibra D, Somaiah N, Torres KE, Ravi V, Ludwig JA, Kleinerman ES, et al. Universal Marker and Detection Tool for Human Sarcoma Circulating Tumor Cells. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitra A, Mishra L, Li S. Technologies for deriving primary tumor cells for use in personalized cancer therapy. Trends in biotechnology. 2013;31:347–54. doi: 10.1016/j.tibtech.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–84. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 14.Yu B, He ZY, You P, Han QW, Xiang D, Chen F, Wang MJ, Liu CC, Lin XW, Borjigin U, Zi XY, Li JX, et al. Reprogramming fibroblasts into bipotential hepatic stem cells by defined factors. Cell Stem Cell. 2013;13:328–40. doi: 10.1016/j.stem.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Mitra A, Ross JA, Rodriguez G, Nagy ZS, Wilson HL, Kirken RA. Signal transducer and activator of transcription 5b (Stat5b) serine 193 is a novel cytokine-induced phospho-regulatory site that is constitutively activated in primary hematopoietic malignancies. The Journal of biological chemistry. 2012;287:16596–608. doi: 10.1074/jbc.M111.319756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy ZS, LeBaron MJ, Ross JA, Mitra A, Rui H, Kirken RA. STAT5 regulation of BCL10 parallels constitutive NFkappaB activation in lymphoid tumor cells. Molecular cancer. 2009;8:67. doi: 10.1186/1476-4598-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May CD, Sphyris N, Evans KW, Werden SJ, Guo W, Mani SA. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast cancer research : BCR. 2011;13:202. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, Wei LX. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer letters. 2014;352:160–8. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, Sphyris N, Shariati M, Kumar SV, Battula VL, Herschkowitz JI, Guerra R, et al. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73:1981–92. doi: 10.1158/0008-5472.CAN-12-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell death & disease. 2013;4:e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Fan J, Qin LX, Zhou J, Sun HC, Qiu SJ, Ye QH, Wang L, Tang ZY. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18:1955–63. doi: 10.1245/s10434-010-1540-z. [DOI] [PubMed] [Google Scholar]
- 22.Ho CM, Lee PH, Shau WY, Ho MC, Wu YM, Hu RH. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery. 2012;151:700–9. doi: 10.1016/j.surg.2011.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.