Abstract
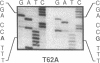
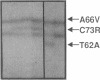
Congenital erythropoietic porphyria (CEP), an inborn error of heme biosynthesis, results from the deficient activity of uroporphyrinogen III synthase (URO-synthase). This autosomal recessive disorder is heterogeneous; patients with severe disease are often transfusion dependent, while milder patients primarily have cutaneous involvement. To investigate this phenotypic heterogeneity, exonic point mutations in the URO-synthase gene were identified in unrelated CEP patients. Four missense mutations were identified: (a) an A to G transition of nucleotide (nt) 184 that predicted a Thr to Ala substitution at residue 62 (designated T62A); (b) a C to T transition of nt 197 that encoded an Ala to Val replacement at residue 66 (A66V); (c) a T to C transition of nt 217 that predicted a Cys to Arg substitution at residue 73 (C73R); and (d) a C to T transition of nt 683 that resulted in a Thr to Met replacement at residue 228 (T228M). In addition, a G to A transition of nt 27 that did not change the encoded amino acid (A9A) was detected in an African patient. The T62A, C73R, and T228M alleles did not express detectable enzymatic activity, while the A66V allele expressed residual, but unstable activity. The C73R allele was present in eight of 21 unrelated CEP patients (21% of CEP alleles). In three patients, identification of both alleles permitted genotype-phenotype correlations; the A66V/C73R, T228M/C73R, and C73R/C73R genotypes had mild, moderately severe, and severe disease, respectively. These findings provide the first genotype-phenotype correlations and permit molecular heterozygote detection in this inherited porphyria.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J., Kunkel L., Bruns G., Tantravahi U., Lalande M., Brewster T., Moreau E., Wilson M., Bromley W., Roderick T. A strategy to reveal high-frequency RFLPs along the human X chromosome. Am J Hum Genet. 1984 May;36(3):546–564. [PMC free article] [PubMed] [Google Scholar]
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Astrin K. H., Warner C. A., Yoo H. W., Goodfellow P. J., Tsai S. F., Desnick R. J. Regional assignment of the human uroporphyrinogen III synthase (UROS) gene to chromosome 10q25.2----q26.3. Hum Genet. 1991 May;87(1):18–22. doi: 10.1007/BF01213085. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Kytzia H. J., Navon R., Sandhoff K. Ganglioside GM2 N-acetyl-beta-D-galactosaminidase activity in cultured fibroblasts of late-infantile and adult GM2 gangliosidosis patients and of healthy probands with low hexosaminidase level. Am J Hum Genet. 1983 Sep;35(5):900–913. [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Deybach J. C., Grandchamp B., Grelier M., Nordmann Y., Boué J., Boué A., de Berrianger P. Prenatal exclusion of congenital erythropoietic porphyria (Günther's disease) in a fetus at risk. Hum Genet. 1980 Feb;53(2):217–221. doi: 10.1007/BF00273499. [DOI] [PubMed] [Google Scholar]
- Deybach J. C., de Verneuil H., Boulechfar S., Grandchamp B., Nordmann Y. Point mutations in the uroporphyrinogen III synthase gene in congenital erythropoietic porphyria (Günther's disease). Blood. 1990 May 1;75(9):1763–1765. [PubMed] [Google Scholar]
- Deybach J. C., de Verneuil H., Phung N., Nordmann Y., Puissant A., Boffety B. Congenital erythropoietic porphyria (Günther's disease): enzymatic studies on two cases of late onset. J Lab Clin Med. 1981 Apr;97(4):551–558. [PubMed] [Google Scholar]
- Horiguchi Y., Horio T., Yamamoto M., Tanaka T., Seki Y., Imamura S. Late onset erythropoietic porphyria. Br J Dermatol. 1989 Aug;121(2):255–262. doi: 10.1111/j.1365-2133.1989.tb01808.x. [DOI] [PubMed] [Google Scholar]
- Levran O., Desnick R. J., Schuchman E. H. Niemann-Pick disease: a frequent missense mutation in the acid sphingomyelinase gene of Ashkenazi Jewish type A and B patients. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3748–3752. doi: 10.1073/pnas.88.9.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann Y., Deybach J. C. Congenital erythropoietic porphyria. Semin Liver Dis. 1982 May;2(2):154–163. doi: 10.1055/s-2008-1040705. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Romeo G., Kaback M. M., Levin E. Y. Uroporphyrinogen 3 cosynthetase activity in fibroblasts from patients with congenital erythropoietic porphyria. Biochem Genet. 1970 Dec;4(6):659–664. doi: 10.1007/BF00486379. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Coupled-enzyme and direct assays for uroporphyrinogen III synthase activity in human erythrocytes and cultured lymphoblasts. Enzymatic diagnosis of heterozygotes and homozygotes with congenital erythropoietic porphyria. Anal Biochem. 1987 Oct;166(1):120–133. doi: 10.1016/0003-2697(87)90554-9. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Human uroporphyrinogen III synthase: molecular cloning, nucleotide sequence, and expression of a full-length cDNA. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7049–7053. doi: 10.1073/pnas.85.19.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Purification and properties of uroporphyrinogen III synthase from human erythrocytes. J Biol Chem. 1987 Jan 25;262(3):1268–1273. [PubMed] [Google Scholar]
- Wang L. M., Weber D. K., Johnson T., Sakaguchi A. Y. Supercoil sequencing using unpurified templates produced by rapid boiling. Biotechniques. 1988 Oct;6(9):839, 841-3. [PubMed] [Google Scholar]
- Youssoufian H., Kazazian H. H., Jr, Phillips D. G., Aronis S., Tsiftis G., Brown V. A., Antonarakis S. E. Recurrent mutations in haemophilia A give evidence for CpG mutation hotspots. 1986 Nov 27-Dec 3Nature. 324(6095):380–382. doi: 10.1038/324380a0. [DOI] [PubMed] [Google Scholar]
- von Scheidt W., Eng C. M., Fitzmaurice T. F., Erdmann E., Hübner G., Olsen E. G., Christomanou H., Kandolf R., Bishop D. F., Desnick R. J. An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med. 1991 Feb 7;324(6):395–399. doi: 10.1056/NEJM199102073240607. [DOI] [PubMed] [Google Scholar]