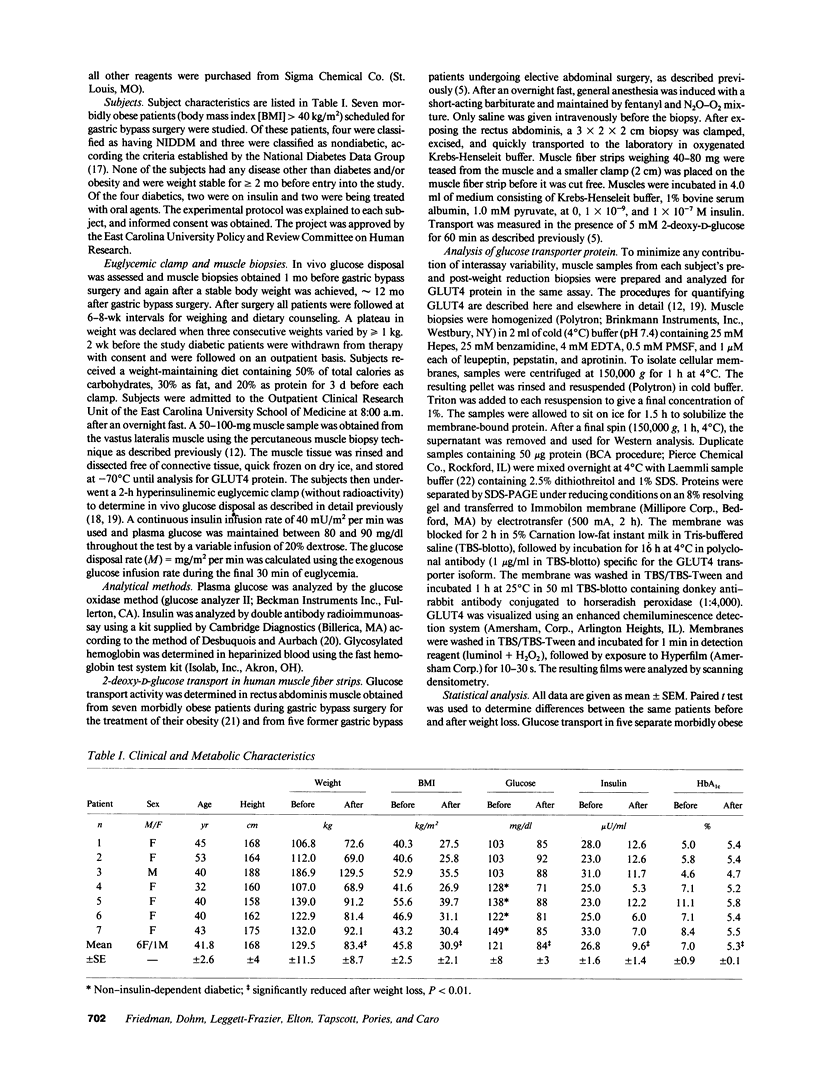
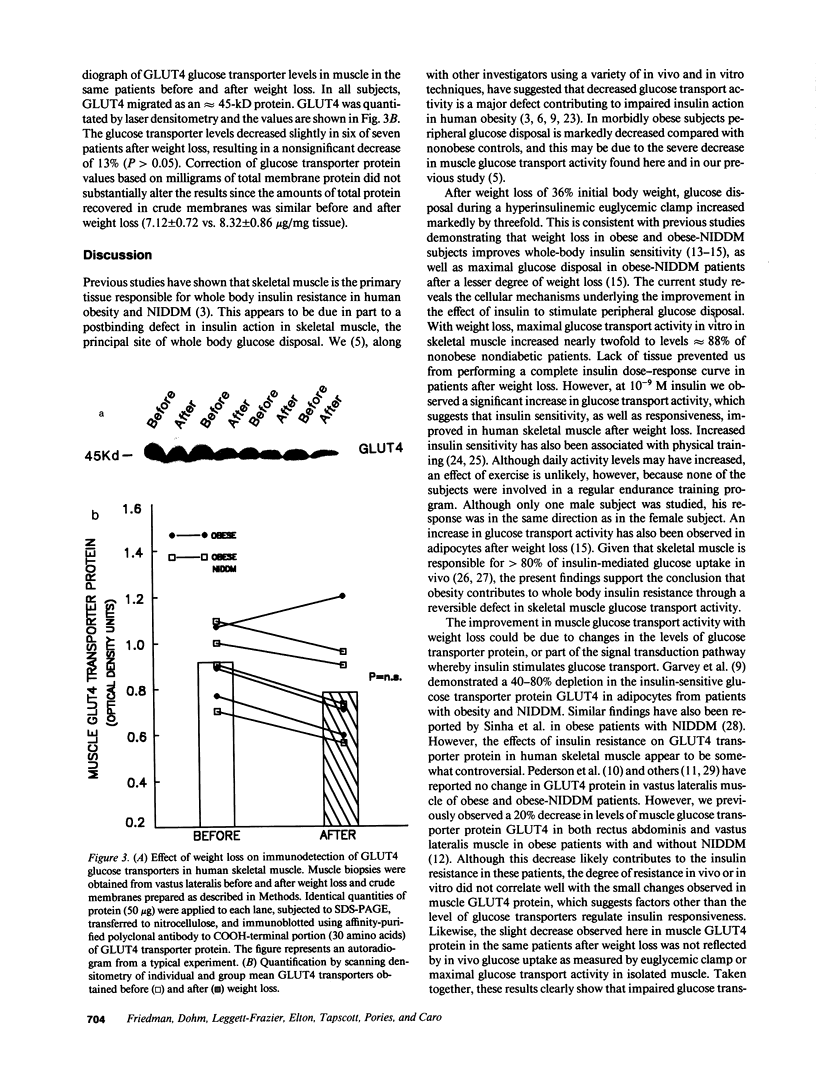
Abstract
A major defect contributing to impaired insulin action in human obesity is reduced glucose transport activity in skeletal muscle. This study was designed to determine whether the improvement in whole body glucose disposal associated with weight reduction is related to a change in skeletal muscle glucose transport activity and levels of the glucose transporter protein GLUT4. Seven morbidly obese (body mass index = 45.8 +/- 2.5, mean +/- SE) patients, including four with non-insulin-dependent diabetes mellitus (NIDDM), underwent gastric bypass surgery for treatment of their obesity. In vivo glucose disposal during a euglycemic clamp at an insulin infusion rate of 40 mU/m2 per min was reduced to 27% of nonobese controls (P less than 0.01) and improved to 78% of normal after weight loss of 43.1 +/- 3.1 kg (P less than 0.01). Maximal insulin-stimulated glucose transport activity in incubated muscle fibers was reduced by approximately 50% in obese patients at the time of gastric bypass surgery but increased twofold (P less than 0.01) to 88% of normal in five separate patients after similar weight reduction. Muscle biopsies obtained from vastus lateralis before and after weight loss revealed no significant change in levels of GLUT4 glucose transporter protein. These data demonstrate conclusively that insulin resistance in skeletal muscle of mobidly obese patients with and without NIDDM cannot be causally related to the cellular content of GLUT4 protein. The results further suggest that morbid obesity contributes to whole body insulin resistance through a reversible defect in skeletal muscle glucose transport activity. The mechanism for this improvement may involve enhanced transporter translocation and/or activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner P., Pollare T., Lithell H., Livingston J. N. Defective insulin receptor tyrosine kinase in human skeletal muscle in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1987 Jun;30(6):437–440. doi: 10.1007/BF00292549. [DOI] [PubMed] [Google Scholar]
- Beck-Nielsen H., Pedersen O., Sørensen N. S. Effects of dietary changes on cellular insulin binding and in vivo insulin sensitivity. Metabolism. 1980 May;29(5):482–487. doi: 10.1016/0026-0495(80)90174-2. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J., Flickinger E. G., Meelheim D., Dohm G. L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987 May;79(5):1330–1337. doi: 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Scarlett J. A., Kao M., Olefsky J. M. Role of glucose transport in the postreceptor defect of non-insulin-dependent diabetes mellitus. Diabetes. 1982 Nov;31(11):1016–1022. doi: 10.2337/diacare.31.11.1016. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Elton C. W., Friedman J. E., Pilch P. F., Pories W. J., Atkinson S. M., Jr, Caro J. F. Decreased expression of glucose transporter in muscle from insulin-resistant patients. Am J Physiol. 1991 Mar;260(3 Pt 1):E459–E463. doi: 10.1152/ajpendo.1991.260.3.E459. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidenberg G. R., Reichart D., Olefsky J. M., Henry R. R. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988 Oct;82(4):1398–1406. doi: 10.1172/JCI113744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. E., Dohm G. L., Elton C. W., Rovira A., Chen J. J., Leggett-Frazier N., Atkinson S. M., Jr, Thomas F. T., Long S. D., Caro J. F. Muscle insulin resistance in uremic humans: glucose transport, glucose transporters, and insulin receptors. Am J Physiol. 1991 Jul;261(1 Pt 1):E87–E94. doi: 10.1152/ajpendo.1991.261.1.E87. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Huecksteadt T. P., Birnbaum M. J., Molina J. M., Ciaraldi T. P. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest. 1991 Mar;87(3):1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainault I., Guerre-Millo M., Guichard C., Lavau M. Differential regulation of adipose tissue glucose transporters in genetic obesity (fatty rat). Selective increase in the adipose cell/muscle glucose transporter (GLUT 4) expression. J Clin Invest. 1991 Mar;87(3):1127–1131. doi: 10.1172/JCI115077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handberg A., Vaag A., Damsbo P., Beck-Nielsen H., Vinten J. Expression of insulin regulatable glucose transporters in skeletal muscle from type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1990 Oct;33(10):625–627. doi: 10.1007/BF00400207. [DOI] [PubMed] [Google Scholar]
- Henry R. R., Wallace P., Olefsky J. M. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986 Sep;35(9):990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- James D. E., Kraegen E. W., Chisholm D. J. Effects of exercise training on in vivo insulin action in individual tissues of the rat. J Clin Invest. 1985 Aug;76(2):657–666. doi: 10.1172/JCI112019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Jimenez J., Zuniga-Guajardo S., Zinman B., Angel A. Effects of weight loss in massive obesity on insulin and C-peptide dynamics: sequential changes in insulin production, clearance, and sensitivity. J Clin Endocrinol Metab. 1987 Apr;64(4):661–668. doi: 10.1210/jcem-64-4-661. [DOI] [PubMed] [Google Scholar]
- Koranyi L., James D., Mueckler M., Permutt M. A. Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. J Clin Invest. 1990 Mar;85(3):962–967. doi: 10.1172/JCI114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lockwood D. H., Amatruda J. M. Cellular alterations responsible for insulin resistance in obesity and type II diabetes mellitus. Am J Med. 1983 Nov 30;75(5B):23–31. doi: 10.1016/0002-9343(83)90250-4. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Ciaraldi T. P., Kolterman O. G. Mechanisms of insulin resistance in non-insulin-dependent (type II) diabetes. Am J Med. 1985 Sep 20;79(3B):12–22. doi: 10.1016/s0002-9343(85)80003-6. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Pories W. J., Caro J. F., Flickinger E. G., Meelheim H. D., Swanson M. S. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg. 1987 Sep;206(3):316–323. doi: 10.1097/00000658-198709000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E. A., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982 Apr;69(4):785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M. K., Raineri-Maldonado C., Buchanan C., Pories W. J., Carter-Su C., Pilch P. F., Caro J. F. Adipose tissue glucose transporters in NIDDM. Decreased levels of muscle/fat isoform. Diabetes. 1991 Apr;40(4):472–477. doi: 10.2337/diab.40.4.472. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Fukumoto H., Koh G., Yano H., Yasuda K., Masuda K., Ikeda H., Imura H., Seino Y. Liver and muscle-fat type glucose transporter gene expression in obese and diabetic rats. Biochem Biophys Res Commun. 1991 Mar 29;175(3):995–1002. doi: 10.1016/0006-291x(91)91663-w. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Young A. A., Lamkin C., Foley J. E. Kinetics of glucose disposal in whole body and across the forearm in man. J Clin Invest. 1987 Jun;79(6):1713–1719. doi: 10.1172/JCI113011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziel F. H., Venkatesan N., Davidson M. B. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988 Jul;37(7):885–890. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]




