Summary
The trade-off between current reproductive effort and survival is a key concept of life history theory. A variety of studies support the existence of this trade-off but the underlying physiological mechanisms are not well-understood. Oxidative stress has been proposed as a potential mechanism underlying the observed inverse relationship between reproductive investment and lifespan. Prolonged fasting is associated with oxidative stress including increases in the production of reactive oxygen species, oxidative damage and inflammation.
Northern elephant seals (NES) undergo prolonged fasts while maintaining high metabolic rates during breeding. We investigated NES of both sexes to assess oxidative stress associated with extended breeding fasts. We measured changes in the plasma activity or concentrations of markers for oxidative stress in 30 adult male and 33 adult female northern elephant seals across their 1–3 month breeding fasts. Markers assessed included a pro-oxidant enzyme, several antioxidant enzymes, markers for oxidative damage to lipids, proteins and DNA, and markers for systemic inflammation.
Plasma xanthine oxidase (XO), a pro-oxidant enzyme that increases production of oxidative radicals, and several protective antioxidant enzymes increased over breeding in both sexes. Males showed increased oxidative damage to lipids and DNA and increased systemic inflammation, while oxidative damage to proteins declined across breeding. In contrast, females showed no oxidative damage to lipids or DNA or changes in inflammation, but showed increases in oxidative damage to proteins. XO activity, antioxidant enzymes, oxidative damage markers, and inflammatory markers were strongly correlated in males but these relationships were weaker or non-existent in females.
NES provide evidence for oxidative stress as a physiological cost of reproduction in a capital breeding mammal. Both sexes strongly up-regulated antioxidant defenses during breeding. Despite this response, and in contrast to similar duration non-breeding fasts in previous studies on conspecifics, there was evidence of oxidative damage to tissues. These data demonstrate the utility of using plasma markers to examine oxidative stress but also suggest the necessity of measuring a broad suite of plasma markers to assess systemic oxidative stress.
Keywords: reproduction, life-history theory, pinnipeds, fasting, lipid peroxidation
Introduction
Evolutionary explanations for life history patterns assume that successful reproduction carries potential fitness costs through reduced survival and future reproduction (Stearns 1992; Roff 2002). Although it is widely assumed that physiological processes underlie these trade-offs (Zera & Harshman 2001; Speakman 2008), evidence for proximate mechanisms mediating trade-offs between reproductive effort and fitness have been rare. These trade-offs are thought to originate from the reduction of energy allocation to vital functions such as cellular maintenance, somatic function, future reproduction and survival (Zera and Harshman 2001). Energetically demanding activities such as male-male combat, ornamentation, harem/territory defense and lactation are undertaken by organisms during reproduction. In the wild, income breeding animals may increase energy intake by 60% – 190% above normal levels to faciltate increased energy expenditure for reproduction (Gittleman & Thompson 1988). In capital breeders, the trade-off between energetic investment in current reproduction and body condition is distinct, as reproductive effort is supported exclusively from body reserves (Jönsson 1997; Stephens et al. 2009).
Oxidative stress has been theorized to be a potential physiological consequence of reproductive effort that may limit investment in other important life history components (Costantini 2008; Dowling & Simmons 2009; Monaghan, Metcalfe & Torres 2009; Metcalfe & Alonso-Alvarez 2010; Isaksson, Sheldon & Uller 2011; Speakman & Garratt 2014). This theory suggests that energetic trade-offs between reproductive effort and maintenance may impact fitness costs through oxidative insult. Normal metabolism produces a variety of reactive oxygen species (ROS) that can cause damage to biomolecules unless regulated by enzymatic and other antioxidants (Balaban, Nemoto & Finkel 2005). Oxidative stress occurs when ROS production exceeds the capacity of antioxidants to control their negative effects. Because the majority of ROS are produced through normal energy metabolism, elevated energy expenditure associated with reproduction may increase ROS production (Balaban, Nemoto & Finkel 2005). However, organisms can still limit the occurrence of oxidative stress by up-regulating antioxidant responses (Garratt et al. 2011, 2012).
To date there is little evidence supporting oxidative stress as a potential fitness cost to reproduction. Studies on invertebrates (Salmon, Marx & Harshman 2001; Wang, Salmon & Harshman 2001), lizards (Olsson et al. 2012), captive birds (Alonso-Alvarez et al. 2004, 2006; Wiersma et al. 2004; Bertrand et al. 2006) and free ranging birds (Casagrande et al. 2011; van de Crommenacker et al. 2012) found links between reproduction and reduced antioxidant protection resulting in increased oxidative stress. However, evidence for increased damage to biomolecules associated with reproduction is rare. Several studies that suggested oxidative damage related to reproductive effort found strong sex differences in this feature (Heiss & Schoech 2012; Olsson et al. 2012). Studies in mammals have been similarly equivocal, with both increased and decreased antioxidant capacity associated with reproduction and little or no evidence for systemic oxidative damage (Speakman and Garratt 2013; Ołdakowski et al. 2012; Garratt et al. 2011, Bergeron et al. 2011). Recent studies have shown that evidence for oxidative damage varied widely with markers and tissue type (Xu et al. 2013; Yang et al. 2013; Garatt et al. 2012). Importantly, all of these studies were undertaken in income breeding species. One study that showed a relationship between reproductive effort and oxidative damage in free-ranging squirrels, found these effects were reduced by food supplementation (Fletcher et al. 2013).
Most air-breathing marine predators face unique energetic constraints while breeding due to the temporal separation of marine foraging and terrestrial breeding. Several groups of animals, including pinnipeds, fast for highly variable periods of time during breeding events (Champagne et al. 2012). Extended fasting is associated with the depletion of antioxidant stores and increased production of reactive oxygen species (ROS; Mårtensson 1986). Fasting promotes the production of hydrogen peroxide and lipid hydroperoxides and the depletion of antioxidant defenses which can cause oxidative damage and inflammation (Crescimanno et al. 1989; Di Simplicio et al. 1997; Grattagliano et al. 2000). ROS such as oxygen free radicals and hydrogen peroxides are produced through the activity of enzymes such as xanthine oxidase (XO) which increase during fasting (Hille & Nishino 1995). Because oxidative stress is a direct consequence of extended fasting, capital breeders may be especially prone to oxidative damage from reproductive effort.
Northern elephant seals (NES; Mirounga angustirostris; Fig. 1) undertake extended fasts during breeding. Adult male elephant seals arrive at the beach and compete to establish dominance hierarchies used to control access to estrus females (Haley, Deutsch & Le Boeuf 1994). Combat, terrestrial locomotion and multiple breeding events lead to an average metabolic rate of 3.1 times Kleiber’s predicted SMR (Crocker, Houser & Webb 2012). This variation in adult male metabolic rate impacts mating success, with the most successful males having higher metabolic rates (Deutsch, Haley & Le Boeuf 1990; Crocker, Houser & Webb 2012). During the same period, female elephant seals give birth to a single pup and provision it with one of the highest energy density milks found in nature for ~25 days (Crocker et al. 2001). Female elephant seals sustain a metabolic rate on average of 2.4 times predicted SMR during breeding. When milk energy output is included, female elephant seals incur rates of energy output that are approaching 6 times Kleiber’s predicted SMR for one month during the breeding fast (Crocker et al. 2001). The strong sex differences in metabolic rates, energy output, and fasting duration in this species provide an ideal study system with which to compare potential impacts of reproduction on oxidative stress.
Fig. 1.

Adult male elephant seals compete for dominance during breeding. Photo Credit: Tanguy de Tillesse.
To investigate the potential for oxidative stress impacts of breeding in elephant seals, we examined differences in plasma markers for oxidative damage to lipids, protein and DNA. We measured changes in the oxidant-producing enzyme (XO), antioxidant enzymes and markers of systemic inflammation. We compared these variables between genders early in breeding and as close as possible to the end of breeding. Our objective was to examine evidence for oxidative stress as a potential fitness cost to reproduction and to compare this feature between the sexes.
Materials and Methods
STUDY SITE AND SUBJECTS
All procedures were carried out under National Marine Fisheries Services marine mammal permit # 14636 and approved by the Sonoma State University IACUC. This study was conducted at Año Nuevo State Reserve, San Mateo County, CA, during the elephant seal breeding season from January through March. Males were evaluated as an adult from specific secondary sexual characteristics and body mass (Deutsch, Haley & Le Boeuf 1990). Females were aged using flipper tags applied to them previously as weaned pups. Fifteen males were sampled early in breeding (1–2 weeks fasted) and 15 males were sampled late in breeding (9–10 weeks fasted) with 6 of the males being sampled at both sample periods. Fourteen females were sampled early (5 days post-partum) and a separate group of 19 females were sampled late in lactation (22 days post-partum). During the initial procedure, seals were marked with hair dye (Stamford, Conn.) for future identification.
SAMPLE COLLECTION
An initial intramuscular injection of Telazol (~0.3mg/kg for males and ~1mg/kg for females) was used to immobilize each seal. To maintain immobilization, ketamine and diazepam were given intravenously as needed (all drugs from Fort Dodge Laboratories, Ft. Dodge, IA). Blood samples were collected from the extradural vein into pre-chilled EDTA-treated collection tubes containing 10 μl ml−1 protease inhibitor cocktail and 0.005% butylated hydroxytoluene (Sigma-Aldrich, St Louis, MO, USA). Blood tubes were centrifuged immediately in the field for 15 minutes at 3,300 RPM. Plasma was collected in 1 ml aliquots in cryogenic vials, and then frozen on dry ice until they were transferred to a −80°c freezer for storage. Standard length and axillary girth measurements were taken to estimate the body mass of the adult males (Le Boeuf et al. 2000; Crocker, Houser & Webb 2012). Female mass was determined using a tripod and scale (MSI, Seattle, WA; ± 2 kg).
SAMPLE ANALYSIS
Plasma markers analyzed and their meanings are summarized in Table 1. The plasma activity of xanthine oxidase, an enzyme that generates superoxide and hydrogen peroxide, was quantified using a flourometric enzyme immuno-assay (EIA; Cayman Chemical, Ann Arbor, MI, USA). The plasma activity of antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase were quantified using colorimetric assays (Cayman Chemical). SOD catalyzes the alteration of superoxides to hydrogen peroxide and water, while catalase and GPx catalyze the alteration of hydrogen peroxide to water and oxygen (Zelko, Mariani & Folz 2002). Plasma 8-isoprostane (8-iso-PGF2α) and 4-Hydroxynonenal (4HNE) concentrations were measured as markers for lipid peroxidation using enzyme-linked immune-sorbent assay (canine ELISA; Oxford Biomedical Research Rochester Hills, MI; Blue Gene Biotec, Shanghai China). Plasma nitrotyrosine was measured by ELISA as a marker for protein oxidation/nitration (Alpco Diagnostics, Salem, NH). Plasma 8-hydroxy-2-deoxy Guanosine (8-OHG) was measured using EIA as a marker for DNA oxidation (Cayman Chemical). Tumor Necrosis Factor α (TNF-α) and C-reactive protein (CRP) concentrations were quantified in plasma as markers for inflammation using EIA (Cayman Chemical).
Table 1.
Markers for oxidative stress measured in plasma samples early and late in breeding in male and female northern elephant seals.
| Marker | Purpose | Category |
|---|---|---|
| Xanthine oxidase (XO) | Pro-oxidant Enzyme | Oxidative stress |
| Superoxide dismutase (SOD) | Antioxidant enzyme | Oxidative defense |
| Catalase | Antioxidant enzyme | Oxidative defense |
| Glutathione peroxidase (GPx) | Antioxidant enzyme | Oxidative defense |
| 8-isoprostanes (8-iso-PGF2α) | Lipid peroxidation | Oxidative damage |
| 4-hydroxynoneal (4-HNE) | Lipid peroxidation | Oxidative damage |
| Nitrotyrosine | Protein nitrosylation | Oxidative damage |
| 8-hydroxy-2-deoxyguanosine (8-OHG) | DNA oxidation | Oxidative damage |
DATA ANALYSIS
Differences between early and late breeding samples and relationships between oxidative stress markers in males were compared using linear mixed models to account for repeat sampling with individual seal as a random effect. For all mixed models, a variance components covariance structure was used for the random effect and model residuals were assessed for approximate normality. An r2 for mixed models regressions was calculated (Edwards et al. 2008) and regressions were visualized using the slope and intercept parameters from the mixed model. Differences between early and late female samples and between sexes were compared using one way ANOVA. In females, relationships between oxidative stress markers were evaluated using simple linear regression. Results were considered significant at p < 0.05. Statistical analyses were performed using JMP 10 (SAS Institute Inc., Raleigh, NC).
Results
Samples of both sexes showed impacts of breeding on body reserves. In males, body mass late in breeding (1040±34 kg) was 27% lower than early in breeding (1430±33 kg). In females, body mass late in breeding (332±12) was 31% lower than early in breeding (478±15).
CHANGES IN PLASMA ENZYMES WITH BREEDING
Plasma enzymes suggested higher levels of pro-oxidant stress in response to breeding that were countered by robust upregulation of antioxidant enzymes in all individuals. Both sexes exhibited a significant increase in XO activity across the breeding fast (F1, 20.7 = 8.5, p < 0.0082; F1,32 = 14.2, p < 0.0007; Table 2). Males showed significant increases in SOD (F1,23.3 = 11.4, p < 0.0027), GPx (F1,12 = 8.6, p = 0.01) and catalase activities (F1,15.6 = 7.1, p = 0.02) between early and late samples (Table 2). Females exhibited increased GPx (F1,32 = 6.8, p < 0.014) and catalase activities (F1,32 = 4.7, p = 0.038) late in breeding while SOD values were similar at both samples. (p > 0.05; Table 2).
Table 2.
Plasma level means (Mean ± S.E.M.) for the pro-oxidant and antioxidant enzymes between early breeding (1–2 weeks males, 1 week females) and late breeding (9–11 weeks males and 4 weeks females) adult northern elephant seals.
| Early Breeding Males | Late Breeding Males | Early Breeding Females | Late breeding females | |
|---|---|---|---|---|
| XO (μU mL−1) | 42.00 ± 2.45^ | 53.20 ± 2.87*^ | 72.24 ± 2.51^ | 85.93 ± 2.51*^ |
| SOD(U mL−1) | 1.17 ± 0.10^ | 1.89 ± 0.17* | 1.80 ± 0.09^ | 1.94 ± 0.12 |
| Catalase (nmol min−1 ml−1) | 11.1 ± 0.9 | 16.9 ± 1.7* | 14.4 ± 1.5 | 23.0 ± 3.2* |
| GPx (nmol min−1 ml−1) | 129.3 ± 11.9 | 229.7 ± 27.7* | 165.3 ± 16.4 | 236.4 ± 20.1* |
XO: xanthine oxidase; SOD: superoxide dismutase; GPx: glutathione peroxidase.
denotes significant changes across breeding.
Denotes significant differences between sexes within early and late sample periods.
When comparing sexes, female XO activities were higher than that of males at both early and late sample periods (F1,28 = 74.1, p = 0.0001; F1,33 = 73.8, p = 0.0001, respectively). Plasma SOD activity was higher in females than in males in the early breeding samples (F1,28 = 20.10, p = 0.0001). All other enzyme activities were similar between the sexes within sampling periods (p > 0.05).
CHANGES IN OXIDATIVE DAMAGE WITH BREEDING
Males exhibited evidence for increased oxidative damage to lipids in response to breeding. This change was not present in females. Males exhibited significant increases in 8-iso-PGF2α across the breeding fast (F1,5.0 = 7.8, p = 0.02), while 4HNE concentrations were similar between samples (F1,13.5 = 3.4, p = 0.09; Table 3). Female elephant seals showed no significant differences in either 8-iso-PGF2α (F1,31 = 0.94, p = 0.34) or 4HNE (F1,32 = 0.11, p = 0.74) across the breeding fast (Table 3). Despite this difference lipid peroxidation markers were similar between the sexes at both time points (p > 0.05).
Table 3.
Plasma level means (S.E.M.) for the markers of lipid, protein and DNA oxidation between early breeding (1–2 weeks males, 1 week females) and late breeding (9–11 weeks males and 4 weeks females) adult northern elephant seals.
| Early Breeding Males | Late Breeding Males | Early Breeding Females | Late breeding females | |
|---|---|---|---|---|
| Damage markers
|
||||
| 8-iso-PGF2α (ng ml−1) | 15.81 ± 3.10 | 23.67 ± 3.92* | 12.26 ± 3.20 | 16.93 ± 3.39 |
| 4-HNE (μg ml−1) | 21.51 ± 2.34 | 27.68 ± 1.93* | 22.40 ± 4.30 | 24.19 ± 3.32 |
| Nitrotyrosine (nM) | 593.7 ± 21.5 | 470.6 ± 20.2*^ | 589.3 ± 23.4 | 795.1 ± 80.5*^ |
| 8-OHG (ng ml−1) | 3.01 ± 0.02^ | 3.22 ± 0.04*^ | 2.64 ± 0.05^ | 2.55 ± 0.08^ |
| Inflammation markers
|
||||
| TNF-α (pg mL−1) | 13.38 ± 2.59 | 23.24 ± 2.59*^ | 14.03 ± 4.07 | 16.24 ± 3.08^ |
| CRP (μg mL−1) | 0.29 ± 0.02 | 0.26 ± 0.02 | 0.27 ± 0.01 | 0.27 ± 0.02 |
8-iso-PGF2α: 8-isoprostanes; 4-HNE: 4-hydroxynoneal; 8-OHG: 8-hydroxy-2-deoxyguanosine; TNF-α: Tumor necrosis factor- α; CRP: C-reative protein.
denotes significant changes across breeding.
Denotes significant differences between sexes within early and late sample periods.
Males exhibited evidence for reduced oxidative damage to proteins across breeding that contrasted with evidence for increased protein damage in females. The marker for protein oxidation/nitration showed opposite patterns between the sexes. Plasma NT significantly decreased across the breeding fast in male seals (F1,21.6 = 15.73, p < 0.001), while females demonstrated a significant increase in NT (F1,32 = 4.6, p = 0.04) across lactation (Table 3). This resulted in females having higher levels of NT than males late in breeding (F1,33 = 12.30, p = 0.001).
Males exhibited evidence for increased damage to DNA in response to breeding that was not present in females. The plasma marker for DNA oxidation (8-OHG) significantly increased across the breeding fast in male northern elephant seals (F1,5.8 = 6.9, p = 0.04), while female northern elephant 8-OHG concentrations were similar between samples (p = 0.40; Table 3). When comparing sexes, males had higher concentrations of 8-OHG at both early and late samples (F1,28 = 97.6, p = 0.0001, F1,33 = 36.9, p = 0.0001, respectively).
INFLAMMATORY MARKERS
Males exhibited evidence for increased systemic inflammation in response to breeding that was not present in females. TNF-α showed significant increases across the breeding fast in males (F1, 11.8 = 7.97, p = 0.02; Table 3). In contrast, CRP levels were low and did not vary between male sampling periods (F1, 11.4 = 0.95, p = 0.35; Table 3). Females showed no significant differences in the concentrations of either TNF-α (F1, 32 = 0.20, p = 0.66) or CRP (F1,32 = 0.03, p = 0.87) across the breeding fast (Table 3). Despite this difference neither inflammation marker differed between the sexes at either time point.
ASSOCIATIONS OF OXIDATIVE STRESS MARKERS
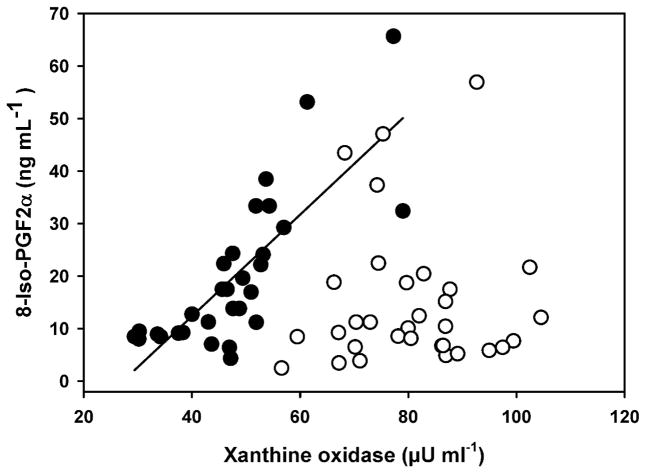
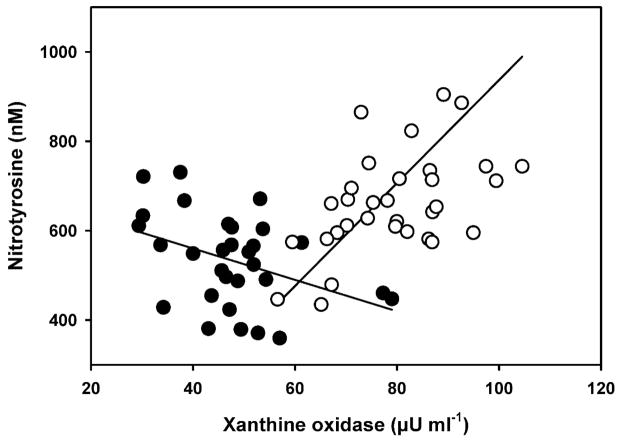
Many of the indices of oxidative stress (XO, antioxidant enzymes, markers of oxidative damage, and markers of inflammation) showed significant associations. As XO levels increased in males, there was a parallel increase in 8-iso-PGF2α (r2 = 0.54, F1,26 = 31.12, p < 0.0001) but that effect was lacking in females (p = 0.90; Figure 2). Plasma XO activity was associated with NT levels in males and females, but in opposite fashions. Male exhibited decreasing NT concentrations with increasing XO activities (r2 = 0.16, F1,27 = 5.42, p = 0.03) while NT concentrations in females increased (r2 = 0.24, F1,32 = 9.81, p = 0.004) (Figure 3).
Figure 2.
Relationship between plasma concentrations of xanthine oxidase and lipid peroxidation marker 8-Isoprostane (8-Iso-PGF2α) in breeding adult male (closed circles) and adult female (open circle) northern elephant seals. 8-isoprostane increases as xanthine oxidase activity for males but not for females. (y = −20.35 + 0.85x), r2 = 0.54, F1,26.2 = 31.12, p<0.001). Regression slope and intercept for males is from mixed model regression.
Figure 3.
Relationship between the plasma concentrations of xanthine oxidase and nitrotyrosine (NT) in adult breeding male (closed circles) and female (open circles) northern elephant seals. Males: y = 692.27 −3.5x, r2 = 0.16, F1,27.7 = 5.42, p = 0.03; females:y=−215.21 + 11.52x, r2 = 0.24, F1,31 = 9.8, p = 0.004. Regression slope and intercept for males is from mixed model regression.
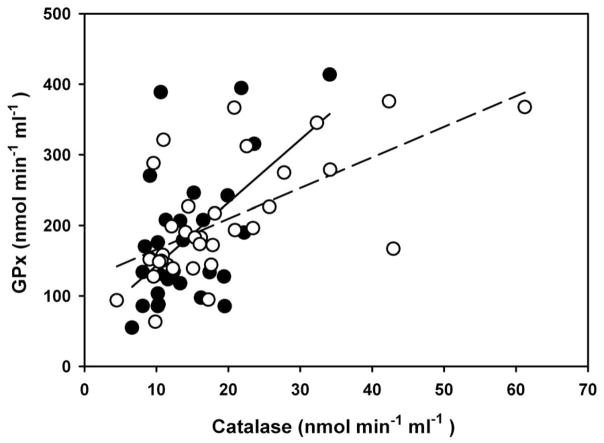
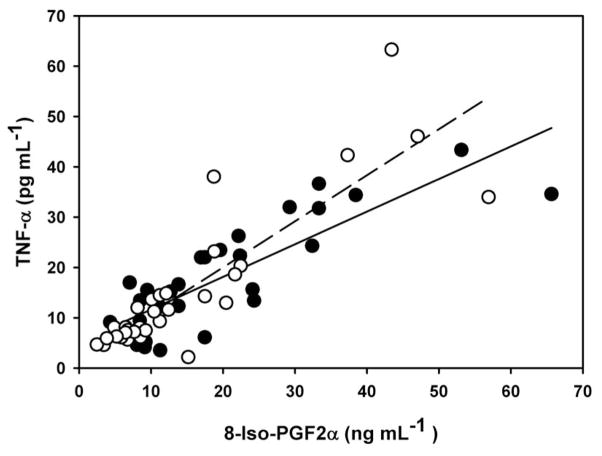
Plasma levels of antioxidant enzymes covaried within the sexes with a strong association of catalase and GPx activities for males (r2 = 0.67, F1,11 = 23.20, P = 0.0005) and females (r2 = 0.38, F1,32 = 18.76, p < 0.0001) (Figure 4). Plasma levels of 8-iso-PGF2α were strong predictors of TNF-α in adult males (r2 = 0.70, F1,27 = 67.68, p < 0.0001) and females (r2 = 0.74, F1,31 = 85.59, p < 0.0001) (Figure 5).
Figure 4.
Relationship between plasma concentrations of antioxidant enzymes catalase and GPx in breeding adult male (closed circles, solid line) and female (open circles, dashed line) northern elephant seals. Males: y = 42.44 + 9.96x, r2 = 0.67, F1,11.3 = 23.16, p = 0.0005; females:y = 122.34 + 4.34x, r2 = 0.38, F1,31 = 18.76, p = 0.0001. Regression slope and intercept for males is from mixed model regression.
Figure 5.
Relationship between plasma concentrations of 8-isoprostane (8-Iso-PGF2α) and tumor-necrosis factor- α (TNF-α) for breeding male (closed circles, solid line) and female (open circles, dashed line) adult northern elephant seals. Males: y = 4.80+0.66x, r2 = 0.70, F1,27 = 67.68, p<0.0001; females: y = 1.72+0.92x, r2 = 0.74, F1,31 = 85.59, p<0.0001. Regression slope and intercept for males is from mixed model regression.
In males, CRP levels increased with body mass (r2 = 0.27, F1,13.2 = 4.97, p < 0.044). In females, variation in body mass was associated with several variables. Catalase activity (r2 = 0.18, F1,31 = 6.50, p = 0.02), GPx activity (r2 = 0.24, F1,31 = 9.38, p = 0.005) and XO activity (r2 = 0.22, F1,31 = 8.43, p = 0.007) increased with declining body mass.
Discussion
NES provide evidence for oxidative stress and inflammation as a cost of breeding in a free-ranging mammal. Both sexes exhibited strong up-regulation in antioxidant enzyme activities. Despite these robust responses, male NES exhibited increased plasma levels of several markers of oxidative stress and inflammation and females exhibited increases in protein oxidative/nitrosative damage across their breeding fasts. These changes suggest that oxidative stress may result in damage to tissue and contribute to fitness costs of reproduction in this species.
PRO-OXIDANT ENZYME LEVELS INCREASE ACROSS BREEDING
Both male and female NES exhibited significant increases in the plasma activity of pro-oxidant XO across the breeding fast. This suggests increased ROS production due to the purine salvage/recycling process undertaken by NES (Soñanez-Organis et al. 2012). XO is involved in the purine degradation process by catalyzing the oxidation of hypoxanthine to xanthine then to uric acid (Hille & Nishino 1995). Purine recycling contributes to an increase in ATP supply under the potential detrimental conditions of prolonged fasting. Under specific conditions such as tissue ischemia or prolonged fasting, XO enzyme activity can increase, resulting in increased superoxide and hydrogen peroxide production (Parks, Bulkley & Granger 1983). Females had significantly higher levels of XO activity at both the early and late sample periods. This indicates that despite higher metabolic rates and fasting durations in males, females are incurring potentially higher rates of ROS production from purine degradation than males. The difference may reflect the high nutrient requirements of lactation in females, where nutrient delivery to the mammary gland may be prioritized over other processes. In addition to XO activity, it has been shown that fasting is associated with strong up-regulation of the renin–angiotensin system (RAS) (Ortiz et al. 2006) with resultant angiotensin-II mediated up-regulation of NADPH oxidase 4 (Nox4) (Vázquez-Medina et al. 2010, 2013). This together with XO may contribute to increased oxidant production over the breeding fast.
CHANGES IN ANTIOXIDANT ENZYMES
In response to increased XO activity (and potentially Nox enzymes) there was a strong upregulation in plasma concentrations of three key antioxidant enzymes (SOD, GPx and catalase). These three antioxidant enzymes work in unison to scavenge ROS produced as a byproduct of many cellular functions including the activity of XO, Nox and cellular respiration. As evident in plasma, male NES significantly up-regulated the activity of all three key antioxidants while females significantly increased the activity of Catalase and GPx across the breeding fast. SOD activities in females were significantly higher at the early breeding sample period when compared with males suggesting that they maintained higher levels of SOD prior to the early sampling. Increased plasma enzymatic activities of all three key antioxidant enzymes indicate a robust response to prevent oxidative damage and inflammation during the breeding fast for both sexes. In general, antioxidant responses were correlated among individuals of both sexes, suggesting coordinated up-regulation.
OXIDATIVE DAMAGE TO LIPIDS
An increase in ROS production coupled to an insufficient antioxidant response can result in oxidative damage. One important target for free radical injury is the peroxidation of lipids. A widely used marker of lipid peroxidation is the free radical catalyzed peroxidation of membrane arachidonic acid, the prostaglandin 8-iso-PGF2α (Morrow et al. 1990). Male NES had a significant increase in the concentration of isoprostanes while female values did not change across breeding, suggesting greater oxidative stress in males. The 50% increase in 8-iso-PGF2α in late breeding males is similar to the effects sizes for plasma values in association with human disease states like asthma or coronary artery disease (Wang et al. 2006; Wood et al. 2000). There was a strong relationship between XO and lipid peroxidation in the male NES, suggesting a direct relationship between ROS production and the peroxidation of lipids during the breeding fast. This interaction may result from the high sustained rates of energy expenditure while fasting in breeding males.
Another important marker of lipid peroxidation is the final product of membrane lipid peroxidation 4HNE (Esterbauer, Schaur & Zollner 1991). This marker of lipid peroxidation did not increase with breeding in females but showed a marginal increase in males (p = 0.09). Thus there appeared to be greater impacts of lipid peroxidation due to breeding in plasma markers for males. Circulating fatty acid concentrations triple across lactation in females reaching concentrations that exceed 3mM (McDonald & Crocker 2006; Houser, Champagne & Crocker 2007) while males exhibit lower stable fatty acid concentrations across breeding (Crocker et al. 2012). Thus enhanced lipid peroxidation in plasma does not likely reflect changes in circulating fatty acids but rather oxidative damage to tissue cell membrane lipids.
OXIDATIVE DAMAGE TO PROTEINS
Male and female NES both had significant changes in the marker for oxidative damage to protein across the breeding fast, but in opposite directions. NT concentrations decreased significantly in males, where females exhibited significant increases across the breeding fast. Females showed significantly higher levels of NT near the end of breeding when compared to males. This indicates that females are incurring oxidative/nitrosative damage to proteins where males are incurring oxidative damage to lipids and DNA. Nitrotyrosine is a product of tyrosine nitration mediated by reactive nitrogen species (RNS). In healthy humans, NT is usually undetectable under normal conditions (Kaur & Halliwell 1994; Fukuyama et al. 1997). The 35% increase in NT levels between early and late lactation females is similar to the elevations seen in human patients with septic shock (Ohya et al. 2002).
One possible explanation for the sex difference in NT concentrations would be simply the circulating levels of tyrosine available for nitration. Breeding males exhibit low levels of protein catabolism (Crocker, Houser & Webb 2012), while females mobilize large amounts of protein for milk synthesis and exhibit increased levels of protein catabolism late in the fast (Crocker et al. 1998). However, females exhibit plasma tyrosine concentrations that decline over lactation and are significantly lower than males (Houser & Crocker 2004). Thus sex differences in plasma NT are not likely due to tyrosine availability and more likely reflect differences in systemic RNS and NO production and potentially increased superoxide production by endothelial Nox proteins.
Protein damage marker NT varied with pro-oxidant XO activity in both sexes but in opposite directions. Males exhibited decreasing NT concentrations with plasma XO suggesting that associated increases in antioxidant enzymes were sufficient to prevent nitrosative damage to proteins. The strong positive association of plasma XO and NT in females suggests that XO influenced ROS production was a significant contributor to nitrosative stress in this group.
OXIDATIVE DAMAGE TO DNA
Increased oxidative DNA damage evident in the plasma of males may reflect the high sustained metabolic rates necessary for breeding (Crocker, Houser & Webb 2012) and in combination with changes in lipid peroxidation markers and TNF-α represent strong evidence for oxidative stress in breeding males. Studies in humans have suggested that increases in plasma 8-OHG, similar to that of NES, were found during sustained aerobic exercise and that oxidative DNA damage correlated with rates of oxygen consumption (Loft et al. 1994). The 7% increase in plasma 8-OHG across breeding in males is similar to the magnitude of changes seen after hypoxic exercise in humans (Møller et al. 2001) and the increased levels of DNA damage found in males compared to females reflect differences in rates of metabolism measured in previous studies (Crocker et al. 2001, 2012)
INFLAMMATORY MARKERS
Adult male NES showed evidence of increased systemic inflammation across breeding. Cumulative impacts of oxidative damage can promote inflammation and immune challenges that result in oxidative stress (Schneeberger, Czirják & Voigt 2013). TNF-α has a principle function of initiating the inflammatory reactions in the immune system as well as being an activator molecule in the cell apoptotic pathway (Micheau & Tschopp 2003). Male NES had a significant increase (74%)in the plasma concentrations of TNF-α, whereas values were stable in females.
In both male and female NES there was a strong positive relationship between 8-iso-PGF2α and TNF-α. This relationship was present in lactating females despite lack of consistent changes across breeding in either marker. These relationships suggest that oxidative damage is associated with up regulation of TNF-α in adipose and other tissue in NES. In obesity models increased ROS in adipose tissue precedes up-regulation of cytokines that have profound impacts on immune function and insulin signaling (Eriksson 2007). The association of membrane lipid peroxidation with TNF- α suggests that oxidative stress directly promotes production of this important inflammatory cytokine.
IMPACTS OF BODY RESERVES
Body mass was significantly related to several markers of oxidative stress. Variation in body mass was inversely associated with systemic inflammation in males. Smaller females exhibited higher plasma XO activity and greater antioxidant responses as evidenced by plasma catalase and GPx. These relationships are important to note because they suggest that variation in available energy reserves for reproduction were related to oxidative stress and inflammation. Several previous studies have shown strong relationships between body reserves and the level of reproductive effort in NES (Crocker et al. 2001; Crocker, Houser & Webb 2012). These relationships may be mediated in part by impacts of body reserves on oxidative stress during breeding.
USE OF PLASMA MARKERS
The ability to measure plasma changes in several markers suggests the utility of plasma markers for assessing oxidative stress in wildlife systems. Oxidative stress and response may vary widely in different tissues and individuals may allocate defenses in a tissue specific manner (Xu et al. 2013; Yang et al. 2013). While plasma markers are commonly used in the detection of severe pathologies in the clinical literature, their use for assessing natural variation in oxidative stress is less well established. The use of plasma markers facilitates research in a wide variety of systems where destructive or tissue sampling is not possible. The strong association of several markers in the males gives insight into systemic levels of oxidative stress. Several recent studies have stressed the variability in oxidative stress responses measured in plasma and various tissues (Xu et al. 2013; Yang et al. 2013). Many published studies select an individual marker for assessment of oxidative stress or damage (e.g. Nussey et al. 2009). In the present study, use of a single plasma marker would have resulted in differing assessments of the potential for oxidative damage. The variability in evidence for damage among the markers stresses the importance of measuring a suite of markers to assess oxidative stress, particularly when making measurements in plasma. Future studies should attempt, when possible, to assess tissue-specific variation in oxidative stress markers and their relationship to plasma measurements to better understand the mechanisms underlying potential fitness costs associated with oxidative stress.
LIFE HISTORY IMPLICATIONS
These data provide evidence that oxidative stress associated with breeding is a potential physiological cost of reproduction. These effects may constitute underlying mechanisms of fitness costs to reproduction. Previous studies yielding equivocal or negative results used income breeders as research systems. Compensatory increases in energy intake may help avoid some of the effects evident in an extreme capital breeder like the NES. The direct allocation trade-offs associated with capital breeding and the high energy costs associated with polygyny and abbreviated lactation may increase fitness costs to reproduction in marine vertebrates that breed on land. These mechanisms may underlie strong relationships between body reserves and reproductive effort as well as apparent impacts of foraging success on natality (Crocker et al. 2006). The wide variation in reproductive success among males and the high reproductive payoff for successful males may select for levels of sustained energy expenditure while fasting despite negative physiological impacts that potentially influence health and survival. In contrast, females may mediate rates of milk delivery and terminate lactation relative to body reserves (Crocker et al. 2001), thus mitigating oxidative stress and tissue damage when compared to males.
While the NES is extreme in its level of sustained fasting energy expenditure during breeding it is also well adapted to defend against oxidative damage. NES exhibit near complete depletion of blood oxygen stores while diving (Meir et al. 2009) and exhibit a robust ability to avoid oxidative damage despite extreme hypoxia and extended fasting when not breeding. (Vázquez-Medina et al. 2010, 2011a, 2011b, 2013). Given these responses, our results provide strong evidence for systemic oxidative damage as a cost of reproduction in polygynous male NES and suggest the possibility of tissue specific impacts of reproductive effort in other species.
Acknowledgments
We thank Dorian Houser, Cory Champagne, Mike Tift, Luis Huckstadt, Sarah Peterson, Patrick Robinson and Dan Costa for help with sample collection. This research was funded by by NHLBI R01-HL091767 (R.M.O., D.E.C.) and a COAST student research grant to J.T.S.
Footnotes
Data Accessibility
Data for this article are deposited in the Dryad Digital Respository: http://doi.org/10.5061/dryad.q32td
References
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecology Letters. 2004;7:363–368. [Google Scholar]
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Chastel O, Sorci G. An experimental manipulation of life-history trajectories and resistance to oxidative stress. Evolution. 2006;60:1913–1924. [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bergeron P, Careau V, Humphries MM, Reale D, Speakman JR, Garant D. The energetic and oxidative costs of reproduction in a free-ranging rodent. Functional Ecology. 2011;25:1063–1071. [Google Scholar]
- Bertrand S, Alonso-Alvarez C, Devevey G, Faivre B, Prost J, Sorci G. Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia. 2006;147:576–584. doi: 10.1007/s00442-005-0317-8. [DOI] [PubMed] [Google Scholar]
- Casagrande S, Dell’Omo G, Costantini D, Tagliavini J, Groothuis T. Variation of a carotenoid-based trait in relation to oxidative stress and endocrine status during the breeding season in the Eurasian kestrel: a multi-factorial study. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2011;160:16–26. doi: 10.1016/j.cbpa.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Champagne C, Crocker D, Fowler M, Houser D. Fasting Physiology of the Pinnipeds: The Challenges of Fasting While Maintaining High Energy Expenditure and Nutrient Delivery for Lactation. In: McCue MD, editor. Comparative Physiology of Fasting, Starvation, and Food Limitation. Springer; Berlin Heidelberg: 2012. pp. 309–336. [Google Scholar]
- Costantini D. Oxidative stress in ecology and evolution: lessons from avian studies. Ecology Letters. 2008;11:1238–1251. doi: 10.1111/j.1461-0248.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- Crescimanno M, Armata MG, Rausa L, Gueli MC, Nicotra C, D’alessandro N. Cardiac peroxisomal enzymes and starvation. Free Radical Research. 1989;7:67–72. doi: 10.3109/10715768909087925. [DOI] [PubMed] [Google Scholar]
- Crocker DE, Costa DP, Le Boeuf BJ, Webb PM, Houser DS. Impact of El Niño on the foraging behavior of female northern elephant seals 2006 [Google Scholar]
- Crocker DE, Houser DS, Webb PM. Impact of body reserves on energy expenditure, water flux, and mating success in breeding male northern elephant seals. Physiological and Biochemical Zoology. 2012;85:11–20. doi: 10.1086/663634. [DOI] [PubMed] [Google Scholar]
- Crocker DE, Ortiz RM, Houser DS, Webb PM, Costa DP. Hormone and metabolite changes associated with extended breeding fasts in male northern elephant seals (Mirounga angustirostris) Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2012;161:388–394. doi: 10.1016/j.cbpa.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Crocker DE, Webb PM, Costa DP, Le Boeuf BJ. Protein catabolism and renal function in lactating northern elephant seals. Physiological and Biochemical Zoology. 1998;71:485–491. doi: 10.1086/515971. [DOI] [PubMed] [Google Scholar]
- Crocker DE, Williams JD, Costa DP, Le Boeuf BJ. Maternal traits and reproductive effort in northern elephant seals. Ecology. 2001;82:3541–3555. [Google Scholar]
- Deutsch CJ, Haley MP, Le Boeuf BJ. Reproductive effort of male northern elephant seals: estimates from mass loss. Canadian Journal of Zoology. 1990;68:2580–2593. [Google Scholar]
- Di Simplicio P, Rossi R, Falcinelli S, Ceserani R, Formento M. Antioxidant status in various tissues of the mouse after fasting and swimming stress. European journal of applied physiology and occupational physiology. 1997;76:302–307. doi: 10.1007/s004210050252. [DOI] [PubMed] [Google Scholar]
- Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O. An R2 statistic for fixed effects in the linear mixed model. Statistics in medicine. 2008;27:6137–6157. doi: 10.1002/sim.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JW. Metabolic stress in insulin’s target cells leads to ROS accumulation–a hypothetical common pathway causing insulin resistance. FEBS letters. 2007;581:3734–3742. doi: 10.1016/j.febslet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Fletcher QE, Selman C, Boutin S, McAdam AG, Woods SB, Seo AY, Leeuwenburgh C, Speakman JR, Humphries MM. Oxidative Damage Increases With Reproductive Energy Expenditure And Is Reduced By Food-Supplementation. Evolution. 2013;67:1527–1536. doi: 10.1111/evo.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama N, Takebayashi Y, Hida M, Ishida H, Ichimori K, Nakazawa H. Clinical evidence of peroxynitrite formation in chronic renal failure patients with septic shock. Free Radical Biology and Medicine. 1997;22:771–774. doi: 10.1016/s0891-5849(96)00401-7. [DOI] [PubMed] [Google Scholar]
- Garratt M, Vasilaki A, Stockley P, McArdle F, Jackson M, Hurst JL. Is oxidative stress a physiological cost of reproduction? An experimental test in house mice. Proceedings of the Royal Society B: Biological Sciences. 2011;278:1098–1106. doi: 10.1098/rspb.2010.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt M, McArdle F, Stockley P, Vasilaki A, Beynon RJ, Jackson MJ, Hurst JL. Tissue-dependent changes in oxidative damage with male reproductive effort in house mice. Functional Ecology. 2012;26:423–433. [Google Scholar]
- Gittleman JL, Thompson SD. Energy allocation in mammalian reproduction. American Zoologist. 1988;28:863–875. [Google Scholar]
- Grattagliano I, Vendemiale G, Caraceni P, Domenicali M, Nardo B, Cavallari A, Trevisani F, Bernardi M, Altomare E. Starvation impairs antioxidant defense in fatty livers of rats fed a choline-deficient diet. The Journal of nutrition. 2000;130:2131–2136. doi: 10.1093/jn/130.9.2131. [DOI] [PubMed] [Google Scholar]
- Haley MP, Deutsch CJ, Le Boeuf BJ. Size, dominance and copulatory success in male northern elephant seals, Mirounga angustirostris. Animal Behaviour. 1994;48:1249–1260. [Google Scholar]
- Heiss RS, Schoech SJ. Oxidative cost of reproduction is Sex specific and correlated with reproductive effort in a cooperatively breeding bird, the florida scrub Jay. Physiological and Biochemical Zoology. 2012;85:499–503. doi: 10.1086/666840. [DOI] [PubMed] [Google Scholar]
- Hille R, Nishino T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. The FASEB journal. 1995;9:995–1003. [PubMed] [Google Scholar]
- Houser DS, Champagne CD, Crocker DE. Lipolysis and glycerol gluconeogenesis in simultaneously fasting and lactating northern elephant seals. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007;293:R2376–R2381. doi: 10.1152/ajpregu.00403.2007. [DOI] [PubMed] [Google Scholar]
- Houser DS, Crocker DE. Age, sex, and reproductive state influence free amino acid concentrations in the fasting elephant seal. Physiological and Biochemical Zoology. 2004;77:838–846. doi: 10.1086/422055. [DOI] [PubMed] [Google Scholar]
- Isaksson C, Sheldon BC, Uller T. The challenges of integrating oxidative stress into life-history biology. BioScience. 2011;61:194–202. [Google Scholar]
- Jönsson KI. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos. 1997:57–66. [Google Scholar]
- Kaur H, Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS letters. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- Le Boeuf B, Crocker D, Costa D, Blackwell S, Webb P, Houser D. Foraging ecology of northern elephant seals. Ecological monographs. 2000;70:353–382. [Google Scholar]
- Loft S, Astrup A, Buemann B, Poulsen HE. Oxidative DNA damage correlates with oxygen consumption in humans. The FASEB journal. 1994;8:534–537. doi: 10.1096/fasebj.8.8.8181672. [DOI] [PubMed] [Google Scholar]
- Mårtensson J. The effect of fasting on leukocyte and plasma glutathione and sulfur amino acid concentrations. Metabolism. 1986;35:118–121. doi: 10.1016/0026-0495(86)90110-1. [DOI] [PubMed] [Google Scholar]
- McDonald BI, Crocker DE. Physiology and behavior influence lactation efficiency in northern elephant seals (Mirounga angustirostris) Physiological and Biochemical Zoology. 2006;79:484–496. doi: 10.1086/501056. [DOI] [PubMed] [Google Scholar]
- Meir JU, Champagne CD, Costa DP, Williams CL, Ponganis PJ. Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2009;297:R927–R939. doi: 10.1152/ajpregu.00247.2009. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Alonso-Alvarez C. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Functional Ecology. 2010;24:984–996. [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Møller P, Loft S, Lundby C, Olsen NV. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. The FASEB Journal. 2001;15:1181–1186. doi: 10.1096/fj.00-0703com. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecology Letters. 2009;12:75–92. doi: 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts Ln. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proceedings of the National Academy of Sciences. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussey DH, Pemberton JM, Pilkington JG, Blount JD. Life history correlates of oxidative damage in a free-living mammal population. Functional Ecology. 2009;23:809–817. [Google Scholar]
- Ohya M, Marukawa S, Inoue T, Ueno N, Hosohara K, Terada N, Kosaka H. Plasma nitrotyrosine concentration relates to prognosis in human septic shock. Shock. 2002;18:116–118. doi: 10.1097/00024382-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Ołdakowski Ł, Piotrowska Ż, Chrząścik KM, Sadowska ET, Koteja P, Taylor JR. Is reproduction costly? No increase of oxidative damage in breeding bank voles. The Journal of Experimental Biology. 2012;215:1799–1805. doi: 10.1242/jeb.068452. [DOI] [PubMed] [Google Scholar]
- Olsson M, Healey M, Perrin C, Wilson M, Tobler M. Sex-specific SOD levels and DNA damage in painted dragon lizards (Ctenophorus pictus) Oecologia. 2012;170:917–924. doi: 10.1007/s00442-012-2383-z. [DOI] [PubMed] [Google Scholar]
- Ortiz RM, Crocker DE, Houser DS, Webb PM. Angiotensin II and aldosterone increase with fasting in breeding adult male northern elephant seals (Mirounga angustirostris) Physiological and Biochemical Zoology. 2006;79:1106–1112. doi: 10.1086/505996. [DOI] [PubMed] [Google Scholar]
- Parks D, Bulkley G, Granger D. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983;94:415–422. [PubMed] [Google Scholar]
- Roff DA. Life history evolution. Sinauer Associates; Sunderland: 2002. [Google Scholar]
- Salmon AB, Marx DB, Harshman LG. A cost of reproduction in Drosophila melanogaster: stress susceptibility. Evolution. 2001;55:1600–1608. doi: 10.1111/j.0014-3820.2001.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Schneeberger K, Czirják GÁ, Voigt CC. Inflammatory challenge increases measures of oxidative stress in a free-ranging, long-lived mammal. The Journal of Experimental Biology. 2013 doi: 10.1242/jeb.090837. jeb. 090837. [DOI] [PubMed] [Google Scholar]
- Sharick JT, Vazquez-Medina JP, Ortiz RM, Crocker DE. Data from: Oxidative stress is a potential cost of breeding in male and female northern elephant seals. Dryad Digital Repository. 2014 doi: 10.1111/1365-2435.12330. http://doi.org/10.5061/dryad.q32td. [DOI] [PMC free article] [PubMed]
- Soñanez-Organis JG, Vázquez-Medina JP, Zenteno-Savín T, Aguilar A, Crocker DE, Ortiz RM. Prolonged fasting increases purine recycling in post-weaned northern elephant seals. The Journal of Experimental Biology. 2012;215:1448–1455. doi: 10.1242/jeb.067173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR. The physiological costs of reproduction in small mammals. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Garratt M. Oxidative stress as a cost of reproduction: Beyond the simplistic trade-off model. Bioessays. 2014;36:93–106. doi: 10.1002/bies.201300108. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories 1992 [Google Scholar]
- Stephens PA, Boyd IL, McNamara JM, Houston AI. Capital breeding and income breeding: their meaning, measurement, and worth. Ecology. 2009;90:2057–2067. doi: 10.1890/08-1369.1. [DOI] [PubMed] [Google Scholar]
- van de Crommenacker J, Richardson DS, Koltz AM, Hutchings K, Komdeur J. Parasitic infection and oxidative status are associated and vary with breeding activity in the Seychelles warbler. Proceedings of the Royal Society B: Biological Sciences. 2012;279:1466–1476. doi: 10.1098/rspb.2011.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. The Journal of Experimental Biology. 2010;213:2524–2530. doi: 10.1242/jeb.041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Soñanez-Organis JG, Rodriguez R, Viscarra JA, Nishiyama A, Crocker DE, Ortiz RM. Prolonged fasting activates Nrf2 in postweaned elephant seals. The Journal of Experimental Biology. 2013 doi: 10.1242/jeb.081927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Zenteno-Savín T, Forman HJ, Crocker DE, Ortiz RM. Prolonged fasting increases glutathione biosynthesis in postweaned northern elephant seals. The Journal of Experimental Biology. 2011a;214:1294–1299. doi: 10.1242/jeb.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Zenteno-Savín T, Tift MS, Forman HJ, Crocker DE, Ortiz RM. Apnea stimulates the adaptive response to oxidative stress in elephant seal pups. The Journal of Experimental Biology. 2011b;214:4193–4200. doi: 10.1242/jeb.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Salmon AB, Harshman LG. A cost of reproduction: oxidative stress susceptibility is associated with increased egg production in Drosophila melanogaster. Experimental Gerontology. 2001;36:1349–1359. doi: 10.1016/s0531-5565(01)00095-x. [DOI] [PubMed] [Google Scholar]
- Wang B, Pan J, Wang L, Zhu H, Yu R, Zou Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis. 2006;184:425–430. doi: 10.1016/j.atherosclerosis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Wiersma P, Selman C, Speakman JR, Verhulst S. Birds sacrifice oxidative protection for reproduction. Proceedings of the Royal Society of London Series B: Biological Sciences. 2004;271:S360–S363. doi: 10.1098/rsbl.2004.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LG, Fitzgerald DA, Gibson PC, Cooper DM, Garg ML. Lipid peroxidation as determined by plasma isoprostanes is related to disease severity in mild asthma. Lipids. 2000;35:967–974. doi: 10.1007/s11745-000-0607-x. [DOI] [PubMed] [Google Scholar]
- Xu YC, Yang DB, Speakman JR, Wang DH. Oxidative stress in response to natural and experimentally elevated reproductive effort is tissue dependent. Functional Ecology 2013 [Google Scholar]
- Yang DB, Xu YC, Wang DH, Speakman JR. Effects of reproduction on immuno-suppression and oxidative damage, and hence support or otherwise for their roles as mechanisms underpinning life history trade-offs, are tissue and assay dependent. The Journal of Experimental Biology. 2013;216:4242–4250. doi: 10.1242/jeb.092049. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biology and Medicine. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Harshman LG. The physiology of life history trade-offs in animals. Annual Review of Ecology and Systematics. 2001;32:95–126. [Google Scholar]