Abstract
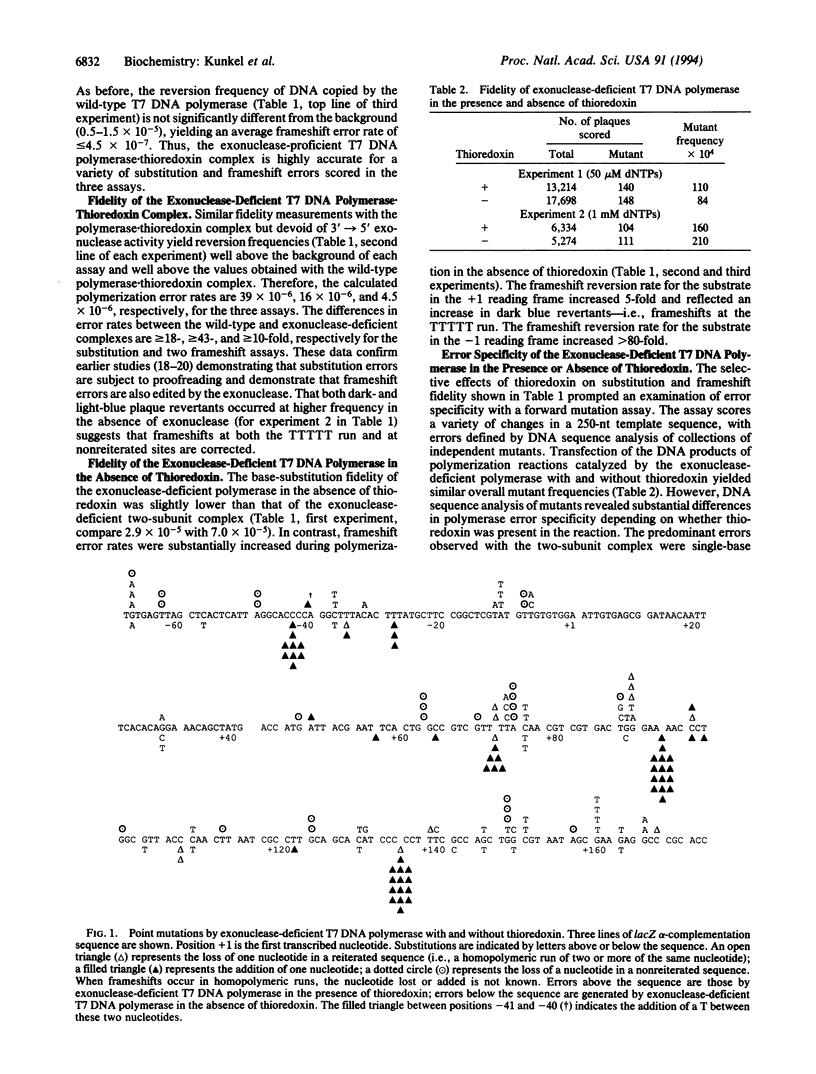
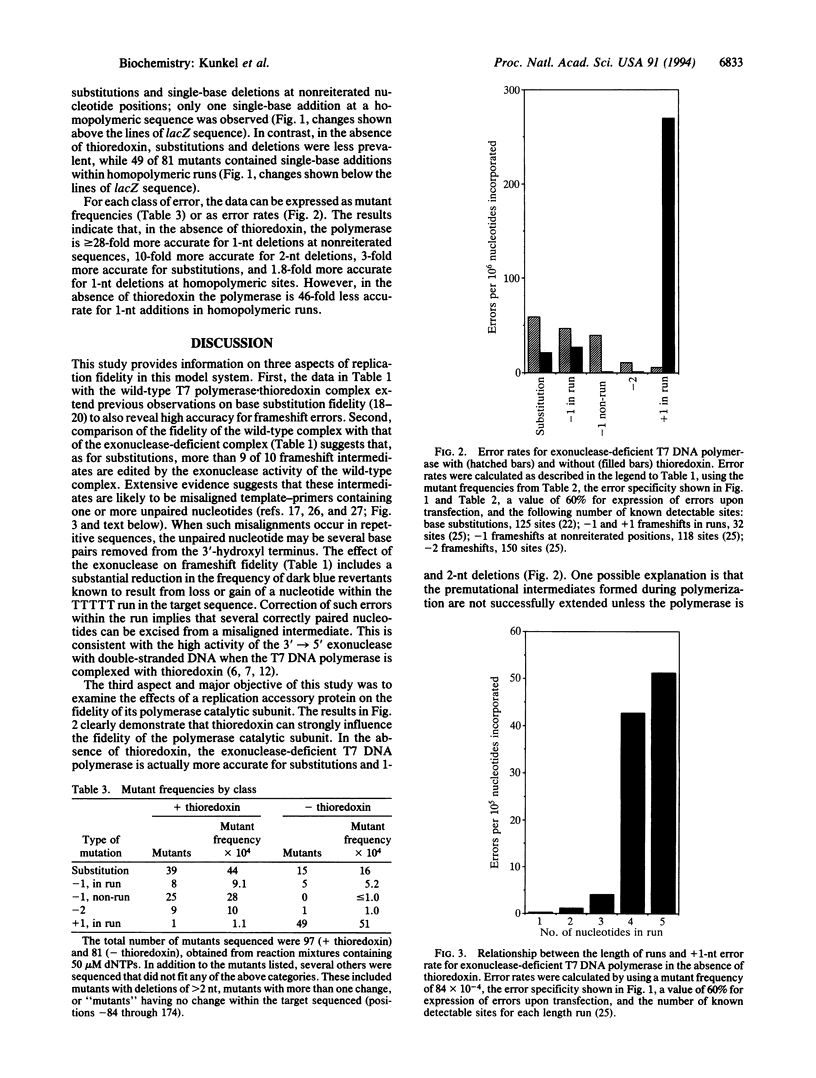
We have examined the effect of thioredoxin, an accessory protein that confers high processivity to bacteriophage T7 DNA polymerase, on the fidelity of DNA synthesis. In the presence of thioredoxin, exonuclease-proficient T7 DNA polymerase is highly accurate. In fidelity assays that score errors that revert M13mp2 lacZ alpha-complementation mutants, error rates are < or = 2.2 x 10(-6) for base substitution and < or = 3.7 x 10(-7) and < or = 4.5 x 10(-7) for frameshifts that revert mutations in the +1 and -1 reading frames, respectively. Rates are more than 10-fold higher during synthesis by polymerase.thioredoxin complex lacking 3'-->5' exonuclease activity, demonstrating that frameshift as well as substitution errors are subject to proofreading. The contribution of thioredoxin to accuracy has been examined by comparing the fidelity of the exonuclease-deficient polymerase in the presence or absence of the accessory protein. Thioredoxin either enhances or reduces fidelity, depending on the type of error considered. In the absence of thioredoxin, T7 DNA polymerase is 3-fold more accurate for base substitutions and > or = 27-fold and 9-fold more accurate, respectively, for 1- and 2-nt deletion errors at nonreiterated nucleotide sequences. Higher fidelity for all three errors may reflect the inability of the polymerase to continue synthesis from the premutational intermediates in the absence of the accessory protein. In marked contrast, the rate for frameshift errors wherein one or more nucleotides has been added to a repeated DNA sequence increases 46-fold when thioredoxin is absent from the polymerization reaction. The error rate increases as the length of the repeated sequence increases, consistent with a model where strand slippage creates misaligned template-primers. Thus, replicative expansion of repetitive sequences occurs in the absence of a replication accessory protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Abbotts J., Bebenek K., Kunkel T. A., Wilson S. H. Mechanism of HIV-1 reverse transcriptase. Termination of processive synthesis on a natural DNA template is influenced by the sequence of the template-primer stem. J Biol Chem. 1993 May 15;268(14):10312–10323. [PubMed] [Google Scholar]
- Adler S., Modrich P. T7-induced DNA polymerase. Characterization of associated exonuclease activities and resolution into biologically active subunits. J Biol Chem. 1979 Nov 25;254(22):11605–11614. [PubMed] [Google Scholar]
- Bebenek K., Abbotts J., Roberts J. D., Wilson S. H., Kunkel T. A. Specificity and mechanism of error-prone replication by human immunodeficiency virus-1 reverse transcriptase. J Biol Chem. 1989 Oct 5;264(28):16948–16956. [PubMed] [Google Scholar]
- Bebenek K., Abbotts J., Wilson S. H., Kunkel T. A. Error-prone polymerization by HIV-1 reverse transcriptase. Contribution of template-primer misalignment, miscoding, and termination probability to mutational hot spots. J Biol Chem. 1993 May 15;268(14):10324–10334. [PubMed] [Google Scholar]
- Donlin M. J., Patel S. S., Johnson K. A. Kinetic partitioning between the exonuclease and polymerase sites in DNA error correction. Biochemistry. 1991 Jan 15;30(2):538–546. doi: 10.1021/bi00216a031. [DOI] [PubMed] [Google Scholar]
- Grippo P., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6867–6873. [PubMed] [Google Scholar]
- Hori K., Mark D. F., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. Characterization of the exonuclease activities of the gene 5 protein and the reconstituted polymerase. J Biol Chem. 1979 Nov 25;254(22):11598–11604. [PubMed] [Google Scholar]
- Hori K., Mark D. F., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. Purification and properties of the phage-encoded subunit, the gene 5 protein. J Biol Chem. 1979 Nov 25;254(22):11591–11597. [PubMed] [Google Scholar]
- Huber H. E., Russel M., Model P., Richardson C. C. Interaction of mutant thioredoxins of Escherichia coli with the gene 5 protein of phage T7. The redox capacity of thioredoxin is not required for stimulation of DNA polymerase activity. J Biol Chem. 1986 Nov 15;261(32):15006–15012. [PubMed] [Google Scholar]
- Huber H. E., Tabor S., Richardson C. C. Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J Biol Chem. 1987 Nov 25;262(33):16224–16232. [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Johnson K. A. Conformational coupling in DNA polymerase fidelity. Annu Rev Biochem. 1993;62:685–713. doi: 10.1146/annurev.bi.62.070193.003345. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kuhl D. P., Caskey C. T. Trinucleotide repeats and genome variation. Curr Opin Genet Dev. 1993 Jun;3(3):404–407. doi: 10.1016/0959-437x(93)90112-3. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Frameshift mutagenesis by eucaryotic DNA polymerases in vitro. J Biol Chem. 1986 Oct 15;261(29):13581–13587. [PubMed] [Google Scholar]
- Kunkel T. A., Hamatake R. K., Motto-Fox J., Fitzgerald M. P., Sugino A. Fidelity of DNA polymerase I and the DNA polymerase I-DNA primase complex from Saccharomyces cerevisiae. Mol Cell Biol. 1989 Oct;9(10):4447–4458. doi: 10.1128/mcb.9.10.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Misalignment-mediated DNA synthesis errors. Biochemistry. 1990 Sep 4;29(35):8003–8011. doi: 10.1021/bi00487a001. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Soni A. Exonucleolytic proofreading enhances the fidelity of DNA synthesis by chick embryo DNA polymerase-gamma. J Biol Chem. 1988 Mar 25;263(9):4450–4459. [PubMed] [Google Scholar]
- Kunkel T. A. The mutational specificity of DNA polymerase-beta during in vitro DNA synthesis. Production of frameshift, base substitution, and deletion mutations. J Biol Chem. 1985 May 10;260(9):5787–5796. [PubMed] [Google Scholar]
- Kunkel T. A. The mutational specificity of DNA polymerases-alpha and -gamma during in vitro DNA synthesis. J Biol Chem. 1985 Oct 15;260(23):12866–12874. [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Richardson C. C. Bacteriophage T7 Deoxyribonucleic acid replication in vitro. A protein of Escherichia coli required for bacteriophage T7 DNA polymerase activity. J Biol Chem. 1975 Jul 25;250(14):5508–5514. [PubMed] [Google Scholar]
- Modrich P., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication invitro. Bacteriophage T7 DNA polymerase: an an emzyme composed of phage- and host-specific subunits. J Biol Chem. 1975 Jul 25;250(14):5515–5522. [PubMed] [Google Scholar]
- Oey J. L., Strätling W., Knippers R. A DNA polymerase induced by bacteriophage T7. Eur J Biochem. 1971 Dec 10;23(3):497–504. doi: 10.1111/j.1432-1033.1971.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Patel S. S., Wong I., Johnson K. A. Pre-steady-state kinetic analysis of processive DNA replication including complete characterization of an exonuclease-deficient mutant. Biochemistry. 1991 Jan 15;30(2):511–525. doi: 10.1021/bi00216a029. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Tabor S., Huber H. E., Richardson C. C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987 Nov 25;262(33):16212–16223. [PubMed] [Google Scholar]
- Wong I., Patel S. S., Johnson K. A. An induced-fit kinetic mechanism for DNA replication fidelity: direct measurement by single-turnover kinetics. Biochemistry. 1991 Jan 15;30(2):526–537. doi: 10.1021/bi00216a030. [DOI] [PubMed] [Google Scholar]



