Abstract
Background:
As new therapies emerge which increase the risk of autoimmune disease it is increasingly important to understand the incidence of autoimmune disease in multiple sclerosis (MS).
Objective:
The purpose of this review is to estimate the incidence and prevalence of comorbid autoimmune disease in MS.
Methods:
The PUBMED, EMBASE, SCOPUS and Web of Knowledge databases, conference proceedings, and reference lists of retrieved articles were searched, and abstracts were independently screened by two reviewers. The data were abstracted by one reviewer using a standardized data collection form, and the findings were verified by a second reviewer. We assessed quality of the included studies using a standardized approach and conducted meta-analyses of population-based studies.
Results:
Sixty-one articles met the inclusion criteria. We observed substantial heterogeneity with respect to the populations studied, methods of ascertaining comorbidity, and reporting of findings. Based solely on population-based studies, the most prevalent autoimmune comorbidities were psoriasis (7.74%) and thyroid disease (6.44%). Our findings also suggest an increased risk of inflammatory bowel disease, likely uveitis and possibly pemphigoid.
Conclusion:
Fewer than half of the studies identified were of high quality. Population-based studies that report age, sex and ethnicity-specific estimates of incidence and prevalence are needed in jurisdictions worldwide.
Keywords: Multiple sclerosis, comorbidity, autoimmune disease, incidence, prevalence
Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system which is believed to be immune-mediated. Although multiple putative etiologic factors have been identified for MS, the etiology of the disease remains unknown.1 The co-occurrence of autoimmune disease has thus been of substantial interest, as associations between MS and other autoimmune diseases may highlight common genetic or environmental exposures. Further, as new therapies emerge that raise the risk of autoimmune diseases such as thyroid disease and idiopathic thrombocytopenic purpura it has become increasingly important to understand the incidence of these conditions in the MS population.2 Unfortunately, findings of published studies have been inconsistent.3–6
We conducted a systematic review to estimate the incidence and prevalence of autoimmune comorbidity in MS, and to assess the quality of included studies. We also aimed to identify the most prevalent autoimmune comorbidities in the MS population.
Methods
This review was part of a larger study on the worldwide incidence and prevalence of comorbidity in MS; we have divided these studies to allow for more detailed examination and discussion of findings.
Search strategy and study selection
As delineated in detail elsewhere, we developed a comprehensive search strategy for autoimmune comorbidities (Supplemental Appendix I). Briefly, the autoimmune comorbidities evaluated included alopecia areata, ankylosing spondylitis, autoimmune thyroid disease, bullous pemphigoid, celiac disease, dermatomyositis, idiopathic thrombocytopenic purpura, inflammatory bowel disease, myasthenia gravis, pemphigus vulgaris, pernicious anemia, polymyositis, primary adrenocortical insufficiency, primary biliary cirrhosis, psoriasis, rheumatoid arthritis, Sjogren’s syndrome, systemic lupus erythematosus, systemic sclerosis, uveitis, vitiligo, and Wegener’s granulomatosis. The autoimmune disorders investigated varied from one study to another, as did the classification schema. Therefore the results are presented anatomically as follows: overall, gastrointestinal system; endocrine; eye; hair and skin; connective tissue disorders; and other. The search included reviews of the electronic databases PUBMED, EMBASE, SCOPUS, and Web of Knowledge for all years available through 15 November 2013, and manual reviews of the reference lists of studies identified during electronic searches.
Two reviewers (RAM, NR) independently assessed whether the unique abstracts identified met the inclusion criteria: (a) MS population; (b) include original data; (c) specify the autoimmune comorbidity of interest; (d) report incidence or prevalence of the comorbidity; and (e) English language publication. If either reviewer selected the abstract it underwent full-text review, during which stage the articles were independently reviewed by the two reviewers. Disagreements were resolved by consensus.
Data extraction and study quality
One reviewer abstracted the data using a standardized data collection form and the findings were verified by the second reviewer. As detailed elsewhere,7 general study characteristics as well as incidence and prevalence estimates were captured. We critically appraised each study and awarded quality scores based on yes or no responses to nine questions.7 These steps supported a qualitative assessment of study heterogeneity.
Statistical analysis
Quantitative analysis was restricted to population-based studies that aimed to estimate the incidence or prevalence of autoimmune comorbidity. We used the I2 test to assess heterogeneity. We conducted random effects meta-analyses of these studies using a Microsoft excel spreadsheet developed for this purpose.8 For studies in which zero events were recorded we employed a continuity correction of 0.5.9
Results
Search
After duplicates were removed the search identified 1476 unique citations (Supplemental, Figure 1). After abstract screening and hand searching of reference lists, 84 articles met the criteria for full-text review, of which we excluded 23. Sixty-one studies were the subject of this review.3–6,10–66 Quality scores are summarized in Supplemental Table 1 of the “Overview” manuscript.7 Incidence and prevalence estimates for all disorders are summarized in Supplemental Tables 1–72. More detailed results are presented below.
Study characteristics
The studies were conducted from 1905–2012 and most were conducted in Europe (32, 52.4%), followed by North America (21, 34.4%), Asia (6, 9.8%) and Australia (2, 3.3%). The data sources used included clinical interview and diagnostic tests (21, 34.4%), medical records (19, 31.1%), self-report (14, 22.9%), and administrative data (10, 16.4%). Some studies used more than one data source. Quality scores varied widely from 0/9 to 8/8 overall, but among population-based studies were higher and less variable ranging from 4/8 to 8/8. Some of the observed limitations included lack of population-based designs, absence of validated approaches to the identification of MS or comorbid disease when using administrative databases, lack of confidence intervals for estimates of incidence and prevalence. Few age or sex-specific estimates were reported.
Any autoimmune disorder (overall)
One population-based study reported the incidence of autoimmune disease (based on hospital contacts) to be 1.26% (Supplemental Table 1), but this did not exceed expectations for the general population (Supplemental Table 2).48
Four studies reported the prevalence of autoimmune disease after MS diagnosis to range from 3–26.1%,3–6 but the autoimmune disorders included varied across studies (Supplemental Table 3). None of these studies was population-based. Two studies reported the prevalence of autoimmune disease at or before MS diagnosis to range from 0.66–1.5%.4,25
Three studies reported that the prevalence of autoimmune disease was higher in the MS population than in other age- and sex-matched populations.4–6 The findings were not statistically significant in the only study that used controls clearly drawn from the general population (Supplemental Table 2).
Gastrointestinal
Autoimmune hepatitis
The incidence of autoimmune hepatitis, in one clinic-based study, was 0.17%; this did not differ from published findings for the general population (Supplemental Table 4).17 Two studies, neither population-based, reported the prevalence of autoimmune hepatitis to range from 0.06% to approximately 0.2% (Supplemental Table 5).25,62 One study compared the prevalence of hepatitis in the MS population before diagnosis to that of age, sex and regionally matched controls, and found no difference (Supplemental Table 6).33
Celiac disease
The incidence of celiac disease in the sole, population-based study, was 0.01% (Supplemental Table 7).48 The prevalence of celiac disease in six studies ranged from 0.0–11.1%;13,21,22,36,54,57 none was truly population-based (Supplemental Table 8). Three of these studies reported comparisons to findings in blood donors, family members or the published literature and found no differences (Supplemental Table 9).13,22,54
Inflammatory bowel disease
Two studies, one population-based, reported the incidence of inflammatory bowel disease to range from 0.33–1.0% (Supplemental Table 10).38,48 Twelve studies reported the prevalence after MS diagnosis to range from 0.36–4.66%.3,5,16,21,22,27,29,37,39,53,55,63 The prevalence in the sole population-based study that captured inpatients and outpatients with MS was 0.78% (Supplemental Table 11).39
At or before MS diagnosis the prevalence of inflammatory bowel disease ranged from 0.10–1.6%.25,33,37 The lower prevalence estimate may have been an underestimate as only individuals with MS diagnoses before age 45 years, and whose inflammatory bowel disease was severe enough to require registration for government programs were captured.25
Findings regarding inflammatory bowel disease were mixed, but most studies reported that the incidence and prevalence were higher in the MS population than in the general population before and after MS diagnosis (Supplemental Table 12).22,27,33,38,39,48,53
Primary biliary cirrhosis
The incidence of primary biliary cirrhosis, in a population-based Danish study, was 0.01% (Supplemental Table 13).48 Five studies reported the prevalence of primary biliary cirrhosis to range from 0–0.12% (Supplemental Table 14).18,25,44,45,63 Of these, three studies reported comparisons to other populations; none observed a difference (Supplemental Table 15).5,27,33
Endocrine
Adrenocortical insufficiency
One population-based study reported the incidence of adrenocortical insufficiency to be 0% (Supplemental Table 16).48 Four studies, none population-based, reported the prevalence to range from 0–0.31% (Supplemental Table 17).5,11,21,22 In the population-based incidence study from Denmark the incidence of adrenocortical insufficiency did not differ in the MS and general populations (Supplemental Table 18). Among the two prevalence studies that reported comparisons, one reported no disease in either the MS population or controls who were drawn from clinic personnel rather than the underlying source population (Supplemental Table 18). The second study reported a substantially increased prevalence of disease in the MS population, but based on a comparison to published data from the same region rather than concurrent controls evaluated using similar methods.
Diabetes
No studies reported the incidence of type I diabetes. Eighteen studies reported the prevalence of type I diabetes to range from 0–9.4%.3,5,16,21,22,24,27,28,30,33,34,40,41,43,52,53,64,65 Only four of these studies were truly population-based (Supplemental Table 19). Heterogeneity of the estimates was substantial (I2=95.3). The summary estimate was 0.016% (95% confidence interval (CI): 0.01–0.21%) (Figure 1).
Figure 1.

Forest plot of the prevalence of diabetes in multiple sclerosis in population-based studies.
Twelve studies compared the prevalence of type I diabetes in the MS population to a comparator population,5,22,24,27,28,30,33,40,43,53,64 with most (eight studies) reporting no difference (Supplemental Table 20).24
Thyroid disease
Four studies reported the incidence of autoimmune thyroid disease.38,48,65,66 Three of these captured both hypo- and hyper-thyroidism, reporting estimates of 0.15–0.42%. The fourth study reported an incidence of Hashimoto’s thyroiditis of 0% (Supplemental Table 21).48 Heterogeneity of the three population-based estimates was substantial (I2=89.9). The summary incidence estimate was 0.17% (95% CI: 0–0.40%) (Figure 2). Removing the earliest study (published 1990) did not reduce heterogeneity (I2=94.1) nor change the summary estimate (0.19%; 95% CI: 0–0.59%).
Figure 2.

Forest plot of the incidence of autoimmune thyroid disease in multiple sclerosis in population-based studies.
Twenty-four studies reported the prevalence of thyroid disease, of which 21 specifically reported the prevalence of autoimmune thyroid disease (Supplemental Table 22).3,5,11,14,16,20–22,27–29,32,33,37,45–47,50,51,53,59,65,66 Some studies reported hypo- and hyper-thyroidism together, while others reported these separately. The prevalence of thyroid disease overall ranged from 2.08–10%, of Hashimoto’s thyroiditis ranged from 0–16.1%, and of Grave’s disease ranged from 0–2.56%. Of the three population-based studies, two reported the prevalence of autoimmune thyroid disease overall.65,66 Heterogeneity of these estimates was substantial (I2=95.4). The summary estimate was 6.44% (95% CI: 0.19–12.7%) (Figure 3).
Figure 3.

Forest plot of the prevalence of autoimmune thyroid disease in multiple sclerosis in population-based studies.
Three studies compared the incidence of thyroid disease in the MS population to that in the general population (Supplemental Table 23);48,65,66 none found a statistically significant difference. Thirteen studies compared the prevalence of thyroid disease in the MS population to that in the general population,5,22,27,28,32,34,38,46,47,53,59,66 but some studies did not use concurrent controls while others used spousal controls, potentially leading to overmatching. Of these 13 studies, eight found that the prevalence of disease was similar in the MS population.
Eye
Two studies reported the incidence of uveitis (Supplemental Table 24),19,38 while 12 reported prevalence (Supplemental Table 25).3,5,15,21,22,31,33,35,37,56 The incidence of uveitis ranged from 0–0.11%, and prevalence ranged from 0–9.33%.3,5,15,21,22,31,33,35,37,56 None of these studies was population-based. The study with the highest reported prevalence conducted a complete ophthalmological examination on every participant, potentially improving ascertainment but likely capturing some asymptomatic cases.
In three studies the prevalence of uveitis reported at or prior to MS diagnosis ranged from 0.41–1.95%.33,35,67
Six studies compared the prevalence of uveitis in the MS population and the general population (Supplemental Table 26).5,15,22,33,38 Only three of these studies used concurrent control populations, two of which noted a higher than expected prevalence of uveitis in the MS population.
Hair and skin
Alopecia areata
No study reported the incidence of alopecia. Four studies reported the prevalence of alopecia areata to be less than 1%.3,5,16,21 Three of the studies were hospital-based; the other study was not population-based (Supplemental Table 27). One of the hospital-based studies reported that the prevalence of alopecia did not differ between the MS population and clinic personnel (Supplemental Table 28).5
Pemphigoid/pemphigus
Two studies reported the incidence of bullous pemphigoid or pemphigus vulgaris (Supplemental Table 29).19,48 The incidence of pemphigoid was 0.11% in a large population-based study from Denmark.48 The incidence of pemphigus vulgaris ranged from 0–0.51%. Four studies reported the prevalence of these conditions (Supplemental Table 30).11,21,33,62 The prevalence of pemphigoid was 0.08% in one study from Europe and one from North America.21,33 The prevalence of pemphigus vulgaris ranged from 0.02–0.62%.11, 21, 62 One Danish population-based study reported that the incidence of pemphigoid and pemphigus foliaceous were increased as compared to matched population controls (Supplemental Table 31).48 A North American study using Kaiser Permanente-linked administrative and clinical records reported an increased prevalence of pemphigoid after MS diagnosis.33
Psoriasis
Two studies reported the incidence of psoriasis (Supplemental Table 32),19,48 while 10 others reported the prevalence (Supplemental Table 33).3,15,21,22,27,33,34,43,53,62 The incidence of psoriasis in two European populations ranged from 0.17–1.63%;12,42 the lower estimate was a population-based study.48 The prevalence of psoriasis ranged from 0.39–7.74%.3,15,21,22,27,33,34,43,53,62 The highest estimate was reported by the sole population-based study.43 The prevalence of psoriasis before MS diagnosis was 1.19% in one case-control study from the USA.33
One study reported a higher incidence of psoriasis in the MS population than expected for the Danish general population (Supplemental Table 34).48 Among seven studies that compared the prevalence of psoriasis in the MS population with another population,15,22,27,33,34,43,53 five used concurrent control populations and did not note differences. One study reported increased odds of psoriasis in the MS population as compared to published data for the general population.22
Vitiligo
One population-based study reported the incidence of vitiligo to be 0% (Supplemental Table 35),48 while seven reported prevalence to range from 0–0.70% (Supplemental Table 36).18,25,26,39,44,45,63 The incidence of vitiligo did not differ between the MS population and expected values calculated for the general population (Supplemental Table 37).48 Among five studies that compared the prevalence of vitiligo in MS to a comparator group, none found an increased burden of vitiligo.25,26,39,44,45
Connective tissue disorders
Ankylosing spondylitis
One population-based Danish study reported the incidence of ankylosing spondylitis to be 0.01% (Supplemental Table 38); this did not differ from expectations in the general population (Supplemental Table 39).48 Five studies reported the prevalence of ankylosing spondylitis or the general group of inflammatory spondyloarthropathies to range from 0.12–1.98% (Supplemental Table 40).3,5,16,26,27,30 One of these studies was population-based, from Taiwan, and reported a prevalence of 1.78%.30 This study also reported that this prevalence was three-fold higher than matched general population controls (Supplemental Table 39). Two other small studies did not report a difference between the prevalence in the MS and general populations.5,27
Dermatomyositis/polymyositis
One population-based Danish study reported the incidence of dermatomyositis to be 0% (Supplemental Table 41).48 One hospital-based study reported the prevalence of dermatopolymyositis to be 0.03%, and the prevalence of myositis more generally to be 3.33% (Supplemental Table 42).21 The latter prevalence exceeded the prevalence of myositis reported in a small clinic-based study of 0.62% (Supplemental Table 43).62 A third study reported the prevalence of polymyositis to be approximately 0.2%.3
Polymyalgia rheumatica
No studies reported the incidence of polymyalgia rheumatica. Two studies reported the case- prevalence of polymyalgia rheumatica to be 0.12–0.15% (Supplemental Table 44),16,33 and one of these also reported that the prevalence of the condition did not differ between the MS population and matched controls (Supplemental Table 45).33
Rheumatoid arthritis
Three studies reported the incidence of rheumatoid arthritis to range from 0.14–1.28% (Supplemental Table 46).38,48,65 Two of the studies were population-based,48,65 and reported that the incidence of rheumatoid arthritis was not significantly higher in the MS population than in the general population (Supplemental Table 47).43,61 The summary estimated incidence was 0.21% (95% CI: 0.087–0.33%) (Figure 4).
Figure 4.
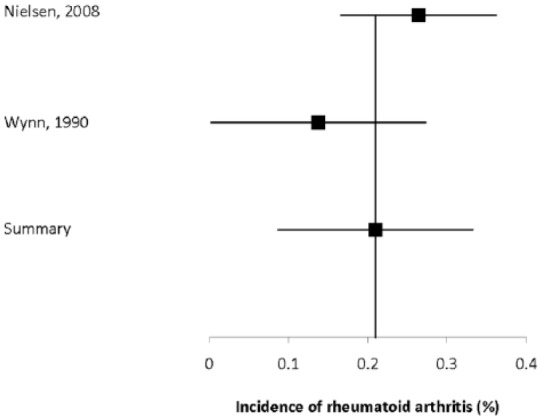
Forest plot of the incidence of rheumatoid arthritis in multiple sclerosis in population-based studies.
Seventeen studies reported the prevalence of rheumatoid arthritis to range from 0.30–3.64% (Supplemental Table 48).3,5,11,16,19,21,22,29,30,33,34,37,43,53,63,65 Generally, studies that used questionnaires reported higher prevalence estimates than those relying on medical records review or administrative databases. The prevalence estimate from two population-based studies30,43 was 2.92% (95% CI: 1.8–4.0%) (Figure 5). Two studies reported the prevalence of rheumatoid arthritis to be 0.22–1.17% before MS diagnosis.33,67 Of 10 comparative studies,5,22,27,30,33,34,43,53,63,67 eight found no differences or did not make comparisons, while two reported that the prevalence of rheumatoid arthritis was higher in the MS population than in matched controls or spouses (Supplemental Table 47).30,53
Figure 5.

Forest plot of the prevalence of rheumatoid arthritis in multiple sclerosis in population-based studies.
Sjogren’s syndrome
Two studies reported the incidence of Sjogren’s syndrome to range from 0.02–0.22% (Supplemental Table 49).38,48 The lower estimate was drawn from a largely population-based study that relied on hospital discharge abstracts, potentially missing cases of Sjogren’s syndrome that did not require hospitalization or outpatient specialty visits.48 Thirteen studies, none population-based, reported the prevalence of Sjogren’s syndrome to range from 0–16.7% (Supplemental Table 50).10,18,19,21,29,33,37,42,44,49,58,60,62 The highest estimate was in a study limited to persons with primary progressive MS.18 The incidence and prevalence of Sjogren’s syndrome did not differ between the MS and comparator populations (Supplemental Table 51).
Systemic lupus erythematosus
Two studies reported the incidence of systematic lupus erythematosus to range from 0.02–0.35% (Supplemental Table 52), but the methods used differed substantially.38,48 The study with the lower estimate used population-based administrative data,48 while the higher estimate used a volunteer sample reporting diagnoses using a validated self-report questionnaire.38 The former study reported that the incidence of systematic lupus erythematosus did not differ from expectations for the general population (Supplemental Table 53).
Nine studies reported the prevalence of systematic lupus erythematosus to range from 0.14–2.90% (Supplemental Table 54).3,16,21,23,27,29,30,33,37,53 The highest estimate was drawn from a population-based study using administrative data in Taiwan,30 while the other studies were conducted in Caucasian populations largely from North America and Europe. Two studies reported the prevalence at or before MS diagnosis to be 0.43–0.56%.33,67 Of five studies that reported comparisons of the prevalence of systematic lupus erythematosus in the MS population with the general population (or with the published literature),27,30,33,53,67 only one reported the prevalence to be increased in the MS population (Supplemental Table 53).30
Systemic sclerosis
One population-based study reported the incidence of systemic sclerosis to be 0.34% (Supplemental Table 55).48 Three studies reported the prevalence of systemic sclerosis to range from 0.06–0.85% (Supplemental Table 56);21,27,33 none was truly population-based. The incidence of systemic sclerosis did not differ from expectations when compared to the general population (Supplemental Table 57).48 In two studies the prevalence did not differ in the MS population as compared to the general population.27,33
Wegener’s granulomatosis
One population-based study reported the incidence of Wegener’s granulomatosis to be 0.01% (Supplemental Table 58), and this did not differ from expectations for the general population (Supplemental Table 59).48 Only two studies reported the prevalence of this condition with similar findings ranging from 0.02–0.03% (Supplemental Table 60).21,33 Of these two studies, the American study compared the findings in the MS population to a matched population and did not identify a difference (Supplemental Table 59).33
Other
Myasthenia gravis
One population-based study reported the incidence of myasthenia gravis to be 0.01% (Supplemental Table 61), and this did not differ from expectations for the general population (Supplemental Table 63).48 Eight studies reported the prevalence of myasthenia gravis to range from 0–0.56%.5,12,15,19,21,33,53,61 One of these studies used population-based data and reported a prevalence of 0.20% (Supplemental Table 62).61
Five studies reported the prevalence of myasthenia in the MS and comparator populations.5,12,15,33,53 Three studies did not report a difference between groups, while two of these studies used published data and did not report formal statistical comparisons but the findings suggested a higher prevalence of myasthenia in the MS population.12,15 However, the only study with controls that appeared to be selected from the same underlying source population reported a nearly statistically significant increase in prevalence in the MS population (OR 2.5; 0.9–6.6, Supplemental Table 63).33
Guillain-Barre syndrome
One Danish study reported the incidence of Guillain-Barre syndrome to be 0% (Supplemental Table 64), and this did not differ from expectations for the general population (Supplemental Table 65).48 Four studies reported the prevalence to range from 0.11–1.66%.3,21,22,33 The highest estimate was produced by a Danish study that was hospital-based,21 while the other estimates were drawn from outpatient, or inpatient and outpatient records. One American study reported that the prevalence of Guillain-Barre syndrome before MS diagnosis was 0% (Supplemental Table 66).33
Two studies reported the prevalence of Guillain-Barre syndrome was higher in the MS population than in a comparator population;22,33 however, only one had findings that were statistically significant (Supplemental Table 65).33
Autoimmune hematologic disorders
One population-based study reported the incidence of pernicious anemia as 0.03% (Supplemental Table 67), and this did not differ from expectations for the general population (Supplemental Table 68).48 Seven studies reported the prevalence of pernicious anemia to range from 0–2.44% (Supplemental Table 69),3,5,11,15,16,33,53 while two studies reported vitamin B12 deficiency without recording whether this was due to pernicious anemia or not (6.82– 12.0%).29, 37 None of these studies was population-based. Of the seven studies, four reported comparisons of the prevalence of pernicious anemia in the MS population with another population or published data.5,22,33,53 Of these, half found the burden of pernicious anemia to be higher in the MS population22,33 while half found no difference (Supplemental Table 68).5,53
One population-based study reported the incidence of autoimmune hemolytic anemia as 0% (Supplementary Table 67), and this did not differ from expectations for the general population (Supplementary Table 68).48 Only two studies reported the prevalence of this condition to range from 0.02–1.11% (Supplementary Table 69).15,33 Of these, one compared the findings in the MS population to a matched population and did not identify a difference (Supplementary Table 68).33
One population-based Danish study reported the incidence of idiopathic thrombocytopenic purpura to be 0.02% (Supplemental Table 70), and this was four-fold higher than expected for the general population (Supplemental Table 71).48 Three other studies reported the prevalence of idiopathic thrombocytopenic purpura to range from 0.11–0.13% (Supplemental Table 72);15,21,33 none was truly population-based, although one covered the entire hospitalized MS population.21 Of these studies, only one reported a comparison to the matched population and did not find a difference (Supplementary Table 71).33 That study focused on the occurrence of purpura prior to MS diagnosis.
Discussion
In this comprehensive systematic review, we evaluated 61 studies reporting the incidence or prevalence or both, of comorbid autoimmune disease in the MS population. Several consistent findings emerged across all conditions studied. While multiple studies were conducted in Europe and North America, most world regions have not been adequately studied. Within the investigations from Europe and North America, most of the information available is restricted to a small number of regions within those continents. Limitations in study quality were common, with few using population-based designs. Among the population-based studies overall quality was better although still variable with scores of 4/8 to 8/8. Incidence was rarely reported (six out of 61 studies). Even among studies reporting prevalence, detailed age and sex-specific estimates were lacking. Although it was out of the scope of this review, we also noted the relative absence of information indicating whether the characteristics of autoimmune disease such as severity or age at symptom onset are different in the MS population versus the general population.
We identified population-based studies of ankylosing spondylitis, inflammatory bowel disease, myasthenia gravis, psoriasis, rheumatoid arthritis, systemic lupus erythematosus, thyroid disease, and type I diabetes. Based on these studies the most prevalent comorbid autoimmune diseases were psoriasis and thyroid disease. Considering all studies, most autoimmune conditions affected fewer than 5% of individuals with MS with the possible exceptions of celiac disease, type I diabetes, thyroid disease, uveitis and psoriasis for which at least one estimate was over 5%.
Among those studies that evaluated the incidence or prevalence of autoimmune disease in MS and a comparator population, few used concurrent controls that were clearly drawn from the same underlying source population. Our findings did not support a non-specific increase in the risk of comorbid autoimmune disease. However, the findings support an increased risk of inflammatory bowel disease, likely uveitis, and possibly pemphigoid. Findings are inconsistent for other conditions such as thyroid disease, type I diabetes, psoriasis, systemic lupus erythematosus, and rheumatoid arthritis, and warrant further investigation. The shared risks of these diseases may reflect shared genetic susceptibility, shared environmental exposures such as smoking,38 or both.
Limitations of this systematic review include the inclusion of only English language articles. However, we excluded only seven of 1476 abstracts identified on this basis, suggesting that any potential bias is likely to be small. We have not reported detailed demographic or clinical descriptions of the MS populations studied because of the absence of this information in many studies, and lack of any consistent approach to reporting this information in others. The absence of information regarding exposure to disease-modifying therapy in many studies deserves particular note, as existing and emerging therapies may influence the risk of autoimmune comorbidities.68 Differential exposure to such therapies may account for some of the heterogeneity observed.
Despite studies spanning more than 100 years, we still lack good estimates of the incidence or prevalence of autoimmune comorbidity in MS. The lack of such estimates hampers the ability to identify temporal trends in the risk of autoimmune disease risk, or to determine the magnitude of increased risk associated with exposure to novel therapies. Ideally future studies will use common approaches to minimize heterogeneity due to differences in study design, and allow evaluation of true heterogeneity across populations.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
Thanks to Tania Gottschalk (Librarian, University of Manitoba), who provided assistance regarding the development of the search strategies for this review. This study was conducted under the auspices of the International Advisory Committee on Clinical Trials of New Drugs in Multiple Sclerosis whose members include Jeffrey Cohen (Cleveland Clinic Foundation, Cleveland, USA), Laura J Balcer (NYU Langone Medical Center, New York City, USA), Brenda Banwell (The Children’s Hospital of Philadelphia, Philadelphia, USA), Michel Clanet (Federation de Neurologie, Toulouse, France), Giancarlo Comi (University Vita-Salute San Raffaele, Milan, Italy), Gary R Cutter (University of Alabama at Birmingham, Birmingham, USA), Andrew D Goodman (University of Rochester Medical Center, Rochester, USA), Hans-Peter Hartung (Heinrich-Heine-University, Duesseldorf, DE), Bernhard Hemmer (Technical University of Munich, Munich, DE), Catherine Lubetzki (Fédération des maladies du système nerveux et INSERM 71, Paris, France), Fred D Lublin (Mount Sinai School of Medicine, New York, USA), Ruth A Marrie (Health Sciences Centre, Winnipeg, Canada), Aaron Miller (Mount Sinai School of Medicine, New York, USA), David H Miller (University College London, London, UK), Xavier Montalban (Hospital Universitari Vall d’Hebron, Barcelona, Spain), Paul O’Connor (St Michael’s Hospital, Toronto, Canada), Daniel Pelletier (Yale University School of Medicine, New Haven, USA), Stephen C. Reingold (Scientific & Clinical Review Assoc., LLC, Salisbury, USA), Alex R Cañellas (Hospital Universitari Vall d’Hebron, Barcelona, Spain), Per S Sørensen (Copenhagen University Hospital, Copenhagen, Denmark), Maria P Sormani (University of Genoa, Genoa, Italy), Olaf Stuve (University of Texas Health Sciences Center, Dallas, USA), Alan J Thompson (University College London, London, UK), Maria Trojano (University of Bari, Bari, Italy), Bernard Uitdehaag (VU University Medical Center, Amsterdam, Netherlands), Emmaunelle Waubant (University of California- San Francisco, San Francisco, USA), Jerry S Wolinsky (University of Texas HSC, Houston, USA).
Footnotes
Conflict of interest: Ruth A Marrie receives research funding from: Canadian Institutes of Health Research, Public Health Agency of Canada, Manitoba Health Research Council, Health Sciences Centre Foundation, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Rx & D Health Research Foundation, and has conducted clinical trials funded by Sanofi-Aventis.
Jeffrey Cohen reports personal compensation for consulting from EMD Serono, Genentech, Genzyme, Innate Immunotherapeutics, Novartis, and Vaccinex. Jeffrey Cohen receives research support paid to his institution from Biogen Idec, Consortium of MS Centers, US Department of Defense, Genzyme, US National Institutes of Health, National MS Society, Novartis, Receptos, Synthon, Teva, and Vaccinex.
Olaf Stuve is an associate editor of JAMA Neurology, and he serves on the editorial boards of the Multiple Sclerosis Journal, Clinical and Experimental Immunology, and Therapeutic Advances in Neurological Disorders. He has participated in data and safety monitoring committees for Pfizer and Sanofi. Olaf Stuve has received grant support from Teva Pharmaceuticals.
Per S Sorensen has received personal compensation for serving on scientific advisory boards, steering committees, independent data monitoring boards in clinical trials, or speaking at scientific meetings from Biogen Idec, Merck Serono, Novartis, Genmab, TEVA, GSK, Genzyme, Bayer Schering, Sanofi-aventis and MedDay pharmaceuticals. His research unit has received research support from Biogen Idec, Merck Serono, TEVA, Sanofi-aventis, Novartis, RoFAR, Roche, and Genzyme.
Gary Cutter has served on scientific advisory boards for and/or received funding for travel from Innate immunity, Klein-Buendel Inc., Genzyme, Medimmune, Novartis, Nuron Biotech, Spiniflex Pharmaceuticals, Somahlution, Teva pharmaceuticals; receives royalties from publishing Evaluation of health promotion and disease prevention (McGraw Hill, 1984); has received honoraria from Glaxo-SmithKline, Novartis, Advanced Health Media Inc., Biogen Idec, EMD Serono Inc., EDJ Associates, Inc., the National Heart, Lung, and Blood Institute, National Institute of Neurological Diseases and Stroke, National Marrow Donor Program, Consortium of Multiple Sclerosis Centers; Mt. Sinai School of Medicine and Teva Pharmaceuticals; has served on independent data and safety monitoring committees for Apotek, Ascendis, Biogen-Idec, Cleveland Clinic, Glaxo Smith Klein Pharmaceuticals, Gilead Pharmaceuticals, Modigenetech/Prolor, Merck/Ono Pharmaceuticals, Merck, Neuren, PCT Bio, Teva, Vivus, NHLBI (Protocol Review Committee), NINDS, NMSS, NICHD (OPRU oversight committee).
Stephen Reingold reports personal consulting fees from the National Multiple Sclerosis Society (NMSS) and the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), during the conduct of this work; and over the past three years, personal consulting fees from Bayer HealthCare, Biogen Idec, Coronado Biosciences Inc., the Cleveland Clinic Foundation, Eli Lilly & Company, from EMD Serono and Merck Serono, Genentech, F Hoffmann-LaRoche, Ironwood Pharmaceuticals Inc., ISIS Pharmaceuticals Inc., Medimmune Inc., Novartis Pharmaceuticals Corporation, Observatoire Français de la Sclérosis en Plaques, Opexa Therapeutics, Sanofi-Aventis, SK Biopharmaceuticals, Synthon Pharmaceuticals Inc., TEVA Pharmaceutical Industries, and Fondation pour l’aide à la Recherche sur la Sclérosis en Plaques, for activities outside of the submitted work.
Maria Trojano has served on scientific Advisory Boards for Biogen Idec, Novartis and Merck Serono; has received speaker honoraria from Biogen-Idec, Sanofi Aventis, Merck-Serono, Teva and Novartis; has received research grants from Biogen-Idec, Merck-Serono, and Novartis.
Nadia Reider reports no disclosures.
Funding: This study was supported (in part) by the National Multiple Sclerosis Society and a Don Paty Career Development Award from the MS Society of Canada.
Contributor Information
Ruth Ann Marrie, Departments of Internal Medicine and Community Health Sciences, University of Manitoba, Canada.
Nadia Reider, Departments of Internal Medicine and Community Health Sciences, University of Manitoba, Canada.
Jeffrey Cohen, Mellen Center for MS Treatment and Research, Cleveland Clinic, USA.
Olaf Stuve, Department of Neurology and Neurotherapeutics, University of Texas Southwestern, USA.
Per S Sorensen, Department of Neurology, Copenhagen University Hospital Rigshospitalet, Denmark.
Gary Cutter, Department of Biostatistics, University of Alabama at Birmingham, USA.
Stephen C Reingold, Scientific and Clinical Review Associates, LLC, USA.
Maria Trojano, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari, Italy.
References
- 1. Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol 2004; 3: 709–718. [DOI] [PubMed] [Google Scholar]
- 2. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing–remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012; 380: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 3. Barcellos LF, Kamdar BB, Ramsay PP, et al. Clustering of autoimmune diseases in families with a high-risk for multiple sclerosis A descriptive study. Lancet Neurol 2006; 5: 924–931. [DOI] [PubMed] [Google Scholar]
- 4. Ghadirian P, Dadgostar B, Azani R, et al. A case-control study of the association between sociodemographic, lifestyle and medical history factors and multiple sclerosis. Can J Public Health 2001; 92: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seyfert S, Klapps P, Meisel C, et al. Multiple sclerosis and other immunologic diseases. Acta Neurol Scand 1990; 81: 37–42. [DOI] [PubMed] [Google Scholar]
- 6. Zorzon M, Zivadinov R, Nasuelli D, et al. Risk factors of multiple sclerosis: A case-control study. Neurol Sci 2003; 24: 242–247. [DOI] [PubMed] [Google Scholar]
- 7. Marrie R, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. 2015; 21(3): 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neyeloff J, Fuchs S, Moreira L. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cox DR. The continuity correction. Biometrika 1970; 57: 217–219. [Google Scholar]
- 10. Annunziata P, De Santi L, Di Rezze S, et al. Clinical features of Sjogren’s syndrome in patients with multiple sclerosis. Acta Neurol Scand 2011; 124: 109–114. [DOI] [PubMed] [Google Scholar]
- 11. Baker HWG, Balla JI, Burger HG, et al. Multiple sclerosis and autoimmune diseases. Aust N Z J Med 1972; 2: 256–260. [DOI] [PubMed] [Google Scholar]
- 12. Basiri K, Etemadifar M, Maghzi AH, et al. Frequency of myasthenia gravis in multiple sclerosis: Report of five cases from Isfahan, Iran. Neurol India 2009; 57: 638–640. [DOI] [PubMed] [Google Scholar]
- 13. Borhani Haghighi A, Ansari N, Mokhtari M, et al. Multiple sclerosis and gluten sensitivity. Clin Neurol Neurosurg 2007; 109: 651–653. [DOI] [PubMed] [Google Scholar]
- 14. Buchanan RJ, Schiffer R, Stuifbergen A, et al. Demographic and disease characteristics of people with multiple sclerosis living in urban and rural areas. Int J MS Care 2006; 8: 89–98. [Google Scholar]
- 15. Cendrowski W. Multiple sclerosis and diseases of autoimmune or related origin. Mater Med Pol 1989; 21: 327–329. [PubMed] [Google Scholar]
- 16. De Keyser J. Autoimmunity in multiple sclerosis. Neurology 1988; 38: 371–374. [DOI] [PubMed] [Google Scholar]
- 17. De Seze J, Canva-Delcambre V, Fajardy I, et al. Autoimmune hepatitis and multiple sclerosis: A coincidental association? Mult Scler 2005; 11: 691–693. [DOI] [PubMed] [Google Scholar]
- 18. De Seze J, Devos D, Castelnovo G, et al. The prevalence of Sjogren syndrome in patients with primary progressive multiple sclerosis. Neurology 2001; 57: 1359–1363. [DOI] [PubMed] [Google Scholar]
- 19. Deretzi G, Kountouras J, Koutlas E, et al. Familial prevalence of autoimmune disorders in multiple sclerosis in Northern Greece. Mult Scler 2010; 16: 1091–1101. [DOI] [PubMed] [Google Scholar]
- 20. Durelli L, Oggero A, Verdun E, et al. Thyroid function and anti-thyroid antibodies in MS patients screened for interferon treatment. A multicenter study. J Neurol Sci 2001; 193: 17–22. [DOI] [PubMed] [Google Scholar]
- 21. Eaton WW, Rose NR, Kalaydjian A, et al. Epidemiology of autoimmune diseases in Denmark. J Autoimmun 2007; 29: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edwards LJ, Constantinescu CS. A prospective study of conditions associated with multiple sclerosis in a cohort of 658 consecutive outpatients attending a multiple sclerosis clinic. Mult Scler 2004; 10: 575–581. [DOI] [PubMed] [Google Scholar]
- 23. Fanouriakis A, Mastorodemos V, Pamfil C, et al. Coexistence of systemic lupus erythematosus and multiple sclerosis: Prevalence, clinical characteristics, and natural history. Semin Arthritis Rheum 2014; 43: 751–758. [DOI] [PubMed] [Google Scholar]
- 24. Fleming ST, Blake Jr RL. Patterns of comorbidity in elderly patients with multiple sclerosis. J Clin Epidemiol 1994; 47: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 25. Fromont A, Binquet C, Rollot F, et al. Comorbidities at multiple sclerosis diagnosis. J Neurol 2013; 260: 2629–2637. [DOI] [PubMed] [Google Scholar]
- 26. Hanrahan PS, Russell AS, McLean DR. Ankylosing spondylitis and multiple sclerosis: An apparent association? J Rheumatol 1988; 15: 1512–1514. [PubMed] [Google Scholar]
- 27. Henderson RD, Bain CJ, Pender MP. The occurrence of autoimmune diseases in patients with multiple sclerosis and their families. J Clin Neurosci 2000; 7: 434–437. [DOI] [PubMed] [Google Scholar]
- 28. Hoppenbrouwers IA, Cortes LM, Aulchenko YS, et al. Familial clustering of multiple sclerosis in a Dutch genetic isolate. Mult Scler 2007; 13:17–24. [DOI] [PubMed] [Google Scholar]
- 29. Horton M, Rudick RA, Hara-Cleaver C, et al. Validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology 2010; 35: 83–90. [DOI] [PubMed] [Google Scholar]
- 30. Kang J-H, Chen Y-H, Lin H-C. Comorbidities amongst patients with multiple sclerosis: A population-based controlled study. Eur J Neurol 2010; 17: 1215–1219. [DOI] [PubMed] [Google Scholar]
- 31. Karara AM, Macky TA, Sharawy MH. Pattern of uveitis in an Egyptian population with multiple sclerosis: A hospital-based study. Ophthalmic Res 2013; 49: 25–29. [DOI] [PubMed] [Google Scholar]
- 32. Karni A, Abramsky O. Association of MS with thyroid disorders. Neurology 1999; 53: 883–885. [DOI] [PubMed] [Google Scholar]
- 33. Langer-Gould A, Albers K, Van Den Eeden S, et al. Autoimmune diseases prior to the diagnosis of multiple sclerosis: A population-based case-control study. Mult Scler 2010; 16: 855–861. [DOI] [PubMed] [Google Scholar]
- 34. Laroni A, Calabrese M, Perini P, et al. Multiple sclerosis and autoimmune diseases: Epidemiology and HLA-DR association in North-east Italy. J Neurol 2006; 253: 636–639. [DOI] [PubMed] [Google Scholar]
- 35. Le Scanff J, Seve P, Renoux C, et al. Uveitis associated with multiple sclerosis. Mult Scler 2008; 14: 415–417. [DOI] [PubMed] [Google Scholar]
- 36. Levinthal DJ, Rahman A, Nusrat S, et al. Adding to the burden: Gastrointestinal symptoms and syndromes in multiple sclerosis. Mult Scler Int 2013; 2013: 319201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marrie RA, Horwitz R, Cutter G, et al. Comorbidity, socioeconomic status, and multiple sclerosis. Mul Scler 2008; 14: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 38. Marrie RA, Horwitz RI, Cutter G, et al. Smokers with multiple sclerosis are more likely to report comorbid autoimmune diseases. Neuroepidemiology 2011; 36: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marrie RA, Yu BN, Leung S, et al. The utility of administrative data for surveillance of comorbidity in multiple sclerosis: a validation study. Neuroepidemiology 2013; 40: 85–92. [DOI] [PubMed] [Google Scholar]
- 40. Marrosu MG, Cocco E, Lai M, et al. Patients with multiple sclerosis and risk of type 1 diabetes mellitus in Sardinia, Italy: a cohort study. Lancet 2002; 359: 1461–1465. [DOI] [PubMed] [Google Scholar]
- 41. Marrosu MG, Motzo C, Murru R, et al. The co-inheritance of type 1 diabetes and multiple sclerosis in Sardinia cannot be explained by genotype variation in the HLA region alone. Hum Mol Genet 2004; 13: 2919–2924. [DOI] [PubMed] [Google Scholar]
- 42. Metz LM, Seland P, Fritzler M. An analysis of the frequency of Sjogren’s syndrome in a population of multiple sclerosis patients. J Clin Lab Immunol 1989; 30: 121–125. [PubMed] [Google Scholar]
- 43. Midgard R, Gronning M, Riise T, et al. Multiple sclerosis and chronic inflammatory diseases. A case-control study. Acta Neurol Scand 1996; 93: 322–328. [DOI] [PubMed] [Google Scholar]
- 44. Miró J, Peña-Sagredo JL, Berciano J, et al. Prevalence of primary Sjögren’s syndrome in patients with multiple sclerosis. Ann Neurol 1990; 27: 582–584. [DOI] [PubMed] [Google Scholar]
- 45. Monzani F, Caraccio N, Meucci G, et al. Effect of 1-year treatment with interferon-beta1b on thyroid function and autoimmunity in patients with multiple sclerosis. Eur J Endocrinol 1999; 141: 325–331. [DOI] [PubMed] [Google Scholar]
- 46. Munteis E, Cano JF, Flores JA, et al. Prevalence of autoimmune thyroid disorders in a Spanish multiple sclerosis cohort. Eur J Neurol 2007; 14: 1048–1052. [DOI] [PubMed] [Google Scholar]
- 47. Niederwieser G, Buchinger W, Bonelli RM, et al. Prevalence of autoimmune thyroiditis and non-immune thyroid disease in multiple sclerosis. J Neurol 2003; 250: 672–675. [DOI] [PubMed] [Google Scholar]
- 48. Nielsen N, Frisch M, Rostgaard K, et al. Autoimmune diseases in patients with multiple sclerosis and their first-degree relatives: A nationwide cohort study in Denmark. Mult Scler 2008; 14: 823–829. [DOI] [PubMed] [Google Scholar]
- 49. Noseworthy JH, Bass BH, Vandervoort MK, et al. The prevalence of primary Sjögren’s syndrome in a multiple sclerosis population. Ann Neurol 1989; 25: 95–98. [DOI] [PubMed] [Google Scholar]
- 50. Nuyen J, Schellevisa FG, Satarianob WA, et al. Comorbidity was associated with neurologic and psychiatric diseases: A general practice-based controlled study. J Clin Epidemiol 2006; 59: 1274–1284. [DOI] [PubMed] [Google Scholar]
- 51. Percy AK, Noberga FT, Okazaki H, et al. Multiple sclerosis in Rochester, Minn. A 60-year appraisal. Arch Neurol 1971; 25: 105–111. [DOI] [PubMed] [Google Scholar]
- 52. Pitzalis M, Zavattari P, Murru R, et al. Genetic loci linked to type 1 diabetes and multiple sclerosis families in Sardinia. BMC Med Genet 2008; 9: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ramagopalan SV, Dyment DA, Valdar W, et al. The occurrence of autoimmune disease in Canadian families with multiple sclerosis. Lancet Neurol 2007; 6: 604–610. [DOI] [PubMed] [Google Scholar]
- 54. Rodrigo L, Hernandez-Lahoz C, Fuentes D, et al. Prevalence of celiac disease in multiple sclerosis. BMC Neurol 2011; 11: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sadovnick A, Paty D, Yannakoulias G. Concurrence of multiple sclerosis and inflammatory bowel disease. N Engl J Med 1989; 321: 762–763. [PubMed] [Google Scholar]
- 56. Salter AR, Tyry T, Vollmer T, et al. ‘Seeing’ in NARCOMS: A look at vision-related quality of life in the NARCOMS registry. Mult Scler 2013; 19: 953–960. [DOI] [PubMed] [Google Scholar]
- 57. Salvatore S, Finazzi S, Ghezzi A, et al. Multiple sclerosis and celiac disease: Is there an increased risk? Mult Scler 2004; 10: 711–712. [DOI] [PubMed] [Google Scholar]
- 58. Sandberg-Wollheim M, Axell T, Hansen BU, et al. Primary Sjogren’s syndrome in patients with multiple sclerosis. Neurology 1992; 42: 845–847. [DOI] [PubMed] [Google Scholar]
- 59. Sloka JS, Pryse-Phillips WEM, Stefanelli M, et al. Co-occurrence of autoimmune thyroid disease in a multiple sclerosis cohort. J Autoimmune Dis 2005; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Solomon AJ, Hills W, Chen Z, et al. Autoantibodies and Sjogren’s Syndrome in multiple sclerosis, a reappraisal. PLoS One 2013; 8: e65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Somer H, Muller K, Kinnunen E. Myasthenia gravis associated with multiple sclerosis. Epidemiological survey and immunological findings. J Neurol Sci 1989; 89: 37–48. [DOI] [PubMed] [Google Scholar]
- 62. Tourbah A, Clapin A, Gout O, et al. Systemic autoimmune features and multiple sclerosis: A 5-year follow-up study. Arch Neurol 1998; 55: 517–521. [DOI] [PubMed] [Google Scholar]
- 63. Tremlett HL, Evans J, Wiles CM, et al. Asthma and multiple sclerosis: An inverse association in a case-control general practice population. QJ Med 2002; 95: 753–756. [DOI] [PubMed] [Google Scholar]
- 64. Wertman E, Zilber N, Abramsky O. An association between multiple sclerosis and type I diabetes mellitus. J Neurol 1992; 239: 43–45. [DOI] [PubMed] [Google Scholar]
- 65. Wynn DR, Rodriguez M, O’Fallon WM, et al. A reappraisal of the epidemiology of multiple sclerosis in Olmsted County, Minnesota. Neurology 1990; 40: 780–786. [DOI] [PubMed] [Google Scholar]
- 66. Marrie RA, Yu BN, Leung S, et al. The incidence and prevalence of thyroid disease do not differ in the multiple sclerosis and general populations: A validation study using administrative data. Neuroepidemiology 2012; 39: 135–142. [DOI] [PubMed] [Google Scholar]
- 67. Marrie RA, Horwitz RI, Cutter G, et al. Association between comorbidity and clinical characteristics of MS. Acta Neurol Scand 2011; 124: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cossburn M, Pace AA, Jones J, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology 2011; 77: 573–579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


