Abstract
Background:
Several studies have suggested that comorbid neurologic disorders are more common than expected in multiple sclerosis (MS).
Objective:
To estimate the incidence and prevalence of comorbid seizure disorders and sleep disorders in persons with MS and to evaluate the quality of studies included.
Methods:
The PUBMED, EMBASE, Web of Knowledge, and SCOPUS databases, conference proceedings, and reference lists of retrieved articles were searched. Two reviewers independently screened abstracts to identify relevant articles, followed by full-text review of selected articles. We assessed included studies qualitatively and quantitatively (I2 statistic), and conducted meta-analyses among population-based studies.
Results:
We reviewed 32 studies regarding seizure disorders. Among population-based studies the incidence of seizure disorders was 2.28% (95% CI: 1.11–3.44%), while the prevalence was 3.09% (95% CI: 2.01–4.16%). For sleep disorders we evaluated 18 studies; none were population-based. The prevalence ranged from 0–1.6% for narcolepsy, 14.4–57.5% for restless legs syndrome, 2.22–3.2% for REM behavior disorder, and 7.14–58.1% for obstructive sleep apnea.
Conclusion:
This review suggests that seizure disorders and sleep disorders are common in MS, but highlights gaps in the epidemiological knowledge of these conditions in MS worldwide. Other than central-western Europe and North America, most regions are understudied.
Keywords: Multiple sclerosis, comorbidity, sleep, epilepsy, seizures, incidence, prevalence
Introduction
Multiple sclerosis (MS) is a chronic disabling disease of the central nervous system.1 Although comorbid physical and psychiatric comorbidities are increasingly recognized as relevant to clinical outcomes,2–4 gaps in the understanding of the epidemiology of comorbidity in MS remain. Several studies have suggested that comorbid neurologic disorders such as migraine, seizure disorders and sleep disorders (such as restless legs syndrome) are more common than expected in the MS population when compared to the general population.5,6 The impact of these conditions on disability, survival, and quality of life is poorly understood but existing studies suggest some neurologic comorbidities worsen these outcomes.7,8 Even though knowledge of the burden of these conditions is relevant to clinicians, the estimates of the frequencies of these conditions are variable and study populations have often been small.
We aimed to systematically review the literature regarding the incidence and prevalence of comorbid seizure disorders and sleep disorders in MS. A secondary aim was to evaluate the quality of all included studies to make recommendations for future research.
Methods
We conducted this review as part of a larger study on the worldwide incidence and prevalence of comorbidity in MS, but have divided these studies to allow for more detailed examination and discussion of findings. Herein we describe the findings for seizure disorders and sleep disorders.
The sleep disorders included were narcolepsy, periodic limb movements of sleep, restless legs syndrome, REM behavior disorder and sleep apnea. We did not review studies regarding sleep quality or insomnia due to challenges regarding the definitions of these conditions. We did not evaluate migraine as a recent systematic review and meta-analysis of headache was already available.9 We reviewed the incidence and prevalence of cerebrovascular disease but these studies are reviewed in conjunction with those regarding comorbid cardiovascular and peripheral vascular disease.
As detailed elsewhere,10 we developed separate search strategies for each comorbidity (Supplemental Appendix I). We reviewed the published literature and conference proceedings using PUBMED, EMBASE, SCOPUS, and Web of Knowledge for all years available through 20 November 2013. We also manually reviewed the reference lists of studies identified during electronic searches.
After review of the study objectives, two reviewers (RAM, NR) independently assessed whether unique abstracts identified met the inclusion criteria. We considered studies that were conducted in an MS population, included original data, specified the comorbidity of interest, clearly reported the incidence or prevalence of the comorbidity, and were published in English. If either reviewer selected the abstract it underwent full-text review, during which stage the articles were independently assessed by the two reviewers. We resolved disagreements by consensus.
One reviewer abstracted the data using a standardized data collection form and the findings were verified by the second reviewer. The data collection form (described in detail elsewhere) captured general study characteristics, as well as incidence and prevalence estimates.10 We critically appraised each study using a standardized assessment tool utilized in another systematic review of the incidence and prevalence of MS, and awarded quality scores based on yes or no responses to 9 questions.10 This process supported a qualitative assessment of study heterogeneity.
Statistical analysis
For the quantitative analysis we used the I2 test to assess heterogeneity, and restricted this analysis to population-based studies. We conducted meta-analyses of these studies using a Microsoft excel spreadsheet developed for this purpose.11 For studies in which zero events were recorded we employed a continuity correction of 0.5.12
Results
Seizure disorder (epilepsy)
Search
We identified 490 unique citations (Supplemental Figure 1). After abstract screening and hand searching of reference lists, 49 articles met the criteria for full-text review, of which we excluded 17. Thirty-two unique studies were the subject of this review.5,7,13–42
Study characteristics
The characteristics of the 32 included studies are shown in Supplemental Tables 2 and 3.5,7,13–42 The studies were conducted from 1935 to 2012. Most of the studies were conducted in Europe (central and Western) (16, 50%), followed by Asia (6, 18.7%), North America (5, 15.6%), and South America (2, 6.2%). Most studies relied on review of medical records including EEGs or clinical databases to establish the diagnosis of epilepsy, although a few relied on self-report or administrative data. Some studies did not distinguish between single seizures and epilepsy. Quality scores varied substantially from study to study, ranging from 0/9 to 8/8 (Supplemental Table 1, “Overview”)10 overall, but most studies had scores of greater than 5/8. Among population-based studies quality scores varied from 4/8 to 8/8. The most common limitations were the lack of a population-based design and confidence intervals for incidence and prevalence estimates.
Incidence
Eighteen studies reported the incidence of epilepsy to range from 0.65% to 5.97% after MS onset in adult-onset MS.5,13,15,18,21,22,25,28,30,33–36,38,39,41,42 In pediatric-onset MS the incidence of seizures was 5.98%.23 Among eight population-based studies the incidence ranged from 0.64% to 7.45% (Figure 1, Supplemental Table 1). Heterogeneity among these studies was substantial (I2 = 98.2). The summary estimate of incidence was 2.28% (95% CI: 1.11–3.44%). Heterogeneity did not improve when the early study published in 1986 was removed (I2 = 98.5), and the summary estimate did not change appreciably (2.44%; 95% CI: 1.17–3.71%).
Figure 1.
Forest plot of the incidence of epilepsy in multiple sclerosis in population-based studies.
Prevalence
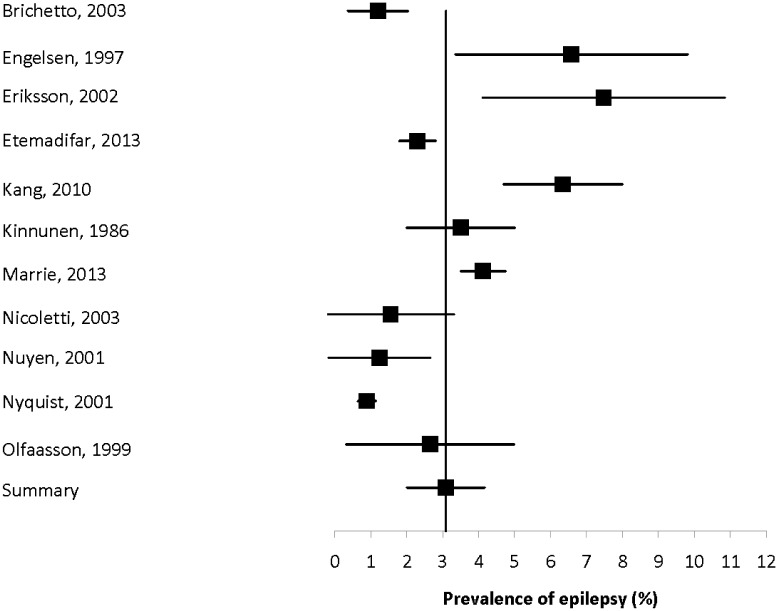
Twenty-four studies reported the prevalence of epilepsy to range from 0.89% to 8.06% (Supplemental Table 2).5,7,14–17,19–22,24–29,31,32,34–36,38,40,42 Among the 11 population-based studies the prevalence ranged from 0.89% to 7.48% (Figure 2). Heterogeneity among these studies was substantial (I2 = 93.9); the summary estimate of prevalence was 3.09% (95% CI: 2.01–4.16%). Heterogeneity did not improve when the early study published in 1986 was removed (I2 = 94.3), and the summary estimate did not change appreciably (3.04%; 95% CI: 1.91–4.18%).
Figure 2.
Forest plot of the prevalence of epilepsy in multiple sclerosis in population-based studies.
Comparisons
Four studies compared the incidence of epilepsy in the MS population with that in a comparator population (Supplemental Table 3).5,13,33,35 Of these, three studies found that the incidence of epilepsy was higher in the MS population while the fourth found no difference. Three studies compared the prevalence of epilepsy in the MS population with that in a comparator population.5,27,29 All found that the prevalence of epilepsy was higher in the MS population.
Sleep disorders
Search
We identified 144 unique citations (Supplemental Figure 2), of which 37 articles met the criteria for full-text review. Eighteen studies were the subject of this review.43–60
Study characteristics
The characteristics of the 18 included studies are shown in (Supplemental Table 4).43–60 The time periods when the studies were conducted ranged from 1989 to 2011. Most of the studies were conducted in Europe (8, 44.4%), followed by North America (6, 33.3%), South America (2, 11.1%) and Asia (2, 11.1%). The studies used questionnaires (7, 38.9%), clinician-conducted interviews (7, 38.9%), and polysomnography (3, 16.7%) to identify sleep disorders, generally in MS clinic populations. None of the identified studies reported the incidence of these disorders, and none of the studies were population-based. Quality scores ranged from 2/9 to 7/9.
Narcolepsy
Two studies reported the prevalence of narcolepsy to range from 0% to 1.6% (Supplemental Table 4).49,56
Periodic limb movements of sleep
One Italian study reported the prevalence of periodic limb movements of sleep to be 36% in an ambulatory MS population (Supplemental Table 5).46
Restless legs syndrome
Twelve studies reported the prevalence of restless legs syndrome to range from 14.4% to 57.5% (Supplemental Table 4).44,47–55,57,59,60 Although the prevalence estimates varied widely, all studies consistently used the same diagnostic criteria from the International Restless Legs Syndrome Study Group, and the exclusion criteria used were similar in seven of the 12 studies. Three of these studies were conducted in Italy by the same group of investigators, two involving the same center and one involving multiple centers.51–53
Seven studies compared the prevalence of restless legs syndrome in the MS population to a control population, and all found a higher prevalence of the syndrome in the MS population (Supplemental Table 5).44,46–50,52,57,60 However, one study reported that the prevalence of restless legs syndrome did not differ between the MS population and a rheumatoid arthritis population.60
REM behavior disorder
Two studies reported the prevalence of REM behavior disorder to range from 2.22% to 3.2% (Supplemental Table 5).48,49 The study with the lower estimate reported that this prevalence did not differ from the general population (0%), however the number of affected individuals in both populations was quite small (Supplemental Table 6).
Sleep apnea
Five studies reported the prevalence of sleep apnea to range from 7.14% to 58.1% (Supplemental Table 5).43,45,47,49,58 One of these studies used a questionnaire to identify those at high risk for obstructive sleep apnea (41.7%) but did not confirm this with polysomnography.45 The other four studies confirmed diagnoses of obstructive sleep apnea with polysomnography, but one did so in a selected population referred for clinical evaluation of sleep disorders, thus this does not represent the prevalence of OSA in the general MS population.43 This latter study also reported the prevalence of central sleep apnea to be 4.17%.
Discussion
We reviewed the world literature regarding the incidence and prevalence of seizure disorders and sleep disorders. The incidence and prevalence of seizure disorders were evaluated in a large number of studies, eight of which were population-based. We also identified a large number of studies regarding sleep disorders, but most of these focused on the prevalence of restless legs syndrome, followed by sleep apnea. Most of the studies on seizure disorders were well-designed. Quality of the studies of sleep disorders was more variable, but the approaches to evaluating the most commonly assessed sleep disorder, restless legs syndrome, were consistent across studies.
In population-based studies, we found that the incidence of seizure disorders was 2.28%, while the prevalence was 3.09%. This exceeded the expected incidence and prevalence in the general population. Several of these studies reported seizures to occur at the onset of MS, and described the occurrence of both partial and generalized seizures. The increased risk of seizures may reflect the effects of inflammation or glial reactions around demyelinating lesions, or the direct effects of demyelinating lesions. The location of the demyelinating lesions appears to be an important factor.24
Sleep disorders are common in the general population,61 and have many adverse effects on other aspects of health.62 Obstructive sleep apnea, for example, is associated with increased risks of hypertension, myocardial infarction, cerebrovascular disease, and daytime drowsiness. Persons with chronic medical conditions tend to be at increased risk of sleep disorders, and may be at risk of under-diagnosis.63 People with MS are more likely to report symptoms of disrupted or inadequate sleep,64 and poor sleep is associated with fatigue and reduced quality of life.65 Thus interest is growing in the role of sleep disorders in MS.49,65
In this review, we focused on specific sleep disorders but found that knowledge of the prevalence and incidence of these disorders remains limited. Only one study reported the prevalence of narcolepsy in a clinic population, while only two reported the prevalence of REM behavior disorder. We found a wide range of prevalence estimates for the best studied disorder, restless legs syndrome, reaching as high as 57.5%. In the absence of population-based studies, the true prevalence of this disorder in the MS population remains uncertain. While some studies suggested a higher prevalence in association with spinal cord disease,53 variation in prevalence across sociodemographic and clinical characteristics remains poorly studied. Findings were consistent with respect to the increased risk of restless legs syndrome in the MS population as compared to the general population; this may not differ from other chronic disease populations. Estimates for the prevalence of obstructive sleep apnea were quite high, but this may reflect selection bias as studies were conducted in clinic-based populations and sometimes in populations with a clinical indication for polysomnography. Population-based studies are needed.
This review suggests that seizure disorders and sleep disorders are common in MS, but also highlights important gaps that exist in the epidemiological knowledge of these conditions in MS worldwide. Other than central-western Europe and North America most world regions are understudied. Future studies could be improved by using population-based designs, reporting age, sex and ethnicity-specific estimates of incidence and prevalence, and by standardizing findings to a common population.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
Thanks to Tania Gottschalk, BA, MEd, MSc (Librarian, University of Manitoba), who provided assistance regarding the development of the search strategies for this review. This study was conducted under the auspices of the International Advisory Committee on Clinical Trials of New Drugs in Multiple Sclerosis whose members include Jeffrey Cohen, MD (Cleveland Clinic Foundation, Cleveland, United States), Laura J Balcer, MD, MSCE (NYU Langone Medical Center, New York City, United States), Brenda Banwell, MD (The Children’s Hospital of Philadelphia, Philadelphia, United States), Michel Clanet, MD (Federation de Neurologie, Toulouse, France), Giancarlo Comi, MD (University Vita-Salute San Raffaele, Milan, Italy), Gary R Cutter, PhD (University of Alabama at Birmingham, Birmingham, United States), Andrew D Goodman, MD (University of Rochester Medical Center, Rochester, United States), Hans-Peter Hartung, MD (Heinrich-Heine-University, Duesseldorf, DE), Bernhard Hemmer, MD (Technical University of Munich, Munich, DE), Catherine Lubetzki, MD, PhD (Fédération des maladies du système nerveux et INSERM 71, Paris, France), Fred D Lublin, MD (Mount Sinai School of Medicine, New York, United States), Ruth Ann Marrie, MD, PhD (Health Sciences Centre, Winnipeg, Canada), Aaron Miller, MD (Mount Sinai School of Medicine, New York, United States), David H Miller, MD (University College London, London, United Kingdom), Xavier Montalban, MD (Hospital Universitari Vall d’Hebron, Barcelona, Spain), Paul O’Connor, MD (St Michael’s Hospital, Toronto, Canada), Daniel Pelletier, MD (Yale University School of Medicine, New Haven, United States), Stephen C Reingold, PhD (Scientific & Clinical Review Assoc, LLC, Salisbury, United States), Alex Rovira Cañellas, MD (Hospital Universitari Vall d’Hebron, Barcelona, Spain), Per Soelberg Sørensen, MD, DMSc (Copenhagen University Hospital, Copenhagen, Denmark), Maria Pia Sormani, PhD (University of Genoa, Genoa, Italy), Olaf Stuve, MD, PhD (University of Texas Health Sciences Center, Dallas, United States), Alan J Thompson, MD (University College London, London, United Kingdom), Maria Trojano, MD (University of Bari, Bari, Italy), Bernard Uitdehaag, MD, PhD (VU University Medical Center, Amsterdam, Netherlands), Emmaunelle Waubant, MD, PhD (University of California-San Francisco, San Francisco, United States), and Jerry S Wolinsky, MD (University of Texas HSC, Houston, United States)
Footnotes
Conflict of interest: Ruth Ann Marrie receives research funding from: Canadian Institutes of Health Research, Public Health Agency of Canada, Manitoba Health Research Council, Health Sciences Centre Foundation, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Rx & D Health Research Foundation, and has conducted clinical trials funded by Sanofi-Aventis.
Nadia Reider reports no disclosures.
Olaf Stuve is an associate editor of JAMA Neurology, and he serves on the editorial boards of the Multiple Sclerosis Journal, Clinical and Experimental Immunology, and Therapeutic Advances in Neurological Disorders. He has participated in data and safety monitoring committees for Pfizer and Sanofi. Dr. Stuve has received grant support from Teva Pharmaceuticals.
Jeffrey Cohen reports personal compensation for consulting from EMD Serono, Genentech, Genzyme, Innate Immunotherapeutics, Novartis, and Vaccinex. Dr. Cohen receives research support paid to his institution from Biogen Idec, Consortium of MS Centers, US Department of Defense, Genzyme, US National Institutes of Health, National MS Society, Novartis, Receptos, Synthon, Teva, and Vaccinex.
Per Soelberg Sorensen has received personal compensation for serving on scientific advisory boards, steering committees, independent data monitoring boards in clinical trials, or speaking at scientific meetings from Biogen Idec, Merck Serono, Novartis, Genmab, TEVA, GSK, Genzyme, Bayer Schering, Sanofi-aventis, and MedDay Pharmaceuticals. His research unit has received research support from Biogen Idec, Merck Serono, TEVA, Sanofi-aventis, Novartis, RoFAR, Roche, and Genzyme.
Maria Trojano has served on scientific Advisory Boards for Biogen Idec, Novartis, and Merck Serono; has received speaker honoraria from Biogen-Idec, Sanofi Aventis, Merck-Serono, Tev,a and Novartis; has received research grants from Biogen-Idec, Merck-Serono, and Novartis.
Gary Cutter has served on scientific advisory boards for and/or received funding for travel from Innate Immunity, Klein-Buendel Incorporated, Genzyme, Medimmune, Novartis, Nuron Biotech, Spiniflex Pharmaceuticals, Somahlution, Teva Pharmaceuticals; receives royalties from publishing Evaluation of Health Promotion and Disease Prevention (The McGraw Hill Companies, 1984); has received honoraria from GlaxoSmithKline, Novartis, Advanced Health Media Inc., Biogen Idec, EMD Serono Inc., EDJ Associates, Inc., the National Heart, Lung, and Blood Institute, National Institute of Neurological Diseases and Stroke, National Marrow Donor Program, Consortium of Multiple Sclerosis Centers; Mt. Sinai School of Medicine and Teva Pharmaceuticals; has served on independent data and safety monitoring committees for Apotek, Ascendis, Biogen-Idec, Cleveland Clinic, Glaxo Smith Klein Pharmaceuticals, Gilead Pharmaceuticals, Modigenetech/Prolor, Merck/Ono Pharmaceuticals, Merck, Neuren, PCT Bio, Teva, Vivus, NHLBI (Protocol Review Committee), NINDS, NMSS, NICHD (OPRU oversight committee).
Stephen Reingold reports personal consulting fees from the National Multiple Sclerosis Society (NMSS) and the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), during the conduct of this work; and over the past three years, personal consulting fees from Bayer HealthCare, Biogen Idec, Coronado Biosciences Inc, the Cleveland Clinic Foundation, Eli Lilly & Company, from EMD Serono and Merck Serono, Genentech, F. Hoffmann-LaRoche, Ironwood Pharmaceuticals Inc, ISIS Pharmaceuticals Inc, Medimmune Inc, Novartis Pharmaceuticals Corporation, Observatoire Français de la Sclérosis en Plaques, Opexa Therapeutics, Sanofi-Aventis, SK Biopharmaceuticals, Synthon Pharmaceuticals Inc, TEVA Pharmaceutical Industries, and Fondation pour l’aide à la Recherche sur la Sclérosis en Plaques, for activities outside of the submitted work.
Funding: This study was supported (in part) by the National Multiple Sclerosis Society and a Don Paty Career Development Award from the MS Society of Canada.
Contributor Information
Ruth Ann Marrie, Department of Internal Medicine, University of Manitoba, Winnipeg, Canada/Department of Community Health Sciences, University of Manitoba, Winnipeg, Canada.
Nadia Reider, Department of Internal Medicine, University of Manitoba, Winnipeg, Canada.
Jeffrey Cohen, Mellen Center for MS Treatment and Research, Cleveland Clinic, Cleveland, OH, USA.
Maria Trojano, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari, Italy.
Per Soelberg Sorensen, Department of Neurology, Copenhagen University Hospital Rigshospitalet, Denmark.
Gary Cutter, Department of Biostatistics, University of Alabama at Birmingham, USA.
Stephen Reingold, Scientific and Clinical Review Associates, LLC, Salisbury, CT, USA.
Olaf Stuve, Department of Neurology and Neurotherapeutics, University of Texas Southwestern, Dallas, TX, USA.
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2002; 359: 1221–1231. [DOI] [PubMed] [Google Scholar]
- 2. Weinstock-Guttman B, Zivadinov R, Horakova D, et al. Lipid profiles are associated with lesion formation over 24 months in interferon-β treated patients following the first demyelinating event. J Neurol Neurosurg Psychiatry 2013; 84: 1186–1191. [DOI] [PubMed] [Google Scholar]
- 3. Warren SA, Turpin KV, Pohar SL, et al. Comorbidity and health-related quality of life in people with multiple sclerosis. Int J MS Care 2009; 11: 6–16. [Google Scholar]
- 4. Finlayson M, Preissner K, Cho C. Impact of comorbidity on fatigue management intervention outcomes among people with multiple sclerosis. Int J MS Care 2013; 15: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nicoletti A, Sofia V, Biondi R, et al. Epilepsy and multiple sclerosis in Sicily: a population-based study. Epilepsia 2003; 44: 1445–1448. [DOI] [PubMed] [Google Scholar]
- 6. Nicoletti A, Patti F, Fermo SL, et al. Headache and multiple sclerosis: a population-based case-control study in Catania, Sicily. Cephalalgia 2008; 28: 1163–1169. [DOI] [PubMed] [Google Scholar]
- 7. Krokki O, Bloigu R, Ansakorpi H, et al. Neurological comorbidity and survival in multiple sclerosis. Mult Scler Relat Disorders 2013; 3: 72–77. [DOI] [PubMed] [Google Scholar]
- 8. Villani V, Prosperini L, Pozzilli C, et al. Quality of life of multiple sclerosis patients with comorbid migraine. Neurol Sci 2011; 32: 149–151. [DOI] [PubMed] [Google Scholar]
- 9. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain 2013; 154: 632–642. [DOI] [PubMed] [Google Scholar]
- 10. Marrie R, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scle Vol 21: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neyeloff J, Fuchs S, Moreira L. Meta-analyses and forest plots using a Microsoft Excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cox DR. The continuity correction. Biometrika 1970; 57: 217–219. [Google Scholar]
- 13. Allen AN, Seminog OO, Goldacre MJ. Association between multiple sclerosis and epilepsy: large population-based record-linkage studies. BMC Neurol 2013; 13: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brichetto G, Uccelli MM, Mancardi GL, et al. Symptomatic medication use in multiple sclerosis. Mult Scler 2003; 9: 458–460. [DOI] [PubMed] [Google Scholar]
- 15. Catenoix H, Marignier R, Ritleng C, et al. Multiple sclerosis and epileptic seizures. Mult Scler 2011; 17: 96–102. [DOI] [PubMed] [Google Scholar]
- 16. Cendrowski W, Majkowski J. Epilepsy in multiple sclerosis. J Neurol Sci 1972; 17: 389–398. [DOI] [PubMed] [Google Scholar]
- 17. Cheng MY, Wai YY, Ro LS, et al. Seizures and multiple sclerosis in Chinese patients: a clinical and magnetic resonance imaging study. Epilepsy Res 2012; 101: 166–173. [DOI] [PubMed] [Google Scholar]
- 18. Drake WE, Jr, Macrae D. Epilepsy in multiple sclerosis. Neurology 1961; 11: 810–816. [DOI] [PubMed] [Google Scholar]
- 19. Durmus H, Kurtuncu M, Tuzun E, et al. Comparative clinical characteristics of early- and adult-onset multiple sclerosis patients with seizures. Acta Neurol Belg 2013; 113: 421–426. [DOI] [PubMed] [Google Scholar]
- 20. Engelsen BA, Gronning M. Epileptic seizures in patients with multiple sclerosis. Is the prognosis of epilepsy underestimated? Seizure 1997; 6: 377–382. [DOI] [PubMed] [Google Scholar]
- 21. Eriksson M, Ben-Menachem E, Andersen O. Epileptic seizures, cranial neuralgias and paroxysmal symptoms in remitting and progressive multiple sclerosis. Mult Scler 2002; 8: 495–499. [DOI] [PubMed] [Google Scholar]
- 22. Etemadifar M, Abtahi SH, Roomizadeh P. Epileptic seizures in multiple sclerosis: a population-based survey in Iran. Acta Neurol Belg 2013; 113: 271–278. [DOI] [PubMed] [Google Scholar]
- 23. Etemadifar M, Abtahi SH, Tabrizi N. Epileptic seizures in early-onset multiple sclerosis. Arch Iran Med 2012; 15: 381–383. [PubMed] [Google Scholar]
- 24. Gambardella A, Valentino P, Labate A, et al. Temporal lobe epilepsy as a unique manifestation of multiple sclerosis. Can J Neurol Sci 2003; 30: 228–232. [DOI] [PubMed] [Google Scholar]
- 25. Ghezzi A, Montanini R, Basso PF, et al. Epilepsy in multiple sclerosis. Eur Neurol 1990; 30: 218–223. [DOI] [PubMed] [Google Scholar]
- 26. Horton M, Rudick RA, Hara-Cleaver C, et al. Validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology 2010; 35: 83–90. [DOI] [PubMed] [Google Scholar]
- 27. Kang J-H, Chen Y-H, Lin H-C. Comorbidities amongst patients with multiple sclerosis: a population-based controlled study. Eur J Neurol 2010; 17: 1215–1219. [DOI] [PubMed] [Google Scholar]
- 28. Kinnunen E, Wikstrom J. Prevalence and prognosis of epilepsy in patients with multiple sclerosis. Epilepsia 1986; 27: 729–733. [DOI] [PubMed] [Google Scholar]
- 29. Marrie RA, Yu BN, Leung S, et al. The utility of administrative data for surveillance of comorbidity in multiple sclerosis: a validation study. Neuroepidemiology 2013; 40: 85–92. [DOI] [PubMed] [Google Scholar]
- 30. Martinez-Lapiscina EH, Ayuso T, Lacruz F, et al. Cortico-juxtacortical involvement increases risk of epileptic seizures in multiple sclerosis. Acta Neurol Scand 2013; 128: 24–31. [DOI] [PubMed] [Google Scholar]
- 31. Moreau T, Sochurkova D, Lemesle M, et al. Epilepsy in patients with multiple sclerosis: radiological–clinical correlations. Epilepsia 1998; 39: 893–896. [DOI] [PubMed] [Google Scholar]
- 32. Nuyen J, Schellevisa FG, Satarianob WA, et al. Comorbidity was associated with neurologic and psychiatric diseases: a general practice-based controlled study. J Clin Epidemiol 2006; 59: 1274–1284. [DOI] [PubMed] [Google Scholar]
- 33. Nyquist PA, Cascino GD, McClelland RL, et al. Incidence of seizures in patients with multiple sclerosis: a population-based study. Mayo Clinic Proc 2002; 77: 910–912. [DOI] [PubMed] [Google Scholar]
- 34. Nyquist PA, Cascino GD, Rodriguez M. Seizures in patients with multiple sclerosis seen at Mayo Clinic, Rochester, Minn, 1990–1998. Mayo Clin Proc 2001; 76: 983–986. [DOI] [PubMed] [Google Scholar]
- 35. Olafsson E, Benedikz J, Hauser WA. Risk of epilepsy in patients with multiple sclerosis: a population-based study in Iceland. Epilepsia 1999; 40: 745–747. [DOI] [PubMed] [Google Scholar]
- 36. Shaygannejad V, Ashtari F, Zare M, et al. Seizure characteristics in multiple sclerosis patients. J Res Med Sci 2013; 18: S74–S77. [PMC free article] [PubMed] [Google Scholar]
- 37. Shiraishi K, Higuchi Y, Ozawa K, et al. Clinical course and prognosis of 27 patients with childhood onset multiple sclerosis in Japan. Brain Dev 2005; 27: 224–227. [DOI] [PubMed] [Google Scholar]
- 38. Sokic DV, Stojsavljevic N, Drulovic J, et al. Seizures in multiple sclerosis. Epilepsia 2001; 42: 72–79. [DOI] [PubMed] [Google Scholar]
- 39. Striano P, Orefice G, Brescia Morra V, et al. Epileptic seizures in multiple sclerosis: clinical and EEG correlations. Neurol Sci 2003; 24: 322–328. [DOI] [PubMed] [Google Scholar]
- 40. Trouillas P, Courjon J. Epilepsy with multiple sclerosis. Epilepsia 1972; 13: 325–333. [DOI] [PubMed] [Google Scholar]
- 41. Uribe-San-Martin R, Ciampi-Diaz E, Suarez-Hernandez F, et al. Prevalence of epilepsy in a cohort of patients with multiple sclerosis. Seizure 2014; 23: 81–83. [DOI] [PubMed] [Google Scholar]
- 42. Viveiros CD, Alvarenga RM. Prevalence of epilepsy in a case series of multiple sclerosis patients. Arq Neuropsiquiatr 2010; 68: 731–736. [DOI] [PubMed] [Google Scholar]
- 43. Braley TJ, Segal BM, Chervin RD. Sleep-disordered breathing in multiple sclerosis. Neurology 2012; 79: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deriu M, Cossu G, Molari A, et al. Restless legs syndrome in multiple sclerosis: a case-control study. Mov Disorders 2009; 24: 697–701. [DOI] [PubMed] [Google Scholar]
- 45. Dias RA, Hardin KA, Rose H, et al. Sleepiness, fatigue, and risk of obstructive sleep apnea using the STOP-BANG questionnaire in multiple sclerosis: a pilot study. Sleep Breath. 2012; 16: 1255–65. [DOI] [PubMed] [Google Scholar]
- 46. Ferini-Strambi L, Filippi M, Martinelli V, et al. Nocturnal sleep study in multiple sclerosis: correlations with clinical and brain magnetic resonance imaging findings. J Neurol Sci 1994; 125: 194–197. [DOI] [PubMed] [Google Scholar]
- 47. Fragoso YD, Finkelsztejn A, Gomes S, et al. Restless legs syndrome and multiple sclerosis: a Brazilian multicenter study and meta-analysis of the literature. Arq Neuropsiquiatr 2011; 69: 180–183. [DOI] [PubMed] [Google Scholar]
- 48. Gomez-Choco M, Iranzo A, Blanco Y, et al. Prevalence of restless legs syndrome and REM sleep behavior disorder in multiple sclerosis. Mult Scler 2007; 13: 805–808. [DOI] [PubMed] [Google Scholar]
- 49. Kaminska M, Kimoff RJ, Benedetti A, et al. Obstructive sleep apnea is associated with fatigue in multiple sclerosis. Mult Scler 2012; 18: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 50. Li Y, Munger KL, Batool-Anwar S, et al. Association of multiple sclerosis with restless legs syndrome and other sleep disorders in women. Neurology 2012; 78: 1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manconi M, Fabbrini M, Bonanni E, et al. High prevalence of restless legs syndrome in multiple sclerosis. Eur J Neurol 2007; 14: 534–539. [DOI] [PubMed] [Google Scholar]
- 52. Manconi M, Ferini-Strambi L, Filippi M, et al. Multicenter case-control study on restless legs syndrome in multiple sclerosis: the REMS study. Sleep 2008; 31: 944–952. [PMC free article] [PubMed] [Google Scholar]
- 53. Manconi M, Rocca MA, Ferini-Strambi L, et al. Restless legs syndrome is a common finding in multiple sclerosis and correlates with cervical cord damage. Mult Scler 2008; 14: 86–93. [DOI] [PubMed] [Google Scholar]
- 54. Miri S, Rohani M, Sahraian MA, et al. Restless legs syndrome in Iranian patients with multiple sclerosis. Neurol Sci 2013; 34: 1105–1108. [DOI] [PubMed] [Google Scholar]
- 55. Moreira NC, Damasceno RS, Medeiros CA, et al. Restless leg syndrome, sleep quality and fatigue in multiple sclerosis patients. Braz J Med Biol Res. 2008; 41: 932–937. [DOI] [PubMed] [Google Scholar]
- 56. Poirier G, Montplaisir J, Dumont M, et al. Clinical and sleep laboratory study of narcoleptic symptoms in multiple sclerosis. Neurology 1987; 37: 693–695. [DOI] [PubMed] [Google Scholar]
- 57. Shaygannejad V, Ardestani PE, Ghasemi M, et al. Restless legs syndrome in Iranian multiple sclerosis patients: a case-control study. Int J Prev Med 2013; 4: S189–S193. [PMC free article] [PubMed] [Google Scholar]
- 58. Tachibana N, Howard RS, Hirsch NP, et al. Sleep problems in multiple sclerosis. Eur Neurol 1994; 34: 320–323. [DOI] [PubMed] [Google Scholar]
- 59. Vavrova J, Kemlink D, Sonka K, et al. Restless legs syndrome in Czech patients with multiple sclerosis: an epidemiological and genetic study. Sleep Med 2012; 13: 848–851. [DOI] [PubMed] [Google Scholar]
- 60. Auger C, Montplaisir J, Duquette P. Increased frequency of restless legs syndrome in a French-Canadian population with multiple sclerosis. Neurology 2005; 65: 1652–1653. [DOI] [PubMed] [Google Scholar]
- 61. Lamberg L. Sleep disorders, often unrecognized, complicate many physical illnesses. JAMA 2000; 284: 2173–2175. [DOI] [PubMed] [Google Scholar]
- 62. Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis 2009; 51: 285–293. [DOI] [PubMed] [Google Scholar]
- 63. Hiestand DM, Britz P, Goldman M, et al. Prevalence of symptoms and risk of sleep apnea in the US population: Results from the National Sleep Foundation Sleep in America 2005 Poll. Chest 2006; 130: 780–786. [DOI] [PubMed] [Google Scholar]
- 64. Bamer AM, Johnson KL, Amtmann D, et al. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler 2008; 14:1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lobentanz IS, Asenbaum S, Vass K, et al. Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurol Scand 2004; 110: 6–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.