Abstract
Background:
Studies of cancer incidence and prevalence in multiple sclerosis (MS) have produced conflicting results.
Objective:
To estimate the incidence and prevalence of cancer in persons with MS and review the quality of included studies.
Methods:
We searched the PUBMED, SCOPUS, Web of Knowledge, and EMBASE databases, conference proceedings, and reference lists of all articles retrieved. Abstracts were screened for relevance by two reviewers. Data from included articles were captured using a standardized form, and the abstraction was verified by a second reviewer. We assessed quality of the included studies. We quantitatively assessed studies using the I2 statistic, and conducted meta-analyses for population-based studies.
Results:
We identified 38 studies. Estimates for incidence and prevalence varied substantially for most cancers. In population-based studies, cervical, breast, and digestive cancers had the highest incidence. The risk of meningiomas and urinary system cancers appeared higher than expected, while the risks of pancreatic, ovarian, prostate and testicular cancer were lower than expected.Conclusion: The complexity of understanding cancer risk in MS is augmented by inconsistencies in study design, and the relative paucity of age, sex and ethnicity-specific risk estimates from which the strong impact of age on the incidence of cancers can be assessed.
Keywords: Multiple sclerosis, comorbidity, cancer, incidence, prevalence, systematic review
Introduction
The immune system plays an important role in multiple sclerosis (MS) and cancer,1 making it plausible that the risk of cancer is altered in MS. However, studies of cancer incidence and prevalence have produced conflicting results regarding the relative risk of cancer in MS.2,3 Uncertainty also exists regarding the risk of specific types of cancer.4,5 This issue is clinically relevant as disease-modifying therapies used to treat MS may increase cancer risk.6 Thus accurate estimates of background cancer risk are needed to fully evaluate the safety of such therapies.
The goals of this systematic review were to estimate the incidence and prevalence of cancer in MS based on the world literature, to evaluate the quality of studies included in the review, and in so doing to provide guidance for future studies in this area.
Methods
We conducted this review as part of a comprehensive study of the incidence and prevalence of comorbidity in MS worldwide. We have divided the studies into groups to allow detailed discussion of the findings. The methods of this study are delineated in detail elsewhere,7 and described briefly here.
Search strategy and study selection
As detailed elsewhere, we developed a search strategy for cancer that queried electronic databases of published literature as well as conference proceedings including PUBMED, EMBASE, SCOPUS, and Web of Knowledge for all years available through 4 November 2013 (see Supplemental Appendix I). We also conducted manual reviews of the reference lists of studies identified during electronic searches. After excluding duplicates, the abstracts were independently assessed by two reviewers (RAM, NR) to determine relevance. If either reviewer selected the abstract it underwent full-text review. This was again done independently to determine whether the studies met the inclusion criteria: (i) MS population; (ii) include original data; (iii) specify the cancer of interest; (iv) report incidence or prevalence of the cancer of interest; and (iv) English language publication. Disagreements were resolved by consensus.
Data extraction and study quality
The data for each article selected were abstracted using a standardized form. The findings were verified by the second reviewer. As delineated elsewhere, general study characteristics, incidence and prevalence estimates were captured. The cancers investigated varied from study to study. We describe the findings according to cancer site based on the schema used by the Surveillance, Epidemiology and End Results Program in the United States (http://www.seer.cancer.gov/manuals/2013/appendixc.html). Specifically, cancers were grouped as all cancers; brain and nervous system; breast; bones and joints; digestive system; endocrine system; eye and orbit; female genital system; male genital system; lymphoma, myeloma, hematopoietic or lymphatic cancer; oral cavity and larynx; respiratory system; skin; and urinary system.
Study quality was evaluated using a standardized assessment tool developed for a systematic review of the incidence and prevalence of MS.8 This involved yes or no responses to nine questions.7 This process supported a qualitative assessment of study heterogeneity.
Statistical analysis
In addition to a qualitative assessment of heterogeneity, we conducted a quantitative analysis of heterogeneity using the I2 test. We restricted the quantitative analysis to population-based studies. Some of the studies overlapped in their study populations, data sources and time periods. Therefore, when conducting the meta-analyses we selected solely one of any studies that overlapped, favoring the studies that validated the identification of the MS population, with the longest study periods. Random effects meta-analyses were conducted using a Microsoft Excel spreadsheet developed for this purpose.9 For studies in which zero events were recorded we employed a continuity correction of 0.5.10
Results
Cancer
Search
The search strategy identified 1323 unique citations (Supplemental Figure 1). After abstract screening and hand searching, 58 articles met the criteria for full-text review, of which we excluded 20. Thirty-eight studies were the subject of this review.2–6,11–44
Study characteristics
The studies conducted ranged from 1953 to 2010, and most were conducted in Europe (26, 68.4%), followed by North America (9, 23.7%) and Asia (3, 7.9%). Of the studies from Europe, 21 (80.8%) were conducted in the Nordic countries of Denmark, Norway, Sweden, or Finland. The most common study design involved linkage of administrative data sources (30, 78.9%), typically hospital registers, to cancer registries. In some studies clinical databases were linked to cancer registries. Although cancer registries have a high degree of accuracy in most jurisdictions, few studies using administrative data validated their approach to identifying the MS population or made efforts to identify persons with MS who were not hospitalized. Other common limitations included lack of a description of the target population (16, 42.1%) and lack of a population-based design (10, 26.3%). None of the studies provided confidence intervals for their incidence or prevalence estimates. Quality scores ranged from 2/9 to 7/8 overall, and from 4/8 to 7/8 among population-based studies (Supplemental Table 1, accompanying manuscript).7
Any cancer
Among the 11 studies that reported the incidence of cancer (Supplemental Table 1) over variable periods of follow-up and at varying times in the MS disease course,2,11–21 estimates ranged widely from 0.50% to 10.55%. Sex-specific estimates were reported in some studies, but age-specific estimates were rarely provided.18 Heterogeneity of the incidence estimates in the nine population-based studies was high (I2 = 99.8%).2,11,12,15,30,32,34,39,40 The summary estimate was 4.39% (95% CI: 2.67–6.1%) (Supplemental Figure 2).
When compared to the general population, cancer incidence was most often reported as lower (6) than in the MS population (Supplemental Table 2).
In thirteen studies, the prevalence of cancer ranged from 0.01% to 16.4% (Supplemental Table 3).5,6,14–18,29,32,36–38,42 Among five population-based studies of the prevalence of cancer in the MS population after diagnosis,6,18,21–23 heterogeneity was substantial (I2 = 90.8%). The summary estimate was 2.23% (95% CI: 1.18–3.29%) (Supplemental Figure 3).
Brain and nervous system
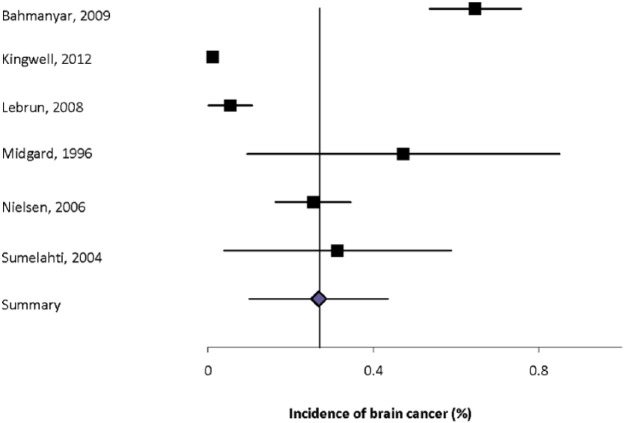
Eight studies reported the incidence of brain tumors (Supplemental Table 4).2,12,20,30,32,34,35,39 Two of these studies were national studies using the Swedish Cancer Registry with overlapping study periods, although the incidence estimates differed slightly.12,20 Two of the studies were Danish studies using the National Cancer Registry, but the MS population was identified in slightly different ways.2,35 The estimated incidence of brain tumors also differed in these studies. These differences highlight the impact of heterogeneity of study design on incidence estimates and the age mix of the patients and the standard population used to standardize the estimates for comparisons. Only two of the incidence studies provided details as to whether the tumors were benign or malignant. The incidence of any brain tumor ranged from 0.035% to 0.65%. Heterogeneity among the incidence estimates was high (I2 = 97.0%). The summary estimate was 0.27% (95%CI: 0.10-0.43%) (Figure 1).
Figure 1.
Forest plot of the incidence of brain cancer in multiple sclerosis in population-based studies.
Two studies reported the prevalence of brain tumors, with similar estimates of 0.17 to 0.18% despite substantial differences in the populations studied (Supplemental Table 5).13,16 Neither study was population-based. The first study population involved insured individuals of all ages, while the second involved hospitalized individuals over the age of 65. Details regarding the type of brain tumor were not provided in either study, nor were sex-specific estimates. The prevalence of brain tumors was the same in the MS and general populations in the insured population,24 while it was lower in the elderly MS population than in the elderly general population (Supplemental Table 6).25
Four of the incidence studies reported that the risk of developing a brain tumor did not differ statistically significantly between the MS and general populations.2,30,34,39 One study reported a reduced risk of brain tumors in the MS population.32 The other studies reported elevated risks of developing brain tumors.12,20,35 However, in one study that reported incidence rates by tumor type, this increased risk appeared to be largely driven by an increased risk of benign tumors (meningiomas) (Supplemental Table 6).26
Breast
Nine studies reported the incidence of breast cancer with estimates ranging from 0.98% to 3.59%, only one of which reported incidence in men (Supplemental Table 7).2,11,12,14,16,17,19,20,27 Heterogeneity among the six population-based estimates was high (I2 = 99.2%).2,11,12,14,16,20 The summary estimate was 1.64% (95% CI: 0.98–2.30%) (Figure 2).
Figure 2.

Forest plot of the incidence of breast cancer in multiple sclerosis in population-based studies.
Six studies reported the prevalence of breast cancer, with estimates ranging from 0.38% to 2.3% (Supplemental Table 8).4,21,24,25,28,29 The sole population-based study from the United States reported a prevalence of 2.01%.21
When compared to the general population, the incidence of breast cancer was variously reported to be the same (4 studies), increased (3), or reduced (2) in the MS population (Supplemental Table 9).2,11,12,22,30,34,35,39,40
Bones and joints
One study reported the prevalence of bone cancer to be 0.46% among hospitalized MS patients over age 65 years (Supplemental Table 10),25 and that this was lower than in hospitalized patients without MS (Supplemental Table 11). A Swedish study reported the incidence of bone cancer to be 0.01%, with a higher incidence in men than women (Supplemental Table 12).12 In this population-based study, the risk of bone cancer did not differ between the MS and general populations (Supplemental Table 11).
Digestive system
Nine studies reported the incidence of digestive system cancers (Supplemental Table 13).2,12,23,30–32,34,35,41 The incidence of digestive cancer overall ranged from 0.02% to 1.74%. Three of these studies used the Danish Cancer Registry, with two of them identifying MS patients using hospital records and one using the Danish MS Registry.2,17,30 Even among these studies incidence estimates varied widely for digestive cancers overall, from 0.02% to 1.52%. Two of the studies used the Swedish Cancer Registry, but reported their incidence estimates based on slightly different classifications of these cancers making comparisons difficult.12,23 Heterogeneity of the population-based estimates was high (I2 = 99.0%). The summary estimate was 1.05% (95% CI: 0.098–2.01%).
The incidence of esophageal cancer ranged from 0.0013% to 0.06% (I2 = 91.1), of stomach cancer 0.0004% to 0.17% (I2 = 97.0), of small intestinal cancer 0% to 0.08%, of large intestinal cancer (I2 = 99.6%), of liver cancer 0.00016% to 0.01%, and pancreatic cancer from 0.0004% to 0.16% (I2 = 96.4%). Summary estimates were 0.028% (95% CI: 0–0.084%) for esophageal cancer (Supplemental Figure 4), 0.082% (95% CI: 0–0.25%) for stomach cancer (Supplemental Figure 5), 0.61% (95% CI: 0–1.78%) for colorectal cancer (Supplemental Figure 6), 0.081% (95% CI: 0–0.22%) for pancreatic cancer (Supplemental Figure 7).
Five studies reported the prevalence of digestive system cancers; one was population-based (Supplemental Table 14).13,16,28,33,42 In one study the prevalence of esophageal cancer was 0.01%. The prevalence of stomach cancer ranged from 0.02% to 0.48% (two studies). The prevalence of liver cancer ranged from 0.02% to 0.31% (two studies). The prevalence of colorectal cancer ranged from 0% to 0.7%.
The incidence of digestive system cancers tended to be lower in the MS population than in the general population, particularly consistent for pancreatic cancer (Supplemental Table 15).2,12,14–17,30–32
Endocrine system
Only one study from Sweden reported the incidence of endocrine cancers in general (0.31%) (Supplemental Table 16).12 Studies reporting the incidence of thyroid cancer were conducted in Sweden, Canada (province of British Columbia), and Taiwan with findings ranging from 0.006% to 0.13% (Supplemental Table 16).12,14,20 Heterogeneity of these findings was substantial (I2 = 92.1%), and the summary estimate was 0.056% (95% CI: 0–0.12%) (Supplemental Figure 8).
One study reported the prevalence of thyroid cancer to be 0.14% in an insured MS population in the United States,24 while a second, population-based, study reported a prevalence of 0.48% (Supplemental Table 17).21
Of the three studies reporting the incidence of thyroid cancer, only one found an increased risk in the MS population as compared to the general population, but this finding was not statistically significant after adjustment for age, sex and comorbidities (Supplemental Table 18).20
Eye and orbit
Three studies reported the incidence of cancer occurring in the eye, with consistent estimates ranging from 0.03% to 0.04% (Supplemental Table 19).2,12,17 Two of these studies, however, were conducted in Denmark with similar study populations and study periods that overlapped somewhat. The first study restricted the analysis to individuals with MS based solely on hospital discharge records,17 while the other identified persons with confirmed diagnoses of MS based on the Danish MS Registry.2 Heterogeneity of the findings was low (I2 = 0), and the summary estimate was 0.033% (95% CI: 0.013–0.053%) (Supplemental Figure 9).
None of these studies found a difference in the risk of ocular cancer in the MS population as compared to the general population (Supplemental Table 20).
Female genital system
Eight studies reported the incidence of female genital cancers, six of which were conducted in Europe (Supplemental Table 21).2,12,22,30,32,34,35,40 Two of the studies were Danish studies using the National Cancer Registry, but the MS population was identified in slightly different ways.2,17 Two of the studies used the Swedish Cancer Registry but identified the MS population in slightly different ways.12,22 The study periods overlapped but reporting of cancer types differed.
The incidence of any female genital cancer was 0.60% to 1.39% (four studies) (Supplemental Table 21). The heterogeneity of these estimates was high (I2 = 95.7%). The summary estimate was 0.95% (95% CI: 0.11–1.80%). The incidence of cervical cancer ranged from 0.01% to 0.56% (five studies), while the incidence of endometrial cancer was 0.65% (one study), of ovarian cancer from 0.01% to 0.42% (three studies), and of uterine cancer from 0.06% to 0.70% (five studies) (Supplemental Table 21). Heterogeneity of all of these estimates was high: (I2 = 98.1% for cervical, 97.3% for ovarian, and 97.4% for uterine cancer). The summary estimate was 0.29% (95% CI: 0.0050–0.58%) for cervical cancer (Supplemental Figure 10), 0.24% (95% CI: 0–0.54%) for ovarian cancer (Supplemental Figure 11), and 0.29% (95% CI: 0.083–0.51%) for uterine cancer (Supplemental Figure 12).
Three American studies reported the prevalence of female genital cancers,13,16,42 one of which was population-based.21 The prevalence of cervical cancer was 0.05% to 0.67% (two studies), of ovarian cancer 0.13% to 1.34% (three studies), and uterine cancer 0.12% to 0.67% (three studies). The prevalence of vulvar cancer was 0.67% (Supplemental Table 22).21
Of the studies that compared the incidence of female genital cancer in the MS population and the general population, all but one found that the risk of cancer was the same or lower in the MS population (Supplemental Table 23).20 The risk of ovarian cancer in particular was reduced.
Male genital system
Seven studies reported the incidence of male genital cancers (Supplemental Table 24).2,12,30,34,35,39,43 Six of these studies were conducted in Scandinavia, and two of the studies used the Danish Cancer Registry during overlapping time periods.2,35 The incidence of prostate cancer ranged from 0.05% to 2.25%. Of note, the estimated incidence of prostate cancer was nearly twice high in one of the Danish studies as in the other study. Heterogeneity of the population-based prostate cancer estimates was high (I2 = 98.7%). The summary estimate was 0.94% (95% CI: 0.021–1.86%) (Supplemental Figure 13). The incidence of testicular cancer ranged from 0.01% to 0.10%. Heterogeneity among population-based estimates was substantial (I2 = 78.4%). The summary estimate was 0.055% (95% CI: 0–0.12%) (Supplemental Figure 14).
Two American studies reported the prevalence of male genital cancers.24,25 The prevalence of prostate cancer ranged from 0.58% to 0.77%. The estimated prevalence of testicular cancer was 0% (Supplemental Table 25).24
Among the incidence studies, the risks of prostate and testicular cancer were consistently lower in the MS population than in the general population, although some of the findings were not statistically significant (Supplemental Table 26).2,12,14,16,17,19,33
Lymphoma, myeloma, hematopoietic, or lymphatic cancer
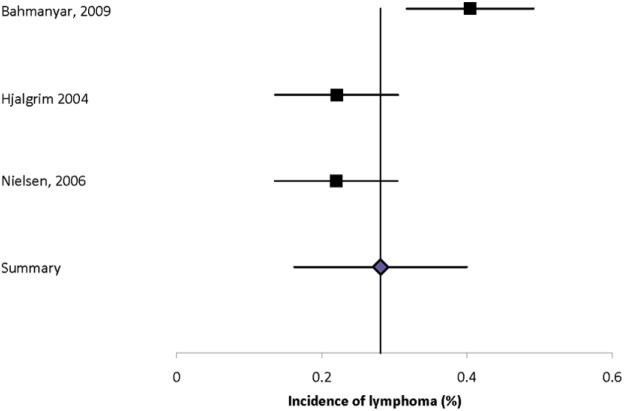
Malignancies evaluated included lymphoma, multiple myeloma, and leukemia. Nine studies reported the incidence of hematologic malignancies (Supplemental Table 27).2,12,15,17,19,27,34–36 The incidence of lymphoma (unspecified) was 0.40%, of myeloma 0.01% to 0.06%, and of leukemia 0.02% to 0.23%. Heterogeneity of the estimates for lymphoma was substantial (I2 = 82.7%) and the summary estimate was 0.28% (95% CI: 0.16–0.40%) (Figure 3).
Figure 3.
Forest plot of the incidence of lymphoma in multiple sclerosis in population-based studies.
Three studies evaluated the prevalence of hematologic malignancy (Supplemental Table 28);13,16,42 one was population-based.21 In one of these studies the prevalence of non-Hodgkin’s lymphoma was 0.12%, of Hodgkin’s lymphoma was 0.02%, of leukemia was 0.06%.35 In three studies the prevalence of multiple myeloma ranged from 0.02% to 0.97%.13,16,42 The population-based study had the highest estimate,21 but had a small sample size (n = 206) and did not link to a cancer registry, potentially reducing the accuracy of the cancer estimates.
Two studies report found no difference or non-significant increases in incidence of hematologic malignancy in the MS population while one reported a statistically significant decreased incidence (Supplemental Table 29).
Oral cavity and larynx
Four studies reported the incidence of such cancers (Supplemental Table 30).2,30,32,35 Two of these studies, however, were conducted in Denmark with similar study populations and study periods that overlapped somewhat, as noted for eye cancer.2,35 The incidence of laryngeal cancer was 0.01% (one study).14 The incidence of salivary gland cancer was 0.02% (one study).17 The incidence of nasopharyngeal cancer ranged from 0.06% to 0.02% (two Danish studies).2,35 The incidence of oral cancer ranged from 0.01% to 0.06% (two studies),30,35 but only one of these was truly population-based.
One study reported the prevalence of cancers affecting the oral cavity and larynx, with findings of 0.01% for laryngeal cancer, 0.03% for lip/oral cavity cancer, and 0.03% for pharyngeal cancer (Supplemental Table 31).24
The incidence of oral cancer did not differ between the MS and general populations (Supplemental Table 32).2,14,15,17
Respiratory system
Ten studies estimated the incidence of lung cancer (Supplemental Table 33).2,12,14–17,19,20,37,38 The incidence of lung cancer ranged from 0.04% to 0.80%. Heterogeneity of the population-based estimates was high (I2 = 94.8%), and the summary estimate was 0.12% (95% CI: 0.069–0.17%) (Supplementary Figure 15).
Five studies reported the prevalence of lung cancer in the MS population; one was population-based.13,16,28,33,42 The prevalence of lung cancer generally ranged from 0.14% to 0.50%. The prevalence in the population-based study was 0.48% (Supplemental Table 34).21
The incidence of lung cancer was reported to be lower in the MS population than in the general population in two studies from Denmark and Sweden,2,12 while the incidence did not differ between populations in seven studies. Only the study from Taiwan suggested that the risk of lung cancer was two-fold higher in the MS population (Supplemental Table 35).20
Skin
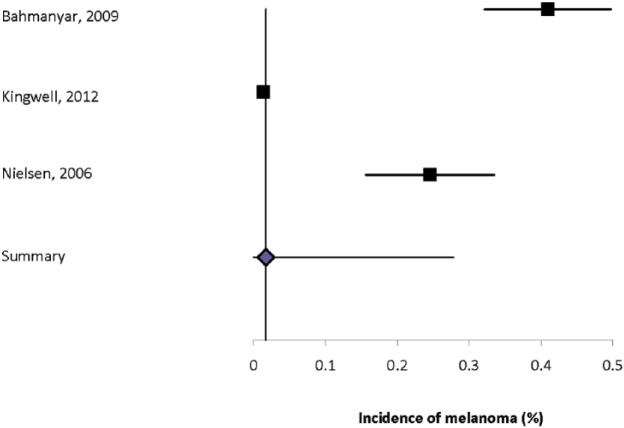
Six studies estimated the incidence of skin cancer, with findings ranging from 0.19% to 1.61% (Supplemental Table 36).2,12,30,32,34,35 All but one of the studies was conducted in Europe, and the study populations in the two Danish studies overlapped. The incidence of melanoma ranged from 0.01% to 0.41%, and of non-melanoma skin cancer was 0.08% to 1.36%. Heterogeneity of the incidence estimates was high (I2 = 98.6% for skin cancer overall, 98.0% for melanoma, 98.9% for non-melanoma). The summary incidence estimate for skin cancer was 0.61% (95% CI: 0.21–1.01%) (Supplemental Figure 16), for melanoma was 0.22% (95% CI: 0–0.41%) (Figure 4), and for non-melanoma skin cancer was 0.60% (95% CI: 0.14–1.06%) (Supplemental Figure 17).
Figure 4.
Forest plot of the incidence of melanoma in multiple sclerosis in population-based studies.
Four studies estimated that the prevalence of skin cancer varied from 0.16% to 3.5% (Supplemental Table 37).4,5,13,28 None of these studies was population-based. The prevalence of melanoma varied from 0.08% to 0.46%.
Two studies reported that the risk of skin cancer overall in the MS population did not differ from that in the general population. All five studies that evaluated the question reported that neither the incidence nor prevalence of melanoma did not differ between the MS and general populations,2,5,12,30,35 while one of the five studies reported that the risk of non-melanoma skin cancers was increased (Supplemental Table 38).
Urinary system
Seven studies reported the incidence of urinary system cancer (Supplemental Table 39).2,12,30,32,34,35,43 The incidence of urinary system cancer in general ranged from 0.09% to 0.77%, and of renal cancer from 0.01% to 0.34%, and of bladder cancer from 0.02% to 0.56%. Heterogeneity of the incidence estimates from population-based studies was high (I2 = 97.9% for urinary cancer, 93.4% for renal cancer, and 96.8% for bladder cancer). The summary estimate for urinary cancer was 0.41% (95% CI: 0.10–0.72%) (Supplemental Figure 18), for renal cancer was 0.16% (95% CI: 0–0.36%) (Supplemental Figure 19), and for bladder cancer was 0.28% (95% CI: 0–0.81%) (Figure 5).
Figure 5.
Forest plot of the incidence of bladder cancer in multiple sclerosis in population-based studies.
One study reported the prevalence of urinary system cancer.24 The prevalence of renal cancer was 0.05% and of bladder cancer was 0.10% (Supplemental Table 40).
The incidence of urinary system cancer was statistically significantly increased in one study and was increased without statistical significance in the other three studies that evaluated this question. The incidence of bladder cancer specifically was not statistically significantly increased in the two studies that evaluated this question (Supplemental Table 41).
Discussion
Thirty-eight studies evaluated the incidence or prevalence of cancer in MS. Based on population-based studies, the cancers with the highest incidence in the MS population are cervical, breast, and digestive system with the caveat that these comparisons are based on crude rates. On meta-analysis the incidence of any cancer was 4.39% but most of these studies were conducted in largely Caucasian populations. Worldwide, the most prevalent cancers affect the breast, colorectal, prostate, lung, and stomach,39 but among women the most prevalent cancers are breast, cervical, and thyroid. The burden of cancer varies by world region and ethnicity, thus the findings in the MS population are not directly comparable to those for the global distribution of cancer. Age-, sex-, and ethnicity-specific estimates, which were largely lacking, would facilitate such comparisons.
Overall the risk of any cancer was most often reported to be lower in MS than in the general population although findings were inconsistent. The case mix of MS patients by age and sex could impact the comparisons across studies as well as the standard used to compute adjusted rates. Conversely, the risk of brain tumors, particularly meningiomas, and possibly urinary system cancer appeared to be slightly higher than expected. This may reflect ascertainment bias due to more frequent referrals to urology or use of brain MRIs in the MS population. The risks of pancreatic, ovarian, prostate and testicular cancer were lower than expected. In the rheumatoid arthritis population the risk of hematologic malignancies and lung cancer is increased,37 while the risk of breast, ovarian and colorectal cancers is decreased.27 Similarly, the systemic lupus erythematosus population has an increased risk of hematologic malignancy and cancers of the lung and vulva,37 but reduced risks of breast, ovarian and endometrial cancers.40 The relationship between MS and the risk of cancer is complex, as observed in other chronic inflammatory diseases.41 Persistent inflammation may promote tumorigenesis. For example persons with inflammatory bowel disease have an increased risk of colorectal cancer.42 Chronic immunosuppression may also increase malignancy risk. However, immune-mediated disorders may have enhanced immunosurveillance due to activation of inflammatory cells, and consequently reduced tumorigenesis. Finally, since cancer incidence increases substantially by age for the major cancers, survival could be biasing comparisons via competing risks (e.g. due to early death to MS).
Limitations of this study included the focus on studies published in English, but this excluded few studies. To ensure a comprehensive assessment of the world literature we did not restrict the studies by time period or the diagnostic criteria employed for MS. This may have contributed to the high degree of heterogeneity observed. We did not evaluate the association of cancer incidence with the use of disease-modifying therapy as this was beyond the scope of this study, but this question warrants future evaluation. This would be facilitated by adoption of a common classification schema for cancer in all studies.
The complexity of understanding cancer risk in MS is augmented by inconsistencies in study design, and the relative paucity of age-, sex-, and ethnicity-specific risk estimates. Ultimately studies evaluating differences in the risk of cancer in the MS population as compared to the general population also need to fully consider common risk factors for cancer such as smoking, physical inactivity, diet, and exposure to immunosuppressive drug therapies. This information would allow us to understand the reasons for the differences in cancer risk observed, and possibly how to reduce cancer risk in the MS population. Finally, Kingwell et al. raised the important concern that patients with MS may experience diagnostic delays that lead to more advanced cancer at diagnosis.14 A European study suggested that survival in multiple myeloma is worse in the MS population.43 These potential disparities deserve immediate attention.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
Thanks to Tania Gottschalk, BA, MEd, MSc (Librarian, University of Manitoba), who provided assistance regarding the development of the search strategies for this review. This study was conducted under the auspices of the International Advisory Committee on Clinical Trials of New Drugs in Multiple Sclerosis whose members include Jeffrey Cohen, MD (Cleveland Clinic Foundation, Cleveland, United States), Laura J. Balcer, MD, MSCE (NYU Langone Medical Center, New York City, United States), Brenda Banwell, MD (The Children’s Hospital of Philadelphia, Philadelphia, United States), Michel Clanet, MD (Federation de Neurologie, Toulouse, France), Giancarlo Comi, MD (University Vita-Salute San Raffaele, Milan, Italy), Gary R Cutter, PhD (University of Alabama at Birmingham, Birmingham, United States), Andrew D Goodman, MD (University of Rochester Medical Center, Rochester, United States), Hans-Peter Hartung, MD (Heinrich-Heine-University, Duesseldorf, DE), Bernhard Hemmer, MD (Technical University of Munich, Munich, DE), Catherine Lubetzki, MD, PhD (Fédération des maladies du système nerveux et INSERM 71, Paris, France), Fred D. Lublin, MD (Mount Sinai School of Medicine, New York, United States), Ruth Ann Marrie, MD, PhD (Health Sciences Centre, Winnipeg, Canada), Aaron Miller, MD (Mount Sinai School of Medicine, New York, United States), David H Miller, MD (University College London, London, United Kingdom), Xavier Montalban, MD (Hospital Universitari Vall d’Hebron, Barcelona, Spain), Paul O’Connor, MD (St Michael’s Hospital, Toronto, Canada), Daniel Pelletier, MD (Yale University School of Medicine, New Haven, United States), Stephen C. Reingold, PhD (Scientific & Clinical Review Assoc, LLC, Salisbury, United States), Alex Rovira Cañellas, MD (Hospital Universitari Vall d’Hebron, Barcelona, Spain), Per Soelberg Sørensen, MD, DMSc (Copenhagen University Hospital, Copenhagen, Denmark), Maria Pia Sormani, PhD (University of Genoa, Genoa, Italy), Olaf Stuve, MD, PhD (University of Texas Health Sciences Center, Dallas, United States), Alan J Thompson, MD (University College London, London, United Kingdom), Maria Trojano, MD (University of Bari, Bari, Italy), Bernard Uitdehaag, MD, PhD (VU University Medical Center, Amsterdam, Netherlands), Emmaunelle Waubant, MD, PhD (University of California-San Francisco, San Francisco, United States), Jerry S Wolinsky, MD (University of Texas HSC, Houston, United States)
Footnotes
Conflict of interest: Ruth Ann Marrie receives research funding from: Canadian Institutes of Health Research, Public Health Agency of Canada, Manitoba Health Research Council, Health Sciences Centre Foundation, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Rx & D Health Research Foundation, and has conducted clinical trials funded by Sanofi-Aventis.
Nadia Reider reports no disclosures.
Olaf Stuve is an associate editor of JAMA Neurology, and he serves on the editorial boards of the Multiple Sclerosis Journal, Clinical and Experimental Immunology, and Therapeutic Advances in Neurological Disorders. He has participated in data and safety monitoring committees for Pfizer and Sanofi. Dr. Stuve has received grant support from Teva Pharmaceuticals.
Jeffrey Cohen reports personal compensation for consulting from EMD Serono, Genentech, Genzyme, Innate Immunotherapeutics, Novartis, and Vaccinex. Dr. Cohen receives research support paid to his institution from Biogen Idec, Consortium of MS Centers, US Department of Defense, Genzyme, US National Institutes of Health, National MS Society, Novartis, Receptos, Synthon, Teva, and Vaccinex.
Per Soelberg Sorensen has received personal compensation for serving on scientific advisory boards, steering committees, independent data monitoring boards in clinical trials, or speaking at scientific meetings from Biogen Idec, Merck Serono, Novartis, Genmab, TEVA, GSK, Genzyme, Bayer Schering, Sanofi-aventis, and MedDay Pharmaceuticals. His research unit has received research support from Biogen Idec, Merck Serono, TEVA, Sanofi-aventis, Novartis, RoFAR, Roche, and Genzyme.
Stephen Reingold reports personal consulting fees from the National Multiple Sclerosis Society (NMSS) and the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), during the conduct of this work; and over the past three years, personal consulting fees from Bayer HealthCare, Biogen Idec, Coronado Biosciences Inc, the Cleveland Clinic Foundation, Eli Lilly & Company, from EMD Serono and Merck Serono, Genentech, F. Hoffmann-LaRoche, Ironwood Pharmaceuticals Inc, ISIS Pharmaceuticals Inc, Medimmune Inc, Novartis Pharmaceuticals Corporation, Observatoire Français de la Sclérosis en Plaques, Opexa Therapeutics, Sanofi-Aventis, SK Biopharmaceuticals, Synthon Pharmaceuticals Inc, TEVA Pharmaceutical Industries, and Fondation pour l’aide à la Recherche sur la Sclérosis en Plaques, for activities outside of the submitted work.
Maria Trojano has served on scientific Advisory Boards for Biogen Idec, Novartis, and Merck Serono; has received speaker honoraria from Biogen-Idec, Sanofi Aventis, Merck-Serono, Teva and Novartis; has received research grants from Biogen-Idec, Merck-Serono, and Novartis.
Gary Cutter has served on scientific advisory boards for and/or received funding for travel from Innate immunity, Klein-Buendel Incorporated, Genzyme, Medimmune, Novartis, Nuron Biotech, Spiniflex Pharmaceuticals, Somahlution, Teva Pharmaceuticals; receives royalties from publishing Evaluation of Health Promotion and Disease Prevention (The McGraw Hill Companies, 1984); has received honoraria from GlaxoSmithKline, Novartis, Advanced Health Media Inc., Biogen Idec, EMD Serono Inc., EDJ Associates, Inc., the National Heart, Lung, and Blood Institute, National Institute of Neurological Diseases and Stroke, National Marrow Donor Program, Consortium of Multiple Sclerosis Centers; Mt. Sinai School of Medicine and Teva Pharmaceuticals; has served on independent data and safety monitoring committees for Apotek, Ascendis, Biogen-Idec, Cleveland Clinic, Glaxo Smith Klein Pharmaceuticals, Gilead Pharmaceuticals, Modigenetech/Prolor, Merck/Ono Pharmaceuticals, Merck, Neuren, PCT Bio, Teva, Vivus, NHLBI (Protocol Review Committee), NINDS, NMSS, NICHD (OPRU oversight committee).
Funding: This work was supported (in part) by the National Multiple Sclerosis Society and a Don Paty Career Development Award from the MS Society of Canada.
Contributor Information
Ruth Ann Marrie, Department of Internal Medicine, University of Manitoba, Winnipeg, Canada/Department of Community Health Sciences, University of Manitoba, Winnipeg, Canada.
Nadia Reider, Department of Internal Medicine, University of Manitoba, Winnipeg, Canada.
Jeffrey Cohen, Mellen Center for MS Treatment and Research, Cleveland Clinic, Cleveland, OH, USA.
Olaf Stuve, Department of Neurology and Neurotherapeutics, University of Texas Southwestern, Dallas, TX, USA.
Maria Trojano, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari, Italy.
Per Soelberg Sorensen, Department of Neurology, Copenhagen University Hospital Rigshospitalet, Denmark.
Stephen C Reingold, Scientific and Clinical Review Associates, LLC, Salisbury, CT, USA.
Gary Cutter, Department of Biostatistics, University of Alabama at Birmingham, USA.
References
- 1. Cools N, Ponsaerts P, Van Tendeloo VF, et al. Regulatory T cells and human disease. Clin Dev Immunol 2007: 89195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nielsen NM, Rostgaard K, Rasmussen S, et al. Cancer risk among patients with multiple sclerosis: a population-based register study. Int J Cancer 2006; 118: 979–984. [DOI] [PubMed] [Google Scholar]
- 3. Söderberg KC, Jonsson F, Winqvist O, et al. Autoimmune diseases, asthma and risk of haematological malignancies: a nationwide case-control study in Sweden. Eur J Cancer. 2006; 42: 3028–3033. [DOI] [PubMed] [Google Scholar]
- 4. Edwards LJ, Constantinescu CS. A prospective study of conditions associated with multiple sclerosis in a cohort of 658 consecutive outpatients attending a multiple sclerosis clinic. Mult Scler 2004; 10: 575–581. [DOI] [PubMed] [Google Scholar]
- 5. Goldacre MJ, Seagroatt V, Yeates D, et al. Skin cancer in people with multiple sclerosis: a record linkage study. J Epidemiol Community Health 2004; 58: 142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lebrun C, Vermersch P, Brassat D, et al. Cancer and multiple sclerosis in the era of disease-modifying treatments. J Neurol 2011; 258: 1304–1311. [DOI] [PubMed] [Google Scholar]
- 7. Marrie R, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. 2015; 21(3): 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans C, Beland S, Kulaga S, et al. Incidence and prevalence of multiple sclerosis in the Americas: a systematic review. Neuroepidemiology 2013; 40: 195–210. [DOI] [PubMed] [Google Scholar]
- 9. Neyeloff J, Fuchs S, Moreira L. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cox DR. The continuity correction. Biometrika. 1970; 57: 217–9. [Google Scholar]
- 11. Achiron A, Barak Y, Gail M, et al. Cancer incidence in multiple sclerosis and effects of immunomodulatory treatments. Breast Cancer Res Treat 2005; 89: 265–270. [DOI] [PubMed] [Google Scholar]
- 12. Bahmanyar S, Montgomery SM, Hillert J, et al. Cancer risk among patients with multiple sclerosis and their parents. Neurology 2009; 72: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 13. Christiansen CF, Christensen S, Farkas DK, et al. Risk of arterial cardiovascular diseases in patients with multiple sclerosis: a population-based cohort study. Neuroepidemiology 2010; 35: 267–274. [DOI] [PubMed] [Google Scholar]
- 14. Kingwell E, Bajdik C, Phillips N, et al. Cancer risk in multiple sclerosis: findings from British Columbia, Canada. Brain 2012; 135: 2973–2979. [DOI] [PubMed] [Google Scholar]
- 15. Lebrun C, Debouverie M, Vermersch P, et al. Cancer risk and impact of disease-modifying treatments in patients with multiple sclerosis. Mult Scler 2008; 14: 399–405. [DOI] [PubMed] [Google Scholar]
- 16. Midgard R, Glattre E, Gronning M, et al. Multiple sclerosis and cancer in Norway. A retrospective cohort study. Acta Neurol Scand 1996; 93: 411–415. [DOI] [PubMed] [Google Scholar]
- 17. Moller H, Kneller RW, Boice JD, Jr, et al. Cancer incidence following hospitalization for multiple sclerosis in Denmark. Acta Neurol Scand 1991; 84: 214–220. [DOI] [PubMed] [Google Scholar]
- 18. Palo J, Duchesne J, Wikstrom J. Malignant diseases among patients with multiple sclerosis. J Neurol 1977; 216: 217–222. [DOI] [PubMed] [Google Scholar]
- 19. Sumelahti M-L, Pukkala E, Hakama M. Cancer incidence in multiple sclerosis: a 35-year follow-up. Neuroepidemiology 2004; 23: 224–227. [DOI] [PubMed] [Google Scholar]
- 20. Sun LM, Lin CL, Chung CJ, et al. Increased breast cancer risk for patients with multiple sclerosis: a nationwide population-based cohort study. Eur J Neurol 2014; 21: 238–244. [DOI] [PubMed] [Google Scholar]
- 21. Wynn DR, Rodriguez M, O’Fallon WM, et al. A reappraisal of the epidemiology of multiple sclerosis in Olmsted County, Minnesota. Neurology 1990; 40: 780–786. [DOI] [PubMed] [Google Scholar]
- 22. Kang J-H, Chen Y-H, Lin H-C. Comorbidities amongst patients with multiple sclerosis: a population-based controlled study. Eur J Neurology 2010; 17: 1215–1219. [DOI] [PubMed] [Google Scholar]
- 23. Nuyen J, Schellevisa FG, Satarianob WA, et al. Comorbidity was associated with neurologic and psychiatric diseases: a general practice-based controlled study. J Clin Epidemiol 2006; 59: 1274–1284. [DOI] [PubMed] [Google Scholar]
- 24. Bloomgren G, Sperling B, Cushing K, et al. Assessment of malignancy risk in patients with multiple sclerosis treated with intramuscular interferon beta-1a: retrospective evaluation using a health insurance claims database and postmarketing surveillance data. Ther Clin Risk Manage 2012; 8: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fleming ST, Blake RL., Jr. Patterns of comorbidity in elderly patients with multiple sclerosis. J Clin Epidemiol 1994; 47: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 26. Hemminki K, Liu X, Forsti A, et al. Subsequent brain tumors in patients with autoimmune disease. Neuro Oncol 2013; 15: 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hemminki K, Liu X, Ji J, Forsti A, et al. Effect of autoimmune diseases on risk and survival in female cancers. Gynecol Oncol 2012; 127: 180–185. [DOI] [PubMed] [Google Scholar]
- 28. Horton M, Rudick RA, Hara-Cleaver C, et al. Validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology 2010; 35: 83–90. [DOI] [PubMed] [Google Scholar]
- 29. Marrie RA, Horwitz R, Cutter G, et al. Comorbidity, socioeconomic status, and multiple sclerosis. Mult Scler 2008; 14: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 30. Sunesen KG, Norgaard M, Thorlacius-Ussing O, et al. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978–2005. Int J Cancer 2010; 127: 675–684. [DOI] [PubMed] [Google Scholar]
- 31. Hemminki K, Liu X, Ji J, Sundquist J, et al. Autoimmune disease and subsequent digestive tract cancer by histology. Ann Oncol 2012; 23: 927–933. [DOI] [PubMed] [Google Scholar]
- 32. Landgren AM, Landgren O, Gridley G, et al. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer 2011; 117: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu X, Ji J, Forsti A, Sundquist K, et al. Autoimmune disease and subsequent urological cancer. J Urol 2013; 189: 2262–2268. [DOI] [PubMed] [Google Scholar]
- 34. Hemminki K, Liu X, Försti A, et al. Subsequent leukaemia in autoimmune disease patients. Br J Haematol 2013; 161: 677–687. [DOI] [PubMed] [Google Scholar]
- 35. Hjalgrim H, Rasmussen S, Rostgaard K, et al. Familial clustering of Hodgkin lymphoma and multiple sclerosis. J Natl Cancer Inst 2004; 96: 780–784. [DOI] [PubMed] [Google Scholar]
- 36. Koshiol J, Lam TK, Gridley G, et al. Racial differences in chronic immune stimulatory conditions and risk of non-Hodgkin’s lymphoma in veterans from the United States. J Clin Oncol 2011; 29: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hemminki K, Liu X, Ji J, et al. Effect of autoimmune diseases on risk and survival in histology-specific lung cancer. Eur Respir J 2012; 40: 1489–1495. [DOI] [PubMed] [Google Scholar]
- 38. Hemminki K, Liu X, Ji J, et al. Subsequent COPD and lung cancer in patients with autoimmune disease. Eur Respir J 2011; 37: 463–465. [DOI] [PubMed] [Google Scholar]
- 39. Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013; 132: 1133–1145. [DOI] [PubMed] [Google Scholar]
- 40. Bernatsky S, Ramsey-Goldman R, Labrecque J, et al. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J Autoimmun 2013; 42: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beyaert R, Beaugerie L, Van Assche G, et al. Cancer risk in immune-mediated inflammatory diseases (IMID). Mol Cancer 2013; 12: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jussila A, Virta LJ, Pukkala E, et al. Malignancies in patients with inflammatory bowel disease: a nationwide register study in Finland. Scand J Gastroenterol 2013; 12: 1405–1413. [DOI] [PubMed] [Google Scholar]
- 43. Hemminki K, Liu X, Forsti A, et al. Effect of autoimmune diseases on incidence and survival in subsequent multiple myeloma. J Hematol Oncol 2012; 5: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.