Abstract
Purpose
Neurotoxic effects of brain irradiation include cognitive impairment in 50% to 90% of patients. Prior studies have suggested that donepezil, a neurotransmitter modulator, may improve cognitive function.
Patients and Methods
A total of 198 adult brain tumor survivors ≥ 6 months after partial- or whole-brain irradiation were randomly assigned to receive a single daily dose (5 mg for 6 weeks, 10 mg for 18 weeks) of donepezil or placebo. A cognitive test battery assessing memory, attention, language, visuomotor, verbal fluency, and executive functions was administered before random assignment and at 12 and 24 weeks. A cognitive composite score (primary outcome) and individual cognitive domains were evaluated.
Results
Of this mostly middle-age, married, non-Hispanic white sample, 66% had primary brain tumors, 27% had brain metastases, and 8% underwent prophylactic cranial irradiation. After 24 weeks of treatment, the composite scores did not differ significantly between groups (P = .48); however, significant differences favoring donepezil were observed for memory (recognition, P = .027; discrimination, P = .007) and motor speed and dexterity (P = .016). Significant interactions between pretreatment cognitive function and treatment were found for cognitive composite (P = .01), immediate recall (P = .05), delayed recall (P = .004), attention (P = .01), visuomotor skills (P = .02), and motor speed and dexterity (P < .001), with the benefits of donepezil greater for those who were more cognitively impaired before study treatment.
Conclusion
Treatment with donepezil did not significantly improve the overall composite score, but it did result in modest improvements in several cognitive functions, especially among patients with greater pretreatment impairments.
INTRODUCTION
Approximately 20% to 40% of the 1,665,450 newly diagnosed patients with cancer in the United States in 2014 will develop brain metastases, and at least 200,000 will likely undergo whole-brain irradiation (WBI). Another approximately 15,000 will undergo partial (PBI) or WBI for treatment of a primary brain tumor.1
Neurotoxic effects of ionizing brain irradiation can include cognitive impairment, leukoencephalopathy, vasculopathies, and secondary neoplasms.2,3 Cognitive impairment associated with primary or metastatic brain tumors and their treatments, including radiation therapy, occurs in 50% to 90% of patients and can have an adverse impact on patients' functioning and quality of life.4–6
Cranial irradiation adversely affects the hippocampus, important in learning, memory, and mood regulation. Animal studies have demonstrated that irradiation of brain cells adversely affects neurochemical and morphologic markers for cholinergic neurons (eg, acetylcholine esterase)7 and blocks the formation of new neurons in the dentate gyrus of the hippocampus, which is associated with impaired performance on short-term memory tasks.8 In clinical studies, hippocampal-sparing cranial radiation therapy results in less memory impairment compared with standard techniques.9 Thus, adverse effects of radiation therapy on neurons in the hippocampus may explain some of the cognitive impairment and mood disturbances commonly reported by patients with brain tumors who undergo cranial irradiation and provide a rationale for the use of cholinergic neurotransmitter modulators in the treatment of radiation-associated neurocognitive symptoms.
In a recent Radiation Therapy Oncology Group study, adult patients with metastatic brain tumors undergoing a course of fractionated WBI who were concurrently treated for 24 weeks with a daily dose of memantine 20 mg showed less decline in memory and a longer time to cognitive decline compared with patients receiving placebo.10 Shaw et al11 reported positive results in an open-label phase II study of 34 adult patients with primary and metastatic brain tumors who had undergone a course of PBI or WBI ≥ 30 Gy at least 6 months before enrollment and who received donepezil (5 mg per day for 6 weeks followed by 10 mg per day for 18 weeks) for 24 weeks. They observed significant improvements in cognitive functioning (attention, concentration, memory, and verbal fluency), self-reported cognitive problems, mood, fatigue, and quality of life.11 Although these results are encouraging, the lack of a control group leaves open the possibility that the observed improvements may be attributable to nontreatment effects (eg, test-taking practice effects, statistical regression).
Donepezil hydrochloride is a piperdine derivative that reversibly inhibits acetylcholine esterase; it is highly selective for acetylcholine esterase and well tolerated.12 Donepezil has demonstrated efficacy in those with moderate to severe Alzheimer's dementia.13,14 Donepezil has also improved cognitive functioning in patients with Parkinson's disease,15 multiple sclerosis,16 and traumatic brain injury17 as well as in healthy young adults.18 In addition to the known direct effects on neuronal function, donepezil also increases cerebral perfusion in brain regions critical to cognitive processing.19
We tested whether 24 weeks of treatment with donepezil improved cognitive functioning in adult brain tumor survivors who had completed a course of either PBI or WBI ≥ 6 months before enrollment compared with placebo. Secondary end points included improvement in specific cognitive functions, fatigue, and quality of life. The protocol was approved by the National Cancer Institute and the Wake Forest University Health Sciences Institutional Review Board.
PATIENTS AND METHODS
Sample
Participants included adult (age ≥ 18 years) primary or metastatic brain tumor survivors who had completed a course of fractionated PBI or WBI of at least 30 Gy ≥ 6 months before enrollment, had no imaging evidence of disease progression within 3 months before enrollment, had a life expectancy > 6 months, had an Eastern Cooperative Oncology Group score ≥ 2, were not using cognition-enhancing medications or undergoing or planning to undergo therapies except hormonal treatments or herceptin for the next 6 months, and were not pregnant. Participants were enrolled at two academic medical centers (Wake Forest University Baptist Medical Center and MD Anderson Cancer Center), 21 community clinical oncology programs (CCOPs) affiliated with the National Cancer Institute–approved Wake Forest CCOP Research Base, and three cancer trial support unit sites.
Design
This was a randomized, double-blind, placebo-controlled clinical trial in which eligible participants were assigned with equal probability to receive a single daily 5-mg dose of donepezil for 6 weeks, which was escalated to 10 mg per day for 18 weeks if well tolerated, or matching placebo (Fig 1). Drug and placebo were overencapsulated and distributed to the study sites by Biologics (Raleigh, NC). Participants were administered the outcome measures before random assignment, 12 weeks random assignment, and 24 weeks random assignment, when active treatment was terminated.
Fig 1.
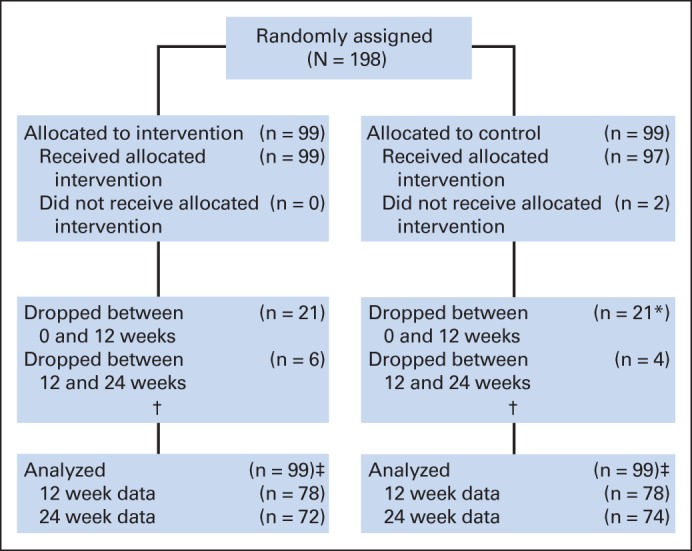
CONSORT diagram. (*) Including two who did not receive intervention. (†) Additional nine patients receiving donepezil and four patients receiving placebo discontinued therapy before 24 weeks but remained in study and completed questionnaires. (‡) All patients were included in some analyses (eg, all patients used in imputation models); those with post–random assignment data (n = 156) were used in primary analyses (repeated measures mixed models).
Measures
Verbal learning and memory were assessed with the Hopkins Verbal Learning Test–Revised (HVLT-R).20 HVLT-R variables included learning (total recall, sum of three learning trials; score range, 0 to 36), memory (delayed recall [DR], trial four; score range, 0 to 12), percent savings ([DR ÷ highest of last two learning trials] × 100; score range, 0-100), recognition memory (true positives [TPs]; score range, 0 to 12), and discrimination (TPs minus false positives; score range, −12 to 12). The modified Rey-Osterreith Complex Figure (mROCF)21 assessed visuomotor skills (mROCF copy; score range, 0 to 24), immediate figural recall (score range, 0 to 24), and delayed figural recall (score range, 0 to 24). The Trail Making Test Parts A (TMT-A) and B (TMT-B)22 assessed attention (TMT-A) and executive function (TMT-B). Verbal fluency was assessed with the Controlled Oral Word Association Test.23 Concentration and working memory were measured with the Digit Span Test (DST),24 with forward (score range, 0 to 14), backward (score range, 0 to 14), and total scores (forward plus backward; score range, 0 to 28). Motor speed and dexterity were measured with the Grooved Pegboard25 for the dominant (GP-D) and nondominant hands. A summary cognitive composite score was computed by standardizing (z score) eight individual test scores representing the major cognitive domains (HVLT-R total recall, HVLT-R DR, mROCF delayed figural recall, DST total, Controlled Oral Word Association Test, TMT-A, TMT-B, and GP-D) using pretreatment overall means and standard deviations. The negatives of the TMT-A, TMT-B, and GP-D standardized scores were used in calculating the composite scores. In addition, log transformations were used for TMT-A and TMT-B scores (before standardizing) because of skewness in the original distributions.
Analysis
The primary objective of this randomized trial was to assess the effect of donepezil on overall cognitive performance after 24 weeks of therapy. We also assessed the effect of donepezil on specific cognitive functions. Patients were stratified by accruing site (academic centers v CCOP sites) and type of irradiation (whole v partial) and assigned within strata to receive donepezil or placebo with equal probability using variably sized permuted-block randomization. The study was powered to detect a 0.23-unit difference in overall objective cognitive function between the two groups, with 90% power at the 5% two-sided level of significance, assuming a standard deviation for the overall cognitive score of 0.82 and a pre–post correlation of 0.87, based on data collected in an earlier pilot study.11 We planned for one interim analysis (after 67 patients had been observed for 24 weeks) using a two-stage group sequential design that was intermediate to the designs of Pocock26 and O'Brien-Fleming27 in its degree of conservativeness.28 The trial would be stopped and the null hypothesis rejected if the two-sided P value comparing the two arms were < .0128. Otherwise, the study would continue and the null hypothesis would be rejected at the final analysis if the two-sided P value were < .0434. With an expected dropout rate of approximately 35%, the required sample size was 100 patients per group. Dropouts were fewer than anticipated, and accrual was stopped after 198 participants.
The χ2, Fisher's exact, and Wilcoxon rank sum tests were used to assess pretreatment group differences in categorical and continuous variables as well as differences between those patients who did and did not complete the study. A repeated measures mixed-effect model was used to assess treatment differences in cognitive function and obtain least squares estimates of the measures over time. An unstructured covariance matrix was used to model the correlation in outcomes over time. Initially, unadjusted models were fitted for each outcome, which included treatment group, time, strata, and pretreatment level of the outcomes. The primary interest was in the effect of donepezil on cognitive function at 24 weeks, and this effect was assessed using a linear contrast within the mixed model. Separate adjusted models were then fitted, which also included the following covariates: age (years), time since diagnosis (years), race (non-Hispanic white v other), sex, and education (≤ high school, some college, or ≥ college). Because we had some dropout (resulting in mostly monotone missing data), multiple imputation was used to assess the sensitivity of our results to the mixed model missing at random assumption. To be conservative, we assumed that all dropouts would follow a pattern similar to that seen among the control patients.29 One hundred data sets were generated using the SAS MI procedure, a repeated measures mixed model was run on each data set, and results were combined using the SAS MIANALYZE procedure (SAS Institute, Cary, NC).30 In addition, covariates related to missing data were included in adjusted mixed models.
The primary outcome was the cognitive composite score. All participants who provided data were included in the analyses, even those who were never treated and those who stopped treatment prematurely. The imputation analyses included all patients, even those with only pretreatment data. Secondary analyses included group comparisons for individual cognitive parameters. Each outcome was assessed at the .05 level of significance; we did not adjust for multiple tests. Pre- and post-treatment (week 24) scores are presented.
RESULTS
Pretreatment Characteristics of Participants
Characteristics for study participants are listed in Table 1. The sample consisted of mostly middle-age, married, non-Hispanic whites with some post–high school education. Most participants had Eastern Cooperative Oncology Group scores of 0 to 1; 66% had primary brain tumors, and 27% had brain metastases; 8% had undergone prophylactic cranial irradiation. The time since diagnosis ranged from 7 to 423 months, with a median of 38 months. Pretreatment patient characteristics did not differ significantly between groups.
Table 1.
Demographic and Clinical Variables by Treatment Group
| Characteristic | Donepezil Group (n = 99) |
Control Group (n = 99) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 56 | 54 | ||
| Range | 19 to 84 | 19 to 81 | ||
| ≥ 50 | 58 | 59 | 61 | 62 |
| Months since diagnosis | ||||
| Median | 37.7 | 39.9 | ||
| Range | 7.3 to 298.4 | 8.8 to 423.2 | ||
| ≥ 36 | 51 | 52 | 55 | 56 |
| BMI, kg/m2 | ||||
| Median | 27.2 | 27.9 | ||
| Range | 17.3 to 49.4 | 18.4 to 41.1 | ||
| < 25 (underweight to normal) | 36 | 36 | 28 | 28 |
| 25 to 30 (overweight) | 31 | 31 | 36 | 36 |
| ≥ 30 (obese) | 32 | 32 | 35 | 35 |
| Strata | ||||
| WBI, WFU | 10 | 10 | 10 | 10 |
| WBI, CCOP | 30 | 30 | 30 | 30 |
| PBI, WFU | 30 | 30 | 30 | 30 |
| PBI, CCOP | 29 | 29 | 29 | 29 |
| ECOG performance status | ||||
| 0 | 49 | 49 | 45 | 45 |
| 1 | 46 | 46 | 48 | 48 |
| 2 | 4 | 4 | 6 | 6 |
| Sex | ||||
| Female | 56 | 57 | 50 | 51 |
| Male | 43 | 43 | 49 | 49 |
| Race | ||||
| Hispanic | 1 | 1 | 0 | 0 |
| Asian | 1 | 1 | 0 | 0 |
| Black | 7 | 7 | 9 | 9 |
| White | 90 | 91 | 90 | 91 |
| Marital status* | ||||
| Single | 12 | 12 | 10 | 10 |
| Married or married-like | 66 | 67 | 73 | 74 |
| Separated, divorced, or widowed | 21 | 21 | 15 | 15 |
| Education* | ||||
| ≤ High school | 29 | 29 | 33 | 34 |
| Vocational or some college | 39 | 39 | 40 | 42 |
| ≥ College graduate | 31 | 31 | 23 | 24 |
| Income, US$* | ||||
| < 20,000 | 31 | 36 | 34 | 42 |
| 20,000 to 50,000 | 31 | 36 | 27 | 33 |
| ≥ 50,000 | 24 | 28 | 20 | 25 |
| Work outside home* | 28 | 28 | 31 | 32 |
| Diagnosis | ||||
| Primary brain tumor | 65 | 66 | 65 | 66 |
| Brain metastasis | 27 | 27 | 26 | 26 |
| PCI | 7 | 7 | 8 | 8 |
| Primary tumor type | n = 65 | n = 65 | ||
| Glioblastoma multiforme | 15 | 23 | 8 | 12 |
| Anaplastic astrocytoma | 4 | 6 | 10 | 15 |
| Anaplastic oligodendroglioma | 8 | 12 | 8 | 12 |
| Anaplastic oligoastrocytoma | 2 | 3 | 1 | 2 |
| Anaplastic ependymoma | 3 | 5 | 1 | 2 |
| Anaplastic mixed glioma | 0 | 0 | 1 | 2 |
| Low-grade astrocytoma | 5 | 8 | 1 | 2 |
| Low-grade oligodendroglioma | 5 | 8 | 8 | 12 |
| Low-grade oligoastrocytoma | 0 | 0 | 1 | 2 |
| Meningioma | 13 | 20 | 9 | 14 |
| Pilocycstic astrocytoma | 2 | 3 | 4 | 6 |
| Other | 8 | 12 | 13 | 20 |
| Metastasis site | n = 34 | n = 34 | ||
| Lung | 19 | 57 | 21 | 62 |
| Breast | 9 | 27 | 7 | 21 |
| Other or unknown | 6 | 18 | 6 | 18 |
| Brain volume, cm3* | ||||
| Median | 1,104 | 1,135 | ||
| Range | 865 to 1,421 | 901 to 1,624 | ||
| ≥ 1,200 | 22 | 22 | 30 | 32 |
| CSF volume, cm3* | ||||
| Median | 211 | 204 | ||
| Range | 101 to 376 | 101 to 367 | ||
| ≥ 200 | 54 | 55 | 48 | 51 |
| Brain or intracranial volume, %* | ||||
| Median | 84.0 | 84.5 | ||
| Range | 74.9 to 91.1 | 74.8 to 92.0 | ||
| Lesion or resection volume, cm3* | ||||
| 0 | 9 | 9 | 14 | 15 |
| < 15 | 64 | 65 | 59 | 61 |
| ≥ 15 | 25 | 26 | 23 | 24 |
| Hippocampus involvement* | 13 | 13 | 10 | 10 |
| Location* | ||||
| Frontal | 29 | 32 | 33 | 39 |
| Parietal | 17 | 19 | 11 | 13 |
| Occipital | 6 | 7 | 5 | 6 |
| Temporal | 19 | 21 | 14 | 16 |
| Basal ganglia | 7 | 8 | 8 | 9 |
| Cerebellum | 9 | 10 | 8 | 9 |
| Brainstem or spinal cord | 3 | 3 | 6 | 7 |
Abbreviations: BMI, body mass index; CCOP, community clinical oncology program; ECOG, Eastern Cooperative Oncology Group; PBI, partial-brain irradiation; PCI, —prophylactic cranial irradiation; WBI, whole-brain irradiation; WFU, Wake Forest University.
Some missing data.
Overall study retention at 24 weeks was 74% and did not differ between groups (P = .75; Appendix Table A1, online only). Self-reported adherence to treatment (mean percent ideal dose) was 92% for participants receiving donepezil and 91% for those receiving placebo (P = .73). Donepezil was well tolerated; the most common toxicity reported was fatigue (donepezil, 58%; placebo, 67%; P = .24), and only diarrhea was significantly different between groups (donepezil, 25%; placebo, 9%; P = .005). Of the 153 patients who returned diaries and remained in the study longer than 6 weeks, only four (3%) did not have their dose escalated.
To characterize the level of cognitive impairment before study treatment in our sample, we calculated the difference between individual test means and mean scores from noncancer normative comparison groups, with the mean difference expressed in standard deviation units (z scores; Table 2). Impairment severity varied across cognitive tests but was worse than the respective comparison group for almost all test parameters. The greatest impairment was observed for verbal memory (HVLT-R), motor speed and dexterity (Grooved Pegboard), attention (TMT-A), and executive function (TMT-B). For each cognitive test, scores ranged from worse than to better than the comparison group, indicating heterogeneity within the sample.
Table 2.
Medians and IQRs for Cognitive Variables at Enrollment
| Variable | No. | Median | IQR |
|
|---|---|---|---|---|
| Q1 | Q3 | |||
| HVLT-R total | 198 | −1.60 | −2.49 | −0.60 |
| HVLT-R delayed recall | 198 | −1.65 | −2.86 | −0.46 |
| HVLT-R percent savings | 198 | −0.95 | −2.50 | 0.04 |
| HVLT-R recognition | 198 | −0.62 | −2.87 | 0.44 |
| HVLT-R discrimination | 198 | −0.61 | −1.97 | 0.14 |
| COWA | 198 | −1.12 | −1.92 | −0.22 |
| DST total | 198 | −0.33 | −1.00 | 0.33 |
| mROCF copy | 197 | −0.14 | −1.10 | 0.81 |
| mROCF immediate recall | 197 | 0.23 | −0.71 | 0.96 |
| mROCF delayed recall | 197 | 0.17 | −0.98 | 0.90 |
| TMT-A | 198 | −1.03 | −2.48 | 0.18 |
| TMT-B | 198 | −2.62 | −5.84 | −0.73 |
| GP dominant | 194 | −3.13 | −5.59 | −1.22 |
| GP nondominant | 194 | −2.86 | −6.88 | −1.24 |
Abbreviations: COWA, Controlled Oral Word Association Test; DST, Digit Span Test; GP, Grooved Pegboard; HVLT-R, Hopkins Verbal Learning Test–Revised; IQR, interquartile range; mROCF, modified Rey-Osterreith Complex Figure; TMT-A, Trail Making Test Part A; TMT-B, Trail Making Test Part B.
Treatment Effects on Cognitive Functioning
Table 3 lists the sample raw scores before study treatment and least squares mean scores (SEs) by group for all cognitive variables after 24 weeks of treatment. Cognitive scores improved over the course of the study in both groups. After 24 weeks of treatment, the cognitive composite scores did not differ significantly between groups during the interim analysis (P = .83) or at the end of the study (P = .48), indicating no overall cognitive benefit of treatment. Significant group differences favoring donepezil were observed for recognition memory (HVLT-R discrimination, P = .007; HVLT-R TP, P = .027) and motor speed and dexterity (GP-D, P = .016). Participants who dropped out of the study were similar to those who remained in the study regarding most characteristics. On average, they were less educated (P = .006) and had lower incomes (P = .019), smaller brain volume (P = .012), and more hippocampal involvement (P = .048). Mixed models run with and without those variables (except income, which was highly related to education and was missing for 16% of participants) produced identical conclusions regarding the treatment effect. The group differences for HVLT-R discrimination and GP-D remained marginally significant when analyses were repeated using multiple imputation assuming all dropouts followed the pattern seen in the control group (P = .046 and .055, respectively). The group difference in HVLT-R TP was reduced from 0.57 to 0.46, with a P value of .090. Groups were not significantly different for other cognitive variables.
Table 3.
Pretreatment Raw Means and Post-Treatment (24 weeks) LS Means for Cognitive Function Scores by Treatment Group
| Measure | Pretreatment |
Post-Treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donepezil Group |
Control Group |
Difference | 95% CI | P† | |||||||
| Mean | SD | No.* | LS Mean | SE | No.* | LS Mean | SE | ||||
| Cognitive composite | 0.00 | 0.73 | 72 | 0.22 | 0.04 | 72 | 0.19 | 0.04 | 0.03 | −0.06 to 0.13 | .484 |
| HVLT-R total | 20.29 | 6.42 | 72 | 22.48 | 0.45 | 73 | 22.16 | 0.45 | 0.32 | −0.93 to 1.56 | .617 |
| HVLT-R delayed recall | 6.23 | 3.47 | 72 | 7.11 | 0.25 | 73 | 6.76 | 0.25 | 0.35 | −0.36 to 1.06 | .334 |
| HVLT-R percent savings | 71.16 | 33.27 | 72 | 78.54 | 3.02 | 73 | 74.58 | 3.02 | 3.95 | −4.48 to 12.39 | .356 |
| HVLT-R recognition | 10.32 | 1.86 | 72 | 10.91 | 0.18 | 73 | 10.34 | 0.18 | 0.57 | 0.07 to 1.07 | .027 |
| HVLT-R discrimination | 9.35 | 2.34 | 72 | 10.10 | 0.24 | 73 | 9.16 | 0.24 | 0.94 | 0.26 to 1.62 | .007 |
| COWA | 29.97 | 12.96 | 72 | 32.27 | 0.82 | 73 | 33.19 | 0.82 | −0.92 | −3.21 to 1.37 | .428 |
| DST forward | 9.56 | 2.52 | 72 | 10.33 | 0.23 | 73 | 10.27 | 0.22 | 0.06 | −0.56 to 0.68 | .851 |
| DST backward | 5.46 | 2.32 | 72 | 5.60 | 0.22 | 73 | 6.00 | 0.22 | −0.39 | −1.01 to 0.22 | .210 |
| DST total | 15.02 | 4.29 | 72 | 15.46 | 0.36 | 73 | 15.62 | 0.35 | −0.16 | −1.16 to 0.83 | .744 |
| mROCF copy | 21.07 | 3.41 | 72 | 20.30 | 0.34 | 72 | 20.99 | 0.34 | −0.70 | −1.64 to 0.25 | .146 |
| mROCF immediate recall | 15.55 | 5.66 | 72 | 17.19 | 0.36 | 72 | 17.69 | 0.36 | −0.50 | −1.50 to 0.51 | .331 |
| mROCF delayed recall | 14.65 | 5.76 | 72 | 16.83 | 0.40 | 71 | 17.22 | 0.40 | −0.39 | −1.50 to 0.72 | .485 |
| TMT-A | 50.70 | 31.32 | 72 | 51.38 | 3.50 | 73 | 53.20 | 3.49 | −1.82 | −11.57 to 7.92 | .713 |
| TMT-B | 144.80 | 88.84 | 72 | 136.74 | 6.30 | 71 | 135.13 | 6.39 | 1.62 | −16.09 to 19.32 | .857 |
| GP dominant | 117.40 | 55.10 | 71 | 105.06 | 3.42 | 70 | 116.99 | 3.49 | −11.92 | −21.58 to −2.27 | .016 |
| GP nondominant | 131.16 | 59.08 | 71 | 127.25 | 3.03 | 71 | 127.26 | 3.02 | −0.01 | −8.45 to 8.42 | .997 |
Abbreviations: COWA, Controlled Oral Word Association Test; DST, Digit Span Test; GP, Grooved Pegboard; HVLT-R, Hopkins Verbal Learning Test–Revised; LS, least squares; mROCF, modified Rey-Osterreith Complex Figure; SD, standard deviation; TMT-A, Trail Making Test Part A; TMT-B, Trail Making Test Part B.
No. with 24-week measurements.
Unadjusted for multiple comparisons.
Given the heterogeneity of cognitive impairment before treatment in our sample, we examined whether treatment effects varied by pretreatment cognitive function level. Significant interaction effects were found for the cognitive composite score (P = .01) and immediate recall (HVLT-R immediate recall, P = .05), delayed recall (HVLT-R percent savings, P = .004), attention (DST forward, P = .01), visuomotor skills (mROCF copy, P = .02), and motor speed and dexterity (GP-D, P < .001). For all interactions except on mROCF copy, as pretreatment cognitive performance level worsened, the donepezil group tended to outperform controls.
DISCUSSION
This randomized placebo-controlled clinical trial assessed the effect of a daily dose of donepezil on cognitive function among long-term adult brain tumor survivors after a course of fractionated WBI or PBI. By the end of study treatment, both groups had performed comparably on a composite measure of overall cognitive functioning. However, examination of group differences for individual cognitive domains revealed a significant but modest benefit of donepezil compared with placebo for memory and motor speed and dexterity. Significant interactions were found between pretreatment cognitive function and treatment for overall cognitive functioning, memory, working memory, motor speed and dexterity, and executive function, indicating that the benefit from donepezil increased as the pretreatment level of cognitive impairment increased. This suggests that treatment with a daily dose of donepezil can provide benefit to some adult long-term brain tumor survivors after PBI or WBI, particularly those with greater pretreatment cognitive impairment.
Results from this study were consistent with those reported by Shaw et al11 in their open-label phase II study of donepezil, which used the same neurocognitive battery, dose regimen of donepezil, and patient population as our study. They found significant pre- to post-treatment improvement in cognitive function after 24 weeks of donepezil treatment. A key difference between the Shaw et al study and ours was our inclusion of a placebo control group. In our study, the improvement in cognitive function occurred in both groups, suggesting that some of the improvements in cognitive functioning in the Shaw et al study might have resulted from practice effects or statistical regression.
Like Shaw et al,11 we enrolled patients regardless of their cognitive functioning. The level of cognitive impairment in our participants relative to noncancer normative groups was quite high; 91% (181 of 198) of participants had at least one test score ≥ 1.5 standard deviations worse than the normal comparison group before study treatment, and 97% (192 of 198) had at least one score ≥ 1.0 standard deviation worse. Interestingly, we had wide variability of scores on most measures, ranging from significantly worse than to better than noncancer comparison groups. This heterogeneity may help to explain why we did not find more robust treatment effects. When we examined whether pretreatment cognitive performance level interacted with treatment, we observed improvement in performance favoring the donepezil group as pretreatment cognitive impairment worsened. This indicates that brain tumors and their treatments, including cranial irradiation, are associated with clinically significant cognitive impairment among some but not all patients. In future studies, demonstrable cognitive impairment should be an inclusion criterion for enrollment.
Correa et al31 reported that patients with low-grade glioma treated with radiation therapy or chemotherapy exhibited deficits in motor speed and executive function compared with untreated patients and that over a 12-month follow-up period, there was no significant improvement. We too found motor speed and dexterity assessed with the same test were impaired at enrollment, but patients treated with donepezil improved significantly more than untreated controls.
Brown et al10 tested whether 24 weeks of memantine, a glutamanergic N-methyl-D-aspartate receptor antagonist affecting cortical and hippocampal neurons, reduced cognitive impairment of patients with brain metastases undergoing WBI compared with placebo. Similar to our study, they found no overall cognitive benefit after 24 weeks of treatment with memantine, but they did observe a modest benefit in memory. Thus, neurotransmitter regulators like memantine and donepezil, which are used widely in the treatment of primary degenerative and vascular dementias,32 can provide some benefit to brain tumor survivors during and after brain irradiation treatment.
There are several limitations to our study. The choices of donepezil dose and duration of treatment were made based on studies of patients with Alzheimer's dementia.32 Greater benefit might have occurred with a higher dose of donepezil or longer treatment duration. In a recent international study, donepezil 23 mg per day was associated with significantly greater cognitive benefit than donepezil 10 mg per day in patients with moderate to severe Alzheimer's dementia.33 This study was not powered to examine differences between patients with different underlying disease properties, tumor types, or tumor locations. The measured cognitive deficits could have resulted from radiation therapy effects, tumor effects, surgery, chemotherapy, or premorbid factors. The impact of the measured benefit on overall quality of life was uncertain, and an analysis of quality-of-life measures is planned. Finally, multiple cognitive outcomes were assessed, which increases the likelihood of false-positive findings. However, the results are consistent in that it was memory and motor speed and dexterity that were improved with donepezil.
The strengths of our study include its size, reasonable retention and adherence rates, geographic diversity of the sample, and use of well-validated cognitive measures.
In conclusion, neurotransmitter regulators like the reversible acetylcholine esterase inhibitor donepezil can play a role in treating cognitive impairment associated with brain cancer and its treatments. In our study, we found that treatment with donepezil did not result in an overall improvement in cognitive function. However, there were modest improvements in several key cognitive functions, especially among patients with greater pretreatment cognitive impairment. With survivorship improving, the identification of effective treatments for the consequences of having and treating primary and metastatic brain tumors is crucial for the protection of patients' quality of life.
Supplementary Material
Appendix
Table A1.
Demographic and Clinical Variables by Completion Status
| Characteristic | Dropped Out (n = 52) |
Completed (n = 146) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Median | 55 | 55 | |||
| Range | 19 to 83 | 20 to 84 | |||
| ≥ 50 | 32 | 62 | 87 | 60 | |
| Months since diagnosis | |||||
| Median | 34.8 | 39.7 | |||
| Range | 8.8 to 228.0 | 7.3 to 423.2 | |||
| ≥ 36 | 26 | 50 | 80 | 55 | |
| BMI, kg/m2 | |||||
| Median | 28.5 | 27.1 | |||
| Range | 17.7 to 38.9 | 17.3 to 49.4 | |||
| < 25 (underweight to normal) | 14 | 27 | 50 | 34 | |
| 25 to 30 (overweight) | 19 | 37 | 48 | 33 | |
| ≥ 30 (obese) | 19 | 37 | 48 | 33 | |
| Strata | |||||
| WBI, WFU | 4 | 8 | 16 | 11 | |
| WBI, CCOP | 20 | 38 | 40 | 27 | |
| PBI, WFU | 14 | 27 | 46 | 32 | |
| PBI, CCOP | 14 | 27 | 44 | 30 | |
| ECOG performance status | |||||
| 0 | 22 | 42 | 72 | 49 | |
| 1 | 25 | 48 | 69 | 47 | |
| 2 | 5 | 10 | 5 | 3 | |
| Sex | |||||
| Female | 33 | 63 | 73 | 50 | |
| Male | 19 | 37 | 73 | 50 | |
| Race | |||||
| Hispanic | 0 | 0 | 1 | 1 | |
| Asian | 1 | 2 | 0 | 0 | |
| Black | 5 | 10 | 11 | 8 | |
| White | 46 | 88 | 90 | 91 | |
| Marital status* | |||||
| Single | 9 | 18 | 13 | 9 | |
| Married or married-like | 34 | 67 | 105 | 72 | |
| Separated, divorced, or widowed | 8 | 16 | 28 | 19 | |
| Education* | .006 | ||||
| ≤ High school | 24 | 48 | 38 | 26 | |
| Vocational or some college | 19 | 38 | 60 | 41 | |
| ≥ College graduate | 7 | 14 | 47 | 32 | |
| Income, US$* | .019 | ||||
| < 20,000 | 23 | 51 | 42 | 34 | |
| 20,000 to 50,000 | 17 | 38 | 41 | 34 | |
| ≥ 50,000 | 5 | 11 | 39 | 32 | |
| Work outside home* | 14 | 27 | 45 | 31 | |
| Diagnosis | |||||
| Primary brain tumor | 31 | 60 | 99 | 68 | |
| Brain metastasis | 15 | 29 | 38 | 26 | |
| PCI | 6 | 12 | 9 | 6 | |
| Primary tumor type | n = 31 | n = 99 | |||
| Glioblastoma multiforme | 4 | 13 | 19 | 19 | |
| Anaplastic astrocytoma | 3 | 10 | 11 | 11 | |
| Anaplastic oligodendroglioma | 6 | 19 | 10 | 10 | |
| Anaplastic oligoastrocytoma | 1 | 3 | 2 | 2 | |
| Anaplastic ependymoma | 2 | 6 | 2 | 2 | |
| Anaplastic mixed glioma | 0 | 0 | 1 | 1 | |
| Low-grade astrocytoma | 1 | 3 | 5 | 5 | |
| Low-grade oligodendroglioma | 0 | 0 | 13 | 13 | |
| Low-grade oligoastrocytoma | 0 | 0 | 1 | 1 | |
| Meningioma | 7 | 23 | 15 | 15 | |
| Pilocycstic astrocytoma | 2 | 6 | 4 | 4 | |
| Other | 5 | 16 | 16 | 16 | |
| Metastasis site | n = 21 | n = 47 | |||
| Lung | 11 | 52 | 29 | 62 | |
| Breast | 8 | 38 | 8 | 17 | |
| Other or unknown | 2 | 10 | 10 | 21 | |
| Brain volume, cm3* | |||||
| Median | 1,086 | 1,144 | |||
| Range | 865 to 1,382 | 901 to 1,624 | |||
| ≥ 1,200 | 8 | 16 | 44 | 31 | |
| CSF volume, cm3* | |||||
| Median | 213 | 206 | |||
| Range | 122 to 315 | 101 to 376 | |||
| ≥ 200 | 27 | 53 | 75 | 53 | |
| Brain or intracranial volume, %* | .012 (continuous); .035 (dichotomous) | ||||
| Median | 83.4 | 84.5 | |||
| Range | 78.1 to 89.5 | 74.8 to 92.0 | |||
| Lesion or resection volume, cm3* | |||||
| 0 | 7 | 14 | 16 | 11 | |
| < 15 | 36 | 71 | 87 | 61 | |
| ≥ 15 | 8 | 16 | 40 | 28 | |
| Hippocampus involvement* | 10 | 19 | 13 | 9 | .048 |
| Location* | |||||
| Frontal | 16 | 36 | 46 | 35 | |
| Parietal | 4 | 9 | 24 | 18 | |
| Occipital | 5 | 11 | 6 | 5 | |
| Temporal | 6 | 13 | 27 | 21 | |
| Basal ganglia | 4 | 9 | 11 | 8 | |
| Cerebellum | 7 | 16 | 10 | 8 | |
| Brainstem or spinal cord | 3 | 7 | 6 | 5 | |
Abbreviations: BMI, body mass index; CCOP, community clinical oncology program; ECOG, Eastern Cooperative Oncology Group; PBI, partial-brain irradiation; PCI, prophylactic cranial irradiation; WBI, whole-brain irradiation; WFU, Wake Forest University.
Some missing data.
Footnotes
See accompanying editorial on page 1633
Supported by Grant No. 5R01NR009675 from the National Institute of Nursing Research, National Institutes of Health; by Grant No. 2U10CA081851 from the National Cancer Institute Division of Cancer Prevention to the Wake Forest Community Clinical Oncology Program Research Base; and by Eisai.
Presented in part at 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00369785.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Stephen R. Rapp, L. Doug Case, Michelle M. Naughton, Edward G. Shaw
Administrative support: Edward G. Shaw
Provision of study materials or patients: Volker W. Stieber, Dennis F. Moore Jr, Steven C. Falchuk, James V. Piephoff, William J. Edenfield, Jeffrey K. Giguere, Monica E. Loghin
Collection and assembly of data: Stephen R. Rapp, Volker W. Stieber, Dennis F. Moore Jr, Steven C. Falchuk
Data analysis and interpretation: Stephen R. Rapp, L. Doug Case, Ann Peiffer, Michelle M. Naughton, Michael D. Chan, Volker W. Stieber, Steven C. Falchuk, James V. Piephoff, William J. Edenfield, Jeffrey K. Giguere, Monica E. Loghin, Edward G. Shaw
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Donepezil for Irradiated Brain Tumor Survivors: A Phase III Randomized Placebo-Controlled Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Stephen R. Rapp
No relationship to disclose
Doug Case
No relationship to disclose
Ann Peiffer
No relationship to disclose
Michelle Naughton
No relationship to disclose
Michael Chan
Expert Testimony: Cooney, Scully, Dowling: Attorneys at Law
Volker Stieber
No relationship to disclose
Dennis Moore
No relationship to disclose
Steven Falchuk
No relationship to disclose
James Piephoff
No relationship to disclose
William Edenfield
Speakers' Bureau: Astellas Pharma, Medivation
Jeffrey Giguere
No relationship to disclose
Monica Loghin
No relationship to disclose
Edward G. Shaw
No relationship to disclose
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: An update and review of the literature. Acta Neuropathol. 2006;111:197–212. doi: 10.1007/s00401-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 3.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment–associated cognitive change: An update on the state of the science. J Clin Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson BE, Becker B, Goff WB, 2nd, et al. Neurologic, neuropsychologic, and computed cranial tomography scan abnormalities in 2- to 10-year survivors of small-cell lung cancer. J Clin Oncol. 1985;3:1659–1667. doi: 10.1200/JCO.1985.3.12.1659. [DOI] [PubMed] [Google Scholar]
- 5.Laukkanen E, Klonoff H, Allan B, et al. The role of prophylactic brain irradiation in limited stage small cell lung cancer: Clinical, neuropsychologic, and CT sequelae. Int J Radiat Oncol Biol Phys. 1988;14:1109–1117. doi: 10.1016/0360-3016(88)90386-0. [DOI] [PubMed] [Google Scholar]
- 6.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: Results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 7.Dimberg Y, Lärkfors L. Effects of irradiation on cholinergic neurons and nerve growth factor mRNA in mouse foetal brain aggregation cultures. Int J Radiat Biol. 1994;66:793–800. [PubMed] [Google Scholar]
- 8.Madsen TM, Kristjansen PE, Bolwig TG, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 9.Pinkham MB, Bertrand KC, Olson S, et al. Hippocampal-sparing radiotherapy: The new standard of care for World Health Organization grade II and III gliomas? J Clin Neurosci. 2014;21:86–90. doi: 10.1016/j.jocn.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw EG, Rosdhal R, D'Agostino RB, Jr, et al. Phase II study of donepezil in irradiated brain tumor patients: Effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 12.Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer's disease: Current status and new perspectives. Lancet Neurol. 2003;2:539–547. doi: 10.1016/s1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- 13.Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev. 2006;1:CD001190. doi: 10.1002/14651858.CD001190.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer's disease. N Engl J Med. 2012;366:893–903. doi: 10.1056/NEJMoa1106668. [DOI] [PubMed] [Google Scholar]
- 15.Leroi I, Brandt J, Reich SG, et al. Randomized placebo-controlled trial of donepezil in cognitive impairment in Parkinson's disease. Int J Geriatr Psychiatry. 2004;19:1–8. doi: 10.1002/gps.993. [DOI] [PubMed] [Google Scholar]
- 16.Krupp LB, Christodoulou C, Melville P, et al. Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology. 2004;63:1579–1585. doi: 10.1212/01.wnl.0000142989.09633.5a. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Plotkin RC, Wang G, et al. Cholinergic augmentation with donepezil enhances recovery in short-term memory and sustained attention after traumatic brain injury. Arch Phys Med Rehabil. 2004;85:1050–1055. doi: 10.1016/j.apmr.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Grön G, Kirstein M, Thielscher A, et al. Cholinergic enhancement of episodic memory in healthy young adults. Psychopharmacology (Berl) 2005;182:170–179. doi: 10.1007/s00213-005-0043-2. [DOI] [PubMed] [Google Scholar]
- 19.Ceravolo R, Volterrani D, Tognoni G, et al. Cerebral perfusional effects of cholinesterase inhibitors in Alzheimer disease. Clin Neuropharmacol. 2004;27:166–170. doi: 10.1097/01.wnf.0000138636.42121.45. [DOI] [PubMed] [Google Scholar]
- 20.Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–142. [Google Scholar]
- 21.Saxton J, Becker JT, Wisniewski SR. The ROCF and dementia. In: Knight JA, Kaplan E, editors. The Handbook of Rey-Osterrieth Complex Figure Usage: Clinical and Research Applications. Lutz, FL: Psychological Assessment Resources; 2003. pp. 659–682. [Google Scholar]
- 22.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 23.Ruff RM, Light RH, Parker SB, et al. Benton Controlled Oral Word Association Test: Reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–338. [PubMed] [Google Scholar]
- 24.Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III) New York, NY: Psychological Corporation/Harcourt; 1996. [Google Scholar]
- 25.Matthews CG, Klove H. Instruction Manual for the Adult Neuropsychological Test Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- 26.Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;64:191–199. [Google Scholar]
- 27.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 28.Spotfire S+ SeqTrial User's Manual. Palo Alto, CA: TIBCO Software; 2014. [Google Scholar]
- 29.Ratitch B, O'Kelly M. Implementation of pattern-mixture models using standard SAS/STAT procedures. http://pharmasug.org/proceedings/2011/SP/PharmaSUG-2011-SP04.pdf.
- 30.SAS/STAT 9.3 SAS User's Guide. Cary, NC: SAS Institute; 2011. [Google Scholar]
- 31.Correa DD, Shi W, Thaler HT, et al. Longitudinal cognitive follow-up in low grade gliomas. J Neurooncol. 2008;86:321–327. doi: 10.1007/s11060-007-9474-4. [DOI] [PubMed] [Google Scholar]
- 32.Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: A randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 33.Salloway S, Mintzer J, Cummings JL, et al. Subgroup analysis of US and non-US patients in a global study of high-dose donepezil (23 mg) in moderate and severe Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2012;27:421–432. doi: 10.1177/1533317512454708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


