Abstract
Purpose
To evaluate the efficacy of maintenance sunitinib after chemotherapy for small-cell lung cancer (SCLC).
Patients and Methods
The Cancer and Leukemia Group B 30504 trial was a randomized, placebo-controlled, phase II study that enrolled patients before chemotherapy (cisplatin 80 mg/m2 or carboplatin area under the curve of 5 on day 1 plus etoposide 100 mg/m2 per day on days 1 to 3 every 21 days for four to six cycles). Patients without progression were randomly assigned 1:1 to placebo or sunitinib 37.5 mg per day until progression. Cross-over after progression was allowed. The primary end point was progression-free survival (PFS) from random assignment for maintenance placebo versus sunitinib using a one-sided log-rank test with α = .15; 80 randomly assigned patients provided 89% power to detect a hazard ratio (HR) of 1.67.
Results
One hundred forty-four patients were enrolled; 138 patients received chemotherapy. Ninety-five patients were randomly assigned; 10 patients did not receive maintenance therapy (five on each arm). Eighty-five patients received maintenance therapy (placebo, n = 41; sunitinib, n = 44). Grade 3 adverse events with more than 5% incidence were fatigue (19%), decreased neutrophils (14%), decreased leukocytes (7%), and decreased platelets (7%) for sunitinib and fatigue (10%) for placebo; grade 4 adverse events were GI hemorrhage (n = 1) and pancreatitis, hypocalcemia, and elevated lipase (n = 1; all in same patient) for sunitinib and thrombocytopenia (n = 1) and hypernatremia (n = 1) for placebo. Median PFS on maintenance was 2.1 months for placebo and 3.7 months for sunitinib (HR, 1.62; 70% CI, 1.27 to 2.08; 95% CI, 1.02 to 2.60; one-sided P = .02). Median overall survival from random assignment was 6.9 months for placebo and 9.0 months for sunitinib (HR, 1.28; 95% CI, 0.79 to 2.10; one-sided P = .16). Three sunitinib and no placebo patients achieved complete response during maintenance. Ten (77%) of 13 patients evaluable after cross-over had stable disease on sunitinib (6 to 27 weeks).
Conclusion
Maintenance sunitinib was safe and improved PFS in extensive-stage SCLC.
INTRODUCTION
Most of the 30,000 patients newly diagnosed each year with small-cell lung cancer (SCLC) in the United States have extensive-stage disease.1 Platinum-based chemotherapy provides response rates of up to 80% and improves survival from approximately 3 to 10 months.2–6 Despite achieving good disease control initially, patients with SCLC usually experience relapse within 6 months of first-line chemotherapy and often do not respond to subsequent chemotherapy. The development of therapy to delay cancer progression and prolong survival after initial chemotherapy for SCLC is an unmet clinical need.
Maintenance chemotherapy after standard platinum-based therapy in SCLC failed to show overall survival (OS) benefit. Topotecan after standard chemotherapy in SCLC improved progression-free survival (PFS), but there was no difference in OS.7 The high frequency of chemotherapy-refractory SCLC after initial chemotherapy may partially explain why chemotherapy maintenance trials in SCLC have failed.
Vascular endothelial growth factor (VEGF) is expressed in approximately 80% of SCLCs.8 Sunitinib is a small-molecule tyrosine kinase inhibitor that inhibits several targets of interest in SCLC such as VEGF receptors (VEGFRs), platelet-derived growth factor receptor, Flt-3, and Kit at levels of 4 to 14 nmol/L.9 In mouse xenograft models, sunitinib inhibited the growth of SCLC.10 Dose-limiting toxicities of sunitinib are reversible grade 3 fatigue, grade 3 hypertension, and grade 2 bullous skin toxicity.10 The standard dose of sunitinib in renal cell cancer is 50 mg per day for 4 of 6 weeks; in pancreatic neuroendocrine tumor, the sunitinib dose is 37.5 mg per day administered continuously.11,12 The Cancer and Leukemia Group B (CALGB) 30504 trial evaluated maintenance sunitinib (37.5 mg per day continuously) compared with placebo after standard chemotherapy for untreated, extensive-stage SCLC as a strategy to improve outcomes in SCLC. CALGB is now part of the Alliance for Clinical Trials in Oncology.
PATIENTS AND METHODS
Eligible patients had histologic documentation of SCLC and extensive stage (extrathoracic metastatic disease, malignant pleural effusion, contralateral supraclavicular adenopathy, or contralateral hilar adenopathy). Eligibility criteria included Eastern Cooperative Oncology Group performance status of 0 to 2 and standard initial laboratory tests. Patients were not eligible if they had recent major surgery, significant hemoptysis (≥ 5 mL), open wounds, or brain metastases present on required pre-enrollment brain imaging or required full-dose anticoagulation. Each participant signed an institutional review board–approved, protocol-specific informed consent in accordance with federal and institutional guidelines. Registration and data collection were managed by the Alliance Statistics and Data Center (Durham, NC). Data quality was ensured by careful review by Alliance Statistics and Data Center staff and the study chairperson following Alliance policies. Analysis of data was performed by Alliance statisticians.
Chemotherapy
In a dose-escalation phase, therapy consisted of cisplatin 80 mg/m2 on day 1 and etoposide 100 mg/m2 per day on days 1 through 3 every 21 days for up to six cycles. Three cohorts of six patients at dose levels of concurrent sunitinib (25, 37.5, or 50 mg per day on days 1 through 14 every 21 days) were planned. Initially, prophylactic granulocyte growth factor support was not allowed. As a result of neutropenia causing treatment delays at dose level 1, cohort 2 added prophylactic granulocyte growth factor at the sunitinib dose level of 25 mg per day. Phase IB was stopped when two of six patients experienced fatal neutropenic sepsis, and the results were reported in abstract form.13
The trial was restructured as a randomized phase II trial of chemotherapy followed by maintenance sunitinib or placebo (Fig 1). Chemotherapy consisted of cisplatin 80 mg/m2 or carboplatin area under the curve of 5 on day 1 and etoposide 100 mg/m2 per day on days 1 through 3 every 21 days for four to six cycles. Cisplatin could be switched to carboplatin at the discretion of the treating physician. Chemotherapy dose modifications were based on treatment day counts; for platelets less than 100,000/μL or granulocytes less than 1,500/μL, day 1 therapy was held. Therapy was discontinued if counts did not return to granulocytes ≥ 1,500/μL and platelets ≥100,000/μL after treatment delay of 3 weeks.
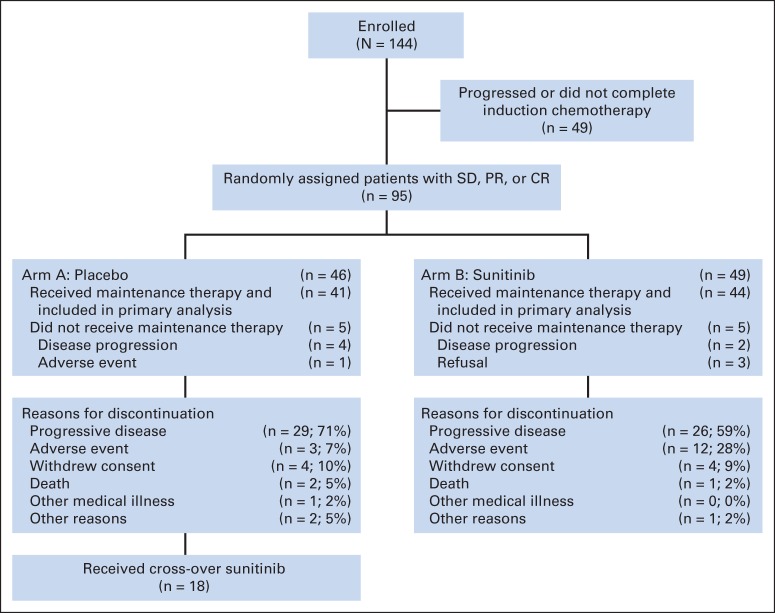
Fig 1.
CONSORT diagram. CR, complete response; PR, partial response; SD, stable disease.
Random Assignment and Masking
Patients who had a best response of complete response (CR), partial response (PR), or stable disease (SD) after completing chemotherapy were randomly assigned double-blind to maintenance sunitinib versus placebo. Patients were stratified at the time of random assignment for six cycles versus less than six cycles of induction chemotherapy and for initial treatment with cisplatin versus carboplatin. Patients were randomly assigned to either sunitinib 150 mg on day 1 (loading dose, a novel strategy of interest when the trial was designed) and then 37.5 mg per day thereafter or placebo until disease progression. Maintenance was initiated at least 3 weeks, but no later than 8 weeks, after day 1 of the last chemotherapy cycle. At the time of progression, patients receiving placebo could cross over to receive open-label sunitinib, at 150 mg on day 1 and then 37.5 mg per day until progression. Patients experiencing reversible grade 3 or 4 toxicity had sunitinib therapy held until toxicity decreased to ≤ grade 2, and sunitinib was restarted or dose-reduced to 25 or 12.5 mg per day. Patients experiencing recurrent or irreversible grade ≥ 3 toxicity, grade 4 hypertension, grade ≥ 3 hemorrhage, grade ≥ 3 cardiac event, or other severe toxicity had sunitinib permanently discontinued.
Prophylactic Cranial Irradiation
The protocol stipulated that prophylactic cranial irradiation (PCI) should be offered to all patients with a CR or PR to chemotherapy. PCI should start approximately 4 to 6 weeks after the final cycle of chemotherapy. Sunitinib was held for at least 2 days before, during, and for 2 days after the completion of radiotherapy. PCI dose was 25 Gy given in 2.5-Gy fractions.
Trial Design and Statistical Considerations
The trial design is a randomized phase II screening design that provides a nondefinitive, screening comparison of sunitinib against placebo. Several authors advocated for such a design for screening promising regimens and detecting reasonably sized efficacy differences between arms at a slightly inflated type I error.14,15 The primary objective was to compare the PFS of the experimental regimen to the PFS of placebo using a stratified one-sided log-rank test with a significance level of P = .15. Computed tomography scan response evaluation using RECIST criteria was performed after every two 3-week cycles. Secondary objectives were to assess the response rates, OS, and toxicity. A total of 80 eligible patients (40 patients per arm) were to be accrued. Sample size was determined to have adequate power to address the primary objective. Using a log-rank test at an inflated one-sided significance level of P = .15, the study has approximately 89% power to reject the null hypothesis of λA/λB = 1 and accept the alternative hypothesis of λA/λB > 1 when the true λA/λB = 1.67. All random assignment was done using a permuted-block scheme with a block size of 6, stratified by cisplatin versus carboplatin and less than six cycles versus six cycles.
PFS was defined as the time from random assignment to disease progression or death from any cause, whichever came first. Progression was defined as at least a 20% increase in the sum of the longest diameter of target lesions, taking as references the smallest sum longest diameter recorded since the treatment started, or the appearance of one or more new lesions. OS was defined as the time from random assignment to death from any cause; living patients were censored at the date of last follow-up.
For efficacy end points, the evaluable population consisted of randomly assigned patients, excluding patients who never received any maintenance therapy or were deemed ineligible (n = 2) based on prespecified eligibility criteria. Kaplan-Meier estimates were used to estimate PFS and OS curves for patients randomly assigned to each treatment arm.16 From these product-limit estimates, median survival times and their 70% or 95% CIs by treatment arm were computed. Cox proportional hazards model was used to estimate the hazard ratios (HRs) and the corresponding 95% CIs of the experimental regimens relative to the control arm.17 The proportion of patients who respond (completely or partially) to each treatment regimen was estimated, including a 95% CI.18 For tabulation of safety data, all patients for whom submitted adverse events during maintenance were reported were included. Alliance Audit Committee members visit all participating institutions at least once every 3 years to review source documents.
RESULTS
CALGB 30504 was activated in March 2007 and closed in December 2011; data lock was October 28, 2013; and median follow-up time for the 18 surviving patients was 17.2 months. Of 144 enrolled patients, 138 received induction chemotherapy. Forty-nine patients failed to achieve a response during initial induction chemotherapy or otherwise were not appropriate for random assignment. Ninety-five patients completed chemotherapy with at least SD and were randomly assigned. All randomly assigned patients were eligible. Ten randomly assigned patients did not receive maintenance therapy (five patients in each arm) as a result of early disease progression, adverse event, or refusal. Of the 85 patients who received maintenance therapy, 41 received placebo on arm A and 44 received sunitinib on arm B. Of these 85 patients, 78 (92%) achieved PR or CR, and seven (8%) had SD after induction chemotherapy. Of the 78 patients with PR or CR, 34 (44%) received PCI. The receipt of PCI was similar in both arms of the study (44% on placebo, 36% on sunitinib). Baseline characteristics for patients who received maintenance are listed in Appendix Table A1 (online only); there were no significant differences between the two arms. Approximately three fourths of maintenance patients received six cycles of chemotherapy, and approximately three fourths received carboplatin rather than cisplatin (Appendix Table A2, online only).
Toxicity
Sunitinib- or placebo-related grade ≥ 3 events for which the rate was more than 5% are listed in Appendix Table A3 (online only). Sunitinib sample size is 43 patients because one patient stopped sunitinib after 2 weeks and no toxicity data were reported. The rate of grade ≥ 3 adverse events was 53.5% on sunitinib and 31.7% on placebo. Adverse events affecting more than 5% of patients were fatigue (19%), decreased neutrophils (14%), decreased leukocytes (7%), and decreased platelets (7%) for sunitinib and fatigue (10%) for placebo. Four patients experienced grade 4 adverse events during maintenance: one patient with GI hemorrhage, one patient with pancreatitis, hypocalcemia, and elevated lipase on sunitinib, one patient with thrombocytopenia, and one patient with hypernatremia on placebo. Twenty-one patients had sunitinib dose modification. There were no treatment-related grade 5 adverse events on maintenance therapy.
Maintenance Efficacy
The median PFS from the time of random assignment was 2.1 months for placebo (95% CI, 1.6 to 2.6 months) versus 3.7 months for sunitinib (95% CI, 1.8 to 4.3 months; Table 1). The stratified one-sided log-rank test P value for PFS is .02 (HR, 1.62; 70% CI, 1.27 to 2.08; 95% CI, 1.02 to 2.60; Table 1, Fig 2). The lower limit of the 70% CI is greater than 1, and the P value is less than the prespecified .15. Therefore, the primary end point of improved PFS for sunitinib compared with placebo was met.
Table 1.
Progression-Free Survival Statistics (time from random assignment)
| Arm | No. of Patients With Treatment Failure/Total No. of Patients | Progression-Free Survival (months) |
Stratified* Hazard Ratio (placebo v sunitinib) |
Stratified* One-Sided P |
||||
|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Hazard Ratio | 95% CI | 70% CI | Log-Rank Test | Cox χ2 Test | ||
| Placebo | 40/41 | 2.1 | 1.6 to 2.6 | 1.62 | 1.02 to 2.60 | 1.27 to 2.08 | .0201 | .0215 |
| Sunitinib | 43/44 | 3.7 | 1.8 to 4.3 | |||||
NOTE. The survival rates were computed using the Kaplan-Meier estimator.
Stratification factors include the number of cycles of chemotherapy (< six v six cycles) and the agent of chemotherapy (cisplatin v carboplatin).
Fig 2.
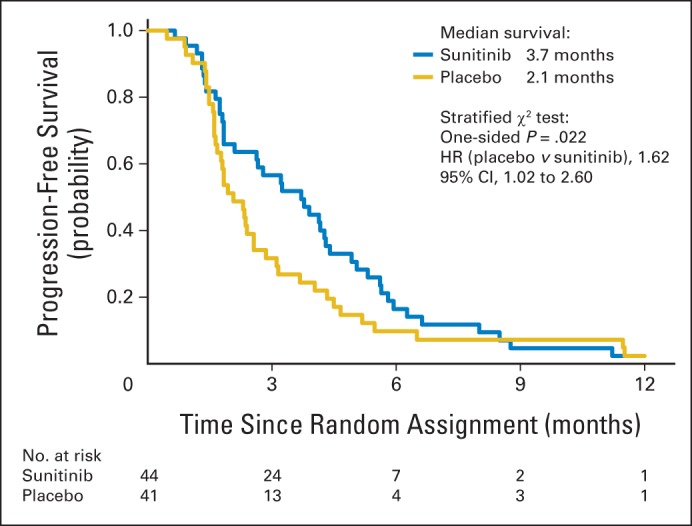
Kaplan-Meier curve for progression-free survival after random assignment to placebo (n = 41) or sunitinib (n = 44). HR, hazard ratio.
The median OS from the time of random assignment was 6.9 months for placebo (95% CI, 5.4 to 11.8 months) versus 9.0 months for sunitinib (95% CI, 8.0 to 12.7 months). The stratified one-sided log-rank test P value is .16 (HR, 1.28; 95% CI, 0.79 to 2.10; Table 2, Fig 3). In an unplanned analysis of outcomes from the time of registration, PFS was 7.7 months for sunitinib (95% CI, 6.5 to 8.6 months) and 6.4 months for placebo (95% CI, 5.9 to 7.1 months), OS was 13.9 months for sunitinib (95% CI, 11.7 to 15.8 months) and 11.0 months for placebo (95% CI, 9.4 to 16.9 months), and 1-year survival rate was 62.6% for sunitinib (95% CI, 46.3% to 75.2%) and 43.9% for placebo (95% CI, 28.6% to 58.2%). There was evidence of single-agent activity of sunitinib, with three patients (6.8%) in the sunitinib arm converting to a CR after the initiation of maintenance, whereas no patients on the placebo arm converted to a CR (Appendix Table A4, online only; Fig 4). The percentage of patients on the sunitinib arm with disease control, defined as SD, PR, or CR, during maintenance was 50% and numerically superior to the percentage of 37% on placebo, although the difference was not statistically significant.
Table 2.
Overall Survival Statistics (time from random assignment)
| Arm | No. of Patients With Treatment Failure/Total No. of Patients | Overall Survival (months) |
12-Month Survival (%) |
Stratified* Hazard Ratio (placebo v sunitinib) |
Stratified* One-Sided P |
||||
|---|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Rate | 95% CI | Hazard Ratio | 95% CI | Log-Rank Test | Cox χ2 Test | ||
| Placebo | 32/41 | 6.9 | 5.4 to 11.8 | 33.6 | 19.7 to 48.1 | 1.28 | 0.79 to 2.10 | .1595 | .1603 |
| Sunitinib | 35/44 | 9.0 | 8.0 to 12.7 | 36.0 | 22.0 to 50.3 | ||||
NOTE. The survival rates were computed using the Kaplan-Meier estimator.
Stratification factors include the number of cycles of chemotherapy (< six v six cycles) and the agent of chemotherapy (cisplatin v carboplatin).
Fig 3.
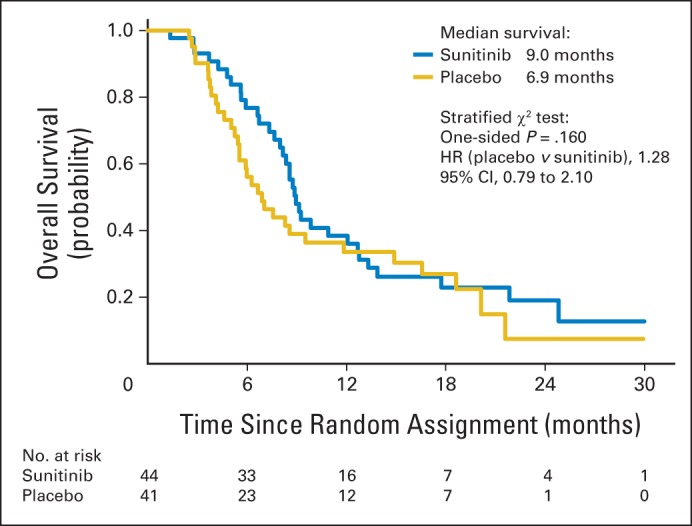
Kaplan-Meier curve for overall survival after random assignment to placebo (n = 41) or sunitinib (n = 44). HR, hazard ratio.
Fig 4.

An example of a partial response to chemotherapy that converted to a complete response on sunitinib. The tumor response to chemotherapy (chemo) plateaued after four cycles, but the tumor then responded to sunitinib for approximately 45 weeks.
Cross-Over Effect
Eighteen (44%) of the 41 patients initially randomly assigned to maintenance placebo received cross-over sunitinib after progression, and 13 patients were evaluable for efficacy. Among the 13 evaluable patients, 10 (77%) had SD. In an unplanned retrospective analysis, among the 13 evaluable patients, PFS on placebo was 2.6 months (95% CI, 1.6 to 4.5 months), whereas on cross-over sunitinib, PFS was 4.9 months (95% CI, 2.9 to 5.9 months). PFS on sunitinib was measured from reregistration until progression or last clinical evaluation date. A spider plot shows tumor growth before and after cross-over for all evaluable patients (Fig 5).
Fig 5.

A spider plot of RECIST tumor measurement on placebo before cross-over and then on sunitinib after cross-over.
PCI
Thirty-four (43%) of the 78 patients responding to chemotherapy received PCI (Appendix Table A2). The median age of patients receiving PCI (58 years) was significantly younger than for patients not receiving PCI (63 years; P = .029). The two maintenance arms were balanced for patients receiving PCI. There was no significant difference in PFS for patients receiving or not receiving PCI.
DISCUSSION
This randomized, double-blind, placebo-controlled trial met its primary objective and showed that sunitinib improved PFS compared with placebo after standard chemotherapy for untreated, extensive-stage SCLC. The median OS for the sunitinib maintenance arm was 2.1 months greater than the placebo arm, but this difference was not statistically significant (Fig 3). In an unplanned analysis, the OS from before the initiation of chemotherapy for patients who received maintenance was 11.0 months for placebo and 13.9 months for sunitinib. Ten of 13 evaluable placebo patients who crossed over to sunitinib at progression had SD, with six having evidence of prolonged disease control receiving 12 to 27 weeks of sunitinib. In addition, in the 13 evaluable patients, PFS on cross-over sunitinib was numerically superior to PFS on placebo (4.9 months [95% CI, 2.9 to 5.9 months] v 2.6 months [95% CI, 1.6 to 4.5 months]). In this trial that was not designed or powered to formally assess OS, median OS was numerically larger for sunitinib compared with placebo despite the apparent favorable sunitinib cross-over effect, but this was not significantly different at the 15% level.
A spider plot that depicts tumor growth rate before and after cross-over to sunitinib showed that, in the majority of patients who crossed over, tumors underwent some degree of objective shrinkage (Fig 5). There was evidence of sunitinib single-agent activity in SCLC that progressed early after the cessation of chemotherapy that would be classified as refractory SCLC. The majority of cross-over patients whose cancer had progressed after only 6 weeks on placebo had disease control and often had objective tumor shrinkage short of PR. Thus, there was evidence of a sunitinib cross-over effect even in refractory SCLC that may have improved OS in the placebo arm.
Continuous sunitinib maintenance dosing at 37.5 mg per day with dose reductions for toxicity was safe and feasible with no tracheoesophageal fistula formation or clinically significant hemoptysis. The only reported nonhematologic grade 3 toxicity of more than 5% incidence was fatigue. Grade 4 toxicities were seen in two patients (4.7%) on the sunitinib arm. In the patients who crossed over to receive sunitinib after progression on placebo and were evaluable for toxicity, 16 patients (94.2%) had grade ≥ 3 toxicity compared with 53.5% of patients (P = .0023, Fisher's exact test) who received immediate sunitinib maintenance therapy. The toxicity for sunitinib after standard chemotherapy may be greater in the setting of actively progressing SCLC compared with immediate maintenance therapy when the disease is stable.
A majority of patients with untreated, extensive-stage SCLC in this study received maintenance therapy using this trial design. Of the 144 patients enrolled before receiving chemotherapy, 85 patients (59%) were randomly assigned and received maintenance therapy, a rate similar to the 57% of patients receiving maintenance on the PARAMOUNT trial.19
Single-arm phase II clinical trials evaluating chemotherapy plus bevacizumab in SCLC showed an acceptable safety profile and favorable PFS and OS compared with historical controls.20,21 The randomized, phase II SALUTE trial found that adding bevacizumab to chemotherapy improved PFS but not OS.22
Multitargeted VEGFR tyrosine kinase inhibitors have been studied in SCLC. Chemotherapy plus concurrent sunitinib or sorafenib in SCLC was not tolerated.13,23 Vandetanib, cediranib, and sorafenib have been studied in SCLC and shown some evidence of disease control or response.24–26
Sunitinib has been studied as therapy for extensive-stage SCLC. A 24-patient, single-arm trial studying second-line sunitinib 50 mg daily for 4 weeks on and 2 weeks off in SCLC reported a 9% PR and 29% SD rate with significant toxicity (63% grade 3 or 4 thrombocytopenia and 25% neutropenia).27 A 16-patient, single-institution, phase II maintenance trial that evaluated sunitinib 50 mg daily for 4 weeks on and 2 weeks off reported 25% grade 3 or 4 thrombocytopenia and 50% discontinuation for toxicity or patient request.28 A 34-patient, single-arm, multi-institution, phase II maintenance trial evaluated sunitinib 25 mg with continuous daily dosing after six cycles of carboplatin and irinotecan.29 Seventeen patients received maintenance sunitinib with grade 3 or 4 toxicities of thrombocytopenia (9%) and fatigue (6%), but no neutropenia. Efficacy was promising, with one patient (5.8%) converting to CR, median time to progression of 8 months, and 1-year OS rate of 85%, similar to CALGB 30504 (6.8% converting to CR, PFS from registration of 7.7 months, and 1-year OS of 63%). Maintenance trials that studied lower dose sunitinib (25 or 37.5 mg continuous daily) found therapy safe and feasible with promising efficacy, whereas sunitinib 50 mg per day was not tolerated.
The results of CALGB 30504 support the strategy of investigating the use of novel therapy in the maintenance setting and the future study of multitargeted VEGFR inhibitors in SCLC. Standard doses of platinum and etoposide are given at close to maximum-tolerated dose, so it is difficult to give additional therapy concurrently, and relapsing SCLC often grows rapidly and causes morbidity that complicates the study of novel agents in the second- or third-line setting. Maintenance sunitinib in CALGB 30504 was safe and significantly less toxic than the same dose of sunitinib given at time of progression, providing a strong rationale for studying novel therapies in a maintenance trial design in SCLC. Sunitinib given after standard chemotherapy in extensive-stage SCLC improved PFS, and OS was promising despite the cross-over design. Three patients converted to CR on maintenance sunitinib, and several patients with rapidly growing refractory SCLC who crossed over to sunitinib had extended disease control, showing a major therapeutic benefit in a small subset of patients. A randomized phase II trial studying maintenance sunitinib with OS as the primary end point would be an appropriate next step, especially if the trial design incorporated a candidate sunitinib benefit predictive biomarker for retrospective validation. These results support the future study of multitargeted VEGFR inhibitors in SCLC, especially as candidate predictive biomarkers are identified and in combination with new therapeutic strategies.
Acknowledgment
Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology, June 4-8, 2010, Chicago, IL; and the 49th Annual Meeting of the American Society of Clinical Oncology, May 31-June 4, 2013, Chicago, IL.
Appendix
The following institutions participated in this study: Christiana Care Health Services, Community Clinical Oncology Program (CCOP), Wilmington, DE, Stephen Grubbs, MD, supported by Grant No. CA45418; Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, MD, PhD, supported by Grant No. CA32291; Duke University Medical Center, Durham, NC, Jeffrey Crawford, MD, supported by Grant No. CA47577; Illinois Oncology Research Association, Peoria, IL, John W. Kugler, MD, supported by Grant No. CA35113; Memorial Sloan-Kettering Cancer Center, New York, NY, Clifford A. Hudis, MD, supported by Grant No. CA77651; Missouri Valley Consortium-CCOP, Omaha, NE, Gamini S. Soori, MD; Mount Sinai Medical Center, Miami, FL, Michael A. Schwartz, MD, supported by Grant No. CA45564; New Hampshire Oncology-Hematology PA, Concord, NH, Douglas J. Weckstein, MD; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN, Rafat Ansari, MD, supported by Grant No. CA86726; The Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, MD, supported by Grant No. CA77658; Southeast Cancer Control Consortium CCOP, Goldsboro, NC, James N. Atkins, MD, supported by Grant No. CA45808; State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, MD, supported by Grant No. CA21060; University of Chicago, Chicago, IL, Hedy L. Kindler, MD, supported by Grant No. CA41287; University of Iowa, Iowa City, IA, Daniel A. Vaena, MD, supported by Grant No. CA47642; University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, MD, supported by Grant No. CA31983; University of Minnesota, Minneapolis, MN, Bruce A. Peterson, MD, supported by Grant No. CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO, Karl E. Freter, MD, supported by Grant No. CA12046; University of Nebraska Medical Center, Omaha, NE, Apar Ganti, MD, supported by Grant No. CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, MD, supported by Grant No. CA47559; University of Vermont, Burlington, VT, Steven M. Grunberg, MD, supported by Grant No. CA77406; Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, MD, supported by Grant No. CA03927; and Washington University School of Medicine, St Louis, MO, Nancy Bartlett, MD, supported by Grant No. CA77440.
Table A1.
Baseline Demographics and Clinical Characteristics for Maintenance Patients Receiving Placebo and Sunitinib
| Demographic or Clinical Characteristic | Placebo (n = 41) |
Sunitinib (n = 44) |
Total (N = 85) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||
| Mean | 60.8 | 59.3 | 60.0 | |||
| Standard deviation | 8.5 | 6.6 | 7.6 | |||
| Median | 61 | 60 | 60 | |||
| Q1-Q3 | 56-67 | 56-64 | 56-66 | |||
| Range | 43-77 | 39-69 | 39-77 | |||
| Race | ||||||
| White | 40 | 97.6 | 41 | 93.2 | 81 | 95.3 |
| Black or African American | 1 | 2.4 | 3 | 6.8 | 4 | 4.7 |
| Sex | ||||||
| Male | 20 | 48.8 | 18 | 40.9 | 38 | 44.7 |
| Female | 21 | 51.2 | 26 | 59.1 | 47 | 55.3 |
| Performance status | ||||||
| 0 | 17 | 41.5 | 20 | 45.5 | 37 | 43.5 |
| 1 | 15 | 36.6 | 19 | 43.2 | 34 | 40.0 |
| 2 | 9 | 22.0 | 5 | 11.4 | 14 | 16.5 |
Abbreviation: Q, quintile.
Table A2.
Therapy Received in the Placebo and Sunitinib Arms for Chemotherapy, Maintenance, and PCI
| Therapy | Placebo (n = 41) |
Sunitinib (n = 44) |
Total (N = 85) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Chemotherapy agent | ||||||
| Cisplatin | 11 | 26.8 | 12 | 27.3 | 23 | 27.1 |
| Carboplatin | 30 | 73.2 | 32 | 72.7 | 62 | 72.9 |
| Cycles of chemotherapy, No. | ||||||
| < 6 | 9 | 22.0 | 12 | 27.3 | 21 | 24.7 |
| 6 | 32 | 78.0 | 32 | 72.7 | 64 | 75.3 |
| Cycles of maintenance, No. | ||||||
| Mean | 3.4 | 3.7 | 3.5 | |||
| SD | 3.5 | 3.1 | 3.3 | |||
| Median | 2.0 | 2.0 | 2.0 | |||
| Q1-Q3 | 2.0-4.0 | 1.5-5.5 | 2.0-5.0 | |||
| Range | 1-17 | 1-15 | 1-17 | |||
| 1-4 | 33 | 80.5 | 30 | 68.2 | 63 | 74.1 |
| 5-8 | 6 | 14.6 | 10 | 22.7 | 16 | 18.8 |
| ≥ 9 | 2 | 4.9 | 4 | 9.1 | 6 | 7.1 |
| PCI | ||||||
| Not received | 23 | 56.1 | 28 | 63.6 | 51 | 60.0 |
| Received | 18 | 43.9 | 16 | 36.4 | 34 | 40.0 |
Abbreviations: PCI, prophylactic cranial irradiation; Q, quintile; SD, standard deviation.
Table A3.
Grade ≥ 3 Sunitinib- or Placebo-Related Adverse Events With > 5% Incidence in Evaluable Patients
| Adverse Event | Grade 3 |
Grade 4 |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Sunitinib (n = 43) | ||||
| Fatigue | 8 | 19 | 0 | 0 |
| Neutrophils | 6 | 14 | 0 | 0 |
| Leukocytes | 3 | 7 | 0 | 0 |
| Platelets | 3 | 7 | 0 | 0 |
| GI hemorrhage | 0 | 0 | 1 | 2 |
| Pancreatitis, hypocalcemia, lipase in same patient | 0 | 0 | 1 | 2 |
| Placebo (n = 41) | ||||
| Fatigue | 4 | 10 | 0 | 0 |
| Platelets | 0 | 0 | 1 | 2 |
| Hypernatremia | 0 | 0 | 1 | 2 |
Table A4.
Best Response to Maintenance Therapy
| Response | Placebo (n = 41) |
Sunitinib (n = 44) |
Total (N = 85) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Complete response | 0 | 0 | 3 | 6.8 | 3 | 3.5 |
| Partial response | 5 | 12.2 | 4 | 9.1 | 9 | 10.6 |
| Stable disease | 10 | 24.4 | 15 | 34.1 | 25 | 29.4 |
| Progression | 26 | 63.4 | 22 | 50.0 | 48 | 56.5 |
| Response rate, % | 12.2 | 15.9 | 14.1 | |||
| 95% CI | 4.1 to 26.2 | 6.6 to 30.1 | 7.5 to 23.4 | |||
See accompanying editorial on page 1637
Written on behalf of the Alliance for Clinical Trials in Oncology.
Support information appears at the end of this article.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00453154.
Support
Supported, in part, by grants from the National Cancer Institute to the Cancer and Leukemia Group B (CALGB; Monica M. Bertagnolli, MD, Chair; Grant No. CA31946) and the CALGB Statistical Center (Daniel J. Sargent, PhD, Statistician; Grant No. CA33601).
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Neal E. Ready, Herbert H. Pang, Jeffrey Bogart, Everett E. Vokes
Administrative support: Everett E. Vokes
Provision of study materials or patients: Maria Baggstrom
Collection and assembly of data: Neal E. Ready, Herbert H. Pang, Lin Gu, Gregory A. Otterson, Sachdev P. Thomas, Antonius A. Miller, Maria Baggstrom, Gregory A. Masters, Stephen L. Graziano
Data analysis and interpretation: Neal E. Ready, Herbert H. Pang, Lin Gu, Jeffrey Crawford, Everett E. Vokes
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive-Stage Small-Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase II Study—CALGB 30504 (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Neal E. Ready
Consulting or Advisory Role: Bristol-Myers Squibb, Onyx, Celgene
Research Funding: Bristol-Myers Squibb, Genentech
Herbert H. Pang
No relationship to disclose
Lin Gu
No relationship to disclose
Gregory A. Otterson
Consulting or Advisory Role: Genentech (Inst), Boehringer Ingelheim (Inst), BIND Biosciences (Inst), Transgene (Inst)
Research Funding: Genentech (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Newlink Genetics (Inst)
Sachdev P. Thomas
Speakers' Bureau: Roche/Genentech, Boehringer Ingelheim, Pharmacyclics
Research Funding: GlaxoSmithKline (Inst), Celgene (Inst), Novartis (Inst), Lilly/ImClone (Inst), Genentech (Inst)
Antonius A. Miller
No relationship to disclose
Maria Baggstrom
Research Funding: Novartis (Inst), Merck (Inst), Wyeth (Inst), ImClone (Inst), Boehringer Ingelheim (Inst), Merrimack (Inst), Lilly (Inst), Bristol-Meyers Squibb (Inst), Oncocyte (Inst), Astex (Inst), Onyx (Inst)
Gregory A. Masters
No relationship to disclose
Stephen L. Graziano
Consulting or Advisory Role: Helsinn Therapeutics
Research Funding: Pfizer, Boehringer Ingelheim
Jeffrey Crawford
Consulting or Advisory Role: Bayer, Boehringer Ingelheim, Lilly, Gilead Sciences, Hospira, Ono Pharmaceutical, Celgene, Roche, Merrimack, Sanofi
Research Funding: Amgen (Inst), AstraZeneca (Inst), GTx (Inst), MedImmune (Inst)
Jeffrey Bogart
No relationship to disclose
Everett E. Vokes
Consulting or Advisory Role: Boehringer Ingelheim, Clovis Oncology, Synta, Venitrx, Abbvie, Merck, AstraZeneca, Celgene, Eisei, GeneCentric, Genentech
REFERENCES
- 1.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75(suppl 1):191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Miller AA, Herndon JE, 2nd, Hollis DR, et al. Schedule dependency of 21 day oral versus 3 day IV etoposide in combination with IV cisplatin in extensive stage small cell lung cancer: A randomized phase III study of the Cancer and Leukemia Group B study group. J Clin Oncol. 1995;13:1871–1879. doi: 10.1200/JCO.1995.13.8.1871. [DOI] [PubMed] [Google Scholar]
- 3.Niell HB, Herndon JE, 2nd, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J Clin Oncol. 2005;23:3752–3759. doi: 10.1200/JCO.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 4.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 5.Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–2535. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 7.Schiller JH, Adak S, Cella D, et al. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593—A phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:2114–2122. doi: 10.1200/JCO.2001.19.8.2114. [DOI] [PubMed] [Google Scholar]
- 8.Dowell JE, Amirkhan RH, Lai WS, et al. Survival in small cell lung cancer is independent of tumor expression of VEGF and COX-2. Anticancer Res. 2004;24:2367–2373. [PubMed] [Google Scholar]
- 9.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 10.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 13.Ready N, Dunphy F, Pang H, et al. Combination chemotherapy with sunitinib (IND74019; NSC 736511) for untreated extensive-stage small cell lung cancer: CALGB 30504 phase 1B safety results. J Clin Oncol. 2010;28(suppl):528s. abstr 7056. [Google Scholar]
- 14.Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 15.Korn EL, Arbuck SG, Pluda JM, et al. Clinical trial designs for cytostatic agents: Are new approaches needed? J Clin Oncol. 2001;19:265–272. doi: 10.1200/JCO.2001.19.1.265. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 18.Agresti A. Categorical Data Analysis. ed 2. New York, NY: Wiley; 2002. [Google Scholar]
- 19.Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 20.Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol. 2009;27:6006–6011. doi: 10.1200/JCO.2009.23.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ready NE, Dudek AZ, Pang HH, et al. Cisplatin, irinotecan, and bevacizumab for untreated extensive-stage small-cell lung cancer: CALGB 30306, a phase II study. J Clin Oncol. 2011;29:4436–4441. doi: 10.1200/JCO.2011.35.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: Results from the SALUTE trial. J Clin Oncol. 2011;29:2215–2222. doi: 10.1200/JCO.2010.29.3423. [DOI] [PubMed] [Google Scholar]
- 23.Sharma N, Pennell N, Nickolich M, et al. Phase II trial of sorafenib in conjunction with chemotherapy and as maintenance therapy in extensive-stage small cell lung cancer. Invest New Drugs. 2014;32:362–368. doi: 10.1007/s10637-013-0061-6. [DOI] [PubMed] [Google Scholar]
- 24.Ramalingam SS, Belani CP, Mack PC, et al. Phase II study of cediranib (AZD 2171), an inhibitor of the vascular endothelial growth factor receptor, for second-line therapy of small cell lung cancer (National Cancer Institute #7097) J Thorac Oncol. 2010;5:1279–1284. doi: 10.1097/JTO.0b013e3181e2fcb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol. 2007;25:4278–4284. doi: 10.1200/JCO.2007.12.3083. [DOI] [PubMed] [Google Scholar]
- 26.Gitlitz BJ, Moon J, Glisson BS, et al. Sorafenib in platinum-treated patients with extensive stage small cell lung cancer: A Southwest Oncology Group (SWOG 0435) phase II trial. J Thorac Oncol. 2010;5:1835–1840. doi: 10.1097/JTO.0b013e3181f0bd78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han JY, Kim HY, Lim KY, et al. A phase II study of sunitinib in patients with relapsed or refractory small cell lung cancer. Lung Cancer. 2013;79:137–142. doi: 10.1016/j.lungcan.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Schneider BJ, Gadgeel SM, Ramnath N, et al. Phase II trial of sunitinib maintenance therapy after platinum-based chemotherapy in patients with extensive-stage small cell lung cancer. J Thorac Oncol. 2011;6:1117–1120. doi: 10.1097/JTO.0b013e31821529c3. [DOI] [PubMed] [Google Scholar]
- 29.Spigel DR, Greco FA, Rubin MS, et al. Phase II study of maintenance sunitinib following irinotecan and carboplatin as first-line treatment for patients with extensive-stage small-cell lung cancer. Lung Cancer. 2012;77:359–364. doi: 10.1016/j.lungcan.2012.03.009. [DOI] [PubMed] [Google Scholar]