Abstract
Mitochondria are cytoplasmic organelles that regulate both metabolic and apoptotic signaling pathways; their most highlighted functions include cellular energy generation in the form of adenosine triphosphate (ATP), regulation of cellular calcium homeostasis, balance between ROS production and detoxification, mediation of apoptosis cell death, and synthesis and metabolism of various key molecules. Consistent evidence suggests that mitochondrial failure is associated with early events in the pathogenesis of ageing-related neurodegenerative disorders including Parkinson's disease and Alzheimer's disease. Mitochondria-targeted protective compounds that prevent or minimize mitochondrial dysfunction constitute potential therapeutic strategies in the prevention and treatment of these central nervous system diseases. This paper provides an overview of the involvement of mitochondrial dysfunction in Parkinson's and Alzheimer's diseases, with particular attention to in vitro and in vivo studies on promising endogenous and exogenous mitochondria-targeted protective compounds.
1. Introduction
Mitochondria are spherical cytoplasmic organelles with a symbiotic origin that are present in all eukaryotic cells. Structurally, mitochondria consist of two compositions and functionally different phospholipid membranes referred to as the outer membrane and the inner membrane and two aqueous compartments, the intermembrane space and the mitochondrial matrix. The outer membrane encloses the entire structure; it has higher content in lipids (over 60%) and it contains porins and a large multiprotein translocase complex allowing the passage to ions and larger molecules. The inner membrane surrounds the mitochondrial matrix and it invaginates to form cristae that increase total surface area. In addition, the inner membrane has lipid content over 20% and it is only permeable to small uncharged molecules. Both membranes are separated by the aqueous compartment intermembrane space, located between them [1, 2]. Moreover, mitochondria contain their own DNA (mDNA) held in the mitochondrial matrix; the human mDNA is a double-stranded circular genome made up of 16,569 base pairs of DNA that encodes 13 proteins, 22 transfer RNAs (tRNAs), and 2 ribosomal RNAs (rRNAs) [3]. Functionally, mitochondria play a vital role in regulating both metabolic and apoptotic signaling pathways. Their main function is to produce energy as adenosine triphosphate (ATP) at the mitochondrial electron transport chain (ETC) in the inner membrane, through the cellular process of oxidative phosphorylation (OXPHOS). The mitochondrial ETC consists of four integral membrane oxidation-reduction electron and proton pump protein complexes (complex I, NADH:ubiquinone oxidoreductase; complex II, succinate dehydrogenase; complex III, ubiquinone-cytochrome c oxidoreductase; complex IV, cytochrome c oxidase) and an ATP synthase (complex V) which catalyzes ADP conversion to form ATP [4]. In addition, mitochondria participate in other series of functions, including regulation of cellular calcium homeostasis, balance between ROS production and detoxification (i.e., superoxide anion (O2 •−) and the highly reactive hydroxyl radical (•OH)), mediation of the process of programmed cell death (apoptosis), and synthesis and metabolism of endogenous compounds such as steroids, heme groups, and fatty acids [5].
Consistent evidence suggests that mitochondrial failure is associated with early events in the pathogenesis of ageing-related neurodegenerative disorders including Parkinson's disease and Alzheimer's disease. Mitochondria-targeted protective compounds that prevent or minimize mitochondrial dysfunction constitute potential therapeutic strategies in the prevention and treatment of these central nervous system diseases [6, 7]. This paper provides an overview of the involvement of mitochondrial dysfunction in Parkinson's and Alzheimer's diseases, with particular attention to in vitro and in vivo studies on promising endogenous and exogenous mitochondria-targeted protective compounds.
2. Parkinson's Disease and Mitochondria-Targeted Protective Compounds
2.1. Parkinson's Disease (PD)
Parkinson's disease is a chronic progressive disorder characterized pathologically by the loss of dopaminergic neurons located in the substantia nigra pars compacta, and, to a lesser extent, in putamen, caudate, and globus pallidus and by the formation of intracellular protein inclusions of mainly alpha-synuclein (named as Lewy bodies) in the remaining neurons [8, 9]. The first clinical description was published in 1817 by the English physician Dr. Parkinson in his work “An Essay on the Shaking Palsy” [10]. Parkinson's disease is the second most common neurodegenerative disorder after Alzheimer's disease which affects more than 6.3 million people over usually the age of 60 worldwide. Regarding epidemiology, this age-related central nervous system disease appears to be slightly more common in whites than blacks and Asian people, in men than in women, and in some geographical regions (i.e., China, India, and USA) [11–13]. The most relevant clinical features include tremor, bradykinesia, rigidity, and dystonia; however, in addition to these characteristic motor signs and symptoms, neuropsychiatric and other nonmotor manifestations such as depression, cognitive impairment, anxiety, and psychosis have been also described [8, 9, 14]. Although the exact causal factors of Parkinson's disease remain unknown, several research studies point to specific genetic mutations and environmental factors [15, 16]. It has been estimated that around 5–10 in every 100 people suffering from Parkinson's disease are associated with gene mutations. Scientifics have identified at least 13 gene mutations, among which one could highlight those in the genes SNCA (synuclein, alpha non-A4 component of amyloid precursor), PARK2 (Parkinson's disease autosomal recessive, juvenile 2), PARK7 (Parkinson's disease autosomal recessive, early-onset 7), PINK1 (PTEN-induced putative kinase 1), and LRRK2 (leucine-rich repeat kinase 2) [15]. The SNCA gene encodes for the protein alpha-synuclein, which is a key component of Lewy bodies; the PARK2 gene encodes for the E3 ubiquitin ligase parkin, which is implied in mitochondrial maintenance; the PARK7 gene encodes for the antioxidant protein DJ-1; PINK 1 gene encodes for a serine/threonine-protein kinase with a protective mitochondrial role. Alterations of SNCA, PARK2, PARK7, and PINK1 genes are involved in the early-onset Parkinson's disease (this is diagnosed before being 50 years old) [17–20]. The LRRK2 gene, which encodes for the protein dardarin, has been associated with the late-onset Parkinson's disease [21]. The rest, around 95%, of diagnosed Parkinson's disease cases are sporadic, in which environmental factors such as pesticides and dietary factors, among others, seem to play a crucial role. Researchers have identified several common pesticides that their exposure may increase the risk of developing Parkinson's disease among which rotenone, paraquat, dithiocarbamates (i.e., maneb, ziram), pyrethroids (i.e., deltamethrin), organochlorine (dieldrin), imidazoles (i.e., triflumizole, benomyl), and 2,2-dicarboximides (i.e., folpet, aptan) are included [22, 23]. Regarding dietary factors, both dietary patterns or/and dietary nutrients that may protect or may increase against to suffer from Parkinson's disease have been reported. As an example, in a case control study performed during ten years for establishing the influence of minerals, vitamins, and fats in the etiology of Parkinson's disease, an association between a high intake of a combination of iron and manganese and the development of Parkinson's disease was found [24]. On the other hand, a large prospective study performed over fifteen years with 49 692 men and 81 676 women revealed that the high intake of fruit, vegetables, legumes, whole grains, nuts, fish, and poultry, the low intake of saturated fat, and the moderate intake of alcohol are protective dietary patterns against Parkinson's disease [25].
2.1.1. Mitochondrial Dysfunction in Familial PD
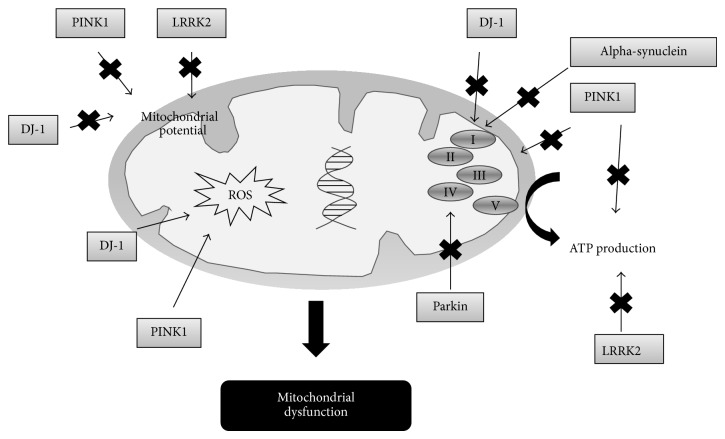
As we have previously commented, around 5–10% of Parkinson's disease cases involve gene products. Mutations in ATP13A2 (PARK9), DJ-1 (PARK7), parkin (PARK2), and PTEN-induced putative kinase 1 (PINK1) (PARK6) are associated with autosomal recessive PD and mutations in α-synuclein gene and leucine-rich repeat kinase 2 gene (LRRK2) are implicated in autosomal dominant PD (see Figure 1) [16].
Figure 1.
The role of gene products in Parkinson's disease.
Mutations in the ATP13A2 gene (PARK9), encoding for a lysosomal type 5P-type ATPase, cause a hereditary rare juvenile onset autosomal recessive Parkinsonism with dementia named as Kufor-Rakeb syndrome. This particular Parkinson's form, characterized by supranuclear gaze palsy, dystonia, pyramidal signs, and cognitive impairment, was first evidenced in 2006 in members of a nonconsanguineous Chilean family. The neuronal damage associated with mutations in this gene is related to alterations in mitochondria and lysosomes functions and divalent cation regulation [26–28].
DJ-1 mutations on chromosome 1p36 cause autosomal recessive early-onset PD and its pathological mechanism seems to be linked with mitochondrial fragmentation and mitochondrial structural damage and consequently defects in the mitochondrial function of dopaminergic cells [29, 30].
Mutations in the parkin gene product, which is an ubiquitin ligase, lead to an early-onset familial Parkinson's disease and its first description dates in the year 1998. Experimental studies have determined that the pathology of parkin is associated with alterations in the mitochondrial recognition, transportation, and ubiquitination and with mitophagy impairment [31, 32].
Mutations in the mitochondrial serine/threonine-protein kinase PINK1 result in alterations in the mitochondrial morphology and function (defects in complex I activity) and they are strongly associated with a form of autosomal recessive early-onset Parkinson's disease [33, 34].
Mutations in the protein α-synuclein, which is the main component of Lewy bodies (it represents 1% of total cytosolic protein of brain cells), have been reported to play a key role in the pathogenesis of autosomal dominant early-onset Parkinson's disease. Particularly, two mutations in the alpha-synuclein gene (A30P and A53T) have been identified which lead to the formation of pathogenic pore-like annular and tubular protofibrils. These mutations inhibit the activity of complex I and induce mitochondrial fragmentation, causing mitochondrial dysfunction [35, 36].
Mutations in the gene encoding leucine-rich repeat kinase 2 (LRRK2) are related to autosomal dominant Parkinson's disease form. The most common mutation is G2019S that accounts for 5-6% of familial cases of Parkinson's disease. Experimental studies have identified different pathogenic mechanisms for altered LRRK2 that involve inflammation processes, oxidative stress, and mitochondrial dysfunction, among others. Focusing on this last pathogenic mechanism, mutations in LRRK2 cause mitochondrial fragmentation and a downregulation in mitochondrial homeostasis (reduction in mitochondrial membrane potential and ATP production) [37, 38].
2.1.2. Mitochondrial Dysfunction in Sporadic PD
Around 95% of diagnosed Parkinson's disease cases are sporadic. One of the proposed mechanisms for the dopaminergic neurons degeneration in sporadic Parkinson's disease cases is related to an excessive production of reactive oxygen species (ROS) that leads to oxidative stress situation. An excess of ROS causes the oxidative modification of macromolecules (lipids, proteins, and DNA) leading to cell damage and even cell death. The pathological effect of ROS is also involved in a reduction of ATP (adenosine triphosphate) production, in an increase of iron levels, and in an increase of intracellular calcium levels and alterations in mitochondrial respiratory chain complexes function. In addition to oxidative stress mechanism, protein misfolding, aggregation, and deposition have been reported as other common pathological mechanisms in Parkinson's disease. A dysfunction in the ubiquitin-proteasome-system (UPS) and the autophagy-lysosomal pathway (ALP) as evidenced in a reduction of proteasome and autophagy activities and in postmortem brains of patients suffering from this neurodegenerative disease has been demonstrated [39–43].
2.1.3. Postmortem PD Brain Tissues, Experimental Models, and Cell-Based Models
Many evidences from postmortem PD brain tissues, experimental models, and cell-based models have demonstrated the involvement of mitochondria dysfunction in the pathogenesis of both familial and sporadic Parkinson's disease.
The first evidence of the relationship between mitochondria and Parkinson's disease dates from the second half of the twentieth century when the postmortem brain analysis of some drug abusers of intravenous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), who have developed a progressive and irreversible parkinsonism, revealed a significant nigrostriatal degeneration. MPTP easily passes through the blood-brain barrier; it is oxidized and transformed into 1-methyl-4-phenylpyridinium (MPP+) and within neurons MPP+ inhibits the complex I (NADH-quinone oxidoreductase) of the electron transport chain, resulting in an enhanced reactive oxygen species (ROS) generation (i.e., hydroxyl radicals, superoxide anion radical) and a decrease in energy supply (ATP production) [44]. Many lines of evidence have further demonstrated complex I deficiency or impairment in the cortical brain tissue, frontal cortex, striatum, skeletal muscle, and platelets of patients with Parkinson's disease [40, 45, 46]. In addition to complex I, other studies have reported that a deficiency in the activity of complex II (succinate ubiquinone oxidoreductase) and complex III (ubiquinol-cytochrome C oxidoreductase) is also associated with the pathogenesis of Parkinson's disease. Complex III inhibition, as what happens with complex I, causes an overproduction of ROS, leading to oxidation of lipids, proteins, and DNA and it finally triggers to cell death [47, 48]. Moreover, ROS mediates the mitochondrial-dependent apoptosis by inducing mitochondrial permeability transition, releasing of cytochrome c, activation of caspase-3 and caspase-9, translocation of Bax to mitochondria, and the activation of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK) in the cytosol [49, 50].
The neurotransmitter dopamine has been also related to the pathogenesis of Parkinson's disease. In vitro experimental researches on neuronal cell types and isolated brain mitochondria and in vivo studies using different animal models and postmortem brain studies in Parkinson's disease have demonstrated that dopamine oxidation and reactive dopamine quinone oxidation products induce mitochondrial respiration uncoupling and cause ATP levels reduction and inactivate proteasomal activity, among other effects, which contribute to mitochondrial dysfunction [51–57]. The role of tetrahydrobiopterin (BH4) in Parkinson's disease etiology is also remarkable; BH4 is an obligatory cofactor for the dopamine synthesis enzyme tyrosine hydroxylase and it is present selectively in monoaminergic neurons in the brain. It has been suggested as an endogenous molecule that contributes to the dopaminergic neurodegeneration through an inhibition of the activities of complexes I and IV of the electron transport chain (ETC), together with a release of mitochondrial cytochrome C and a reduction of mitochondrial membrane potential [58].
There are other studies which involve calcium excitotoxicity and nonexcitotoxicity related mechanisms in the etiology of Parkinson's disease. Alterations in calcium influx in neurons via L-type voltage-dependent channels and N-methyl-D-aspartate (NMDA) receptors may lead to an excitotoxic cellular calcium accumulation that can cause mitochondrial dysfunction by reducing ATP production, activating mitochondrial permeability transition, increasing ROS generation, and inducing mitochondrial-dependent apoptosis [59, 60]. Other circumstances, not ordinarily toxic, have been reported to contribute to mitochondrial dysfunction. Hence, Sheehan et al. (1997) showed using mitochondrially transformed cells (cybrids) that the capacity to sequestrate calcium was lower in patients with Parkinson's disease than in control subjects, suggesting that this homeostasis alteration could increase neurons cell death [61].
Regarding familial Parkinson's disease, mutations in several genes previously reported (Parkin, PINK1, DJ-1, α-synuclein, and LRRK2) which encode for mitochondrial proteins have been identified to contribute to mitochondrial dysfunction [62, 63].
There are different neurotoxins including rotenone, 1-methyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA), and paraquat, among others, which have been extensively used as Parkinson's disease experimental models to mimic the neuropathology of this neurodegenerative disorder in both in vitro (i.e., human neuroblastoma SK-N-SH cells) and in vivo (animals models such as rats, mice) investigations and, consequently, to help establish neuroprotective strategies. Rotenone, an insecticide extracted from the roots of Derris spp. and Lonchocarpus spp. (Leguminosae family), acts by inhibiting the mitochondrial respiratory chain complex I [64]. Paraquat (1,1-dimethyl-4,4′-bipyridinium dichloride), which is a quaternary nitrogen herbicide used to control weed growth, has been reported to increase ROS generation and induce α-synuclein fibril formation [65]. The 1-methyl-1,2,3,6-tetrahydropyridine (MPTP), a byproduct obtained during the chemical synthesis of a meperidine analog, is metabolized in the brain to the toxic compound MPP+ which inhibits complex I of the electron transport chain [44]. The catecholaminergic neurotoxin 6-hydroxydopamine (6-OHDA), via intracerebral infusion, causes the irreversible loss of nigrostriatal dopaminergic neurons by inducing ROS production and inhibiting complex I and complex IV of the electron transport chain [66].
2.2. Mitochondria-Targeted Protective Compounds in PD
Endogenous and exogenous compounds are in continuing investigation as mitochondria-targeted agents to prevent or treat Parkinson's disease (Figure 2). Table 1 reports compounds that have been demonstrated to be promising agents in the protection of mitochondrial dysfunction in different Parkinson's disease models. Hence, among the endogenous compounds investigated so far, the hormone melatonin, the neuropeptide cocaine, and amphetamine regulated transcript (CART), the ursodeoxycholic acid, the mitoQ (mitoquinone mesylate), and the α-lipoic acid can be highlighted. The hormone melatonin has been shown to exert in vivo mitochondrial protective action in MPTP-induced mice model, 6-OHDA rat model, and rotenone-induced rat model by maintaining mitochondrial membrane potential, increasing antioxidant enzymatic (i.e., SOD, CAT) and nonenzymatic levels (i.e., glutathione), inhibiting ROS overproduction, increasing ATP production, decreasing calcium concentration levels, and enhancing mitochondrial complex I activity [67–71]. The neuropeptide cocaine and amphetamine regulated transcript (CART) protected mitochondrial DNA and cellular proteins and lipids of human neuroblastoma SH-SY5Y cells, HEK293 cells, and cultures of cortical and hippocampal neurons exposed to hydrogen peroxide [72]. The ursodeoxycholic acid (one of the secondary bile acids) and the mitoQ (mitoquinone mesylate) acted as antiapoptotic agent in human neuroblastoma SH-SY5Y cells treated with SNP and 6-OHDA, respectively [73, 74]. The α-lipoic acid has been evaluated as mitochondrial-targeted protective compound in several in vitro and in vivo Parkinson's disease models (i.e., PC12 cells, SK-N-MC cells, and rat model; toxins as MPP(+) and rotenone); this organosulfur compound derived from octanoic acid protects mitochondria by inhibiting ROS production, increasing glutathione levels, and maintaining mitochondrial membrane potential [75–78]. Pyruvate has also been demonstrated in in vitro studies that maintains mitochondrial membrane potential and inhibits ROS generation and nuclear translocation of NF-kappaB as well as mitochondrial apoptotic pathway [79, 80].
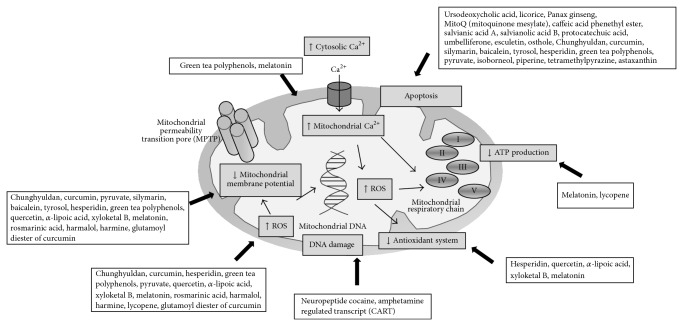
Figure 2.
Mechanisms of mitochondrial dysfunction and mitochondria-targeted drugs that have produced beneficial effect in PD models.
Table 1.
Parkinson's disease and mitochondria-targeted protective compounds.
| Compound | Class of compound | Parkinson's model | Mechanisms | References |
|---|---|---|---|---|
| Acetyl-L-carnitine | Quaternary ammonium | In vitro human neuroblastoma SK-N-MC cells model of rotenone-induced PD | Maintenance of mitochondrial integrity and function: ↑ mitochondrial biogenesis ↓ ROS generation |
[65] |
|
| ||||
| Astaxanthin | Nonprovitamin A carotenoid | MPP(+)-induced mouse model In vitro human neuroblastoma SK-N-SH cells model of MPP+-induced PD |
Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax and α-synuclein ↓ caspase-3 |
[81] |
|
| ||||
| In vitro human neuroblastoma SH-SY5Y cells model of 6-OHDA-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential; maintain mitochondrial redox activity |
[82] | ||
| Baicalein | Flavonoid | In vitro rat adrenal pheochromocytoma PC12 cells model and isolated rat brain mitochondria of rotenone induced PD | Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-3 and caspase-7 Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation ↑ intracellular ATP production |
[83] |
| In vitro human neuroblastoma SH-SY5Y cells model of 6-OHDA-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-3 and caspase-9 ↓ p-c-Jun N-terminal kinase (JNK) |
[84] | ||
|
| ||||
|
Caffeic acid phenethyl ester |
Phenolic compound | 6-OHDA-induced rat PD model | Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-3 and caspase-9 ↓ cytochrome c release Maintenance of mitochondrial integrity and function |
[85] |
| Primary cultures of cerebellar granule neuron 6-OHDA-induced PD | Inhibition of the mitochondrial apoptotic pathway, ↓caspase-3 ↓ cytochrome c release |
[86] | ||
|
| ||||
| CNB-001 | Pyrazole derivative of curcumin | MPTP-induced rodent PD model | Maintenance of normal mitochondrial morphology and size | [87] |
| In vitro human neuroblastoma SK-N-SH cells model of rotenone-induced PD | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax ↓ caspase-3 and caspase-9 ↓ cytochrome c release Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential |
[88] | ||
|
| ||||
| Curcumin | Polyphenol | PC12 cells mutant A53T α-synuclein-induced cell death | Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-3 and caspase-9 ↓ cytochrome c release Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation |
[89] |
| Mouse brain and the 1RB3AN27 (N27) rat dopaminergic neuronal cell line model of buthionine sulfoximine-induced PD | Maintenance of mitochondrial integrity and function: ↑ activities of complex I ↓ oxidative damage to proteins ↑ glutathione |
[90] | ||
|
| ||||
| DL-3-n-butylphthalide (NBP) | Synthetic compound based on L-3-n-butylphthalide | In vitro rat adrenal pheochromocytoma (PC12) cells model of MPP(+)-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation ↑ glutathione |
[91] |
|
| ||||
| Echinacoside | Phenylethanoid glycoside | In vitro rat adrenal pheochromocytoma (PC12) cells model of H2O2-induced PD | Inhibition of the mitochondrial apoptotic pathway | [92] |
|
| ||||
| Edaravone | Pyrazole derivative | Rotenone-induced rat PD model | Inhibition of the mitochondrial apoptotic pathway: ↓ Bax Maintenance of mitochondrial integrity and function: ↓ ROS generation |
[93] |
|
| ||||
| Esculetin | Coumarin | MPTP-induced mice PD model | Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-3 |
[94] |
|
| ||||
| Ginsenoside Re | Panaxatriol saponin | PINK1 null cells | Reversing the deficit in complex IV activity: ↑ LRPPRC, Hsp90, and Hsp60 levels |
[95] |
|
| ||||
| Glutamoyl diester of curcumin | Polyphenol | Mouse brain mitochondria induced-peroxynitrite PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation |
[96] |
|
| ||||
| Harmalol | Beta-carboline | In vitro rat adrenal pheochromocytoma (PC12) cells model of SNAP-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation |
[97] |
|
| ||||
| Harmine | Beta-carboline | In vitro rat adrenal pheochromocytoma (PC12) cells model of SNAP-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation |
[97] |
|
| ||||
| Hesperidin | Polyphenol | In vitro human neuroblastoma SK-N-SH cells model of rotenone-induced PD | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax ↓ caspase-3 and caspase-9 ↓ cytochrome c release Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation ↑ glutathione |
[98] |
|
| ||||
| Isoborneol | Monoterpenoid alcohol | In vitro human neuroblastoma SH-SY5Y cells model of 6-OHDA-induced PD | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax ↓ caspase-3 ↓ cytochrome c release |
[99] |
|
| ||||
| Kaempferol | Flavonoid | In vitro human neuroblastoma SH-SY5Y cells model and primary neurons of rotenone-induced PD | ↑ Autophagy | [100] |
|
| ||||
| α-Lipoic acid | Organosulfur compound derived from octanoic acid | In vitro rat adrenal pheochromocytoma PC12 cells model of MPP(+)-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation |
[75] |
| Rotenone-induced rat PD model | Maintenance of mitochondrial integrity and function: ↑ mitochondrial complex I activity ↑ glutathione |
[76] | ||
| In vitro human neuroblastoma SK-N-MC cells model of rotenone-induced PD | Maintenance of mitochondrial integrity and function: ↑ mitochondrial biogenesis ↓ ROS generation |
[77] | ||
| In vitro rat adrenal pheochromocytoma (PC12) cells model of PD | Maintenance of mitochondrial integrity and function: ↑ mitochondrial complex I activity ↑ glutathione |
[78] | ||
|
| ||||
| Lycopene | Carotenoid | In vitro human neuroblastoma SH-SY5Y cells model of MPP(+)-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation ↓ lipid peroxidation ↑ intracellular ATP production ↑ mitochondrial DNA copy numbers and mitochondrial RNA transcript levels |
[101] |
| Rotenone-induced rat PD model | Inhibition of the mitochondrial apoptotic pathway: ↓ cytochrome c release Maintenance of mitochondrial integrity and function: ↓ lipid peroxidation ↑ SOD activity ↑ glutathione |
[102] | ||
|
| ||||
| MPTP-induced mice PD model | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↑ antioxidant enzyme levels ↑ intracellular ATP production |
[67] | ||
| Rotenone-induced rat PD model | Maintenance of mitochondrial integrity and function: ↓ ROS generation ↑ glutathione ↑ SOD and catalase activities |
[68] | ||
| Melatonin | Hormone | Rotenone-induced isolated rat brain mitochondria PD model | Maintenance of mitochondrial integrity and function: ↓ Ca2+ levels ↓ ROS generation |
[69] |
| 6-OHDA-induced rat PD model | Maintenance of mitochondrial integrity and function: ↑ mitochondrial complex I activity |
[70] | ||
| MPP(+)-induced isolated rat liver mitochondria and striatal synaptosomes PD model | Maintenance of mitochondrial integrity and function: ↑ mitochondrial complex I activity |
[71] | ||
|
| ||||
| MitoQ (mitoquinone mesylate) |
— | In vitro human neuroblastoma SH-SY5Y cells model of 6-OHDA-induced PD | Inhibition of the mitochondrial apoptotic pathway: ↓ Bax ↓ Drp1 |
[73] |
|
| ||||
| N-Acetylcysteine | Amino acid derivative | H2O2 and toxic quinones derived from dopamine-induced rat PD model | Maintenance of mitochondrial integrity and function: ↑ mitochondrial respiratory activity ↑ Na+, K+-ATPase activity |
[103] |
|
| ||||
| Neuropeptide cocaine and amphetamine regulated transcript (CART) | Peptide | In vitro human neuroblastoma SH-SY5Y, HEK293 cells, and cultures of cortical and hippocampal neurons of H2O2-induced PD | Maintenance of mitochondrial integrity and function: protection of mitochondrial DNA (mtDNA), cellular proteins, and lipids | [78] |
|
| ||||
| Osthole | Coumarin | In vitro rat adrenal pheochromocytoma (PC12) cells model of MPP(+)-induced PD | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax ↓ caspase-3 ↓ cytochrome c release |
[104] |
|
| ||||
| Piperine | Alkaloid | 6-OHDA-induced rat PD model | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax ↓ caspase-3 and caspase-9 ↓ cytochrome c release |
[105] |
|
| ||||
| Polyhydroxylated fullerene derivative C(60)(OH)(24) | — | In vitro human neuroblastoma cells model of MPP(+)-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation ↑ activities of complexes I and II ↓ oxidative damage to DNA and proteins |
[106] |
|
| ||||
| Protocatechuic acid | Polyphenol | In vitro rat adrenal pheochromocytoma (PC12) cells model of MPP(+)-induced PD | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ caspase-3 Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation ↑ glutathione |
[107] |
|
| ||||
| Pyruvate | Organic acid | In vitro human neuroblastoma SK-N-SH cells model of H2O2-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation |
[79] |
| In vitro rat adrenal pheochromocytoma (PC12) cells model of dopamine-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation ↓ p53 ↓ nuclear translocation of NF-kappaB Inhibition of the mitochondrial apoptotic pathway |
[80] | ||
|
| ||||
| Quercetin | Bioflavonoid | Rotenone-induced rat PD model | Maintenance of mitochondrial integrity and function: ↓ ROS generation maintain mitochondrial membrane potential |
[108] |
| In vitro glial-neuronal system model of MPP(+)-induced PD | Maintenance of mitochondrial integrity and function: ↓ inducible nitric oxide synthase protein expression ↓ superoxide radicals |
[109] | ||
|
| ||||
| Rapamycin | Macrolide | 6-OHDA-induced rat PD model | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax ↓ caspase-9 ↓ cytochrome c release Maintenance of mitochondrial integrity and function: ↓ lipid peroxidation ↑ SOD and GSH-PX |
[110] |
|
| ||||
| Resveratrol | Polyphenol | In vitro primary fibroblasts cultures from patients with parkin mutations (PARK2) | Maintenance of mitochondrial integrity and function: ↑ complex I activity ↑ citrate synthase activity ↑ basal oxygen consumption ↑ mitochondrial ATP production ↓ lactate content |
[111] |
|
| ||||
| Rosmarinic acid | Polyphenol | In vitro MES23.5 dopaminergic cells model of 6-OHDA-induced PD. | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation |
[112] |
|
| ||||
| Salvianic acid A | Polyphenol | In vitro human neuroblastoma SH-SY5Y cells model of MPP(+)-induced PD | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax |
[113] |
|
| ||||
| Salvianolic acid B | Polyphenol | In vitro human neuroblastoma SH-SY5Y cells model of 6-OHDA-induced PD | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax ↓ caspase-3 ↓ cytochrome c release |
[114] |
|
| ||||
| Sesamin | Lignan | In vitro glial-neuronal system model of MPP(+)-induced PD | Maintenance of mitochondrial integrity and function: ↓ inducible nitric oxide synthase protein expression ↓ superoxide radicals |
[22] |
|
| ||||
| Tetramethylpyrazine | Pyrazine | MPTP-induced rat PD model | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax ↓ caspase-3 ↓ cytochrome c release |
[115] |
|
| ||||
| Tyrosol | Phenolic compound | MPP(+)-induced CATH.a cells PD model | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential; maintain intracellular ATP production Inhibition of the mitochondrial apoptotic pathway: (i) activation of PI3K/Akt signalling pathway |
[116] |
|
| ||||
| Umbelliferone | Coumarin | MPTP-induced mice PD model | Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-3 |
[94] |
|
| ||||
| Ursodeoxycholic acid |
Secondary bile acids | In vitro human neuroblastoma SH-SY5Y cells model of SNP-induced PD | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 ↓ Bax ↓ caspase-3, caspase-7, and caspase-9 ↓ cytochrome c release Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation ↑ glutathione |
[74] |
|
| ||||
| Xyloketal B | Triterpenoid | In vitro rat adrenal pheochromocytoma (PC12) cells model and Caenorhabditis elegans of MPP(+)-induced PD | Maintenance of mitochondrial integrity and function: maintain mitochondrial membrane potential ↓ ROS generation ↑ glutathione |
[117] |
Several natural products from medicinal plants, both isolated compounds and extracts, have been demonstrated in in vitro and in vivo studies to exert promising mitochondrial protection. As extracts, it has been reported that berries rich in anthocyanidins and proanthocyanidins protect mitochondria from rotenone-induced changes in the respiratory chain [118]. The silymarin, which is a standardized extract of the milk thistle seeds, maintained mitochondrial integrity and function and inhibited mitochondrial apoptotic pathway in MPP(+)-induced rat model [119]. Green tea polyphenols have been also evidenced to inhibit mitochondrial apoptotic pathway (increasing Bcl2 and decreasing caspase-3 activity) and to maintain mitochondrial membrane potential, to inhibit ROS production and calcium concentration levels [120]. The licorice (root of Glycyrrhiza glabra) inhibited dopaminergic apoptotic cell death as evidenced in the increase in Bcl2 levels and in the decrease in Bax levels, caspase-3 activity, cytochrome c release, and JNK and MAP activities in a model of 6-OHDA-induced Parkinson's disease [121]. The water extract of Panax ginseng also inhibited apoptosis MPP(+)-induced in the human neuroblastoma SH-SY5Y cells by decreasing Bax levels, caspase-3 activity, and cytochrome release and increasing Bcl2 levels [122].
The herbal medicine Chunghyuldan inhibited caspase-3, ROS generation and maintained mitochondrial membrane potential in 6-OH Parkinson's disease model [123]. Among isolated natural products, highlight those with polyphenol structure. The polyphenol resveratrol has been demonstrated in in vitro primary fibroblasts cultures from patients with parkin mutations (PARK2) to regulate mitochondrial energy homeostasis as evidenced in the increment of complex I activity, citrate synthase activity, basal oxygen consumption, and ATP production and in the decrement of lactate content [111]. The polyphenol hesperidin inhibited mitochondrial apoptotic pathway (increased Bcl2 levels and decreased Bax, caspase-3, and caspase-9 activities and inhibited cytochrome c release), maintained mitochondrial membrane potential, inhibited ROS production, and increased glutathione levels in in vitro human neuroblastoma SK-N-SH cells model of rotenone-induced Parkinson's disease [98]. Quercetin rescued toxic-induced defects in mitochondria in in vitro and in vivo experiments. Quercetin inhibited ROS generation and maintained mitochondria membrane potential in rotenone-induced rat model [108]. Moreover, quercetin decreased the production of superoxide radicals and inhibited the expression of the inducible nitric oxide synthase protein expression in in vitro glial-neuronal system model of MPP(+)-induced Parkinson's disease [109]. The flavonoid baicalein inhibited in vitro apoptotic mitochondrial cell death and maintained mitochondrial integrity and function in both SH-SYTY and PC12 cells in 6-OHDA and rotenone Parkinson's disease models as evidenced in the decrease in caspase-3, caspase-7, caspase-9, and JNK activities and in the maintenance of mitochondrial membrane potential, increment of ATP content and reduction of ROS production [82–84]. The tyrosol protected CATH.a cells against MPP(+)-toxicity by inhibiting apoptotic cell death via activation of PI3K/Akt signaling pathway and by maintaining ATP production and mitochondria membrane potential [116]. The caffeic acid phenethyl ester inhibited 6-OHDA-induced mitochondrial apoptotic pathway in in vitro and in vivo models [85, 86]. The curcumin polyphenol derived from the spice turmeric acts as mitochondrial antiapoptotic agent through the inhibition of caspase-3 and caspase-9 activities and cytochrome c release and it also protects mitochondrial integrity and function via ROS production inhibition and complex I activity enhancement [89, 90]. In addition to curcumin, its synthetic pyrazole derivative compound, CNB-001, has been also studied, which avoids rotenone-induced mitochondrial damage in the human neuroblastoma SK-N-SH cells by inhibiting mitochondrial apoptotic pathway and maintaining mitochondrial structure [87, 88]. Glutamoyl diester of curcumin has also been shown to maintain mitochondrial membrane potential and to inhibit ROS production in mouse brain mitochondria induced-peroxynitrite Parkinson's disease model [96]. Salvianic acid A and salvianolic acid B isolated from Salvia spp. as well as protocatechuic acid afford in vitro protection through antiapoptotic pathway [107, 113, 114]. The flavonoid kaempferol exerts antiparkinsonian effect via autophagy [100] and rosmarinic acid maintains mitochondria protection by maintaining mitochondrial membrane potential and decreasing ROS production [112].
Other natural products with mitochondrial protective effect are the coumarins umbelliferone, esculetin, and osthole which in in vitro and in vivo Parkinson's disease models have been demonstrated to possess antiapoptotic properties on mitochondria [94, 104]. Other compounds that exert protection via inhibition of the mitochondrial apoptotic pathway are the monoterpenoid alcohol isoborneol [99], the alkaloid piperine [105], the pyrazine tetramethylpyrazine [81], and the nonprovitamin A carotenoid astaxanthin [115]. On the other hand, the β-carboline alkaloids harmalol and harmine maintained mitochondria membrane potential and decreased ROS generation in PC12 cells exposed to S-nitroso-N-acetyl-DL-penicillamine (SNAP) [97]. Moreover, the triterpenoid xyloketal B also maintained mitochondria membrane potential and decreased ROS generation and it increased glutathione levels in in vitro rat adrenal pheochromocytoma (PC12) cells and Caenorhabditis elegans of MPP(+)-induced PD [117]. Furthermore, the carotenoid lycopene inhibited macromolecular mitochondrial damage (lipids, DNA, and proteins), overproduction of ROS, ATP failed production, and cytochrome c release in MPP(+)-induced human neuroblastoma SK-N-SH cells and rotenone-induced rat model [101, 102].
3. Alzheimer's Disease and Mitochondrial-Targeted Protective Compounds
3.1. Alzheimer's Disease
Alzheimer's disease (AD) is a neurodegenerative disease characterized by progressive cognitive decline leading to complete need for care within several years after clinical diagnosis [124]. AD is the most common form of dementia, being the most prevalent neurodegenerative disease (followed by Parkinson's disease), and accounts for approximately 65% to 75% of all dementia cases. It has been estimated that Alzheimer's disease affects over 44 million people worldwide, mainly after the age of 65 years [125, 126]. The incidence of AD augments with age in an exponential manner and its prevalence increases from 3% among individuals aged 65–74 to almost 50% among those 85 or older; these numbers can be translated to the extremely high health care costs that AD represents [127]. In addition, because of the aging of the population, it is expected that the prevalence will quadruple by 2050, which means 1 in 85 persons worldwide will be living with the disease [128].
AD is a progressive neurodegenerative disease with a marked late onset (late diagnosis as well) and mainly characterized by progressive decline of cognitive functions, memory, and changes in behavior and personality [129, 130]. The two major pathophysiological hallmarks that have been observed in postmortem brains of AD patients include extracellular β-amyloid protein (Aβ) deposits in the form of senile plaques and intracellular deposition of the microtubule-associated protein tau as neurofibrillary tangles, especially abundant in the regions of the brain responsible for learning and memory. These features have been linked to an abnormally enhanced neuronal loss in this condition, especially affecting cholinergic neurons and consequently leading to a reduction in the levels of the neurotransmitter acetylcholine in the hippocampus and cortex areas of brains of AD patients. Moreover, AD has also been associated with the loss of synapses, synaptic function, inflammatory responses involving glial cells, and mitochondrial abnormalities [131–133].
Considering AD pathogenesis, multiple etiological factors including genetics, environmental factors, diet, and general lifestyles have to be taken into account [134]. Most of the cases of AD are believed to be “sporadic” and their causal factors are still unknown for the vast majority of patients; on the other hand, genetic factors cause about 2% of all AD cases and include mutations in APP (Aβ protein precursor), presenilin-1 and presenilin-2 genes, and polymorphisms in apolipoprotein allele E4 [135, 136].
Due to the complex and not fully understood etiopathology of AD, no available drug has been shown to completely protect neurons in AD patients, and there is a continuous search for new compounds and therapeutic tools. There are two possible conceptual approaches to the treatment of AD. The first one is a symptomatic treatment that tries to minimize tertiary cognitive symptoms and protects from further cognitive decline; it is the most common therapeutic tendency and drugs such as tacrine, donepezil, and rivastigmine have been used with this purpose with limited efficacy. Another approach is the treatment addressed to prevent the onset of the disease by sequestering the primary progenitors or targets, to reduce the secondary pathologies of the disease, to slow disease progression, or to delay onset of disease, by preventing or attenuating neuronal damaging factors [137, 138]. With regard to this, compounds that exert activity against oxidative stress and mitochondrial dysfunction in AD (as discussed below) deserve to be considered as potential therapeutic options.
During the last two decades, consistent evidences have proposed oxidative stress as a crucial pathogenic mechanism underlying AD [139]. Oxidative stress (OS) occurs when the production of reactive oxygen species (ROS) exceeds the antioxidant enzymatic and nonenzymatic cellular mechanisms. Actually, the β-amyloid peptide Aβ 1–42 (insoluble form), which forms the senile plaques, exerts neurotoxicity involving OS in AD. Particularly, this Aβ 1–42 has the ability to produce ROS, mainly hydrogen peroxide, when it reacts with transition metal ions present in senile plaques [140]. As a result of OS, accumulated oxidative damage to lipids, proteins, and nucleic acids in postmortem studies of brains of patients with AD has been identified: advanced glycation end-products (AGEs), advanced lipid peroxidation end-products, nucleic acid oxidation, carbonyl-modified neurofilament protein, and free carbonyls [141]. The brain is more susceptible to OS than other organs because of a low antioxidative protection system, which allows for increased exposure of target molecules to ROS; the higher level of ROS, together with neuroinflammation and excessive glutamate levels, is proposed to contribute to neuronal damage and death in AD [142].
3.1.1. Mitochondrial Dysfunction in AD
Mitochondria are the primary source of ROS, and oxidative damage to mitochondrial components precedes damage to any other cellular component during the development of neurodegenerative diseases [143]. Actually, mitochondrial dysfunction has largely been demonstrated as one of the main key cytopathologies of AD [144, 145]. Numerous evidences suggest the involvement of β-amyloid protein deposits in the mitochondrial dysfunction found in AD as a plausible mechanism for its neurodegenerative effects [146–148]. In support of this, it has been shown that cells depleted of endogenous mDNA lacking functional electron transport chains (ETC) are resistant to Aβ toxicity [149]; also, a reduced respiratory capacity and low cytochrome oxidase activity were found in isolated mitochondria exposed to Aβ [150, 151]; transgenic mice expressing mutant APP (amyloid protein precursor) genes exhibit mitochondrial dysfunction, and an AD transgenic mouse line presents early expression of genes encoding mitochondrial proteins and ETC subunits, as an initial cellular change in AD pathology [152].
Mitochondria have been shown to be a direct site of Aβ accumulation in AD neurons, and various experimental models of AD were used by researchers to verify the effect of that specific accumulation on cell death [153]. Actually, Manczak et al. proved an association between mutant APP derivatives (Aβ monomers and oligomers, such as Aβ 1–40 and Aβ 1–42) and mitochondria in cerebral cortex slices from Tg2576 mice and N2a cells expressing mutant APP. Such accumulation supposes an increase in mitochondrial ROS production together with a reduced Cyt C oxidase activity, thus relating in vivo oxidative stress and impaired mitochondrial metabolism to the toxic effects of Aβ peptides [154]. Further, Devi et al. demonstrated that the mitochondrial dysfunction in human AD brain is associated with the abnormal accumulation of APP across the mitochondrial import channels. In postmortem evaluations, it was evidenced that nonglycosylated full-length and C-terminal truncated APP had been accumulated exclusively in the protein import channel of mitochondria of AD brains (specially higher accumulation in AD-vulnerable regions, such as cortex, hippocampus, and amygdala), by forming stable complexes with the outer membrane translocase and/or the inner membrane translocase; the effect of such association could inhibit the entry of nuclear encoded Cyt C oxidase protein, thus diminishing its activity in mitochondria and increasing the levels of H2O2. The higher the level of arrested mitochondrial APP, the worse the mitochondrial dysfunction [155].
What is more, a recent study indicated that mitochondria-targeted Aβ 1–42 accumulation is the necessary and sufficient condition for Aβ-mediated mitochondrial impairments and derived cellular death. In an in vitro model of mice hippocampal cell line (HT22 cells), an exogenous Aβ 1–42 treatment caused a deleterious alteration in mitochondrial morphology and function, which was blocked by a clathrin-mediated endocytosis blocker; besides, specific mitochondria-targeted accumulation of Aβ 1–42 in HT22 cells using a mitochondria-targeting sequence reproduced the same morphological and functional alterations of mitochondria as those observed in APP mutant mice model and the previous Aβ 1–42-treated HT22 cells. Mitochondria-mediated apoptotic cell death was observed in both models, thus implying that no other signaling alteration induced by Aβ plays a more relevant role in cell death than its mitochondrial toxicity [156].
In general, mitochondrial dysfunction in AD is essentially characterized by diminution in complex IV activity (cytochrome c oxidase), decline in other enzymes of tricarboxylic acids cycle, and mutations to mDNA. The mechanism that underlies the complex IV defect is not clearly known, but a study on SK-N-SH cells exposed to Aβ-induced toxicity showed a decrease in mDNA encoded complex IV subunits, at both the mRNA and protein levels; this finding suggests a possible relationship between decreased complex IV activity and mDNA perturbation [157]. Results from cybrids studies also imply that AD is characterized by specific mDNA mutations that correlate with defects in certain mitochondrial respiratory complexes. These changes generate an increased production of oxidant species and free radicals, such as hydrogen peroxide. In turn, a deficiency in energy metabolism and ATP generation is a serious consequence of impaired mitochondrial function [158, 159]. In addition, deficiency in scavenging mitochondrial free radicals may similarly contribute to the excessive oxidative damage in the affected brain regions in AD. For instance, decreased mitochondrial MnSOD expression level has been found in AD patients as well as decreased Coenzyme Q in peripheral tissues and brains [160, 161]. Therefore, a relationship between the mitochondrial dysfunction and the oxidative stress situation is established.
Neurodegeneration and synaptic degradation in AD are primarily mediated by defective mitochondrial biogenesis and axonal transport of mitochondria [162]. Normal mitochondrial dynamics, an essential function in maintaining cell viability, is likewise impaired in AD. Disturbances affecting the balance of fusion and fission processes trigger serious mitochondrial changes and lead to cellular perturbations, such as apoptosis. Recent studies have found altered levels of mitochondrial fusion (including MNF-1/2 and OPA1) and fission (FIS1) proteins in AD hippocampal tissues, meaning decreased fusion and increased fission processes; mitochondrial fission protein DLP1 has also been found to be decreased in hippocampal neurons [163]. Moreover, mitochondrial calcium overload is another feature of mitochondrial dysfunction in AD; Aβ has been shown to cause calcium overload that then causes increased free radical accumulation and provokes the formation of mitochondrial transition pore (mPTP), thus leading to exacerbation of cytoplasmic calcium and eventual neuronal death [164].
Further, mitochondria play a pivotal role in aging and senescence, contributing to neural dysfunction with age. They are actually the main cellular organelle implicated in the process of neuronal apoptosis, which takes place in an excessive manner in AD brains [165]. The fact that many neurons undergo apoptosis in AD is evidenced by the presence of high levels of activated proapoptotic proteins such as caspase-3 and Bax in neurons that exhibit neurofibrillary tangle pathology [166].
Concerning AD models of study, unfortunately, there is no animal model so far that replicates all the major aspects of AD pathology and symptoms, and models based on postulated disease pathways are widely used to explore biological targets [167]. Regarding the investigation of the effects of compounds on mitochondrial dysfunction, rodent transgenic models are very common for reproducing the mitochondriopathy features in AD. For instance, an APP (amyloid precursor protein) mice transgenic model demonstrated an accelerated upregulation of the apoptotic-related factors involved in mitochondria-mediated apoptosis, such as Bax and caspase-3 [168]. Similarly, isolated mitochondria from APPSW mice (expressing the Swedish familial mutation in APP gene) presented an abnormally reduced mitochondrial respiratory rate, mitochondrial membrane potential (MMP) disruption, increased ROS generation, and lower ATP levels [169]. APP/PS1 transgenic mice include mutations both in APP and in presenilin-1 genes and show similar mitochondrial characteristics [170]. Other models even express more mutations, such as 3xTg-AD mouse model that includes three mutant human genes: APPswe, presenilin-1 (PS1M146V), and tau protein (tau P301L); in this model, MMP loss and higher caspases 3 and 9 activations are observed [171].
However, most of the works assessing mitochondrial defects in AD are still performed on toxin-induced in vitro models. With this respect, rat primary neurons in culture exposed to Aβ 1–42 oligomers reproduced the generation of mPTP in mitochondrial membrane with subsequent calcium overload, the MMP loss, and release of cytochrome C, thus leading to cell death via mitochondrial-mediated apoptosis [172]. Mouse neuroblastoma N2a cells cotransfected with Swedish mutant APP and Δ9 deleted presenilin-1 (N2a/Swe.Δ9) recapitulated similar loss of mitochondrial integrity and function and evidenced increased mitochondrial apoptotic pathway, with a higher Bax/Bcl2 ratio and augmented caspase-3 activity [173]. Recent studies have employed cybrid neurons resulting from incorporating platelet mitochondria from AD patients into mitochondrial DNA-depleted neuronal cells (SH-SY5Y cell line); this model demonstrates changes in length and density of mitochondria, imbalanced mitochondrial fission, and fusion dynamics (altered expression and distribution of DLP1 and Mfn2 proteins), together with reduced mitochondrial function and energy metabolism [174].
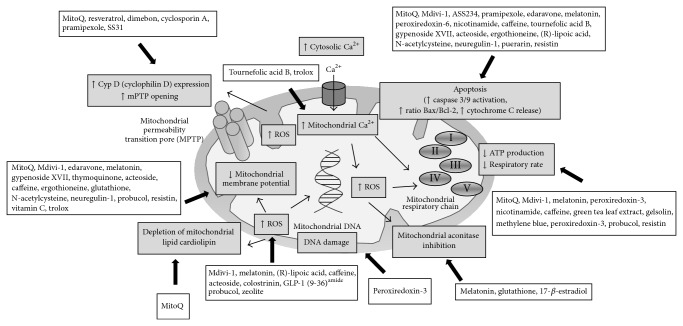
Therefore, it has been suggested that a substance of exogenous or endogenous origin that is able to reverse any of the aforementioned mitochondrial deficits may facilitate a better neuronal health and then be of interest of study as a potential active compound in AD therapy [175]. In fact, mitochondrial medicine is emerging as a field of research focused on the finding of therapeutic strategies to enhance mitochondrial function in aging and in those neurodegenerative diseases in which it has been shown to be impaired [176–178]. This avenue of investigation has led to the discovery of several agents directly targeted to mitochondria that are able to delay or revert the mitochondrial impairments associated to AD; all available information on these compounds is reviewed below and collected in Table 2 and schematized in Figure 3.
Table 2.
Alzheimer's disease and mitochondria-targeted protective compounds.
| Compound | Class of compound | AD model | Mechanism/effect | References |
|---|---|---|---|---|
| 17-β-Estradiol | Estrogen | In vitro isolated mitochondria from postmortem human AD and SFAD brains | Activation of mitochondrial aconitase | [179] |
|
| ||||
| Acetyl-L-carnitine | Amino acid | In vivo F344 rat model | Restoration of intact mitochondrial morphology and prevention from age-related mitochondrial decay | [180] |
|
| ||||
| Acteoside | Glycoside | In vitro Aβ25–35-induced SH-SY5Y human neuroblastoma cells model | Maintenance of mitochondrial integrity and function: ↓ ROS production ↓ MMP loss ↓ CytC release Inhibition of the mitochondrial apoptotic pathway: ↓ Bax/Bcl-2 ratio ↓ caspase-3 cleavage |
[181] |
|
| ||||
| ASS234 | Amine with complex structure | In vitro Aβ 1–42-induced SH-SY5Y human neuroblastoma cells model | Inhibition of the mitochondrial apoptotic pathway: ↓ levels of cleaved caspases 3 and 9 ↓ levels of proteolysed PARP |
[182] |
|
| ||||
| Caffeine | Alkaloid | In vivo APPsw mice model | Maintenance of mitochondrial integrity and function: restoration of mitochondrial respiratory rate, maintenance of MMP ↓ ROS production ↑ ATP levels |
[169] |
|
| ||||
| Colostrinin | Polypeptide | In vitro Aβ-induced human SH-SY5Y and rat PC12 cells model | Maintenance of mitochondrial integrity and function: ↓ mitochondrial H2O2 production |
[183] |
|
| ||||
| Crude caffeine | Alkaloid | In vitro primary neurons from J20 mouse line | Maintenance of mitochondrial integrity and function: ↑ ATP levels ↓ ROS production Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-3 activity |
[184] |
|
| ||||
| Cyclosporin A | — | In vitro isolated cortical mitochondria from mAPP-Ppif−/−transgenic mice | Maintenance of mitochondrial integrity and function: inhibition of mPTP opening ↑ Ca2+ buffering capacity |
[185] |
|
| ||||
| D609 | Tricyclodecan-9-yl-xanthogenate | In vitro Aβ 1–42-induced isolated brain mitochondria model (from gerbils) | Maintenance of mitochondrial integrity and function: ↓ levels of protein carbonyls ↓ levels of protein-bound HNE ↓ levels of 3-NT ↓ CytC release Maintenance of GSH/GSSG ratio ↑ GST, GPx, and GR activities |
[186] |
|
| ||||
| DAPT | Complex structure of butyl ester | In vitro N2a/APP695 and N2a/APPswe cells models | Maintenance of mitochondrial integrity and function: stabilization of normal MMP and ATP levels |
[187] |
|
| ||||
| Dimebon | Tetrahydrocarboline | In vitro Aβ 25–35-exposed isolated rat brain mitochondria | Maintenance of mitochondrial integrity and function: inhibition of mPTP opening |
[188] |
|
| ||||
| Edaravone | Pyrazolinone | In vitro N2a/Swe.Δ9 cells model | Maintenance of mitochondrial integrity and function, ↑ MMP ↓ ROS production ↓ CytC release Inhibition of the mitochondrial apoptotic pathway: ↓ Bax/Bcl2 ratio ↓ Caspase-3 activation |
[173] |
|
| ||||
| Ergothioneine | 2-Mercaptohistidine trimethylbetaine | In vitro Aβ 25–35-induced PC12 rat pheochromocytoma cells model | Maintenance of mitochondrial integrity and function: ↓ MMP loss Inhibition of the mitochondrial apoptotic pathway, ↓ ratio Bax/Bcl-XL ↓ caspase-3 activation |
[189] |
|
| ||||
| Gelsolin | Peptide | In vitro Aβ 1–42-induced rat epithelial cells model | Maintenance of mitochondrial integrity and function: increase in mitochondrial complex IV activity |
[190] |
|
| ||||
| Genistein | Phytoestrogen | Mitochondrial fraction of postmortem human AD brains | ↑ mitochondrial Na/K-ATPase activity | [191] |
|
| ||||
| GLP-1 (9-36)amide | Peptide | In vitro slices of hippocampus from APP/PS1 mice | Maintenance of mitochondrial integrity and function: ↓ abnormal mitochondrial SO levels |
[192] |
|
| ||||
| Glutathione | Tripeptide | In vitro Aβ-induced human HCN-1A cells model | Maintenance of mitochondrial integrity and function: ↓ mitochondrial membrane depolarization |
[193] |
| In vitro isolated mitochondria from postmortem human AD and SFAD brains | Activation of mitochondrial aconitase | [179] | ||
|
| ||||
| Gypenoside XVII | Phytoestrogen | In vitro Aβ 25–35-induced PC12 cells model | Maintenance of mitochondrial integrity and function: restoration of normal MMP ↓ CytC release Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-3 activation and cleavage ↓ PARP cleavage |
[194] |
|
| ||||
| JHX-4, HK-2, and HK-4 | — | In vitro Aβ-induced human SH-SY5Y cells model | Prevention from MnCl2-induced loss of mitochondrial activity | [195] |
|
| ||||
| Lipoic acid | Organosulfur compound derived from octanoic acid | In vivo F344 rat model | Restoration of intact mitochondrial morphology and prevention from age-related mitochondrial decay | [180] |
| In vitro fibroblasts from AD patients | Maintenance of mitochondrial integrity and function: ↓ NMP-induced mitochondrial oxidative stress Inhibition of the mitochondrial apoptotic pathway: ↓ Bax and caspase-9 levels |
[196] | ||
|
| ||||
| In vitro Aβ 25–35-induced mouse microglial BV2 cells model | Inhibition of the mitochondrial apoptotic pathway: activation of Bcl-2 antiapoptotic pathways ↓ Bax mRNA level ↑ Bcl-2 expression ↓ caspase-3 activity |
[197] | ||
| In vitro Aβ 25–35-induced rat hippocampal neurons model | Maintenance of mitochondrial integrity and function: restoration of MMP loss attenuation of respiratory chain complexes ↑ ATP levels |
[198] | ||
| Melatonin | Hormone | In vivo APP/PS1 transgenic mice | Maintenance of mitochondrial integrity and function: restoration of mitochondrial respiratory rates ↑ MMP ↑ ATP levels |
[170] |
| In vivo APPsw mice model | Maintenance of mitochondrial integrity and function, restoration of mitochondrial respiratory rate maintenance of MMP ↓ ROS production ↑ ATP levels |
[169] | ||
| In vitro isolated mitochondria from postmortem human AD and SFAD brains | Activation of mitochondrial aconitase | [179] | ||
| In vivo APP transgenic mice model | Maintenance of mitochondrial integrity and function, ↑ SOD activity Inhibition of the mitochondrial apoptotic pathway, ↓ Bax, caspase-3, and Par-4 expressions |
[168] | ||
|
| ||||
| Methylene blue | Phenothiazine | In vivo C57BL/6 mice | Maintenance of mitochondrial integrity and function, ↑ mitochondrial complex IV (CytC oxidase) activity ↓ MAO activity |
[199] |
|
| ||||
| In vitro Aβ-induced mouse neuroblastoma N2a cells model | Maintenance of mitochondrial integrity and function, ↓ abnormal expression of peroxiredoxins and mitochondrial structural genes normalization in mitochondria number |
[200] | ||
| Mito Q | Methanesulfonate | In vivo AβPP transgenic mice | Maintenance of mitochondrial integrity and function: ↓ CypD expression |
|
| In vivo 3xTg-AD mice | Maintenance of mitochondrial integrity and function: prevention of MMP loss Inhibition of the mitochondrial apoptotic pathway: blockage of caspases-3/7 activation |
[171] | ||
| In vivo Caenorhabditis elegans model overexpressing human Aβ peptides | Maintenance of mitochondrial integrity and function, ↓ depletion of mitochondrial lipid cardiolipin Protection of complexes IV and I of the ETC |
[201] | ||
|
| ||||
| Mitochondrial division inhibitor 1 (Mdivi-1) | Quinazolinone | In vitro Aβ-induced mouse BV-2 cells and primary microglial cells model | Maintenance of mitochondrial integrity and function: ↓ abnormal mitochondrial fission ↓ MMP loss ↓ CytC release Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-3 activation |
[182] |
| In vitro cybrid cells model (mtDNA-depleted neuronal SH5Y5Y cells transfected with platelet mitochondria from AD patient) | Maintenance of mitochondrial integrity and function: ↓ mitochondrial ROS production ↑ MMP ↑ mitochondrial length and density ↑ CytC oxidase activity ↑ SOD activity ↑ ATP levels ↓ impaired mitochondrial fission and fusion dynamics |
[174] | ||
|
| ||||
| N-Acetyl cysteine | Amino acid derivative | In vitroβ-amyloid-induced human HCN-1A cells model | Maintenance of mitochondrial integrity and function: ↓ mitochondrial membrane depolarization |
[193] |
| In vivo APP/PS-1 mice | Amelioration of energy levels and mitochondrial-related proteins | [202] | ||
| In vitro fibroblasts from AD patients | Maintenance of mitochondrial integrity and function: ↓ NMP-induced mitochondrial oxidative stress Inhibition of the mitochondrial apoptotic pathway: ↓ Bax and caspase-9 levels |
[196] | ||
|
| ||||
| Neuregulin-1 | Peptide | In vitro model of SH-SY5Y human neuroblastoma cells transfected with C-terminal fragments of APP | Maintenance of mitochondrial integrity and function: ↓ MMP loss Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 expression |
[203] |
|
| ||||
| Nicotinamide | Amide | In vivo Aβ 1–42-induced rat model | Upregulation of mitochondrial function Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 levels ↓ Bax levels |
[204] |
|
| ||||
| Peroxiredoxin 3 | Enzyme | In vivo APP transgenic mice (Tg2576) and APP/Prdx3 transgenic mice models | Maintenance of mitochondrial integrity and function: ↓ mitochondrial DNA oxidation ↑ activity of mitochondrial complexes I and IV |
[205] |
|
| ||||
| Peroxiredoxin 6 | Enzyme | In vitro Aβ 25–35-induced PC12 cells model | Inhibition of the mitochondrial apoptotic pathway: ↓ caspases 3 and 9 activation ↓ PARP activation ↓ Bcl-2 and Bax dysregulations |
[206] |
|
| ||||
| Probucol | — | In vitro cybrid cells model (mtDNA-depleted neuronal SH5Y5Y cells transfected with platelet mitochondria from AD patient) | Maintenance of mitochondrial integrity and function: ↓ mitochondrial ROS production ↑ MMP ↑ mitochondrial length and density ↑ CytC oxidase activity ↑ SOD activity ↑ ATP levels ↓ impaired mitochondrial fission and fusion dynamics |
[174] |
|
| ||||
| Puerarin | Phytoestrogen | In vivo Aβ 1–42-induced rat model | Inhibition of the mitochondrial apoptotic pathway: ↓ caspase-9 activity and mRNA levels |
[207] |
| In vitro cybrid cells model (mtDNA-depleted neuronal SH-5Y5Y cells transfected with platelet mitochondria from AD patient) | Inhibition of the mitochondrial apoptotic pathway: ↑ Bcl-2 levels ↓ Bax expression ↓ caspase-3 activity |
[208] | ||
|
| ||||
| R(+) and S(−) pramipexoles | — | In vitro Aβ 25–35-induced SH-SY5Y human neuroblastoma cells model | Maintenance of mitochondrial integrity and function: inhibition of mPTP opening Inhibition of the mitochondrial apoptotic pathway: ↓ caspases 3 and 9 activations |
[209] |
|
| ||||
| Resistin | Adipokine protein | In vitro mouse N2a/Swe.Δ9 cells (N2a cells transfected with Sw-APP mutant and presenilin exon 9 deletion mutant) | Maintenance of mitochondrial integrity and function: ↑ ATP levels ↑ MMP Inhibition of the mitochondrial apoptotic pathway: ↓ Bax/Bcl2 ratio ↓ CytC release ↓ caspase-3 activation |
[210] |
|
| ||||
| Resveratrol | Polyphenol | In vitro Aβ-induced mouse neuroblastoma N2a cells model | Maintenance of mitochondrial integrity and function: ↓ abnormal expression of peroxiredoxins and mitochondrial structural genes Normalization in mitochondria number |
[200] |
| In vivo AβPP transgenic mice | Maintenance of mitochondrial integrity and function: ↓ CypD expression |
|||
|
| ||||
| Salicylate Sulindac sulfide Indomethacin Ibuprofen R-flurbiprofen |
NSAIDs | In vitro Aβ1–42- and Aβ 25–35-induced rat cerebellar granule cells and cortical neurons model | Maintenance of mitochondrial integrity and function: ↑ MMP ↓ mitochondrial Ca2+ overload ↓ CytC release |
[172] |
|
| ||||
| SS31 | Peptide | In vitro Aβ 25–35-induced C57BL/6 mice hippocampal neurons model | Maintenance of mitochondrial integrity and function: ↓ levels of mitochondrial fission proteins (Drp1, Fis1), matrix protein, and CypD ↑ number of healthy and intact mitochondria Restoration of mitochondrial transport |
[162] |
| In vitro Aβ-induced mouse neuroblastoma N2a cells model | Maintenance of mitochondrial integrity and function: ↓ abnormal expression of peroxiredoxins and mitochondrial structural genes Normalization in mitochondria number |
[200] | ||
| In vivo AβPP transgenic mice | Maintenance of mitochondrial integrity and function: ↓ CypD expression |
|||
| In vitro Tg2576 mice primary neurons | Maintenance of mitochondrial integrity and function: restoration of mitochondrial transport ↓ percentage of defective mitochondria |
[211] | ||
|
| ||||
| Thymoquinone | Benzoquinone | In vitro Aβ 25–35-induced PC12 cells model | Maintenance of mitochondrial integrity and function: ↑ MMP |
[212] |
| In vitro Aβ 1–42-induced primary rat neurons model | Maintenance of mitochondrial integrity and function: ↑ MMP |
[213] | ||
|
| ||||
| Tournefolic acid B | Polyphenol | In vitro β 25–35-induced rat cortical neurons model | Maintenance of mitochondrial integrity and function: ↓ mitochondrial Ca2+levels delay in CytC release Inhibition of the mitochondrial apoptotic pathway: ↓ tBid levels |
[214] |
|
| ||||
| Trolox | Analog of vitamin E | In vitro dentate granule cells from transgenic mouse AD model (Tg2576) | Maintenance of mitochondrial integrity and function: scavenging of mitochondrial SO restoration of Ca2+ clearance ↑ MMP |
[215] |
|
| ||||
| UPF1 and UPF17 | Peptides | Mitochondrial fraction of postmortem human AD brains | ↑ Mitochondrial Mn-SOD activity | [191] |
|
| ||||
| Vitamin C | Vitamin | In vitro Aβ-induced human HCN-1A cells model | Maintenance of mitochondrial integrity and function: ↓ mitochondrial membrane depolarization |
[193] |
| In vivo 5XFAD Knockout-transgenic mice model | Maintenance of mitochondrial integrity and function: prevention of abnormal mitochondrial morphology |
[216] | ||
|
| ||||
| Wy-14.463 (peroxisome proliferator) | — | In vitro Aβ 1–40-induced primary rat hippocampal neurons model | Maintenance of mitochondrial integrity and function: ↑ MMP |
[217] |
|
| ||||
| Zeolite | — | In vitro H2O2-induced SH-SY5Y cells model | Maintenance of mitochondrial integrity and function: ↓ mitochondrial ROS production |
[218] |
| In vivo APPswePS1dE9 transgenic mice model | Significant increase in SOD activity | |||
ETC: electron transport chain; MMP: mitochondrial membrane potential; CytC: cytochrome C; SO: superoxide; SOD: superoxide dismutase; SwAPP: Swedish amyloid precursor protein; ROS: reactive oxygen species; 4HNE: 4-hydroxy-2-nonenal; AChE: acetylcholine esterase; GSH: glutathione; GR: glutathione reductase; GPx: glutathione peroxidase; SFAD: Swedish Familial Alzheimer's disease; MAO: monoamine oxidase; tBid: BH3 interacting domain death agonist; Bak: Bcl-2 agonist killer 1; mPTP: mitochondrial permeability transition pore; CypD: cyclosporine D; NMP: N-methyl protoporphyrin; SP-04: dimethyl-carbamic acid 2,3-bis-dimethylcarbamoyloxy-6-(4-ethyl-piperazine-1-carbonyl)-phenyl ester; SP-04m: 4-(4-ethyl-piperazin-1-yl)-1-(2,3,4-trihydroxy-phenyl)-butan-1-one; CCCP: carbonyl cyanide 3-chlorophenylhydrazone; FCCP: carbonyl cyanide p-trifluoromethoxy-phenylhydrazone; PAO: phenylarsine oxide; PARP: poly-ADP-ribosyl polymerase; mtDNA: mitochondrial DNA; Par-4: prostate apoptosis response-4; 3-NT: 3-nitrotyrosine; GSH: reduced glutathione; GSSG: oxidized glutathione; GST: glutathione-S-transferase; GPx: glutathione peroxidase; GR: glutathione reductase; ASS234: N-((5-(3-(1-benzylpiperidin-4-yl)propoxy)-1-methyl-1H-indol-2-yl)methyl)-N-methylprop-2-yn-1-amine; APP: amyloid precursor protein; Aβ: β amyloid; NSAIDs: nonsteroidal anti-inflammatory drugs.
Figure 3.
Mechanisms of mitochondrial dysfunction and mitochondria-targeted drugs that have produced beneficial effect in AD models.
3.2. Mitochondria-Targeted Protective Compounds in AD
3.2.1. Synthetic Compounds
Several mitochondria-targeted antioxidants have been designed by conjugating the lipophilic triphenylphosphonium (TPP+) cation to an antioxidant moiety, such as coenzyme Q (CoQ), obtaining as a result compounds like MitoQ [219]. Due to its chemical nature, MitoQ takes advantage of the large MMP for reaching high concentrations in mitochondria and, unlike isolated CoQ, it is an effective antioxidant in the absence of functional ETC [220, 221]. McManus et al. demonstrated that MitoQ is effective in preventing loss of spatial memory and delaying the early neuropathology in a triple transgenic mouse model of AD; they evaluated its effect on mitochondrial deficiency and found that MitoQ avoided the MMP drop and reduced the apoptosis in cortical neurons by a decrease in caspase-3 activity [171]. Another study employed a Caenorhabditis elegans model overexpressing human Aβ end evidenced that MitoQ exerted protective effects on lifespan and Aβ-induced paralysis and markedly ameliorated the depletion of mitochondrial lipid cardiolipin and increased the mitochondrial ETC function by protecting complexes IV and I; however, it was not able to reduce the Aβ-induced mitochondrial DNA oxidative damage [201].
Recently, Szeto developed a series of small, cell-permeable antioxidant peptides (SS peptides) that are known to protect mitochondria from oxidative damage [222]. SS31 (H-D-Arg-Dmt-Lys-Phe-NH2) is one of them and presents a sequence motif that allows it to target mitochondria. In an Aβ 25–35-induced AD model of mice hippocampal neurons, SS31 restored axonal transport of mitochondria and displayed promising protection and maintenance of mitochondrial function, proved by an increase in the number of healthy and intact mitochondria and a reduction in the levels of fission proteins, matrix protein, and CypD [162]. Similar results were found by Calkins et al. [211]. Manczak et al. also revealed positive effects on mitochondria for SS31; it was able to normalize the number of mitochondria and reduce the abnormal expression of peroxiredoxins and mitochondrial structural genes, that was present in an Aβ 25–35-induced mouse N2a cells AD model [200]. Moreover, the mitochondrial division inhibitor 1 (mdivi-1) attenuated the degree of apoptosis in an Aβ-induced model of AD in BV-2 and primary microglial cells, then counteracting another pathological feature of AD, such as neuroinflammation; this effect is probably mediated through its effects on mitochondria, since Mdivi-1 reversed abnormal mitochondrial fission, MMP loss, CytC release, and caspase-3 activation [223]. Besides these actions, in a cybrid cell model, it also maintained mitochondrial integrity and function, via a reduction of mitochondrial ROS production, and increases in CytC oxidase and SOD activities and ATP levels [174]. Another chemically synthesized compound such as complex ASS234, a novel multipotent molecule that combines indolyl propargylamine and benzylpiperidine moieties, has shown protective activities in an Aβ 1–42-induced SH-SY5Y neuroblastoma cells model of AD; ASS234 inhibited the mitochondrial-mediated apoptotic pathway by reducing the levels of cleaved caspases 3 and 9 and the levels of proteolysed PARP [182]. The pyrazolone edaravone reversed the AD-like in vitro mitochondrial insults in the transfected N2a/Swe.Δ9 cell model, in which edaravone treatment increased cell viability, attenuating oxidative stress and CytC release and improving MMP; in addition, it diminished apoptotic rate through a decrease in the Bax/Bcl-2 ratio and a suppression of caspase-3 activation [173]. Also, both R(+) and S(−) stereoisomers of pramipexole exerted restorative effects in another Aβ-induced model of AD; they were able to inhibit mitochondrial-mediated apoptotic process by inhibiting caspases activations [209].
Finally, a very recent work has revealed a mitochondrial-targeted protective action for the well-known acetylcholinesterase inhibitor donepezil, which is clinically used for treating AD. Donepezil displayed ameliorative effects on behavioral deficits in APP/PS1 double transgenic mice and enhanced the resistance of their brain mitochondria to the induction of mPTP by calcium ions; such action may be mediated by its lowering effect on mitochondrial Aβ level in brain of treated animals, which was also later confirmed in vitro in isolated mitochondria from rat brains. Thus, it avoids the Aβ 1–42-induced functional decay in mitochondria in such AD model [224].
3.2.2. Endogenous Compounds
Several compounds with endogenous origin have revealed interesting actions on mitochondrial deficiencies of AD. For instance, the hormone melatonin has been largely studied. Dragicevic et al. showed that a pretreatment with melatonin protected cognitive function in an APPsw mice model of AD. A plausible mechanism for the observed effect via mitochondria was tested in isolated mitochondria from mice, proving that melatonin could completely restore mitochondrial respiratory rate, MMP, ROS production, and energy metabolism; other in vitro assays suggested a possible implication of c-AMP-dependent phosphodiesterase (PDE) 4 or cGMP-dependent PDE5 in the effects displayed by melatonin [169]. The same group of research previously demonstrated similar actions of melatonin in brain mitochondria isolated from the double transgenic APP/PS1 mice model of AD and determined that melatonin receptor signaling is required for its full effect [170]. Furthermore, melatonin evidenced mitochondrial protective activity in in vitro models. Besides acting as an antioxidant activator of mitochondrial aconitase, one of the enzymes of the citric acid cycle that is affected by the oxidative stress in AD [179], melatonin was reported to act as a defensive agent against Aβ-induced cytotoxicity in BV2 microglial cells; it attenuated the cellular apoptosis by activating Bcl-2 antiapoptotic pathways, thus involving higher Bcl-2 expression and reduced Bax mRNA level and caspase-3 activity [197].
The endogenous antioxidant glutathione also acts as a mitochondrial aconitase activator [179] and avoids the Aβ-induced mitochondrial membrane depolarization in human HCN-1A cells [193]. Two mitochondrial metabolites such as the acetyl-L-carnitine and the R-α-lipoic acid (LA) have attracted attention in AD-related research. Aliev et al. confirmed that old rats fed with both compounds presented significantly reduced number of damaged mitochondria and increased number of intact mitochondria in hippocampus, thus preventing from mitochondrial decay associated with age and AD [180]. The organosulfur compound LA was able to decrease the mitochondrial-related oxidative stress in fibroblasts from AD patients; at a concentration of 1 mM, LA attenuated AD-type mitochondrial dysfunction generated by the cytochrome oxidase assembly inhibitor N-methylprotoporphyrin [196].
The neuroprotective role of peroxiredoxins (Prdx) 3 y 6, the key mitochondrial antioxidant defense enzymes in detoxifying ROS such as H2O2, has been evaluated with results suggesting their therapeutic/prophylactic potential to slow and attenuate AD progression and related neuronal death. Rat PC12 cells overexpressing functional Prdx 6 presented diminished Aβ 25–35-induced mitochondrial apoptotic pathway, expressed by an inhibition of PARP inactivation, caspases 3 and 9 activations, and Bcl-2 and Bax dysregulation [206]. Meanwhile, Chen et al. evidenced the role of Prdx 3 in improving cognition, by using two transgenic mice models of AD (APP and APP/Prdx3 models); Prdx 3 activity was correlated with reduced brain amyloid beta level and production and maintenance of mitochondrial integrity and function, showing reduced mitochondrial DNA oxidation and enhanced activity of mitochondrial complexes I and IV [205]. Nicotinamide, as an endogenous inhibitor of poly-ADP-ribose polymerase-1 (PARP-1), is evidenced to display reduction of oxidative stress in an Aβ 1–42-induced rat model, and it upregulated mitochondrial function and downregulated mitochondrial apoptosis, lessening Bax levels and increasing Bcl-2 levels [204].
3.2.3. Natural Products
Finally, numerous natural products deserve to be mentioned for their mitochondrial-targeted antioxidant activities. Alkaloid caffeine is proved to exert similar activities to melatonin [169] and in its crude form, resulting from decaffeination of coffee, it reduced memory impairment and Aβ 1–42 levels in hippocampus of an AD mouse model, the J20 mouse line; crude caffeine maintained mitochondrial function as revealed by increased ATP levels and lower ROS generation and inhibited the apoptosis mediated by mitochondria (reduced caspase-3 activity) [184]. Polyphenol resveratrol, a proven antioxidant with interest in AD [225], when tested in an Aβ-induced N2a mouse cells model, normalized the number of mitochondria and decreased the abnormal expression of peroxiredoxins and mitochondrial structural genes; it also preserved mitochondrial function in vivo, by inhibiting CypD expression [200]. Polyphenolic compound tournefolic acid B (TAB) has been isolated from Tournefortia sarmentosa Lam. (Boraginaceae) and investigated by Chi et al. on an in vitro model of AD, who demonstrated its neuroprotective effect via mitochondrial caspase 8-tBid-cytochrome c pathway. Actually, 50 μM TAB revoked the Aβ protein-induced caspases 8 and 9 activation, significantly reduced the elevation of calcium level in mitochondria, and delayed the release of CytC; reduction in apoptosis was evidenced by an attenuation in mitochondrial tBid elevation, without affecting Aβ-mediated decrease in mitochondrial Bcl-2α [214].
Gypenoside XVII (GP-17) is a novel phytoestrogen isolated from Gynostemma pentaphyllum or Panax notoginseng that, due to its structure, was found to confer neuroprotection against Aβ 25–35-induced neurotoxicity in PC12 cells via estrogen receptor-dependent activation of PI3K/Akt and Nrf2/ARE/HO-1 pathways and inactivation of PI3K/Akt pathway; regarding mitochondrial failures, pretreatment with GP-17 (10 μM) for 12 h is demonstrated to restore normal MMP and reduced CytC release and the enhanced apoptosis, by inhibiting caspase-3 activation and cleavage [194]. The phytoestrogen genistein also showed its potency to upregulate the mitochondrial Na/K-ATPase activity in human AD brains, which is proved to be disturbed in the early phase of the disease; by ameliorating the energy metabolism in the initiation of AD pathology, genistein is presented as an interesting candidate drug [191].
Wang et al. evaluated the potential activity of acteoside, an antioxidant phenylethanoid glycoside first extracted from Verbascum sinuatum and named “verbascoside,” on Aβ 25–35-induced SHSY5Y cell injury. They concluded that a pretreatment with acteoside for 15 h was effective for inhibiting ROS production and mitochondrial dysfunction and apoptotic pathway; this modulation involved decrease in Bax/Bcl-2 ratio, cytochrome c release, and the cleavage of caspase-3 [181]. Thymoquinone is the bioactive and most abundant constituent of the volatile oil of black seed and has been described as a promising antioxidant in in vitro AD models, in part for its restorative action on MMP impairments due to Aβ neurotoxicity [212, 213].
Concerning plant extracts, a green tea leaf extract rich in epicatechin and epigallocatechin gallate, was able to reverse damaging effects of AlCl3 in a rat model, suggesting it might be beneficial in AD. It reduced the aluminium neurotoxicity via antioxidant and mitochondrial protective effects, such as an augment in CytC oxidase activity [226].
Acknowledgment
This work was supported by a doctoral grant from the Spanish Ministry of Education, Culture and Sports (FPU), awarded to Carlos Fernández-Moriano (no. FPU12/03824).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Carlos Fernández-Moriano and Elena González-Burgos contributed equally to this work.
References
- 1.Schenkel L. C., Bakovic M. Formation and regulation of mitochondrial membranes. International Journal of Cell Biology. 2014;2014:13. doi: 10.1155/2014/709828.709828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horvath S. E., Daum G. Lipids of mitochondria. Progress in Lipid Research. 2013;52(4):590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Taylor R. W., Turnbull D. M. Mitochondrial DNA mutations in human disease. Nature Reviews Genetics. 2005;6(5):389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudkina N. V., Kouřil R., Peters K., Braun H.-P., Boekema E. J. Structure and function of mitochondrial supercomplexes. Biochimica et Biophysica Acta. 2010;1797(6-7):664–670. doi: 10.1016/j.bbabio.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Brdiczka D. G., Zorov D. B., Sheu S.-S. Mitochondrial contact sites: their role in energy metabolism and apoptosis. Biochimica et Biophysica Acta. 2006;1762(2):148–163. doi: 10.1016/j.bbadis.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Martin L. J. Biology of mitochondria in neurodegenerative diseases. Progress in Molecular Biology and Translational Science. 2012;107:355–415. doi: 10.1016/B978-0-12-385883-2.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin M. T., Beal M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 8.Beitz J. M. Parkinson's disease: a review. Frontiers in Bioscience. 2014;6:65–74. doi: 10.2741/s415. [DOI] [PubMed] [Google Scholar]
- 9.Shulman J. M., De Jager P. L., Feany M. B. Parkinson's disease: genetics and pathogenesis. Annual Review of Pathology: Mechanisms of Disease. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 10.Parkinson J. An essay on the shaking palsy. The Journal of neuropsychiatry and clinical neurosciences. 2002;14(2):223–236. doi: 10.1176/jnp.14.2.223. [DOI] [PubMed] [Google Scholar]
- 11.Pringsheim T., Jette N., Frolkis A., Steeves T. D. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Movement Disorders. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 12.Wright Willis A., Evanoff B. A., Lian M., Criswell S. R., Racette B. A. Geographic and ethnic variation in Parkinson disease: a population-based study of us medicare beneficiaries. Neuroepidemiology. 2010;34(3):143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai B. C. L., Tsui J. K. C. Epidemiology of Parkinson's disease. British Columbia Medical Journal. 2001;43(3):133–137. [Google Scholar]
- 14.Thanvi B. R., Munshi S. K., Vijaykumar N., Lo T. C. N. Neuropsychiatric non-motor aspects of Parkinson's disease. Postgraduate Medical Journal. 2003;79(936):561–565. doi: 10.1136/pmj.79.936.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi S., Wood N. W. Genome-wide association studies: the key to unlocking neurodegeneration? Nature Neuroscience. 2010;13(7):789–794. doi: 10.1038/nn.2584. [DOI] [PubMed] [Google Scholar]
- 16.Kieburtz K., Wunderle K. B. Parkinson's disease: evidence for environmental risk factors. Movement Disorders. 2013;28(1):8–13. doi: 10.1002/mds.25150. [DOI] [PubMed] [Google Scholar]
- 17.Kim H. Alpha-synuclein expression in patients with Parkinson's disease: a clinician's perspective. Experimental Neurobiology. 2013;22(2):77–83. doi: 10.5607/en.2013.22.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veeriah S., Taylor B. S., Meng S., et al. Somatic mutations of the Parkinson's disease-associated gene PARK2 in glioblastoma and other human malignancies. Nature Genetics. 2010;42(1):77–82. doi: 10.1038/ng.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Björkblom B., Adilbayeva A., Maple-Grødem J., et al. Parkinson disease protein DJ-1 binds metals and protects against metal-induced cytotoxicity. Journal of Biological Chemistry. 2013;288(31):22809–22820. doi: 10.1074/jbc.M113.482091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morais V. A., Verstreken P., Roethig A., et al. Parkinson's disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Molecular Medicine. 2009;1(2):99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan N. L., Jain S., Lynch J. M., et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson's disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128(12):2786–2796. doi: 10.1093/brain/awh667. [DOI] [PubMed] [Google Scholar]
- 22.Moretto A., Colosio C. The role of pesticide exposure in the genesis of Parkinson's disease: epidemiological studies and experimental data. Toxicology. 2013;307:24–34. doi: 10.1016/j.tox.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Fitzmaurice A. G., Rhodes S. L., Cockburn M., Ritz B., Bronstein J. M. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology. 2014;82(5):419–426. doi: 10.1212/wnl.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers K. M., Smith-Weller T., Franklin G. M., Longstreth W. T., Jr., Swanson P. D., Checkoway H. Parkinson's disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology. 2003;60(11):1761–1766. doi: 10.1212/01.WNL.0000068021.13945.7F. [DOI] [PubMed] [Google Scholar]
- 25.Gao X., Chen H., Fung T. T., et al. Prospective study of dietary pattern and risk of Parkinson disease. The American Journal of Clinical Nutrition. 2007;86(5):1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X., Xu Y. Mutations in the ATP13A2 gene and parkinsonism: a preliminary review. BioMed Research International. 2014;2014:9. doi: 10.1155/2014/371256.371256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams D. R., Hadeed A., Najim Al-Din A. S., Wreikat A.-L., Lees A. J. Kufor Rakeb disease: autosomal recessive, levodopa-responsive Parkinsonism with pyramidal degeneration, supranuclear gaze palsy, and dementia. Movement Disorders. 2005;20(10):1264–1271. doi: 10.1002/mds.20511. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez A., Heimbach A., Gründemann J., et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nature Genetics. 2006;38(10):1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Petrie T. G., Liu Y., Liu J., Fujioka H., Zhu X. Parkinson's disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. Journal of Neurochemistry. 2012;121(5):830–839. doi: 10.1111/j.1471-4159.2012.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodson M. W., Guo M. Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson's disease. Current Opinion in Neurobiology. 2007;17(3):331–337. doi: 10.1016/j.conb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Morrison K. E. Parkin mutations and early onset parkinsonism. Brain. 2003;126(6):1250–1251. doi: 10.1093/brain/awg189. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.-Y., Nagano Y., Taylor J. P., Lim K. L., Yao T.-P. Disease-causing mutations in Parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. The Journal of Cell Biology. 2010;189(4):671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song S., Jang S., Park J., et al. Characterization of PINK1 (PTEN-induced putative kinase 1) mutations associated with Parkinson disease in mammalian cells and drosophila . The Journal of Biological Chemistry. 2013;288(8):5660–5672. doi: 10.1074/jbc.m112.430801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazlauskaite A., Muqit M. M. PINK1 and Parkin—mitochondrial interplay between phosphorylation and ubiquitylation in Parkinson's disease. FEBS Journal. 2015;282(2):215–223. doi: 10.1111/febs.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lashuel H. A., Petre B. M., Wall J., et al. α-synuclein, especially the parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. Journal of Molecular Biology. 2002;322(5):1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 36.Stefanis L. α-Synuclein in Parkinson's disease. Cold Spring Harbor Perspectives in Medicine. 2012;2(2) doi: 10.1101/cshperspect.a009399.a009399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J. Q., Tan L., Yu J. T. The role of the LRRK2 gene in Parkinsonism. Molecular Neurodegeneration. 2014;9, article 47 doi: 10.1186/1750-1326-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mata I. F., Wedemeyer W. J., Farrer M. J., Taylor J. P., Gallo K. A. LRRK2 in Parkinson's disease: protein domains and functional insights. Trends in Neurosciences. 2006;29(5):286–293. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Henchcliffe C., Beal M. F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nature Clinical Practice Neurology. 2008;4(11):600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 40.Subramaniam S. R., Chesselet M.-F. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Progress in Neurobiology. 2013;106-107:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauser D. N., Hastings T. G. Mitochondrial dysfunction and oxidative stress in Parkinson's disease and monogenic parkinsonism. Neurobiology of Disease. 2013;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebrahimi-Fakhari D., Wahlster L., McLean P. J. Protein degradation pathways in Parkinson's disease: curse or blessing. Acta Neuropathologica. 2012;124(2):153–172. doi: 10.1007/s00401-012-1004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bir A., Sen O., Anand S., et al. α-synuclein-induced mitochondrial dysfunction in isolated preparation and intact cells: implications in the pathogenesis of Parkinson's disease. Journal of Neurochemistry. 2014;131(6):868–877. doi: 10.1111/jnc.12966. [DOI] [PubMed] [Google Scholar]
- 44.Langston J. W., Ballard P., Tetrud J. W., Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 45.Schapira A. H. V., Cooper J. M., Dexter D., Clark J. B., Jenner P., Marsden C. D. Mitochondrial complex I deficiency in Parkinson's disease. Journal of Neurochemistry. 1990;54(3):823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 46.Dias V., Junn E., Mouradian M. M. The role of oxidative stress in Parkinson's disease. Journal of Parkinson's Disease. 2013;3(4):461–491. doi: 10.3233/jpd-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haas R. H., Nasirian F., Nakano K., et al. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson's disease. Annals of Neurology. 1995;37(6):714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- 48.Rana M., de Coo I., Diaz F., Smeets H., Moraes C. T. An out-of-frame cytochrome b gene deletion from a patient with parkinsonism is associated with impaired complex III assembly and an increase in free radical production. Annals of Neurology. 2000;48(5):774–781. doi: 10.1002/1531-8249(200011)48:560;774::aid-ana1162;3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 49.Rego A. C., Oliveira C. R. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochemical Research. 2003;28(10):1563–1574. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- 50.Budd S. L., Tenneti L., Lishnak T., Lipton S. A. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(11):6161–6166. doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gluck M. R., Zeevalk G. D. Inhibition of brain mitochondrial respiration by dopamine and its metabolites: implications for Parkinson's disease and catecholamine-associated diseases. Journal of Neurochemistry. 2004;91(4):788–795. doi: 10.1111/j.1471-4159.2004.02747.x. [DOI] [PubMed] [Google Scholar]
- 52.Khan F. H., Sen T., Maiti A. K., Jana S., Chatterjee U., Chakrabarti S. Inhibition of rat brain mitochondrial electron transport chain activity by dopamine oxidation products during extended in vitro incubation: implications for Parkinson's disease. Biochimica et Biophysica Acta: Molecular Basis of Disease. 2005;1741(1-2):65–74. doi: 10.1016/j.bbadis.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Jana S., Sinha M., Chanda D., et al. Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: implications in dopamine cytotoxicity and pathogenesis of Parkinson's disease. Biochimica et Biophysica Acta. 2011;1812(6):663–673. doi: 10.1016/j.bbadis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Van Laar V. S., Mishizen A. J., Cascio M., Hastings T. G. Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiology of Disease. 2009;34(3):487–500. doi: 10.1016/j.nbd.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hastings T. G. The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson's disease. Journal of Bioenergetics and Biomembranes. 2009;41(6):469–472. doi: 10.1007/s10863-009-9257-z. [DOI] [PubMed] [Google Scholar]
- 56.Berman S. B., Hastings T. G. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. Journal of Neurochemistry. 1999;73(3):1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 57.Luo Y., Umegaki H., Wang X., Abe R., Roth G. S. Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. The Journal of Biological Chemistry. 1998;273(6):3756–3764. doi: 10.1074/jbc.273.6.3756. [DOI] [PubMed] [Google Scholar]
- 58.Choi H. J., Lee S. Y., Cho Y., No H., Kim S. W., Hwang O. Tetrahydrobiopterin causes mitochondrial dysfunction in dopaminergic cells: implications for Parkinson's disease. Neurochemistry International. 2008;48(4):255–262. doi: 10.1016/j.neuint.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Gunter T. E., Buntinas L., Sparagna G., Eliseev R., Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28(5-6):285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 60.Pivovarova N. B., Andrews S. B. Calcium-dependent mitochondrial function and dysfunction in neurons: minireview. The FEBS Journal. 2010;277(18):3622–3636. doi: 10.1111/j.1742-4658.2010.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheehan J. P., Swerdlow R. H., Parker W. D., Miller S. W., Davis R. E., Tuttle J. B. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson's disease. Journal of Neurochemistry. 1997;68(3):1221–1233. doi: 10.1046/j.1471-4159.1997.68031221.x. [DOI] [PubMed] [Google Scholar]
- 62.Park J., Kim Y., Chung J. Mitochondrial dysfunction and Parkinson's disease genes: insights from Drosophila . Disease Models & Mechanisms. 2009;2(7-8):336–340. doi: 10.1242/dmm.003178. [DOI] [PubMed] [Google Scholar]
- 63.Mandemakers W., Morais V. A., de Strooper B. A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases. Journal of Cell Science. 2007;120(10):1707–1716. doi: 10.1242/jcs.03443. [DOI] [PubMed] [Google Scholar]
- 64.Hien P. P., Gortnizka H., Kraemer R. Rotenone—potential and prospect for sustainable agriculture. Omonrice. 2003;11:83–92. [Google Scholar]
- 65.Manning-Bog A. B., McCormack A. L., Li J., Uversky V. N., Fink A. L., Di Monte D. A. The herbicide paraquat causes up-regulation and aggregation of α-synuclein in mice: paraquat and α-synuclein. The Journal of Biological Chemistry. 2002;277(3):1641–1644. doi: 10.1074/jbc.c100560200. [DOI] [PubMed] [Google Scholar]
- 66.Simola N., Morelli M., Carta A. R. The 6-hydroxydopamine model of Parkinson's disease. Neurotoxicity Research. 2007;11(3-4):151–167. doi: 10.1007/bf03033565. [DOI] [PubMed] [Google Scholar]
- 67.Patki G., Lau Y.-S. Melatonin protects against neurobehavioral and mitochondrial deficits in a chronic mouse model of Parkinson's disease. Pharmacology Biochemistry and Behavior. 2011;99(4):704–711. doi: 10.1016/j.pbb.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saravanan K. S., Sindhu K. M., Mohanakumar K. P. Melatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat model. Journal of Pineal Research. 2007;42(3):247–253. doi: 10.1111/j.1600-079x.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 69.Sousa S. C., Castilho R. F. Protective effect of melatonin on rotenone plus Ca2+-induced mitochondrial oxidative stress and PC12 cell death. Antioxidants and Redox Signaling. 2005;7(9-10):1110–1116. doi: 10.1089/ars.2005.7.1110. [DOI] [PubMed] [Google Scholar]
- 70.Dabbeni-Sala F., di Santo S., Franceschini D., Skaper S. D., Giusti P. Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. Federation of American Societies for Experimental Biology Journal. 2001;15(1):164–170. doi: 10.1096/fj.00-0129com. [DOI] [PubMed] [Google Scholar]
- 71.Absi E., Ayala A., Machado A., Parrado J. Protective effect of melatonin against the 1-methyl-4-phenylpyridinium-induced inhibition of complex I of the mitochondrial respiratory chain. Journal of Pineal Research. 2000;29(1):40–47. doi: 10.1034/j.1600-079x.2000.290106.x. [DOI] [PubMed] [Google Scholar]
- 72.Mao P., Meshul C. K., Thuillier P., Goldberg N. R. S., Reddy P. H. CART peptide is a potential endogenous antioxidant and preferentially localized in mitochondria. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0029343.e29343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solesio M. E., Prime T. A., Logan A., et al. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson's disease. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2013;1832(1):174–182. doi: 10.1016/j.bbadis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 74.Chun H. S., Low W. C. Ursodeoxycholic acid suppresses mitochondria-dependent programmed cell death induced by sodium nitroprusside in SH-SY5Y cells. Toxicology. 2012;292(2-3):105–112. doi: 10.1016/j.tox.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Li D.-W., Li G.-R., Lu Y., et al. α-lipoic acid protects dopaminergic neurons against MPP+-induced apoptosis by attenuating reactive oxygen species formation. International Journal of Molecular Medicine. 2013;32(1):108–114. doi: 10.3892/ijmm.2013.1361. [DOI] [PubMed] [Google Scholar]
- 76.Abdin A. A., Sarhan N. I. Intervention of mitochondrial dysfunction-oxidative stress-dependent apoptosis as a possible neuroprotective mechanism of α-lipoic acid against rotenone-induced parkinsonism and l-dopa toxicity. Neuroscience Research. 2011;71(4):387–395. doi: 10.1016/j.neures.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H., Jia H., Liu J., et al. Combined R-alpha-lipoic acid and acetyl-L-carnitine exerts efficient preventative effects in a cellular model of Parkinson's disease. Journal of Cellular and Molecular Medicine. 2010;14(1-2):215–225. doi: 10.1111/j.1582-4934.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bharath S., Cochran B. C., Hsu M., Liu J., Ames B. N., Andersen J. K. Pre-treatment with R-lipoic acid alleviates the effects of GSH depletion in PC12 cells: implications for Parkinson's disease therapy. NeuroToxicology. 2002;23(4-5):479–486. doi: 10.1016/s0161-813x(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 79.Wang X., Perez E., Liu R., Yan L. J., Mallet R. T., Yang S. H. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Research. 2007;1132(1):1–9. doi: 10.1016/j.brainres.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L.-Z., Sun W.-C., Zhu X.-Z. Ethyl pyruvate protects PC12 cells from dopamine-induced apoptosis. European Journal of Pharmacology. 2005;508(1–3):57–68. doi: 10.1016/j.ejphar.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 81.Lu C., Zhang J., Shi X., et al. Neuroprotective effects of tetramethylpyrazine against dopaminergic neuron injury in a rat model of Parkinson's disease induced by MPTP. International Journal of Biological Sciences. 2014;10(4):350–357. doi: 10.7150/ijbs.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y.-H., Yu H.-T., Pu X.-P., Du G.-H. Baicalein prevents 6-hydroxydopamine-induced mitochondrial dysfunction in SH-SY5Y cells via inhibition of mitochondrial oxidation and up-regulation of DJ-1 protein expression. Molecules. 2013;18(12):14726–14738. doi: 10.3390/molecules181214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X.-X., He G.-R., Mu X., et al. Protective effects of baicalein against rotenone-induced neurotoxicity in PC12 cells and isolated rat brain mitochondria. European Journal of Pharmacology. 2012;674(2-3):227–233. doi: 10.1016/j.ejphar.2011.09.181. [DOI] [PubMed] [Google Scholar]
- 84.Lee H. J., Noh Y. H., Lee D. Y., et al. Baicalein attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. European Journal of Cell Biology. 2005;84(11):897–905. doi: 10.1016/j.ejcb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Barros Silva R., Santos N. A. G., Martins N. M., et al. Caffeic acid phenethyl ester protects against the dopaminergic neuronal loss induced by 6-hydroxydopamine in rats. Neuroscience. 2013;233:86–94. doi: 10.1016/j.neuroscience.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 86.Noelker C., Bacher M., Gocke P., et al. The flavanoide caffeic acid phenethyl ester blocks 6-hydroxydopamine-induced neurotoxicity. Neuroscience Letters. 2005;383(1-2):39–43. doi: 10.1016/j.neulet.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 87.Jayaraj R. L., Elangovan N., Manigandan K., Singh S., Shukla S. CNB-001 a novel curcumin derivative, guards dopamine neurons in MPTP model of Parkinson's disease. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/236182.236182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jayaraj R. L., Tamilselvam K., Manivasagam T., Elangovan N. Neuroprotective effect of CNB-001, a novel pyrazole derivative of curcumin on biochemical and apoptotic markers against rotenone-induced SK-N-SH cellular model of parkinson's disease. Journal of Molecular Neuroscience. 2013;51(3):863–870. doi: 10.1007/s12031-013-0075-8. [DOI] [PubMed] [Google Scholar]
- 89.Liu Z., Yu Y., Li X., Ross C. A., Smith W. W. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacological Research. 2011;63(5):439–444. doi: 10.1016/j.phrs.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 90.Jagatha B., Mythri R. B., Vali S., Bharath M. M. S. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson's disease explained via in silico studies. Free Radical Biology and Medicine. 2008;44(5):907–917. doi: 10.1016/j.freeradbiomed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 91.Huang J.-Z., Chen Y.-Z., Su M., et al. Dl-3-n-Butylphthalide prevents oxidative damage and reduces mitochondrial dysfunction in an MPP+-induced cellular model of Parkinson's disease. Neuroscience Letters. 2010;475(2):89–94. doi: 10.1016/j.neulet.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 92.Kuang R., Sun Y., Yuan W., Lei L., Zheng X. Protective effects of echinacoside, one of the phenylethanoid glycosides, on H2O2-induced cytotoxicity in PC12 cells. Planta Medica. 2009;75(14):1499–1504. doi: 10.1055/s-0029-1185806. [DOI] [PubMed] [Google Scholar]
- 93.Xiong N., Xiong J., Khare G., et al. Edaravone guards dopamine neurons in a rotenone model for Parkinson's disease. PLoS ONE. 2011;6(6):11. doi: 10.1371/journal.pone.0020677.e20677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subramaniam S. R., Ellis E. M. Neuroprotective effects of umbelliferone and esculetin in a mouse model of Parkinson's disease. Journal of Neuroscience Research. 2013;91(3):453–461. doi: 10.1002/jnr.23164. [DOI] [PubMed] [Google Scholar]
- 95.Kim K. H., Song K., Yoon S. H., Shehzad O., Kim Y. S., Son J. H. Rescue of PINK1 protein null-specific mitochondrial complex IV deficits by ginsenoside Re activation of nitric oxide signaling. The Journal of Biological Chemistry. 2012;287(53):44109–44120. doi: 10.1074/jbc.m112.408146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mythri R. B., Harish G., Dubey S. K., Misra K., Srinivas Bharath M. M. Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson's disease. Molecular and Cellular Biochemistry. 2011;347(1-2):135–143. doi: 10.1007/s11010-010-0621-4. [DOI] [PubMed] [Google Scholar]
- 97.Han Y., Kim J., Cho J., Lee C. S., Kim D. Comparison of the protective effect of indole β-carbolines and R-(-)-deprenyl against nitrogen Species-induced cell death in experimental culture model of parkinson's disease. Journal of Clinical Neurology. 2005;1(1):81–91. doi: 10.3988/jcn.2005.1.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tamilselvam K., Braidy N., Manivasagam T., et al. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson's disease. Oxidative Medicine and Cellular Longevity. 2013;2013:11. doi: 10.1155/2013/102741.102741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian L.-L., Zhou Z., Zhang Q., et al. Protective effect of (+/−) isoborneol against 6-OHDA-induced apoptosis in SH-SY5Y cells. Cellular Physiology and Biochemistry. 2007;20(6):1019–1032. doi: 10.1159/000110682. [DOI] [PubMed] [Google Scholar]
- 100.Filomeni G., Graziani I., de Zio D., et al. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson's disease. Neurobiology of Aging. 2012;33(4):767–785. doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 101.Yi F., He X., Wang D. Lycopene protects against MPP+-induced cytotoxicity by maintaining mitochondrial function in SH-SY5Y cells. Neurochemical Research. 2013;38(8):1747–1757. doi: 10.1007/s11064-013-1079-z. [DOI] [PubMed] [Google Scholar]
- 102.Kaur H., Chauhan S., Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson's disease. Neurochemical Research. 2011;36(8):1435–1443. doi: 10.1007/s11064-011-0469-3. [DOI] [PubMed] [Google Scholar]
- 103.Bagh M. B., Maiti A. K., Jana S., Banerjee K., Roy A., Chakrabarti S. Quinone and oxyradical scavenging properties of N-acetylcysteine prevent dopamine mediated inhibition of Na+, K+-ATPase and mitochondrial electron transport chain activity in rat brain: Implications in the neuroprotective therapy of Parkinson's disease. Free Radical Research. 2008;42(6):574–581. doi: 10.1080/10715760802158430. [DOI] [PubMed] [Google Scholar]
- 104.Liu W.-B., Zhou J., Qu Y., et al. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochemistry International. 2010;57(3):206–215. doi: 10.1016/j.neuint.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 105.Shrivastava P., Vaibhav K., Tabassum R., et al. Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson's rat model. Journal of Nutritional Biochemistry. 2013;24(4):680–687. doi: 10.1016/j.jnutbio.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 106.Cai X., Jia H., Liu Z., et al. Polyhydroxylated fullerene derivative C60(OH)24 prevents mitochondrial dysfunction and oxidative damage in an MPP+-induced cellular model of Parkinson's disease. Journal of Neuroscience Research. 2008;86(16):3622–3634. doi: 10.1002/jnr.21805. [DOI] [PubMed] [Google Scholar]
- 107.Guan S., Jiang B., Bao Y. M., An L. J. Protocatechuic acid suppresses MPP+-induced mitochondrial dysfunction and apoptotic cell death in PC12 cells. Food and Chemical Toxicology. 2006;44(10):1659–1666. doi: 10.1016/j.fct.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Karuppagounder S. S., Madathil S. K., Pandey M., Haobam R., Rajamma U., Mohanakumar K. P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson's disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 109.Bournival J., Plouffe M., Renaud J., Provencher C., Martinoli M.-G. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxidative Medicine and Cellular Longevity. 2012;2012:11. doi: 10.1155/2012/921941.921941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang J., Jiang J., Zuo Y., Gu Z. Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson's disease. International Journal of Molecular Medicine. 2013;31(4):825–832. doi: 10.3892/ijmm.2013.1280. [DOI] [PubMed] [Google Scholar]
- 111.Ferretta A., Gaballo A., Tanzarella P., et al. Effect of resveratrol on mitochondrial function: Implications in parkin-associated familiar Parkinson's disease. Biochimica et Biophysica Acta: Molecular Basis of Disease. 2014;1842(7):902–915. doi: 10.1016/j.bbadis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 112.Ren P., Jiang H., Li R., et al. Rosmarinic acid inhibits 6-OHDA-induced neurotoxicity by anti-oxidation in MES23.5 cells. Journal of Molecular Neuroscience. 2009;39(1-2):220–225. doi: 10.1007/s12031-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 113.Wang X.-J., Xu J.-X. Salvianic acid A protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Neuroscience Research. 2005;51(2):129–138. doi: 10.1016/j.neures.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 114.Tian L.-L., Wang X.-J., Sun Y.-N., et al. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents 6-hydroxydopamine induced apoptosis in SH-SY5Y cells. International Journal of Biochemistry & Cell Biology. 2008;40(3):409–422. doi: 10.1016/j.biocel.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 115.Lee D.-H., Kim C.-S., Lee Y. J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro . Food and Chemical Toxicology. 2011;49(1):271–280. doi: 10.1016/j.fct.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dewapriya P., Himaya S. W. A., Li Y.-X., Kim S.-K. Tyrosol exerts a protective effect against dopaminergic neuronal cell death in in vitro model of Parkinson's disease. Food Chemistry. 2013;141(2):1147–1157. doi: 10.1016/j.foodchem.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 117.Lu X.-L., Yao X.-L., Liu Z., et al. Protective effects of xyloketal B against MPP+-induced neurotoxicity in Caenorhabditis elegans and PC12 cells. Brain Research. 2010;1332:110–119. doi: 10.1016/j.brainres.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 118.Strathearn K. E., Yousef G. G., Grace M. H., et al. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Research. 2014;1555:60–77. doi: 10.1016/j.brainres.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Geed M., Garabadu D., Ahmad A., Krishnamurthy S. Silibinin pretreatment attenuates biochemical and behavioral changes induced by intrastriatal MPP+ injection in rats. Pharmacology Biochemistry & Behavior. 2014;117:92–103. doi: 10.1016/j.pbb.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 120.Guo S., Bezard E., Zhao B. Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS-NO pathway. Free Radical Biology and Medicine. 2005;39(5):682–695. doi: 10.1016/j.freeradbiomed.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 121.Hwang C. K., Chun H. S. Isoliquiritigenin isolated from licorice Glycyrrhiza uralensis prevents 6-hydroxydopamine-induced apoptosis in dopaminergic neurons. Bioscience, Biotechnology and Biochemistry. 2012;76(3):536–543. doi: 10.1271/bbb.110842. [DOI] [PubMed] [Google Scholar]
- 122.Hu S., Han R., Mak S., Han Y. Protection against 1-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis by water extract of ginseng (Panax ginseng C.A. Meyer) in SH-SY5Y cells. Journal of Ethnopharmacology. 2011;135(1):34–42. doi: 10.1016/j.jep.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 123.Kim H. G., Ju M. S., Kim D.-H., et al. Protective effects of Chunghyuldan against ROS-mediated neuronal cell death in models of parkinson's disease. Basic & Clinical Pharmacology & Toxicology. 2010;107(6):958–964. doi: 10.1111/j.1742-7843.2010.00612.x. [DOI] [PubMed] [Google Scholar]
- 124.Hampel H., Prvulovic D., Teipel S., et al. The future of Alzheimer's disease: the next 10 years. Progress in Neurobiology. 2011;95(4):718–728. doi: 10.1016/j.pneurobio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 125.Selkoe D. J., Lansbury P. J. Alzheimer’s disease is the most common neurodegenerative disorder. In: Siegel J. G., Agranoff B. W., Albers R. W., et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th. Philadelphia, Pa, USA: Lippincott-Raven; 1999. [Google Scholar]
- 126.Alzheimer's Association. Alzheimer's disease: facts and figures. Alzheimer's & Dementia. 2013;9(2) doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 127.Zhu C. W., Sano M. Economic considerations in the management of Alzheimer's disease. Clinical Interventions in Aging. 2006;1(2):143–154. doi: 10.2147/ciia.2006.1.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H. M. Forecasting the global burden of Alzheimer's disease. Alzheimer's & Dementia. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 129.Selkoe D. J. Alzheimer's disease: genes, proteins, and therapy. Physiological Reviews. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 130.Mattson M. P. Pathways towards and away from Alzheimer's disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blass J. P., Gibson G. E. Correlations of disability and biologic alterations in Alzheimer brain and test of significance by a therapeutic trial in humans. Journal of Alzheimer's Disease. 2006;9(2):207–218. doi: 10.3233/jad-2006-9212. [DOI] [PubMed] [Google Scholar]
- 132.Serrano-Pozo A., Frosch M. P., Masliah E., Hyman B. T. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine. 2011;1(1) doi: 10.1101/cshperspect.a006189.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang S., Li Y., Zhang C., et al. M1 muscarinic acetylcholine receptor in Alzheimer's disease. Neuroscience Bulletin. 2014;30(2):295–307. doi: 10.1007/s12264-013-1406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kalaria R. N., Maestre G. E., Arizaga R., et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. The Lancet Neurology. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tanzi R. E. A brief history of Alzheimer's disease gene discovery. Journal of Alzheimer's Disease. 2013;33(supplement 1):S5–S13. doi: 10.3233/jad-2012-129044. [DOI] [PubMed] [Google Scholar]
- 136.Zhou Q., Peng D. T., Yuan X. R., et al. APOE and APOC1 gene polymorphisms are associated with cognitive impairment progression in Chinese patients with late-onset Alzheimer's disease. Neural Regeneration Research. 2014;9(6):653–660. doi: 10.4103/1673-5374.130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de la Torre J. C. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. The Lancet Neurology. 2004;3(3):184–190. doi: 10.1016/s1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 138.Feng Y., Wang X. Antioxidant therapies for Alzheimer's disease. Oxidative Medicine and Cellular Longevity. 2012;2012:17. doi: 10.1155/2012/472932.472932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yana M. H., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radical Biology and Medicine. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang X., Atwood C. S., Hartshorn M. A., et al. The Aβ peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38(24):7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 141.Perry G., Cash A. D., Smith M. A. Alzheimer disease and oxidative stress. Journal of Biomedicine and Biotechnology. 2002;2(3):120–123. doi: 10.1155/s1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huber A., Stuchbury G., Bürkle A., Burnell J., Münch G. Neuroprotective therapies for Alzheimer's disease. Current Pharmaceutical Design. 2006;12(6):705–717. doi: 10.2174/138161206775474251. [DOI] [PubMed] [Google Scholar]
- 143.Bonda D. J., Lee H.-P., Lee H.-G., et al. Novel therapeutics for Alzheimer's disease: an update. Current Opinion in Drug Discovery & Development. 2010;13(2):235–246. [PMC free article] [PubMed] [Google Scholar]
- 144.Swerdlow R. H. The neurodegenerative mitochondriopathies. Journal of Alzheimer's Disease. 2009;17(4):737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maruszak A., Żekanowski C. Mitochondrial dysfunction and Alzheimer's disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(2):320–330. doi: 10.1016/j.pnpbp.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 146.Vargas T., Ugalde C., Spuch C., et al. Aβ accumulation in choroid plexus is associated with mitochondrial-induced apoptosis. Neurobiology of Aging. 2010;31(9):1569–1581. doi: 10.1016/j.neurobiolaging.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 147.Hauptmann S., Scherping I., Dröse S., et al. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiology of Aging. 2009;30(10):1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 148.Kaminsky Y. G., Tikhonova L. A., Kosenko E. A. Critical analysis of Alzheimer’s amyloid-beta toxicity to mitochondria. Frontiers in Bioscience. 2015;20:173–197. doi: 10.2741/4304. [DOI] [PubMed] [Google Scholar]
- 149.Cardoso S. M., Santos S., Swerdlow R. H., Oliveira C. R. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. The FASEB Journal. 2001;15(8):1439–1441. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- 150.Pereira C., Santos M. S., Oliveira C. Mitochondrial function impairment induced by amyloid β-peptide on PC12 cells. NeuroReport. 1998;9(8):1749–1755. doi: 10.1097/00001756-199806010-00015. [DOI] [PubMed] [Google Scholar]
- 151.Canevari L., Clark J. B., Bates T. E. β-Amyloid fragment 25-35 selectively decreases complex IV activity in isolated mitochondria. FEBS Letters. 1999;457(1):131–134. doi: 10.1016/s0014-5793(99)01028-5. [DOI] [PubMed] [Google Scholar]
- 152.Reddy P. H., McWeeney S., Park B. S., et al. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: Up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Human Molecular Genetics. 2004;13(12):1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 153.Picone P., Nuzzo D., Caruana L., Scafidi V., Di Carlo M. Mitochondrial dysfunction: different routes to Alzheimer's disease therapy. Oxidative Medicine and Cellular Longevity. 2014;2014:11. doi: 10.1155/2014/780179.780179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Manczak M., Anekonda T. S., Henson E., Park B. S., Quinn J., Reddy P. H. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Human Molecular Genetics. 2006;15(9):1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 155.Devi L., Prabhu B. M., Galati D. F., Avadhani N. G., Anandatheerthavarada H. K. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. Journal of Neuroscience. 2006;26(35):9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cha M.-Y., Han S.-H., Son S. M., et al. Mitochondria-specific accumulation of amyloid β induces mitochondrial dysfunction leading to apoptotic cell death. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0034929.e34929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hong W. K., Han E. H., Kim D. G., Ahn J. Y., Park J. S., Han B. G. Amyloid-β-peptide reduces the expression level of mitochondrial cytochrome oxidase subunits. Neurochemical Research. 2007;32(9):1483–1488. doi: 10.1007/s11064-007-9336-7. [DOI] [PubMed] [Google Scholar]
- 158.Mutisya E. M., Bowling A. C., Beal M. F. Cortical cytochrome oxidase activity is reduced in Alzheimer's disease. Journal of Neurochemistry. 1994;63(6):2179–2184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- 159.Atamna H., Kumar R. Protective role of methylene blue in Alzheimer's disease via mitochondria and cytochrome c oxidase. Journal of Alzheimer's Disease. 2010;20(1, supplement 2):S439–S452. doi: 10.3233/jad-2010-100414. [DOI] [PubMed] [Google Scholar]
- 160.Zubenko G. S., Sauer P. SOD-1 activity and platelet membrane fluidity in Alzheimer's disease. Biological Psychiatry. 1989;25(6):671–678. doi: 10.1016/0006-3223(89)90236-9. [DOI] [PubMed] [Google Scholar]
- 161.Moreira P. I., Santos M. S., Sena C., Nunes E., Seiça R., Oliveira C. R. CoQ10 therapy attenuates amyloid beta-peptide toxicity in brain mitochondria isolated from aged diabetic rats. Experimental Neurology. 2005;196(1):112–119. doi: 10.1016/j.expneurol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 162.Reddy P. H., Tripathi R., Troung Q., et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer's disease: implications to mitochondria-targeted antioxidant therapeutics. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2012;1822(5):639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang X., Su B., Lee H.-G., et al. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. Journal of Neuroscience. 2009;29(28):9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Begley J. G., Duan W., Chan S., Duff K., Mattson M. P. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. Journal of Neurochemistry. 1999;72(3):1030–1039. doi: 10.1046/j.1471-4159.1999.0721030.x. [DOI] [PubMed] [Google Scholar]
- 165.Obulesu M., Lakshmi M. J. Apoptosis in Alzheimer's disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochemical Research. 2014;39(12):2301–2312. doi: 10.1007/s11064-014-1454-4. [DOI] [PubMed] [Google Scholar]
- 166.Eckert A., Keil U., Marques C. A., et al. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer's disease. Biochemical Pharmacology. 2003;66(8):1627–1634. doi: 10.1016/S0006-2952(03)00534-3. [DOI] [PubMed] [Google Scholar]
- 167.Götz J., Ittner L. M. Animal models of Alzheimer's disease and frontotemporal dementia. Nature Reviews Neuroscience. 2008;9(7):532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 168.Feng Z., Qin C., Chang Y., Zhang J.-T. Early melatonin supplementation alleviates oxidative stress in a transgenic mouse model of Alzheimer's disease. Free Radical Biology and Medicine. 2006;40(1):101–109. doi: 10.1016/j.freeradbiomed.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 169.Dragicevic N., Delic V., Cao C., et al. Caffeine increases mitochondrial function and blocks melatonin signaling to mitochondria in Alzheimer's mice and cells. Neuropharmacology. 2012;63(8):1368–1379. doi: 10.1016/j.neuropharm.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 170.Dragicevic N., Copes N., O'Neal-Moffitt G., et al. Melatonin treatment restores mitochondrial function in Alzheimer's mice: a mitochondrial protective role of melatonin membrane receptor signaling. Journal of Pineal Research. 2011;51(1):75–86. doi: 10.1111/j.1600-079x.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 171.McManus M. J., Murphy M. P., Franklin J. L. The mitochondria-targeted antioxidant mitoq prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. Journal of Neuroscience. 2011;31(44):15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sanz-Blasco S., Valero R. A., Rodríguez-Crespo I., Villalobos C., Núñez L. Mitochondrial Ca2+ overload underlies Aβ oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS ONE. 2008;3(7) doi: 10.1371/journal.pone.0002718.e2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yan Y., Gong K., Ma T., et al. Protective effect of edaravone against Alzheimer's disease-relevant insults in neuroblastoma N2a cells. Neuroscience Letters. 2012;531(2):160–165. doi: 10.1016/j.neulet.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 174.Gan X., Huang S., Wu L., et al. Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer's disease cybrid cell. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2014;1842(2):220–231. doi: 10.1016/j.bbadis.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu J., Ames B. N. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer's disease, and Parkinson's disease. Nutritional Neuroscience. 2005;8(2):67–89. doi: 10.1080/10284150500047161. [DOI] [PubMed] [Google Scholar]
- 176.Reddy P. H. Role of mitochondria in neurodegenerative diseases: mitochondria as a therapeutic target in Alzheimer's disease. CNS Spectrums. 2009;14(8, supplement 7):6–13. doi: 10.1017/s1092852900024901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Du H., Yan S. S. Mitochondrial medicine for neurodegenerative diseases. International Journal of Biochemistry and Cell Biology. 2010;42(5):560–572. doi: 10.1016/j.biocel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moreira P. I., Zhu X., Wang X., et al. Mitochondria: a therapeutic target in neurodegeneration. Biochimica et Biophysica Acta. 2010;1802(1):212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Raukas M., Rebane R., Mahlapuu R., et al. Mitochondrial oxidative stress index, activity of redox-sensitive aconitase and effects of endogenous anti- and pro-oxidants on its activity in control, Alzheimer's disease and Swedish Familial Alzheimer's disease brain. Free Radical Research. 2012;46(12):1490–1495. doi: 10.3109/10715762.2012.728286. [DOI] [PubMed] [Google Scholar]
- 180.Aliev G., Liu J., Shenk J. C., et al. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. Journal of Cellular and Molecular Medicine. 2009;13(2):320–333. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang H., Xu Y., Yan J., et al. Acteoside protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Brain Research. 2009;1283:139–147. doi: 10.1016/j.brainres.2009.05.101. [DOI] [PubMed] [Google Scholar]
- 182.Bolea I., Gella A., Monjas L., et al. Multipotent, permeable drug ASS234 inhibits Aβ aggregation, possesses antioxidant properties and protects from Aβ-induced apoptosis in vitro . Current Alzheimer Research. 2013;10(8):797–808. doi: 10.2174/15672050113109990151. [DOI] [PubMed] [Google Scholar]
- 183.Bacsi A., Woodberry M., Kruzel M. L., Boldogh I. Colostrinin delays the onset of proliferative senescence of diploid murine fibroblast cells. Neuropeptides. 2007;41(2):93–101. doi: 10.1016/j.npep.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 184.Chu Y.-F., Chang W.-H., Black R. M., et al. Crude caffeine reduces memory impairment and amyloid β 1-42 levels in an Alzheimer's mouse model. Food Chemistry. 2012;135(3):2095–2102. doi: 10.1016/j.foodchem.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 185.Du H., Guo L., Fang F., et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nature Medicine. 2008;14(10):1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ansari M. A., Joshi G., Huang Q., et al. In vivo administration of D609 leads to protection of subsequently isolated gerbil brain mitochondria subjected to in vitro oxidative stress induced by amyloid beta-peptide and other oxidative stressors: relevance to Alzheimer's disease and other oxidative stress-related neurodegenerative disorders. Free Radical Biology and Medicine. 2006;41(11):1694–1703. doi: 10.1016/j.freeradbiomed.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sheng B., Gong K., Niu Y., et al. Inhibition of γ-secretase activity reduces Aβ production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: implications for the treatment of Alzheimer's disease. Free Radical Biology and Medicine. 2009;46(10):1362–1375. doi: 10.1016/j.freeradbiomed.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 188.Bachurin S. O., Shevtsova E. P., Kireeva E. G., Oxenkrug G. F., Sablin S. O. Mitochondria as a target for neurotoxins and neuroprotective agents. Annals of the New York Academy of Sciences. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. [DOI] [PubMed] [Google Scholar]
- 189.Jang J.-H., Aruoma O. I., Jen L.-S., Chung H. Y., Surh Y.-J. Ergothioneine rescues PC12 cells from beta-amyloid-induced apoptotic death. Free Radical Biology and Medicine. 2004;36(3):288–299. doi: 10.1016/j.freeradbiomed.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 190.Vargas T., Antequera D., Ugalde C., Spuch C., Carro E. Gelsolin restores A beta-induced alterations in choroid plexus epithelium. Journal of Biomedicine and Biotechnology. 2010;2010:7. doi: 10.1155/2010/805405.805405 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Kairane C., Mahlapuu R., Ehrlich K., Zilmer M., Soomets U. The effects of different antioxidants on the activity of cerebrocortical MnSOD and Na,K-ATPase from post mortem Alzheimer's disease and age-matched normal brains. Current Alzheimer Research. 2014;11(1):79–85. doi: 10.2174/15672050113106660179. [DOI] [PubMed] [Google Scholar]
- 192.Ma T., Du X., Pick J. E., Sui G., Brownlee M., Klann E. Glucagon-like peptide-1 cleavage product GLP-1(9-36) amide rescues synaptic plasticity and memory deficits in Alzheimer's disease model mice. Journal of Neuroscience. 2012;32(40):13701–13708. doi: 10.1523/JNEUROSCI.2107-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Medina S., Martínez M., Hernanz A. Antioxidants inhibit the human cortical neuron apoptosis induced by hydrogen peroxide, tumor necrosis factor alpha, dopamine and beta-amyloid peptide 1–42. Free Radical Research. 2002;36(11):1179–1184. doi: 10.1080/107157602100006445. [DOI] [PubMed] [Google Scholar]
- 194.Meng X., Wang M., Sun G., et al. Attenuation of Aβ25–35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicology and Applied Pharmacology. 2014;279(1):63–75. doi: 10.1016/j.taap.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 195.Kawada H., Blessing K., Kiyota T., Woolman T., Winchester L., Kador P. F. Effects of multifunctional antioxidants on mitochondrial dysfunction and amyloid-β metal dyshomeostasis. Journal of Alzheimer's Disease. 2015;44(1):297–307. doi: 10.3233/JAD-132471. [DOI] [PubMed] [Google Scholar]
- 196.Moreira P. I., Harris P. L. R., Zhu X., et al. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. Journal of Alzheimer's Disease. 2007;12(2):195–206. doi: 10.3233/jad-2007-12210. [DOI] [PubMed] [Google Scholar]
- 197.Jang M.-H., Jung S.-B., Lee M.-H., et al. Melatonin attenuates amyloid beta25-35-induced apoptosis in mouse microglial BV2 cells. Neuroscience Letters. 2005;380(1-2):26–31. doi: 10.1016/j.neulet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 198.Dong W., Huang F., Fan W., et al. Differential effects of melatonin on amyloid-beta peptide 25–35-induced mitochondrial dysfunction in hippocampal neurons at different stages of culture. Journal of Pineal Research. 2010;48(2):117–125. doi: 10.1111/j.1600-079x.2009.00734.x. [DOI] [PubMed] [Google Scholar]
- 199.Gharib A., Atamna H. Neurodegeneration: Theory, Disorders and Treatments. chapter 3. New York, NY, USA: Nova Science; 2010. Methylene blue induces mitochondrial complex IV and improves cognitive function and grip strength in old mice; pp. 63–86. [Google Scholar]
- 200.Manczak M., Mao P., Calkins M. J., et al. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. Journal of Alzheimer's Disease. 2010;20(2):S609–S631. doi: 10.3233/jad-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ng L. F., Gruber J., Cheah I. K., et al. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radical Biology and Medicine. 2014;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 202.Robinson R. A. S., Joshi G., Huang Q., et al. Proteomic analysis of brain proteins in APP/PS-1 human double mutant knock-in mice with increasing amyloid β-peptide deposition: insights into the effects of in vivo treatment with N-acetylcysteine as a potential therapeutic intervention in mild cognitive impairment and Alzheimer's disease. Proteomics. 2012;11(21):4243–4256. doi: 10.1002/pmic.201000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ryu J., Yu H.-N., Cho H., et al. Neuregulin-1 exerts protective effects against neurotoxicities induced by C-terminal fragments of APP via ErbB4 receptor. Journal of Pharmacological Sciences. 2012;119(1):73–81. doi: 10.1254/jphs.12057FP. [DOI] [PubMed] [Google Scholar]
- 204.Turunc Bayrakdar E., Uyanikgil Y., Kanit L., Koylu E., Yalcin A. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Aβ(1-42)-induced rat model of Alzheimer's disease. Free Radical Research. 2014;48(2):146–158. doi: 10.3109/10715762.2013.857018. [DOI] [PubMed] [Google Scholar]
- 205.Chen L., Na R., Ran Q. Enhanced defense against mitochondrial hydrogen peroxide attenuates age-associated cognition decline. Neurobiology of Aging. 2014;35(11):2552–2561. doi: 10.1016/j.neurobiolaging.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 206.Kim I. K., Lee K. J., Rhee S., Seo S. B., Pak J. H. Protective effects of peroxiredoxin 6 overexpression on amyloid β-induced apoptosis in PC12 cells. Free Radical Research. 2013;47(10):836–846. doi: 10.3109/10715762.2013.833330. [DOI] [PubMed] [Google Scholar]
- 207.Li J., Wang G., Liu J., et al. Puerarin attenuates amyloid-beta-induced cognitive impairment through suppression of apoptosis in rat hippocampus in vivo . European Journal of Pharmacology. 2010;649(1–3):195–201. doi: 10.1016/j.ejphar.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 208.Zhang H., Liu Y., Lao M., Ma Z., Yi X. Puerarin protects Alzheimer's disease neuronal cybrids from oxidant-stress induced apoptosis by inhibiting pro-death signaling pathways. Experimental Gerontology. 2011;46(1):30–37. doi: 10.1016/j.exger.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 209.Abramova N. A., Cassarino D. S., Khan S. M., Painter T. W., Bennett J. P., Jr. Inhibition by R(+) or S(-) pramipexole of caspase activation and cell death induced by methylpyridinium ion or beta amyloid peptide in SH-SY5Y neuroblastoma. Journal of Neuroscience Research. 2002;67(4):494–500. doi: 10.1002/jnr.10127. [DOI] [PubMed] [Google Scholar]
- 210.Liu J., Chi N., Chen H., et al. Resistin protection against endogenous Aβ neuronal cytotoxicity from mitochondrial pathway. Brain Research. 2013;1523:77–84. doi: 10.1016/j.brainres.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 211.Calkins M. J., Manczak M., Mao P., Shirendeb U., Reddy P. H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Human Molecular Genetics. 2011;20(23):4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Khan A., Vaibhav K., Javed H., et al. Attenuation of Aβ-induced neurotoxicity by thymoquinone via inhibition of mitochondrial dysfunction and oxidative stress. Molecular and Cellular Biochemistry. 2012;369(1-2):55–65. doi: 10.1007/s11010-012-1368-x. [DOI] [PubMed] [Google Scholar]
- 213.Alhebshi A. H., Gotoh M., Suzuki I. Thymoquinone protects cultured rat primary neurons against amyloid β-induced neurotoxicity. Biochemical and Biophysical Research Communications. 2013;433(4):362–367. doi: 10.1016/j.bbrc.2012.11.139. [DOI] [PubMed] [Google Scholar]
- 214.Chi C.-W., Lin Y.-L., Wang Y.-H., Chen C.-F., Wang C.-N., Shiao Y.-J. Tournefolic acid B attenuates amyloid β protein-mediated toxicity by abrogating the calcium overload in mitochondria and retarding the caspase 8-truncated Bid-cytochrome c pathway in rat cortical neurons. European Journal of Pharmacology. 2008;586(1–3):35–43. doi: 10.1016/j.ejphar.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 215.Lee S. H., Kim K. R., Ryu S. Y., et al. Impaired short-term plasticity in mossy fiber synapses caused by mitochondrial dysfunction of dentate granule cells is the earliest synaptic deficit in a mouse model of Alzheimer's disease. The Journal of Neuroscience. 2012;32(17):5953–5963. doi: 10.1523/jneurosci.0465-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kook S.-Y., Lee K.-M., Kim Y., et al. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death and Disease. 2014;5(2) doi: 10.1038/cddis.2014.26.e1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Santos M. J., Quintanilla R. A., Toro A., et al. Peroxisomal proliferation protects from β-amyloid neurodegeneration. The Journal of Biological Chemistry. 2005;280(49):41057–41068. doi: 10.1074/jbc.m505160200. [DOI] [PubMed] [Google Scholar]
- 218.Montinaro M., Uberti D., MacCarinelli G., Bonini S. A., Ferrari-Toninelli G., Memo M. Dietary zeolite supplementation reduces oxidative damage and plaque generation in the brain of an Alzheimer's disease mouse model. Life Sciences. 2013;92(17–19):903–910. doi: 10.1016/j.lfs.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 219.Moreira P. I., Cardoso S. M., Pereira C. M., Santos M. S., Oliveira C. R. Mitochondria as a therapeutic target in Alzheimer's disease and diabetes. CNS and Neurological Disorders—Drug Targets. 2009;8(6):492–511. doi: 10.2174/187152709789824651. [DOI] [PubMed] [Google Scholar]
- 220.Smith R. A. J., Kelso G. F., James A. M., Murphy M. P. Targeting coenzyme Q derivatives to mitochondria. Methods in Enzymology. 2004;382:45–67. doi: 10.1016/s0076-6879(04)82003-2. [DOI] [PubMed] [Google Scholar]
- 221.Lu C., Zhang D., Whiteman M., Armstrong J. S. Is antioxidant potential of the mitochondrial targeted ubiquinone derivative MitoQ conserved in cells lacking mtDNA? Antioxidants and Redox Signaling. 2008;10(3):651–660. doi: 10.1089/ars.2007.1865. [DOI] [PubMed] [Google Scholar]
- 222.Szeto H. H. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. The AAPS Journal. 2006;8(3):521–531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xie N., Wang C., Lian Y., Wu C., Zhang H., Zhang Q. Inhibition of mitochondrial fission attenuates Aβ-induced microglia apoptosis. Neuroscience. 2014;256:36–42. doi: 10.1016/j.neuroscience.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 224.Ye C. Y., Lei Y., Tang X. C., Zhang H. Y. Donepezil attenuates Aβ-associated mitochondrial dysfunction and reduces mitochondrial Aβ accumulation in vivo and in vitro . Neuropharmacology. 2015;95:29–36. doi: 10.1016/j.neuropharm.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 225.Rege S. D., Geetha T., Griffin G. D., Broderick T. L., Babu J. R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Frontiers in Aging Neuroscience. 2014;6, article 218 doi: 10.3389/fnagi.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jelenković A., Jovanović M. D., Stevanović I., et al. Influence of the green tea leaf extract on neurotoxicity of aluminium chloride in rats. Phytotherapy Research. 2014;28(1):82–87. doi: 10.1002/ptr.4962. [DOI] [PubMed] [Google Scholar]