Abstract
Neuroimaging comprises a set of tools, which include different types of magnetic resonance imaging such as fMRI, MRS, ASL, and radiotracer imaging such as PET and SPECT. The focus of this review is to address the question whether functional magnetic resonance imaging (fMRI) can contribute to the diagnosis and treatment of anxiety disorders. Key anxiety processes and neural substrates are reviewed. The main findings and shortcomings of fMRI in the context of anxiety are briefly summarized. Finally, the next stages of developing fMRI for diagnosis and treatment are highlighted. The main conclusion of this review is that fMRI could become a clinical tool for the diagnosis and treatment of anxiety disorders but neuroimaging groups will need to better develop its specificity and sensitivity so that fMRI results can be meaningful for an individual patient not just for groups of individuals.
fMRI – what is it and what does it measure?
Functional magnetic resonance imaging (fMRI) is a technique that enables one to map cognitive, affective, and experiential processes onto brain substrates. However, fMRI is not about increased or decreased activation in a certain part of the brain; rather it is a proxy measure about how complex cognitive, emotional, social and other experiential processes are implemented in different neural systems. For example, it is important to realize that it makes little sense to talk about hyperactivity in the amygdala in individuals with anxiety disorder without referencing the process, which is being measured during the amygdala hyperactivity, i.e. the task that individuals are engaged in while the functional images are obtained. Although the human brain comprises only about 2% of the body mass, it accounts for approximately 20% of its total oxygen consumption [1]. Deoxyhemoglobin has paramagnetic effects in the blood upon the nuclear magnetic resonance transverse relaxation times of nearby water protons in the tissue [2]. The fact that changes in the oxygen level in the blood can affect the fraction of hemoglobin in the deoxygenated state can be utilized as an image contrast and was termed as blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) [3]. Recent BOLD fMRI experiments in the awake human visual cortex have shown that the ratio between BOLD-fMRI signal change and baseline signal is linearly proportional to the change in blood flow relative to the baseline blood flow [4]. Moreover, increases in baseline blood flow is thought to be proportional to total deoxyhemoglobin within a voxel [5]. For example, increased baseline cerebral blood flow by breathing CO2 reduces the BOLD response to the same task substantially [6]. Therefore, the BOLD signal reflects the effect of neural activity on dynamic changes in cerebral blood flow (CBF), cerebral blood volume, and the cerebral rate of oxygen metabolism through a process generally referred to as neurovascular coupling. Thus, prior experimental and theoretical work suggests that a measure of baseline CBF in addition to fMRI could also be useful in determining the non-process specific effects. Specifically, baseline CBF measures can be used as covariates in the interpretation of BOLD changes induced by anxiety treatments. In addition, group differences in both baseline CBF and the effect of anxiety treatments on CBF can be interpreted as additional independent factors in a bio-assay. For example, if baseline CBF in amygdala is found to be higher in anxiety-prone subjects, it may turn out that the most promising anxiolytic drug candidates are those that reduce baseline CBF in amygdala to normal levels.
Neural Systems Relevant for Anxiety Disorders
When conducting neuroimaging experiments in the field of anxiety disorders, one is concerned with four issues: (1) What is the process that one wants to measure; (2) what task one wants to use to assess the process; (3) how is brain activation related to the process at hand; and (4) how is this process altered in individuals with anxiety disorders. These issues are complex and there is no clear resolution as to the best processes, the best tasks, or the basic nature of dysfunctional processes in anxiety disorders. Instead, there are several approaches that researchers have taken to map out the functional circuitry of anxiety disorders. Moreover, the situation is complicated by the fact that studies with anxiety disorder subjects are frequently complicated by concomitant medication treatment or other non-anxiety comorbidity. Although, these results are relevant for “real life” patients, they make it difficult to uniquely attribute dysfunctional processes to specific anxiety disorders. Clearly, the amygdala plays a critical role in the functional neurocircuitry of anxiety disorders. The amygdala is involved in normal fear conditioning and is implicated in the pathophysiology of several different anxiety disorders [7,8]. However, this structure is also important for other emotional information processing and behavior [9]. Functional neuroimaging studies have shown amygdala activation in fear conditioning [10], reward related processing [11], encoding of emotionally salient information [12], risk-taking [13], processing positively valenced stimuli [14], and appetitive or aversive olfactory learning [15]. Individuals with social anxiety disorder [16] or posttraumatic stress disorder [17] show amygdala hyperresponsivity to fearful or angry faces. In addition to the amygdala changes, panic disorder patients have decreased benzodiazepine receptor binding in left hippocampal and precuneus [18] and in right orbitofrontal cortex and right insula [19].
In addition to the amygdala, a network of structures which includes the insula, anterior cingulate gyrus and medial prefrontal cortex are important for the identification of the emotional significance of a stimulus, to generate an affective response, and to regulate the affective state [20]. The insula (for review see [21,22]) is one of the paralimbic structures and constitutes the invaginated portion of the cerebral cortex, forming the base of the sylvian fissure. The insular cortex has been considered limbic sensory cortex by some investigators [23]. A central insular sulcus divides the insula into two portions, the anterior and posterior insula. The anterior insula is strongly connected to different parts of the frontal cortex, whereas the posterior insula is connected to both the parietal and temporal cortex [24]. The columnar organization of the insular cortex shows an highly organized anterior inferior to posterior superior gradient (for example see [25]). Specifically, whereas posterior insular is characterized by a granular cortical architecture, the anterior inferior insula has an agranular columnar organization, i.e. lacks layer 4 granular cells. This type of transition is found in other parts of the brain whenever cortical re-representations are based on modulatory or selective feedback circuits [26]. Finally, the discovery of spindle cells within the anterior insular –orbitofrontal transition region [27] has provided a cellular substrate underlying the possibility of widespread cortical integration. Insular cortex appears to be particularly important for subjective feeling states and interoceptive awareness [28,29]. The insula has afferent and efferent connections to medial and orbitofrontal cortex, anterior cingulate and several nuclei of the amygdala [21]. Although insula activation has been frequently associated with disgust [30], there is increasing evidence of a broader role for this brain structure in emotion processing [31]. Insula activation is thought to be involved in differential positive versus negative emotion processing [32], in particular fearful face processing [33], pain perception [34,35], and when individuals were asked to make judgments about emotions [36].
The medial prefrontal cortex, an area that includes various parts of the prefrontal cortex including the superior frontal gyrus, the para-cingulate and the inferior frontal gyrus, in addition to the amygdala and insula has been recognized as increasingly important for the regulation of emotion in general and anxiety-related processing in particular. For example, the correct recognition of self-encoded personality traits engaged dorso-medial prefrontal cortex and lateral prefrontal regions, premotor cortex, parietal and occipital cortex, caudate and cerebellum [37]. Other investigators have shown that activity in medial prefrontal cortex predicted both subsequent memory performance and judgments of self-relevance [38,39]. More specifically, individuals while making judgments about trait adjectives under three experimental conditions (self-relevance, other-relevance, or case judgment) show that the medial prefrontal cortex was selectively engaged during self-referential processing [38], which is consistent with other findings that there is a common area of medial prefrontal activation during the “ME” conditions of self- and other-evaluation versus the baseline semantic positivity-evaluation condition [40].
Others have reported that self and other decisions both activated bilateral medial areas of the frontal and parietal lobes and the bilateral insula in comparison to a letter task [41]. These evaluative judgment are associated with activation in the anterior frontomedian cortex (BA 10/9), the inferior precuneus (BA 23/31), and the left inferior prefrontal cortex (BA 45/47). Some investigators have made a distinction between the anterior frontomedian cortex and in the inferior precuneus. Whereas the latter was found to be activated by episodic retrieval processes, supporting its function as a multimodal association area that integrates the different aspects of retrieved and newly presented information, the anterior frontomedian cortex was mainly involved in evaluative judgments, supporting its role in self-referential processes and in the self-initiation of cognitive processes [42]. Activation in anterior insula and rostral ACC during “self” versus “other” judgments, suggests that the neural substrate for empathic experience not only involves self-relevant processing areas but partially engages the “pain matrix” [43]. Moreover, the nucleus accumbens responds to both increasing emotional intensity and self-relatedness. Finally, activity in the amygdala was specifically related to affective judgments and emotional intensity. The volitional act of appraising the extent of personal association specifically engaged the ventral medial prefrontal cortex (MPFC), and additionally recruited dorsal medial frontal regions and insula as the extent of self-relatedness increased [44]. Taken together, medial prefrontal cortex regions may contribute to the neural instantiation of aspects of the multifaceted “self” [45]. Thus, amygdala, insula, and medial prefrontal cortex are critical for the recognition, anticipation, and expression of emotions as they relate to the self.
Emotional Processes Relevant For Anxiety Disorders
Emotional face processing has been the most often used behavioral paradigm to probe dysfunctional neural systems in anxiety disorder. However, to identify, recognize, and respond to facial emotional stimuli is a complex process. This involves a well-studied neural circuitry, which is altered in individuals with anxiety disorders. Adjacent to extrastriate cortex are cortical areas that are highly specialized for face processing [46]. In particular, bilateral lingual/fusiform gyri and the right parahippocampal gyrus are almost always involved in facial processing [47]. Processing of faces in this area takes place within 165 ms [48] and the amygdala is required to link visual representations of facial expressions with affective representations such as fear [49]. Some groups have suggested that the amygdala is more sensitive to fear relative to other emotional expressions [50], and is involved even in the absence of awareness [51], which may be mediated via subcortical pathway to the right amygdala, via midbrain and thalamus [52]. Moreover, an extended circuitry comprising the amygdala, pulvinar, anterior insula and anterior cingulate activates during the processing of fearful faces [53], which also appears to be engaged whenever an explicit emotion face judgment is required [36]. Some investigators have argued that left and right amygdala and extended limbic areas are differentially involved in negative versus positive emotion processing, respectively. For example, left amygdala activity was associated with stronger activation during negative valenced face presentation. In comparison, right amygdaloid activity was stronger when positive facial expressions were evaluated [54]. Others have found emotional expressions of happiness, fear, and sadness but not anger are recognized more efficiently in the right versus the left hemiface [55]. This notion is consistent with findings of exaggerated left but not right amygdala response to masked faces in depressed subjects [56]. Based on studies with brain lesion individuals, it appears that the right inferior parietal cortex and the right mesial anterior infracalcarine cortex is important for the recognition of an emotion in pictures of faces [57]. Moreover, holding emotional faces in mind is associated with differential activity in left ventral prefrontal cortex, the left anterior cingulate cortex, and the right fusiform gyrus [58]. Recently, some investigators have argued that the amygdala is able to process complex social emotions such as guilt, admiration or flirtatiousness [59]. Therefore, even seemingly “simple” paradigms such as emotional face processing are comprised of complex emotional and cognitive component processes. Thus, it is important to better delineate which components may be dysfunctional in individuals with anxiety disorders.
The neural substrates underlying executive functioning, e.g. the dorsolateral prefrontal cortex and the anterior cingulate, modulate the activation of amygdala and the extended limbic system [60]. Specifically, inversely correlated activation has been observed in these areas in relation to the amygdala and are thought to contribute to conscious evaluation and appraisal [61,62]. These findings are consistent with recent report of an altered relationship between amygdala activation and medial prefrontal cortex [63] and can be disaggregated using multivariate statistical approaches [64]. Others have also found a strong attention related modulation in the orbitofrontal cortex during emotional face processing [65], which may give rise to representations of somatic markers, i.e. “gut feelings”, associated with facial emotions [66].
Several groups have begun to relate emotional face processing to anxiety. For example, low anxiety subjects but not high anxiety subjects were found to show reduced amygdala response to unattended versus attended fearful faces. Moreover, latter group show an increased amygdala response to fearful versus neutral faces regardless of attentional focus [67]. Others have proposed that high trait anxious individuals show enhanced unconscious processing of emotional faces, which has been attributed to activation in the basolateral amygdala [68]. Some have suggested that the insula plays a unique role in the processing of threat signals in subjects with anxiety disorders [69]. In summary, the neural circuitry underlying emotional face processing has been well delineated and consists of limbic and paralimbic “bottom up” processing circuits and cortical “top-down” processing circuits.
Taken together, several key structures are hypothesized to modulate the basic anxiety circuitry. First, the amygdala is critical for assigning valence or salience to environment and internal stimuli. Second, the insular cortex is important for the processing of interoception and predictive interoception, i.e. how the body feels and how it may feel given a predictive internal or environmental stimulus. Third, the medial prefrontal cortex including the anterior cingulate is important for cognitive and affective conflict as well as self-relevant processing and evaluates the degree to which one needs to deploy executive control in response to environmental demands.
Anxiety Phenotypes
Anxiety is a normal emotion if the arousing and motivating interoception is due to significant internal or external stimuli and can be used to deploy new cognitive or behavioral strategies. However, altered levels of anxiety may be due to several different dysfunctional neural circuit processes. First, increased amygdala may drive the insular too much, i.e. normal interoceptive stimuli acquire aversive valence or salience. Second, insular cortex may “overpredict” aversive outcomes and therefore predictive stimuli are associated with hyper-amygdala response. Third, general heightened arousal level may result in aversive “tagging” of predictive stimuli as aversive, which leads to increased anxiety. The neural circuit model, which we have proposed recently, is consistent with recent psychological conceptualizations of anxiety disorders. Together with temperamental vulnerabilities, which can be viewed as diatheses that make certain individuals more susceptible to adverse and stressful experiences, altered learning processes can result in the development of anxiety disorders [70]. Of the anxiety disorders, two are of particular interest because the processes that initiate or maintain them may differ, whereas the neural substrates might be quite overlapping and support the generalizability of our proposed model.
First, the development of panic disorder has been described by some [71] as a process they termed “fear of fear” developing from interoceptive conditioning. In particular, the match-mismatch model of panic states that panic disorder patients tend to overestimate the probability of panic prior to engaging in a fear-provoking situation [71]. This is part of the general mismatch prediction model, which states that people overestimate how frightened they will be when faced by a fear-provoking situation [72]. Second, several psychological theories have proposed that uncontrollable and unpredictable aversive events may play an important role in the development of GAD [73,74]. Specifically, people with GAD have far less tolerance for uncertainty than do nonanxious controls [75] and they are especially disturbed by not being able to predict the future [76]. Therefore, whereas panic disorder may be a form of “bottom up” failure, i.e. may be due to altered modulation of interoceptive signals, generalized anxiety disorder may be due to an altered “top down” modulation. In both cases, however, we predict that these individuals will show altered connectivity in the basic anxiety circuitry.
This altered “bottom up” or “top down” modulation is not unlike processes that have been described in the pain physiology literature as the basis for allodynia, i.e. the perception of innocuous stimuli as being painful and aversive. Interestingly, the same neural circuitry that we propose to comprise the basic anxiety circuit is also involved in allodynia. For example, in a recent study, the intensity of allodynic pain was directly related to the degree of activation in the caudal anterior insular cortex [77], which is an area that has been reported to code for the intensity of perceived pain [78] as opposed to ongoing pain intensity, which has been found to correlate with rostral anterior insula [79].
Thus, one may be able to distinguish an altered “top down” modulation of the basic anxiety circuitry, which will manifest in some individuals, such that the executive, cognitive control system attempts to down-regulate this system by cognitive activity, i.e. worrying. This results in the GAD phenotype. In contrast, altered “bottom up” modulation will be present in individuals who do not use extensive cognitive control (worry) and will therefore experience episodes of unconstrained fear and associated physical symptoms. This is the Panic Disorder (PD) phenotype; many of these individuals will avoid environments that are associated with insula-amygdala hyperactivity. This is the agoraphobia phenotype.
Thus, although many studies have been carried out with specific anxiety disorder groups, it is not clear whether the imaging phenotypes proposed here will follow the somewhat arbitrary conventional distinction of DSM IV-TR categories of anxiety disorders. Nevertheless, it is useful to briefly summarize the main findings in selected anxiety disorders. Individuals with Generalized Social Phobia show significant increased activation during contemptuous face processing in left allocortex, which includes amygdala, uncus, and parahippocampal gyrus [16]. Similarly, relative to happy faces, activation of the amygdala in response to harsh (angry, disgusted, fearful) faces was greater in these patients than in controls, and the extent of amygdala activation was positively correlated with severity of social anxiety symptoms [80]. Generalized Social Phobia patients, however, show reduced neural activation related to implicit learning compared with healthy comparison subjects in the left caudate head, left inferior parietal lobe, and bilateral insula [81]. Post-traumatic Stress Disorder is characterized by an exaggerated amygdala response, which may subserve exaggerated acquisition of fear associations and expression of fear responses, and deficient frontal cortical function, which may mediate deficits in extinction and the capacity to suppress attention/response to trauma-related stimuli, as well as deficient hippocampal function, which may be responsible for deficits in appreciation of safe contexts and explicit learning/memory [82]. In pain-related experiments, patients with PTSD rated temperatures as less painful compared with controls but show increased activation in the left hippocampus and decreased activation in the bilateral ventrolateral prefrontal cortex and the right amygdala [83]. Phobic individuals show early amygdala-related picture processing abnormalities. In particular, amygdalar BOLD responses associated with timing but not magnitude of activation predicted affective responses to phobogenic stimuli [84].
Patients with Panic disorder display less amygdala activation but greater cingulate cortex activation than controls in response to fearful faces [85]. In Obsessive Compulsive Disorder (OCD), color naming OCD-related, but not PD-related, words was found to correlate with increased activation of frontal-striatal and temporal regions. In contrast, an increased frontal-striatal involvement was found during color naming both OCD-related and panic-related words in Panic Disorder patients [86]. Baseline perfusion of the orbitofrontal cortex predicted panic attacks such that lower perfusion was associated with heightened anxiety in response to a pharmacological challenge [87]. Others have found that OCD subjects exhibited a weaker response than control subjects bilaterally across all face conditions versus fixation in the amygdala [88]. Therefore, although there are some distinctions in processing-related activation differences across diagnostic groups, it is not clear how reliable and specific these differences are because of the lack of large studies with multi-diagnostic groups.
Apart from different neural substrate based processing dysfunction derived anxiety phenotypes, one can begin to examine the effect of anti-anxiety treatments on healthy individuals or patients with anxiety disorders. This approach can be useful to determine whether neuroimaging tools could become (1) a bioassay for developing novel treatments for anxiety disorder, or (2) a way of monitoring treatment success during longitudinal studies, or (3) to measure the risk for developing another symptomatic episode of a particular anxiety disorder.
A recent neuroimaging study showed that right amygdala response to aversive faces was attenuated by citalopram [89]. Others have reported that after treatment with citalopram, worry sentences, compared to neutral statements, elicit reduced BOLD responses in prefrontal regions, the striatum, insula and paralimbic regions [90]. Finally, citalopram also reduced responses within the hippocampus and medial prefrontal cortex specifically during the fear-relevant stimuli [91]. Thus, serotonin specific reuptake inhibitors, which are standard treatment for many anxiety disorders, alter process-related all three key neural substrates that were summarized above. Some investigators have argued that individuals whose pretreatment amygdala activity is the strongest may be particularly likely to respond well to such widely used treatments as selective serotonin reuptake inhibitor (SSRI) medications and CBT [92]. Novel treatment approaches may also be good candidates for imaging studies to better understand how they affect anxiety disorders. For example, oxytocin relative to placebo potently reduced activation of the amygdala and reduced coupling of the amygdala to brainstem regions implicated in autonomic and behavioral manifestations of fear [93].
Anxiety-prone subjects had significantly greater bilateral amygdala and insula activation to emotional faces than did the anxiety-normative comparison subjects [94]. Similarly, basolateral amygdala to unconscious stimuli, and subjects’ reaction times, were predicted by individual differences in trait anxiety [68]. Finally, behaviorally inhibited individuals relative to healthy adolescents show an exaggerated amygdala response during subjective fear ratings and deactivation during passive viewing, across all emotion faces [95]. In comparison, neither high- nor low-anxious volunteers showed an increased amygdala response to threat distractors. However, under low perceptual load, elevated state anxiety was associated with a heightened response to threat distractors in the amygdala and superior temporal sulcus, whereas individuals high in trait anxiety showed a reduced prefrontal response to these stimuli, consistent with weakened recruitment of control mechanisms used to prevent the further processing of salient distractors [96]. Taken together, there are several studies that show individuals who are at risk for an anxiety disorder show brain processing differences that are quantitatively similar to those observed in anxiety disorder patients.
Other groups have investigated the role of specific candidate genes to alter anxiety-related processing and therefore potentially serve as vulnerability genes. For example, the 5-HTTLPR (Serotonin Transporter) gene polymorphism has a powerful effect on amygdala reactivity to environmental threat. Although, the 5-HTTLPR gene is not specifically related to an anxiety or mood disorder, it may represent a classic susceptibility factor [97]. Others have pointed to the dopamine neurotransmission associated with the met allele of the COMT polymorphism, which is associated with heightened reactivity and connectivity in corticolimbic circuits [98]. Functional analysis of those regions during perceptual processing of fearful stimuli demonstrated tight coupling as a feedback circuit implicated in the extinction of negative affect. Finally, short-allele carriers of the 5-HTTLPR gene show relative uncoupling of the medial prefrontal cortex amygdala circuit [63].
One of the major challenges for neuroimaging to play a critical clinical role is to determine its sensitivity and specificity. Thus far, most imaging studies have revealed intriguing systems neuroscience results on a group level, however, these findings are insufficient to help move imaging forward clinically. On the other hand, most imaging studies have demonstrated surprisingly large effect sizes, which would support the idea that differences across individuals and across time within individuals may be large enough to be meaningfully measured on a subject by subject basis. To be useful as an illness severity marker, neuroimaging measures need to closely track disease state both when it is symptomatic as well as when the disorder is asymptomatic. Thus, it is not sufficient to show that ill individuals differ from healthy subjects but also that recovered or asymptomatic anxiety disorder individuals have altered processing levels implemented in specific brain structures when compared to those individuals without an anxiety disorder. The latter will enable one to make clinical predictions about individuals who are at high risk for experiencing exacerbation of their anxiety symptoms sometimes in the future. As pointed out above, neuroimaging is not useful in isolation but needs to be considered within the context of the process that the brain substrates are carrying out. Here, again, results from studies examining both amygdala and insular cortex function offer some insight into the direction of the clinical use of neuroimaging. Clearly, functional neuroimaging will play an important role in anxiety disorder research, however, in order for this modality to be useful for defining diagnostic categories or monitoring treatment success, one will need to push the limits of this technology to clearly show its ability on a single-subject basis.
Figure 1.
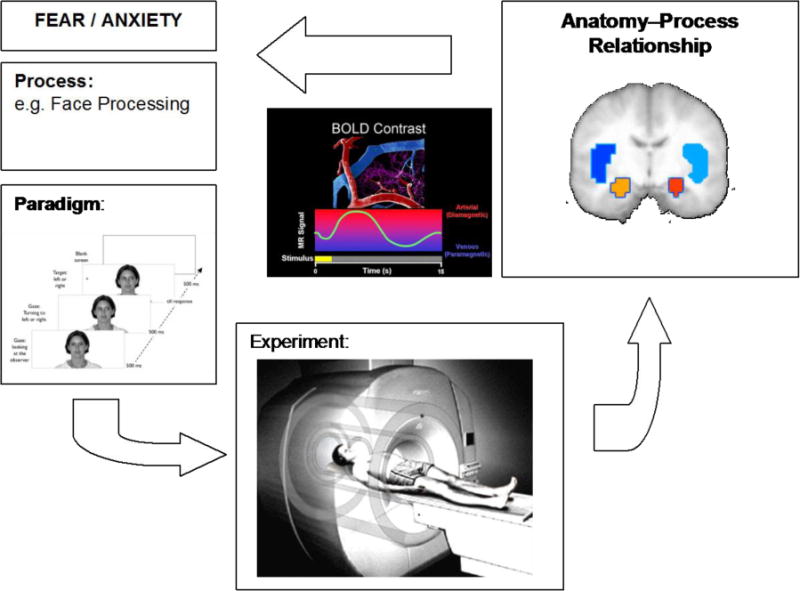
This figure shows the process of the role of brain imaging in anxiety disorders. Specifically, we propose that functional neuroimaging is not about a particular brain area but about the interaction between the process and the brain system. Therefore, it is important to clarify the role of brain structure involvement in relation to the process that is being tested.
Acknowledgments
This research was supported by grants from NIDA (R01DA016663, R01DA018307) and by a VA Merit Grant.
References
- 1.Shulman RG, Hyder F, Rothman DL. Biophysical basis of brain activity: implications for neuroimaging. Q Rev Biophys. 2002;35:287–325. doi: 10.1017/s0033583502003803. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa S, Lee TM. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magnetic Resonance in Medicine. 1990;16:9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magnetic Resonance in Medicine. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 4.Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- 5.Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Kastrup A, Kruger G, Glover GH, Neumann-Haefelin T, Moseley ME. Regional variability of cerebral blood oxygenation response to hypercapnia. Neuroimage. 1999;10:675–681. doi: 10.1006/nimg.1999.0505. [DOI] [PubMed] [Google Scholar]
- 7.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 8.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 9.LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 10.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 11.Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- 12.Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a Risk-taking Task. A PET Study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- 14.Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12:2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- 15.Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 17.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 18.Bremner JD, Innis RB, White T, Fujita M, Silbersweig D, Goddard AW, Staib L, Stern E, Cappiello A, Woods S, Baldwin R, Charney DS. SPECT [I-123]iomazenil measurement of the benzodiazepine receptor in panic disorder. Biol Psychiatry. 2000;47:96–106. doi: 10.1016/s0006-3223(99)00188-2. [DOI] [PubMed] [Google Scholar]
- 19.Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry. 1998;55:715–720. doi: 10.1001/archpsyc.55.8.715. [DOI] [PubMed] [Google Scholar]
- 20.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 21.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 22.Augustine JR. The insular lobe in primates including humans. Neurol Res. 1985;7:2–10. doi: 10.1080/01616412.1985.11739692. [DOI] [PubMed] [Google Scholar]
- 23.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 24.Ture U, Yasargil DC, Al Mefty O, Yasargil MG. Topographic anatomy of the insular region. J Neurosurg. 1999;90:720–733. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- 25.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 26.Shipp S. The importance of being agranular: a comparative account of visual and motor cortex. Philos Trans R Soc Lond B Biol Sci. 2005;360:797–814. doi: 10.1098/rstb.2005.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci U S A. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 29.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 30.Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA. Neural responses to facial and vocal expressions of fear and disgust. Proc R Soc Lond B Biol Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 32.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 33.Morris JS, Friston KJ, Bèuchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 34.Gelnar PA, Krauss BR, Sheehe PR, Szeverenyi NM, Apkarian AV. A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage. 1999;10:460–482. doi: 10.1006/nimg.1999.0482. [DOI] [PubMed] [Google Scholar]
- 35.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 36.Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, Nicoletti R, Umita C, Nichelli P. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- 37.Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22:1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 38.Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 39.Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22:941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Seger CA, Stone M, Keenan JP. Cortical Activations during judgments about the self and an other person. Neuropsychologia. 2004;42:1168–1177. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage. 2002;15:983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
- 43.Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 44.Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 45.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. Journal of Neuroscience. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapur N, Friston KJ, Young A, Frith CD, Frackowiak RS. Activation of human hippocampal formation during memory for faces: a PET study. Cortex. 1995;31:99–108. doi: 10.1016/s0010-9452(13)80108-6. [DOI] [PubMed] [Google Scholar]
- 48.Halgren E, Raij T, Marinkovic K, Jousmaki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cereb Cortex. 2000;10:69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- 49.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 51.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating ‘unseen’ fear. Proc Natl Acad Sci U S A. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 54.Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, Sadato N. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. J Cogn Neurosci. 2001;13:1035–1047. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- 55.Indersmitten T, Gur RC. Emotion processing in chimeric faces: hemispheric asymmetries in expression and recognition of emotions. J Neurosci. 2003;23:3820–3825. doi: 10.1523/JNEUROSCI.23-09-03820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 57.Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. J Neurosci. 1996;16:7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dolan RJ, Fletcher P, Morris J, Kapur N, Deakin JF, Frith CD. Neural activation during covert processing of positive emotional facial expressions. Neuroimage. 1996;4:194–200. doi: 10.1006/nimg.1996.0070. [DOI] [PubMed] [Google Scholar]
- 59.Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 60.Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, Yonekura Y. Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fMRI study. Neuroimage. 2004;21:352–363. doi: 10.1016/j.neuroimage.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 61.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 62.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 63.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005 doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 64.Keightley ML, Winocur G, Graham SJ, Mayberg HS, Hevenor SJ, Grady CL. An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia. 2003;41:585–596. doi: 10.1016/s0028-3932(02)00199-9. [DOI] [PubMed] [Google Scholar]
- 65.Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 66.Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 67.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 69.Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: An event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 70.Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. Am Psychol. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- 71.de Beurs E, Chambless DL, Goldstein AJ. The match-mismatch model and panic patients’ accuracy in predicting naturally occurring panic attacks. Depress Anxiety. 2002;16:172–181. doi: 10.1002/da.10073. [DOI] [PubMed] [Google Scholar]
- 72.Rachman S. The overprediction of fear: a review. Behav Res Ther. 1994;32:683–690. doi: 10.1016/0005-7967(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 73.Mineka S, Hendersen RW. Controllability and predictability in acquired motivation. Annu Rev Psychol. 1985;36:495–529. doi: 10.1146/annurev.ps.36.020185.002431. [DOI] [PubMed] [Google Scholar]
- 74.Barlow DH, Blanchard EB, Vermilyea JA, Vermilyea BB, DiNardo PA. Generalized anxiety and generalized anxiety disorder: description and reconceptualization. Am J Psychiatry. 1986;143:40–44. doi: 10.1176/ajp.143.1.40. [DOI] [PubMed] [Google Scholar]
- 75.Dugas MJ, Freeston MH, Ladouceur R, Rheaume J, Provencher M, Boisvert JM. Worry themes in primary GAD, secondary GAD, and other anxiety disorders. J Anxiety Disord. 1998;12:253–261. doi: 10.1016/s0887-6185(98)00013-9. [DOI] [PubMed] [Google Scholar]
- 76.Roemer L, Molina S, Borkovec TD. An investigation of worry content among generally anxious individuals. J Nerv Ment Dis. 1997;185:314–319. doi: 10.1097/00005053-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Schweinhardt P, Glynn C, Brooks J, McQuay H, Jack T, Chessell I, Bountra C, Tracey I. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 78.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 79.Petrovic P, Petersson KM, Hansson P, Ingvar M. A regression analysis study of the primary somatosensory cortex during pain. Neuroimage. 2002;16:1142–1150. doi: 10.1006/nimg.2002.1069. [DOI] [PubMed] [Google Scholar]
- 80.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 81.Sareen J, Campbell DW, Leslie WD, Malisza KL, Stein MB, Paulus MP, Kravetsky LB, Kjernisted KD, Walker JR, Reiss JP. Striatal Function in Generalized Social Phobia: A Functional Magnetic Resonance Imaging Study. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 82.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Geuze E, Westenberg HG, Jochims A, de Kloet CS, Bohus M, Vermetten E, Schmahl C. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76–85. doi: 10.1001/archpsyc.64.1.76. [DOI] [PubMed] [Google Scholar]
- 84.Larson CL, Schaefer HS, Siegle GJ, Jackson CA, Anderle MJ, Davidson RJ. Fear is fast in phobic individuals: amygdala activation in response to fear-relevant stimuli. Biol Psychiatry. 2006;60:410–417. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 85.Pillay SS, Gruber SA, Rogowska J, Simpson N, Yurgelun-Todd DA. fMRI of fearful facial affect recognition in panic disorder: the cingulate gyrus-amygdala connection. J Affect Disord. 2006;94:173–181. doi: 10.1016/j.jad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 86.van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, van Balkom AJ, van Oppen P, van Dyck R. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- 87.Kent JM, Coplan JD, Mawlawi O, Martinez JM, Browne ST, Slifstein M, Martinez D, Abi-Dargham A, Laruelle M, Gorman JM. Prediction of panic response to a respiratory stimulant by reduced orbitofrontal cerebral blood flow in panic disorder. Am J Psychiatry. 2005;162:1379–1381. doi: 10.1176/appi.ajp.162.7.1379. [DOI] [PubMed] [Google Scholar]
- 88.Cannistraro PA, Wright CI, Wedig MM, Martis B, Shin LM, Wilhelm S, Rauch SL. Amygdala responses to human faces in obsessive-compulsive disorder. Biol Psychiatry. 2004;56:916–920. doi: 10.1016/j.biopsych.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 89.Del Ben CM, Deakin JF, McKie S, Delvai NA, Williams SR, Elliott R, Dolan M, Anderson IM. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30:1724–1734. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- 90.Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 2004;131:11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant Drug Treatment Modifies the Neural Processing of Nonconscious Threat Cues. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 92.McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Leibenluft E, Ernst M, Pine DS. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl) 2006 doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- 93.Kirsch P, Reuter M, Mier D, Lonsdorf T, Stark R, Gallhofer B, Vaitl D, Hennig J. Imaging gene-substance interactions: the effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neurosci Lett. 2006;405:196–201. doi: 10.1016/j.neulet.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 94.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 95.Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bishop SJ, Jenkins R, Lawrence AD. Neural Processing of Fearful Faces: Effects of Anxiety are Gated by Perceptual Capacity Limitations. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- 97.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 98.Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]


