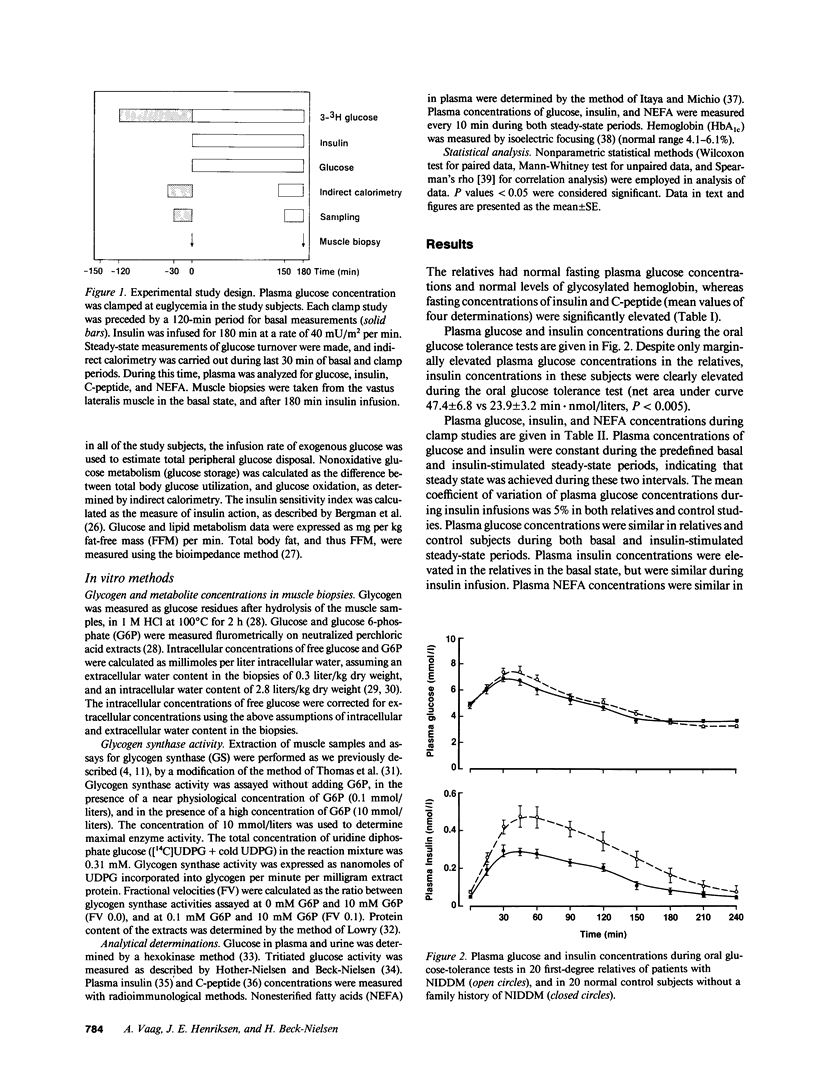
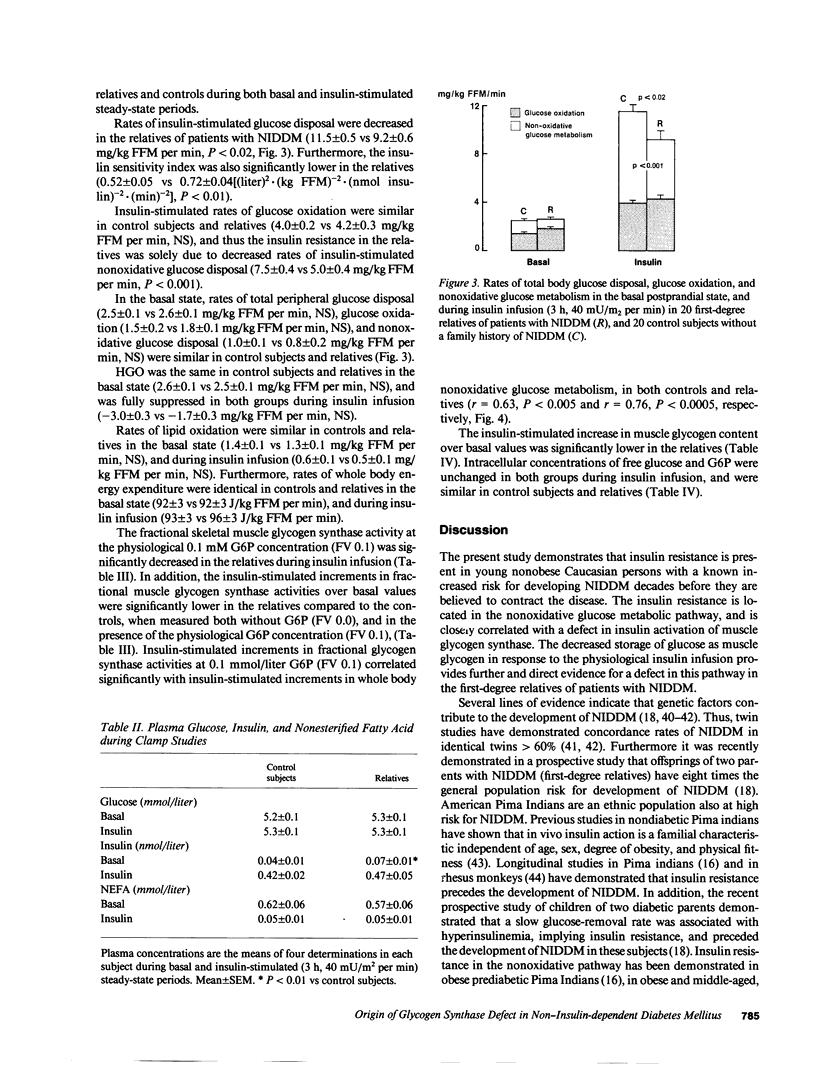
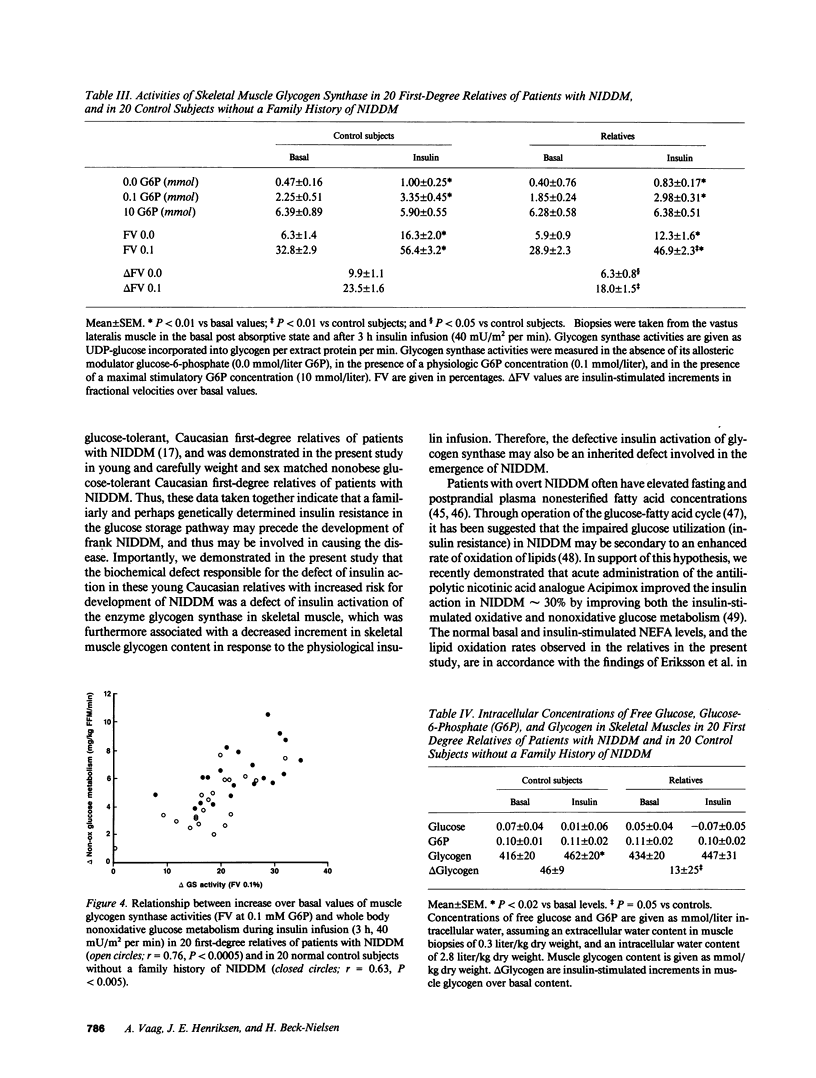
Abstract
Insulin resistance in non-insulin-dependent diabetes is associated with a defective insulin activation of the enzyme glycogen synthase in skeletal muscles. To investigate whether this may be a primary defect, we studied 20 young (25 +/- 1 yr) Caucasian first-degree relatives (children) of patients with non-insulin-dependent diabetes, and 20 matched controls without a family history of diabetes. Relatives and controls had a normal oral glucose tolerance, and were studied by means of the euglycemic hyperinsulinemic clamp technique, which included performance of indirect calorimetry and muscle biopsies. Insulin-stimulated glucose disposal was decreased in the relatives (9.2 +/- 0.6 vs 11.5 +/- 0.5 mg/kg fat-free mass per (FFM) min, P less than 0.02), and was due to a decreased rate of insulin-stimulated nonoxidative glucose metabolism (5.0 +/- 0.5 vs 7.5 +/- 0.4 mg/kg fat-free mass per min, P less than 0.001). The insulin-stimulated, fractional glycogen synthase activity (0.1/10 mmol liter glucose-6-phosphate) was decreased in the relatives (46.9 +/- 2.3 vs 56.4 +/- 3.2%, P less than 0.01), and there was a significant correlation between insulin-stimulated, fractional glycogen synthase activity and nonoxidative glucose metabolism in relatives (r = 0.76, P less than 0.001) and controls (r = 0.63, P less than 0.01). Furthermore, the insulin-stimulated increase in muscle glycogen content over basal values was lower in the relatives (13 +/- 25 vs 46 +/- 9 mmol/kg dry wt, P = 0.05). We conclude that the defect in insulin activation of muscle glycogen synthase may be a primary, possibly genetically determined, defect that contributes to the development of non-insulin-dependent diabetes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett A. H., Eff C., Leslie R. D., Pyke D. A. Diabetes in identical twins. A study of 200 pairs. Diabetologia. 1981 Feb;20(2):87–93. doi: 10.1007/BF00262007. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Hope I. D., Yang Y. J., Watanabe R. M., Meador M. A., Youn J. H., Ader M. Assessment of insulin sensitivity in vivo: a critical review. Diabetes Metab Rev. 1989 Aug;5(5):411–429. doi: 10.1002/dmr.5610050501. [DOI] [PubMed] [Google Scholar]
- Boden G., Ray T. K., Smith R. H., Owen O. E. Carbohydrate oxidation and storage in obese non-insulin-dependent diabetic patients. Effects of improving glycemic control. Diabetes. 1983 Nov;32(11):982–987. doi: 10.2337/diab.32.11.982. [DOI] [PubMed] [Google Scholar]
- Bodkin N. L., Metzger B. L., Hansen B. C. Hepatic glucose production and insulin sensitivity preceding diabetes in monkeys. Am J Physiol. 1989 May;256(5 Pt 1):E676–E681. doi: 10.1152/ajpendo.1989.256.5.E676. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobelli C., Mari A., Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol. 1987 May;252(5 Pt 1):E679–E689. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- Damsbo P., Vaag A., Hother-Nielsen O., Beck-Nielsen H. Reduced glycogen synthase activity in skeletal muscle from obese patients with and without type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991 Apr;34(4):239–245. doi: 10.1007/BF00405082. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Eriksson J., Franssila-Kallunki A., Ekstrand A., Saloranta C., Widén E., Schalin C., Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989 Aug 10;321(6):337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- Felber J. P., Ferrannini E., Golay A., Meyer H. U., Theibaud D., Curchod B., Maeder E., Jequier E., DeFronzo R. A. Role of lipid oxidation in pathogenesis of insulin resistance of obesity and type II diabetes. Diabetes. 1987 Nov;36(11):1341–1350. doi: 10.2337/diab.36.11.1341. [DOI] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Frayn K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Freidenberg G. R., Reichart D., Olefsky J. M., Henry R. R. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988 Oct;82(4):1398–1406. doi: 10.1172/JCI113744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay A., DeFronzo R. A., Ferrannini E., Simonson D. C., Thorin D., Acheson K., Thiébaud D., Curchod B., Jéquier E., Felber J. P. Oxidative and non-oxidative glucose metabolism in non-obese type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1988 Aug;31(8):585–591. doi: 10.1007/BF00264764. [DOI] [PubMed] [Google Scholar]
- Greenfield M., Kolterman O., Olefsky J., Reaven G. M. Mechanism of hypertriglyceridaemia in diabetic patients with fasting hyperglycaemia. Diabetologia. 1980 Jun;18(6):441–446. doi: 10.1007/BF00261698. [DOI] [PubMed] [Google Scholar]
- Haffner S. M., Stern M. P., Hazuda H. P., Mitchell B. D., Patterson J. K. Increased insulin concentrations in nondiabetic offspring of diabetic parents. N Engl J Med. 1988 Nov 17;319(20):1297–1301. doi: 10.1056/NEJM198811173192001. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia. 1972 Aug;8(4):260–266. doi: 10.1007/BF01225569. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- Hother-Nielsen O., Beck-Nielsen H. On the determination of basal glucose production rate in patients with type 2 (non-insulin-dependent) diabetes mellitus using primed-continuous 3-3H-glucose infusion. Diabetologia. 1990 Oct;33(10):603–610. doi: 10.1007/BF00400204. [DOI] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Johnson A. B., Argyraki M., Thow J. C., Broughton D., Jones I. R., Taylor R. Effects of intensive dietary treatment on insulin-stimulated skeletal muscle glycogen synthase activation and insulin secretion in newly presenting type 2 diabetic patients. Diabet Med. 1990 Jun;7(5):420–428. doi: 10.1111/j.1464-5491.1990.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Mandarino L. J. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990 Dec;86(6):1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennington A. S., Hill C. R., Craig J., Bogardus C., Raz I., Ortmeyer H. K., Hansen B. C., Romero G., Larner J. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1990 Aug 9;323(6):373–378. doi: 10.1056/NEJM199008093230603. [DOI] [PubMed] [Google Scholar]
- Kida Y., Esposito-Del Puente A., Bogardus C., Mott D. M. Insulin resistance is associated with reduced fasting and insulin-stimulated glycogen synthase phosphatase activity in human skeletal muscle. J Clin Invest. 1990 Feb;85(2):476–481. doi: 10.1172/JCI114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida Y., Nyomba B. L., Bogardus C., Mott D. M. Defective insulin response of cyclic adenosine monophosphate-dependent protein kinase in insulin-resistant humans. J Clin Invest. 1991 Feb;87(2):673–679. doi: 10.1172/JCI115045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk R. L., Serjeantson S. W., King H., Zimmet P. The genetic epidemiology of diabetes mellitus. Prog Clin Biol Res. 1985;194:119–146. [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LELOIR L. F., OLAVARRIA J. M., GOLDEMBERG S. H., CARMINATTI H. Biosynthesis of glycogen from uridine diphosphate glucose. Arch Biochem Biophys. 1959 Apr;81(2):508–520. doi: 10.1016/0003-9861(59)90232-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leslie R. D., Volkmann H. P., Poncher M., Hanning I., Orskov H., Alberti K. G. Metabolic abnormalities in children of non-insulin dependent diabetics. Br Med J (Clin Res Ed) 1986 Oct 4;293(6551):840–842. doi: 10.1136/bmj.293.6551.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Howard B. V., Bennett P. H., Yki-Järvinen H., Freymond D., Nyomba B. L., Zurlo F., Swinburn B., Bogardus C. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988 May 12;318(19):1217–1225. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Zawadzki J. K., Young A. A., Abbott W. G., Knowler W. C., Bennett P. H., Moll P., Bogardus C. In vivo insulin action is familial characteristic in nondiabetic Pima Indians. Diabetes. 1987 Nov;36(11):1329–1335. doi: 10.2337/diab.36.11.1329. [DOI] [PubMed] [Google Scholar]
- Lukaski H. C., Johnson P. E., Bolonchuk W. W., Lykken G. I. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985 Apr;41(4):810–817. doi: 10.1093/ajcn/41.4.810. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Wright K. S., Verity L. S., Nichols J., Bell J. M., Kolterman O. G., Beck-Nielsen H. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest. 1987 Sep;80(3):655–663. doi: 10.1172/JCI113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H. U., Curchod B., Maeder E., Pahud P., Jequier E., Felber J. P. Modifications of glucose storage and oxidation in nonobese diabetics, measured by continuous indirect calorimetry. Diabetes. 1980 Sep;29(9):752–756. doi: 10.2337/diab.29.9.752. [DOI] [PubMed] [Google Scholar]
- Mortensen H. B. Quantitative determination of hemoglobin A1c by thin-layer isoelectric focusing. J Chromatogr. 1980 Jun 13;182(3-4):325–333. doi: 10.1016/s0378-4347(00)81481-4. [DOI] [PubMed] [Google Scholar]
- Newman B., Selby J. V., King M. C., Slemenda C., Fabsitz R., Friedman G. D. Concordance for type 2 (non-insulin-dependent) diabetes mellitus in male twins. Diabetologia. 1987 Oct;30(10):763–768. doi: 10.1007/BF00275741. [DOI] [PubMed] [Google Scholar]
- Nyomba B. L., Ossowski V. M., Bogardus C., Mott D. M. Insulin-sensitive tyrosine kinase: relationship with in vivo insulin action in humans. Am J Physiol. 1990 Jun;258(6 Pt 1):E964–E974. doi: 10.1152/ajpendo.1990.258.6.E964. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes. 1991 Feb;40(2):166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Greenfield M. S. Diabetic hypertriglyceridemia: evidence for three clinical syndromes. Diabetes. 1981;30(Suppl 2):66–75. doi: 10.2337/diab.30.2.s66. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G., Adams R. P., Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985 Feb;248(2 Pt 2):R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G., Saltin B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am J Physiol. 1982 Sep;243(3):R271–R280. doi: 10.1152/ajpregu.1982.243.3.R271. [DOI] [PubMed] [Google Scholar]
- Tappy L., Owen O. E., Boden G. Effect of hyperinsulinemia on urea pool size and substrate oxidation rates. Diabetes. 1988 Sep;37(9):1212–1216. doi: 10.2337/diab.37.9.1212. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Brechtel G., Henry R. R. Multiple defects in muscle glycogen synthase activity contribute to reduced glycogen synthesis in non-insulin dependent diabetes mellitus. J Clin Invest. 1991 Feb;87(2):489–495. doi: 10.1172/JCI115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaag A., Skött P., Damsbo P., Gall M. A., Richter E. A., Beck-Nielsen H. Effect of the antilipolytic nicotinic acid analogue acipimox on whole-body and skeletal muscle glucose metabolism in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1991 Oct;88(4):1282–1290. doi: 10.1172/JCI115432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warram J. H., Martin B. C., Krolewski A. S., Soeldner J. S., Kahn C. R. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990 Dec 15;113(12):909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]




