Abstract
Background
Race-ethnic differences exist in the epidemiology of heart failure, with blacks experiencing higher incidence and worse prognosis. Left ventricular (LV) systolic dysfunction (LVSD) detected by speckle-tracking global longitudinal strain (GLS) is a predictor of cardiovascular events including heart failure. It is not known whether race-ethnic differences in GLS-LVSD exist in subjects without overt LV dysfunction.
Methods
Participants from a tri-ethnic community-based study underwent two-dimensional echocardiography with assessment of LV ejection fraction (LVEF) and GLS by speckle-tracking. Participants with LVEF<50% were excluded. GLS-LVSD was defined as GLS > 95% percentile in a healthy sample (−14.7%).
Results
Of the 678 study participants (mean age 71±9 years, 61% women), 114 were blacks, 464 Hispanics, and 100 whites. GLS was significantly lower in blacks (−16.5±3.5%) than in whites (−17.5±3.0%) and Hispanics (−17.3±2.9%) in both univariate (p=0.015) and multivariate analysis (p=0.011), whereas LVEF was not significantly different between the 3 groups (64.3±4.6%, 63.4±4.9%, 64.7±4.9% respectively, univariate p=0.064, multivariate p=0.291). GLS-LVSD was more frequent in blacks (27.2%) than in whites (19.0%) and Hispanics (14.9%, p=0.008). In multivariate analysis adjusted for confounders and cardiovascular risk factors, blacks were significantly more likely to have GLS-LVSD (adjusted odds ratio=2.6, 95% confidence intervals=1.4–4.7, p=0.002) compared to the other groups.
Conclusions
Among participants from a tri-ethnic community cohort, black race was associated with greater degree of subclinical LVSD by GLS than other race-ethnic groups. This difference was independent of confounders and cardiovascular risk factors.
Keywords: Race-ethnicity, left ventricular dysfunction, global longitudinal strain, speckletracking, echocardiography
INTRODUCTION
Heart failure is a leading cause of morbidity and mortality in the U.S., affects an estimated 5.1 million adult Americans, and is projected to undergo a 25% increase in prevalence by 2030.1 Several studies showed that race-ethnic differences exist in heart failure epidemiology.2–5 Data from population studies have shown that the incidence and prevalence of heart failure are greater in the black population. Once the diagnosis is made, heart failure progression is more rapid in black than in white patients, with associated higher risk of hospitalizations.6–8 This imbalance between races possibly reflects the higher prevalence in black subjects of cardiovascular risk factors such as hypertension and diabetes mellitus and their worse profile of cardiovascular health metrics.9–12 Left ventricular systolic dysfunction (LVSD), a predictor of cardiovascular events and incident heart failure, has been documented in 2% to 14% of the general population, even without a prior diagnosis of myocardial infarction or heart failure.13–16 Despite the higher incidence of heart failure in blacks, it is not clear whether LVSD is more frequent in blacks, especially after accounting for their worse cardiovascular risk profile.17–19 In previous studies LVSD was assessed by LV ejection fraction (LVEF); however, LV global longitudinal strain (GLS) measured by echocardiographic speckle-tracking imaging is emerging as a more sensitive measure of LV function, able to detect subclinical LVSD in a significant number of subjects with normal LVEF.20 Furthermore, because GLS is a powerful predictor of cardiovascular outcome, independent of and additive to LVEF,20–22 its assessment might be of help in the early identification of subjects at high risk of future cardiovascular events, especially in race-ethnic groups that more frequently experience overt heart failure. Accordingly, we investigated race-ethnic differences in subclinical LVSD measured by echocardiographic speckle-tracking GLS in a tri-ethnic sample from a community-based cohort study.
METHODS
Study population
The Cardiovascular Abnormalities and Brain Lesion (CABL) study is a community-based epidemiologic study designed to investigate the cardiovascular predictors of silent brain disease in the community. CABL based its recruitment on the Northern Manhattan Study (NOMAS), a population-based prospective study investigating the epidemiology and risk factors for stroke and cardiovascular disease. The study design and recruitment details of NOMAS have been described previously.23 CABL inclusion criteria were: 1) age >50 years; 2) no contraindications to MRI; and 3) no prior stroke. Of the 1,004 participants enrolled between 2005 and 2010, 276 had no raw digital echocardiographic data or had suboptimal image quality for speckle-tracking analysis, and were therefore excluded from the present analysis. Additional exclusions were LVEF<50% and race-ethnicities other than black, white or Hispanic. Written informed consent was obtained from all study participants. The study protocol was approved by the Institutional Review Boards of Columbia University Medical Center and of the University of Miami. This study was supported by grants from the National Institute of Neurological Disorders and Stroke [grant number R01 NS36286 to MDT and R37 NS29993 to RLS/MSE]. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Risk factors assessment
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, or self-reported history of hypertension or use of anti-hypertensive medication. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or self-reported history of diabetes or use of diabetes medications. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, self-report of hypercholesterolemia or use of lipid-lowering treatment. Cigarette smoking, either at the time of the interview or in the past, was recorded. Coronary artery disease was defined as a history of myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention. The race-ethnicity classification was based on self-identification, and modeled after the U.S. Census. Self-reported race-ethnicity was categorized as non-Hispanic white (white), non-Hispanic black (black), and Hispanic. Participants of other race-ethnicities (n=22) were excluded.
Echocardiographic assessment
Two-dimensional echocardiography
Transthoracic echocardiography was performed using a commercially available system (iE 33, Philips, Andover, MA) by a trained, registered cardiac sonographer according to a standardized protocol. LV wall thickness and diameters, and left atrial antero-posterior diameter were measured from a parasternal long-axis view according to current guidelines.24 LV end-diastolic diameter (LVEDi) and left atrial diameter were indexed by body surface area. LVEF was calculated using the biplane modified Simpson’s rule. LV mass was calculated with a validated method25 and indexed by body surface area (LV mass index). LV relative wall thickness was calculated as: 2 × posterior wall thickness/LV enddiastolic diameter. LV diastolic function was assessed as previously described by trans-mitral Doppler and mitral annulus tissue Doppler.26 Peak early (E) and late velocity (A) of mitral inflow were measured, and the E/A ratio was calculated. Peak early diastolic velocity (e′) of the lateral and septal mitral annulus were measured and averaged.
Speckle-tracking strain imaging
Speckle-tracking analysis was performed off-line using commercially available software (QLAB Advanced Quantification Software version 8.1, Philips) as previously described.27 Briefly, speckle-tracking analysis of LV myocardial deformation over the longitudinal axis was performed from two-dimensional gray-scale loops recorded at a frame rate ≥ 45 fps. GLS was calculated averaging the negative peak of longitudinal strain from 12 ventricular segments from the apical 4-chamber and 2-chamber views. Abnormal GLS was defined as GLS >−14.7%, corresponding to the value separating the lowest 5 percent of the distribution in a healthy subgroup from the same cohort.20
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables and as percentage for categorical variables. One-way ANOVA, and Chi-square tests were used to assess differences between race-ethnic groups. Linear and logistic models were used to assess differences in systolic function between groups, and odds ratios (OR) and 95% confidence intervals (CI) were calculated. Covariates were selected based on their univariate association with race-ethnicity with p-value <0.1. For all statistical analyses, a two-tailed p<0.05 was considered significant. Statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of the study population by race-ethnicity
Study sample included 678 participants, of whom 464 were Hispanics (68.4%), 114 blacks (16.8%), and 100 whites (14.8%). Mean age of the study population was 71±9 years, and 61% were women. Table I shows the demographics and clinical characteristics of the study population by race-ethnicity. Significant differences between the 3 groups were found for age, body mass index, sex distribution, level of education, SBP, DBP, prevalence of hypertension, diabetes, and cigarette smoking (all p<0.05). Echocardiographic parameters of LV structure and function by race-ethnicity are shown in Table II. Significant differences between the three groups were found in LVEDi, LV mass index (both p<0.01), and left atrial size (p<0.05).
Table I.
Clinical and echocardiographic characteristics of the study population by race-ethnicity.
| Black N=114 |
White N=100 |
Hispanic N=464 |
P value | |
|---|---|---|---|---|
| Age, years | 75.1±9.9 | 74.4±10.3 | 69.6±8.6 | <0.001 |
| Body mass index, kg/m2 | 27.7±4.5 | 26.3±5.1 | 28.2±4.5 | 0.001 |
| Women, n (%) | 73 (64.0) | 50 (50.0) | 299 (64.4) | 0.024 |
| High school education, n (%) | 88 (77.2) | 88 (88.0) | 126 (27.2) | <0.001 |
| SBP, mmHg | 137.2±16.5 | 131.8±18.2 | 135.7±16.1 | 0.046 |
| DBP, mmHg | 79.3±10.1 | 76.0±10.5 | 78.5±8.8 | 0.022 |
| Hypertension, n (%) | 93 (81.3) | 65 (65.0) | 366 (78.9) | 0.005 |
| Anti-hypertensive treatment, n (%) | 88 (77.2) | 54 (54.0) | 334 (72.0) | 0.001 |
| Diabetes mellitus, n (%) | 29 (25.4) | 9 (9.0) | 154 (33.2) | <0.001 |
| Hypercholesterolemia, n (%) | 63 (55.3) | 67 (67.0) | 312 (67.2) | 0.066 |
| History of cigarette smoking, n (%) | 67 (58.8) | 59 (59.0) | 229 (49.4) | 0.070 |
| Coronary artery disease, n (%) | 9 (7.9) | 6 (6.0) | 25 (5.4) | 0.595 |
SBP: Systolic blood pressure. DBP: Diastolic blood pressure.
Table II.
Echocardiographic variables of LV structure by race-ethnicity.
| Black | White | Hispanic | P value | |
|---|---|---|---|---|
| LV septal thickness, mm | 11.6±1.8 | 11.2±1.6 | 11.3±1.8 | 0.163 |
| LVEDi, mm/m2 | 24.3±2.7 | 25.1±2.9 | 25.8±2.9 | <0.001 |
| LV posterior wall thickness, mm | 11.2±1.6 | 11.2±1.7 | 11.0±1.5 | 0.413 |
| LV mass index, g/m2.7 | 47.0±12.8 | 46.3±12.1 | 50.8±13.0 | 0.001 |
| Relative wall thickness | 0.52±0.09 | 0.50±0.10 | 0.50±0.08 | 0.150 |
| Left atrial diameter, cm/m2 | 21.9±3.2 | 22.3±3.3 | 22.7±3.1 | 0.040 |
| E/A | 0.88±0.34 | 0.87±0.26 | 0.82±0.22 | 0.052 |
| e′, cm/s | 7.2±2.0 | 7.6±1.7 | 7.3±1.6 | 0.124 |
LV: Left ventricular. LVEDi: Left ventricular diastolic dimension index.
We tested GLS differences between men and women in each race-ethnic group. In the overall population, no significant difference in GLS was present between men and women (−17.0±2.8% vs. −17.4±3.2%, p=0.09), despite a significantly higher LVEF in women (65.4±4.6% vs. 62.8±4.9% in men, p<0.001). GLS was not significantly different between men and women in whites (−17.0±2.7% vs. −18.1±3.3%, p=0.07), blacks (−16.5±3.4% vs. −16.5±3.5%, p=0.94), and Hispanics (−17.1±2.7% vs. −17.5±3.0%, p=0.19). Table III shows the age- and sex-adjusted correlations between clinical risk factors and GLS in different race-ethnic groups. GLS was significantly lower in presence of hypertension in blacks (B=2.3, p=0.010) and in presence of diabetes in Hispanics (B=0.9, p=0.001).
Table III.
Age- and sex-adjusted relationship of cardiovascular risk factors with GLS by race-ethnicity.
| Black | White | Hispanic | ||||
|---|---|---|---|---|---|---|
| B (SE) | p | B (SE) | P | B (SE) | p | |
| Hypertension | 2.3 (0.9) | 0.010 | 0.2 (0.7) | 0.756 | 0.6 (0.3) | 0.063 |
| Diabetes | 0.7 (0.7) | 0.332 | 0.9 (1.1) | 0.416 | 0.9 (0.3) | 0.001 |
| Obesity | 0.2 (0.7) | 0.785 | 0.5 (0.8) | 0.544 | 0.3 (0.3) | 0.259 |
| Hypercholesterolemia | 0.4 (0.7) | 0.514 | 0.001 (0.7) | 0.999 | 0.3 (0.3) | 0.310 |
| Cigarette smoking | 0.3 (0.7) | 0.677 | −0.5 (0.6) | 0.402 | 0.2 (0.3) | 0.562 |
Values in table are parameter estimates (B) and standard errors (SE).
LV systolic function and race-ethnicity
In univariate analysis, GLS was significantly lower in blacks (−16.5±3.5%) than in whites (− 17.5±3.0%, p<0.05 vs. blacks) and Hispanics (−17.3±2.9%, p<0.01 vs. blacks), whereas LVEF did not significantly differ between the three groups. After adjusting for relevant clinical and echocardiographic confounders, GLS remained significantly lower in blacks compared to the other two groups (p=0.011 for overall comparison; see Table IV for pairwise comparisons). In a subanalysis stratified by sex, a significantly lower GLS in blacks than whites and Hispanics was found among women (p=0.028 for overall comparison, Table IV), whereas a similar but nonsignificant trend was found among men (p=0.361, Table IV).
Table IV.
LV systolic function by race-ethnicity.
| Black | White | Hispanic | P value | |
|---|---|---|---|---|
| Overall | ||||
| GLS, % | −16.5 (0.3) | −17.5 (0.3)† | −17.5 (0.1)‡ | 0.011 |
| LVEF, % | 64.0 (0.5) | 63.9 (0.5) | 64.7 (0.2) | 0.291 |
|
| ||||
| Men | ||||
| GLS, % | −16.3 (0.5) | −16.9 (0.4) | −17.2 (0.2) | 0.361 |
| LVEF, % | 62.0 (0.8) | 62.8 (0.8) | 63.1 (0.4) | 0.578 |
|
| ||||
| Women | ||||
| GLS, % | −16.6 (0.4) | −18.0 (0.5)† | −17.6 (0.2)† | 0.028 |
| LVEF, % | 65.2 (0.6) | 64.0 (0.8) | 65.8 (0.3) | 0.106 |
Values in table are adjusted means (standard deviation).
Covariates: Age, sex (only in overall population model), body mass index, high school education, hypertension, anti-hypertensive treatment, SBP, DBP, diabetes, cigarette smoking, hypercholesterolemia, LV mass index, left atrial size, and E/A.
p<0.05 and
p<0.01 vs. blacks.
GLS: Global longitudinal strain. LVEF: Left ventricular ejection fraction.
Prevalence of LVSD by GLS (GLS-LVSD) was 14.9% in Hispanics, 19.0% in whites, and 27.2% in blacks (p for trend=0.008; blacks vs. others p=0.003) (Figure). In multivariate analysis adjusted for age, sex, body mass index, SBP, DBP, hypertension, anti-hypertensive medication, diabetes, hypercholesterolemia, cigarette smoking, education level, LV mass index, left atrial size, and E/A, black race remained significantly associated with GLS-LVSD with an adjusted OR=2.6 (95% CI=1.4–4.7, p=0.002) compared to the other groups. The significant association of black race with GLS-LVSD was present in both men (adjusted OR=3.5, 95% CI=1.3–9.6, p=0.016) and women (adjusted OR=2.4, 95% CI=1.1–5.4, p=0.029).
Figure. Prevalence of GLS-LVSD by race-ethnicity.
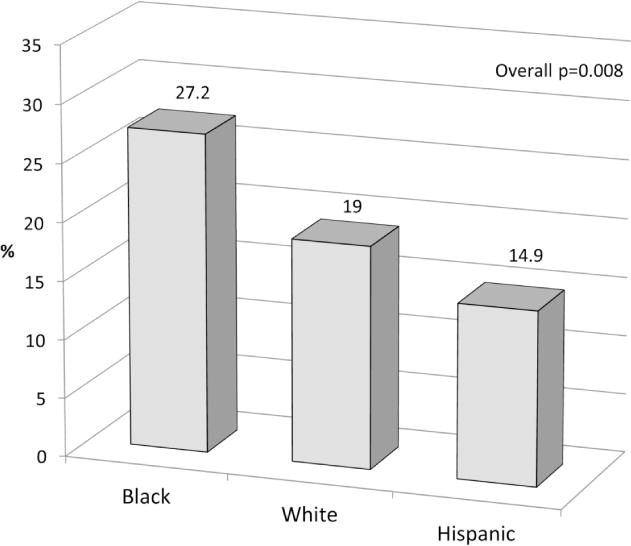
GLS-LVSD was more frequent in blacks than whites and Hispanics (overall p=0.008; blacks vs. other groups p=0.003).
DISCUSSION
In this study, we assessed subclinical LVSD assessed by speckle-tracking GLS in participants with normal LVEF from a tri-ethnic community-based cohort. We showed that GLS, an indicator of systolic myocardial deformation over the longitudinal direction, was significantly lower in black study participants compared to whites and Hispanics. Subclinical LVSD detected by GLS was also significantly more frequent in blacks than in the other groups. The prevalence of cardiovascular risk factors such as hypertension and diabetes in our population was greater in blacks and Hispanics than in whites, a finding consistent with previous reports.9,12,28,29 It is known that hypertension and diabetes are associated with increased LV mass, myocardial fibrosis and endothelial dysfunction, all factors that might be at least in part responsible for an impairment of LV function by GLS.30–32 We also found that cardiovascular risk factors showed different strength of association with GLS in different race-ethnic groups. In particular, hypertension was more strongly associated with lower GLS in blacks, whereas diabetes was more strongly associated with lower GLS in Hispanics. However, the significant differences in risk factors among race-ethnicities did not entirely explain the observed differences in GLS, suggesting that a genetic influence on LV structure and systolic function and on the cardiac adaptive mechanisms to hypertension may be at play for individuals of African ancestry.33–36 Furthermore, hypertension duration, severity, and degree of pharmacologic control known difficulties in the management of hypertension in blacks,37 and may have contributed to our findings.
Previous studies investigated race-ethnic differences in LV systolic function, with conflicting results. In the HyperGEN study, black race was not associated with lower LV function than whites in univariate analysis, but it was in a multivariate model17 and in non-hypertensive subjects.38 In the Multiethnic Study of Atherosclerosis (MESA), African Americans showed higher prevalence of cardiovascular risk factors, greater LV mass, and no differences in LVEF compared to other race-ethnicities, but also showed lower segmental circumferential strain (assessed by magnetic resonance imaging) in some ventricular areas.18 In another report from the same study, no differences were found in LVEF between blacks, whites and Hispanics after excluding participants with cardiovascular risk factors.19 Our study adds new data to the existing literature, documenting race-ethnic differences in subclinical LVSD using an established, prognostically validated parameter of global LV function, readily available in most modern echocardiographic systems. Furthermore, among myocardial deformation parameters, GLS is considered to be affected in the earlier stages of LV dysfunction, whereas circumferential strain can be expression of more advanced dysfunction and greater extension of wall damage.39,40 Lower GLS is associated with increased arterial stiffness, subclinical atherosclerosis and small vessel alterations (medial hyperplasia, perivascular and interstitial fibrosis) that have been described in hypertensive patients in various vascular territories,41,42 and is expression of the contraction of the longitudinally oriented myofibers that are predominant in the subendocardium, an area particularly vulnerable to injury because exposed to higher wall stress.43
Because GLS has been demonstrated to predict unfavorable cardiovascular outcomes including development of HF,20,21,27 our study provides a prognostically validated estimate of cardiovascular risk in different race-ethnic groups, and shows for the first time that blacks, a racial group particularly susceptible of developing HF and experiencing worse prognosis, have early evidence of significantly lower GLS and more frequent subclinical LVSD than other groups.6–8 The use of GLS might therefore have a role in identifying subjects with LV dysfunction at high cardiovascular risk that are not detected by traditional methods such as LVEF. Whether treatment options in subjects with lower GLS may improve subsequent outcome is not known, and studies specifically designed with that purpose are needed.
Strengths and limitations
Strengths of our study are the large number of subjects from a tri-ethnic community-based cohort, the use of advanced imaging techniques, and the wide range of cardiovascular risk profiles present in our study population. However, our study also has limitations. The study sample included subjects over 50 years old with high prevalence of cardiovascular risk factors, which might not allow generalization of our findings to populations with different demographic composition and risk profile. The smaller sample size of the white and black groups might have prevented the possibility of detecting significant differences between these groups for some variables. In particular, this may apply to sex-stratified analyses because of the smaller race-ethnic differences in GLS observed among men than among women together with the lower number of men than women in our cohort, possibly resulting in insufficient power to detect a smaller effect size. Finally, although we accounted for several confounders and performed multivariate analyses adjusting for established cardiovascular risk factors, we cannot rule out the possibility of unmeasured confounders playing a role in the observed associations.
CONCLUSIONS
In a tri-ethnic community-based population with normal LVEF, black participants had lower GLS and higher prevalence of GLS-LVSD than whites and Hispanics, independent of confounders and cardiovascular risk factors. These differences in subclinical LVSD might portend the higher incidence of heart failure and cardiovascular disease in blacks observed in previous studies. Further studies are needed to assess the effect on GLS and cardiovascular prognosis of a tighter control of risk factors in subjects with subclinical LVSD.
Acknowledgments
The authors wish to thank Janet De Rosa, MPH (project manager), Rui Liu, MD, Rafi Cabral, MD, Michele Alegre, RDCS, and Palma Gervasi-Franklin (collection and management of the data).
Grant support: This work was supported by the National Institute of Neurological Disorders and Stroke [grant number R01 NS36286 to MDT and R37 NS29993 to RLS/MSE].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown DW, Haldeman GA, Croft JB, et al. Racial or ethnic differences in hospitalization for heart failure among elderly adults: Medicare, 1990 to 2000. Am Heart J. 2005;150:448–54. doi: 10.1016/j.ahj.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loehr LR, Rosamond WD, Chang PP, et al. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–22. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 5.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander M, Grumbach K, Selby J, et al. Hospitalization for congestive heart failure. Explaining racial differences. JAMA. 1995;274:1037–42. [PubMed] [Google Scholar]
- 7.Philbin EF, DiSalvo TG. Influence of race and gender on care process, resource use, and hospital-based outcomes in congestive heart failure. Am J Cardiol. 1998;82:76–81. doi: 10.1016/s0002-9149(98)00233-1. [DOI] [PubMed] [Google Scholar]
- 8.Philbin EF, Weil HF, Francis CA, et al. Race-related differences among patients with left ventricular dysfunction: observations from a biracial angiographic cohort. Harlem-Bassett LP(A) Investigators. J Card Fail. 2000;6:187–93. doi: 10.1054/jcaf.2000.9677. [DOI] [PubMed] [Google Scholar]
- 9.Carnethon MR, Lynch EB, Dyer AR, et al. Comparison of risk factors for cardiovascular mortality in black and white adults. Arch Intern Med. 2006;166:1196–202. doi: 10.1001/archinte.166.11.1196. [DOI] [PubMed] [Google Scholar]
- 10.Gillum RF, Mussolino ME, Madans JH. Coronary heart disease risk factors and attributable risks in African-American women and men: NHANES I epidemiologic follow-up study. Am J Public Health. 1998;88:913–7. doi: 10.2105/ajph.88.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hozawa A, Folsom AR, Sharrett AR, et al. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects–Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–9. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 12.Thomas AJ, Eberly LE, Davey SG, et al. Race/ethnicity, income, major risk factors, and cardiovascular disease mortality. Am J Public Health. 2005;95:1417–23. doi: 10.2105/AJPH.2004.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang TJ, Evans JC, Benjamin EJ, et al. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 14.Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 15.Yeboah J, Rodriguez CJ, Stacey B, et al. Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the multi-ethnic study of atherosclerosis (MESA) Circulation. 2012;126:2713–9. doi: 10.1161/CIRCULATIONAHA.112.112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hays AG, Sacco RL, Rundek T, et al. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke. 2006;37:1715–9. doi: 10.1161/01.STR.0000227121.34717.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devereux RB, Bella JN, Palmieri V, et al. Left ventricular systolic dysfunction in a biracial sample of hypertensive adults: The Hypertension Genetic Epidemiology Network (HyperGEN) Study. Hypertension. 2001;38:417–23. doi: 10.1161/01.hyp.38.3.417. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes VR, Cheng S, Cheng YJ, et al. Racial and ethnic differences in subclinical myocardial function: the Multi-Ethnic Study of Atherosclerosis. Heart. 2011;97:405–10. doi: 10.1136/hrt.2010.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 20.Russo C, Jin Z, Elkind MS, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–9. doi: 10.1002/ejhf.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 22.Motoki H, Borowski AG, Shrestha K, et al. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012;60:2074–81. doi: 10.1016/j.jacc.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 23.Sacco RL, Khatri M, Rundek T, et al. Improving Global Vascular Risk Prediction With Behavioral and Anthropometric Factors The Multiethnic NOMAS (Northern Manhattan Cohort Study) J Am Coll Cardiol. 2009;54:2303–11. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 26.Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–74. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo C, Jin Z, Homma S, et al. Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Circulation. 2013;128:1105–11. doi: 10.1161/CIRCULATIONAHA.113.001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 29.Feinstein M, Ning H, Kang J, et al. Racial differences in risks for first cardiovascular events and noncardiovascular death: the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Multi-Ethnic Study of Atherosclerosis. Circulation. 2012;126:50–9. doi: 10.1161/CIRCULATIONAHA.111.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishizu T, Seo Y, Kameda Y, et al. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension. 2014;63:500–6. doi: 10.1161/HYPERTENSIONAHA.113.02149. [DOI] [PubMed] [Google Scholar]
- 31.Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398–401. doi: 10.1016/j.amjcard.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 32.Hare JL, Brown JK, Marwick TH. Association of myocardial strain with left ventricular geometry and progression of hypertensive heart disease. Am J Cardiol. 2008;102:87–91. doi: 10.1016/j.amjcard.2008.02.101. [DOI] [PubMed] [Google Scholar]
- 33.Fox ER, Musani SK, Barbalic M, et al. Genome-wide association study of cardiac structure and systolic function in African Americans: the Candidate Gene Association Resource (CARe) study. Circ Cardiovasc Genet. 2013;6:37–46. doi: 10.1161/CIRCGENETICS.111.962365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnett DK, Devereux RB, Kitzman D, et al. Linkage of left ventricular contractility to chromosome 11 in humans: The HyperGEN Study. Hypertension. 2001;38:767–72. doi: 10.1161/hy1001.092650. [DOI] [PubMed] [Google Scholar]
- 35.Meyers KJ, Mosley TH, Fox E, et al. Genetic variations associated with echocardiographic left ventricular traits in hypertensive blacks. Hypertension. 2007;49:992–9. doi: 10.1161/HYPERTENSIONAHA.106.081265. [DOI] [PubMed] [Google Scholar]
- 36.Fox ER, Klos KL, Penman AD, et al. Heritability and genetic linkage of left ventricular mass, systolic and diastolic function in hypertensive African Americans (From the GENOA Study) Am J Hypertens. 2010;23:870–5. doi: 10.1038/ajh.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper R, Rotimi C. Hypertension in blacks. Am J Hypertens. 1997;10:804–12. doi: 10.1016/s0895-7061(97)00211-2. [DOI] [PubMed] [Google Scholar]
- 38.Glasser SP, Lynch AI, Devereux RB, et al. Hemodynamic and echocardiographic profiles in African American compared with White offspring of hypertensive parents: the HyperGEN study. Am J Hypertens. 2014;27:21–6. doi: 10.1093/ajh/hpt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sengupta PP, Narula J. Reclassifying heart failure: predominantly subendocardial, subepicardial, and transmural. Heart Fail Clin. 2008;4:379–82. doi: 10.1016/j.hfc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Chan J, Hanekom L, Wong C, et al. Differentiation of subendocardial and transmural infarction using two-dimensional strain rate imaging to assess short-axis and long-axis myocardial function. J Am Coll Cardiol. 2006;48:2026–33. doi: 10.1016/j.jacc.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 41.Russo C, Jin Z, Takei Y, et al. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J Hypertens. 2011;29:574–82. doi: 10.1097/HJH.0b013e328342ca56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson CS, Hakim AM. Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke. 2009;40:e322–e330. doi: 10.1161/STROKEAHA.108.542266. [DOI] [PubMed] [Google Scholar]
- 43.Reimer KA, Lowe JE, Rasmussen MM, et al. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–94. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]


