Sir,
Jalili syndrome1 is a rare autosomal-recessive disorder, which is caused by mutations in the CNNM4 gene.2, 3 Two phenotypes are proposed: associated with bull's eye maculopathy and peripheral retinal degeneration (type A), or with minor retinal dystrophy (type B).4
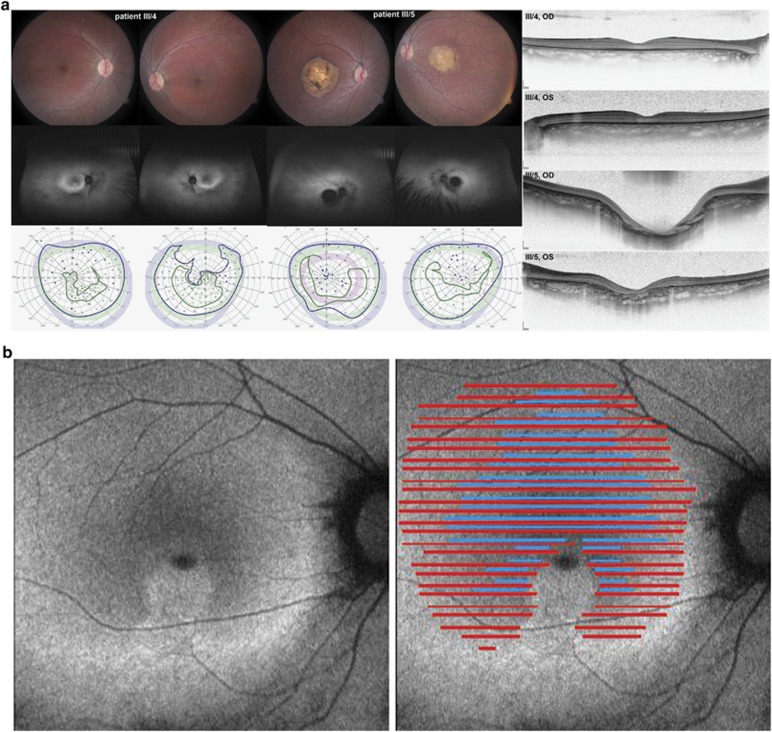
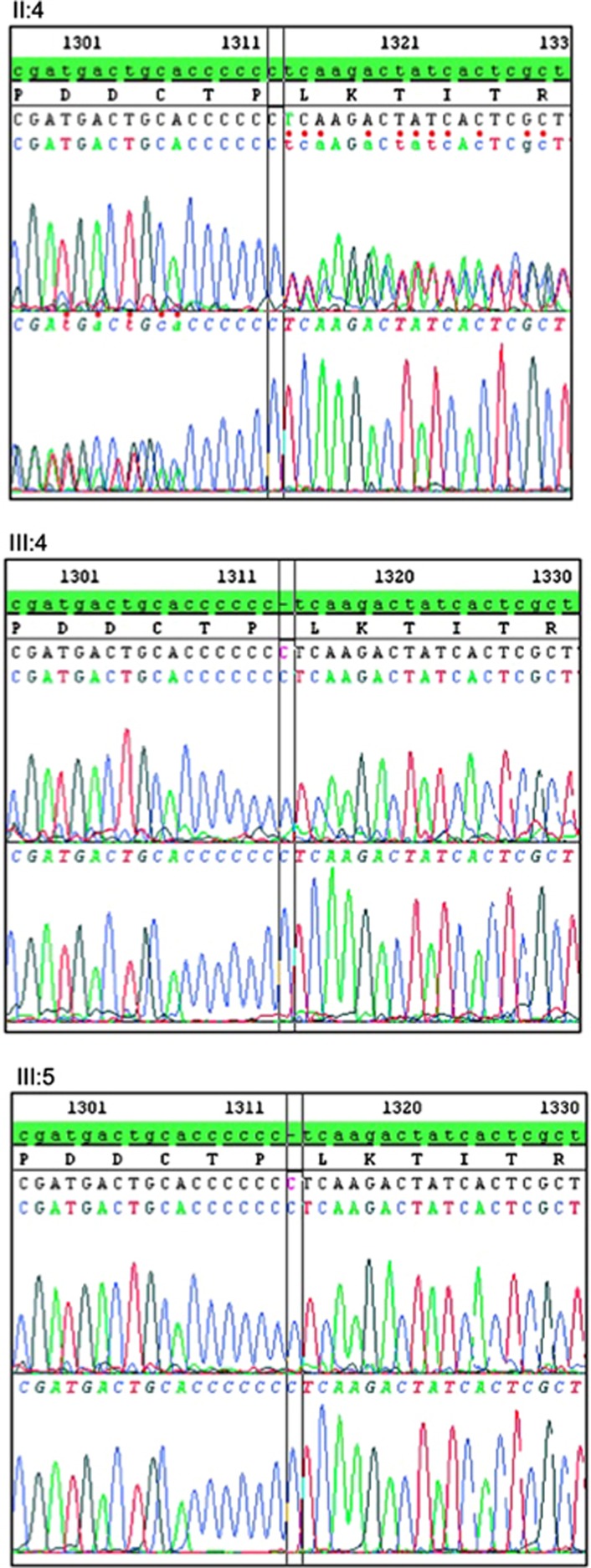
We report two new cases (siblings of 15 and 16 years of age) with different retinal findings, and their relationship with the existing phenotype classification. Fundus examination revealed severe bull's eye maculopathy in one sibling and diffuse retinal dystrophy in the other. ERG showed cone–rod dysfunction. OCT demonstrated thinning of the outer retinal layers, in particular the outer nuclear layer and the outer photoreceptor segments. Both patients showed amelogenesis imperfecta (Figure 1). Phenotype details in comparison with the reported patients with CNNM4 mutations are listed in Table 1. The same homozygous mutation c.1312dup; p.Leu438Profs*9 was found in the affected patients (III:4, III:5). The mother (II:4) was heterozygous for this sequence alteration in CNNM4 (Figure 2). The mutation within the cystathionine beta-synthase domain most likely results in a premature termination codon and nonsense-mediated mRNA decay of the mutant transcript. No mutation in the ABCA4 gene was identified.
Figure 1.
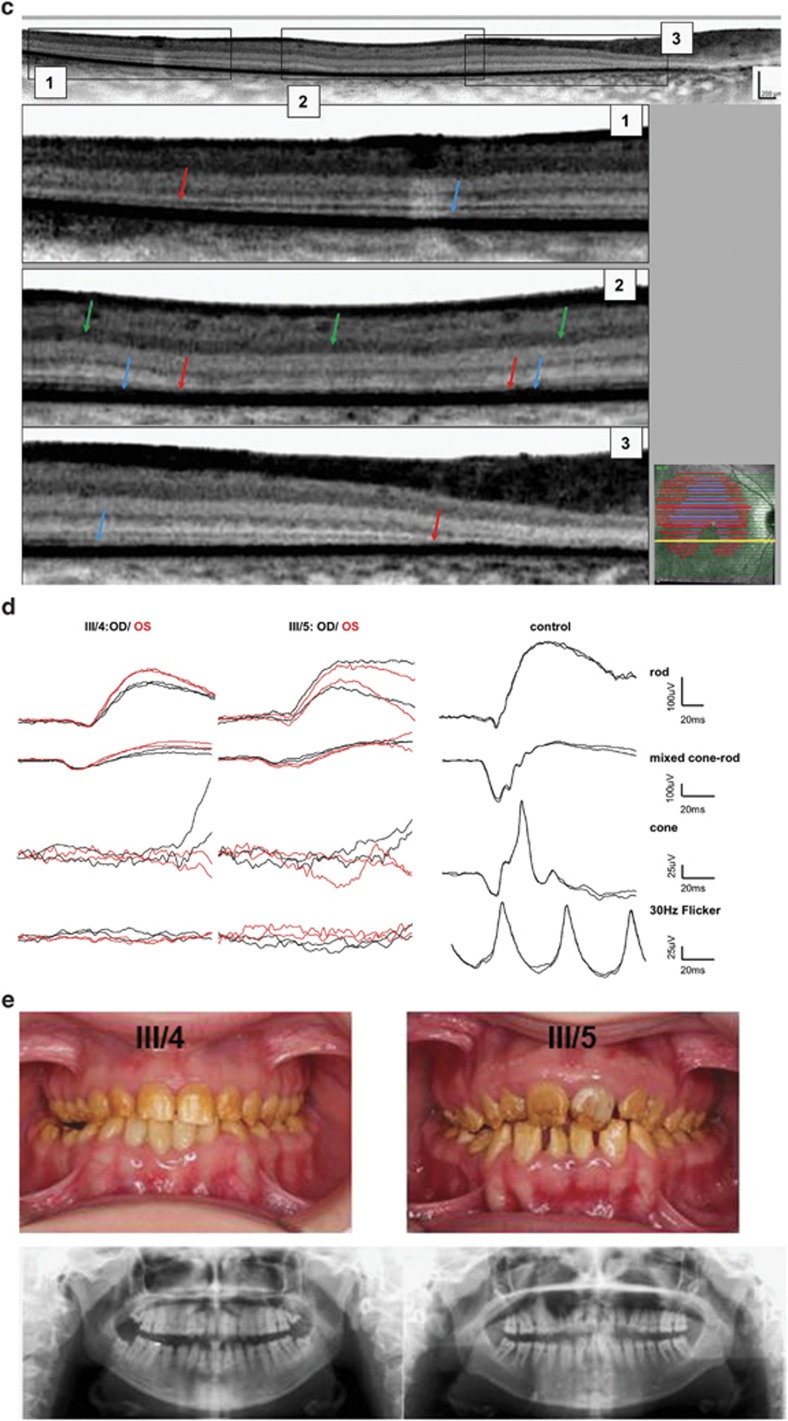
(a–e) Ocular and dental phenotype: ocular phenotype is illustrated by color fundus images and corresponding autofluoresence images, which show mild (III/4) to severe (III/5) maculopathy and retinopathy. Kinetic visual fields show a central scotoma and retained peripheral field sensitivity to large isopters (blue: V:4e; green: I:4e) in both patients, with the exception of the left eye of III/4 (lower panel). Horizontal line scans through the center of the macular are shown at the right. (a) Detailed retinal OCT analysis of the horizontal scans in this area of increased AF shows loss of the inner segment ellipsoid (illustrated by the absence of blue lines) and severe reduction of the outer retinal layers with merging of the outer limiting membrane (OLM) and the retinal pigment epithelium (RPE)/Bruch's membrane complex (absence of red lines). (b) Three stages of retinal layer changes are demonstrated on one horizontal line scan (yellow line, inset): absent inner segment ellipsoid (blue arrow), enhanced visibility of the inner plexiform layer (green arrow), and merging of the OLM and the RPE (red arrows). (c) Full-field ERG waveforms of the two patients and one age-matched control show severely reduced cone and rod function. (d) Color dental images and radiography show typical signs of AI (e).
Table 1. Review of published CNNM4 mutations and associated phenotype.
| Mutation 1 | Mutation 2 | Author | N | M | F | Age (years) | VA | Nystagmus | Refraction (SE) (Dpt) | Fundus | ERGa | Origin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.1312dupC: p.L438Pfs*9 | c.1312dupC: p.L438Pfs*9 | Present study | 2 | 0 | 2 | 15, 16 | 20/200, 20/400 (1/2 progressive) | Fine pendular (1/2) | −0.5 to +2.0 | Optic atrophy Macular atrophy of minor to severe extent | Scotopic: reduced, delayed Photopic: NR | Kosovo |
| c.1312dupC: p.L438Pfs*9 | c.1312dupC: p.L438Pfs*9 | Polok et al3 | 2 | 1 | 1 | 7, 14 | 20/100 to 20/320 | Pendular | Highly hypermetropic | Optic atrophy Macular atrophy w/ pigment mottling, periphery: white dots, 1/2: bone spiculae | Scotopic: b-wave reduced, slightly delayed Photopic: NR Repeat after 7 yrs: progressive deterioration | Kosovo |
| c.1312dupC: p.L438Pfs*9 | c.1312dupC: p.L438Pfs*9 | Parry et al2 Michaelides et al6 | 2 | 2 | 0 | 8, 10 | 3/60 | Fine pendular | Hypermetropic astigmatism | Macular atrophy and pigmentation | Rod response: abnormal Cone response: NR Repeat after 4 yrs: progressive deterioration | Kosovo |
| c.1312dupC: p.L438Pfs*9 | c.1312dupC: p.L438Pfs*9 | Zobor et al7 | 1 | 0 | 1 | 9 | 0.05, 0.125 | Slight myopia, astigmatism | Optic atrophy RPE atrophy macula | Scotopic: slightly delayed Cone response: NR | Kosovo | |
| c.1312dupC: p.L438Pfs9* | c.1312dupC: p.L438Pfs9* | Luder et al5 | 2 | 2 | 3, 4 | 20/200 | Fine pendular to jerky | +8.0, +9.0 | Optic atrophy Macular atrophy | Scotopic: reduced Photopic: NR | Kosovo | |
| c.599C>A: p.S200Y | c.599C>A: p.Ser200Y | Parry et al2 Jalili and Smith1 Jalili4 | 31 | 17 | 14 | 0.25–50 | 6/36 to NLP | Fine pendular to jerky | average +3.0 | RPE macular atrophy, normal optic disc (early) Chorioretinal atrophy, optic atrophy (late) | Scotopic (n=3): slightly to severely reduced b-wave Flicker response: NR | Gaza A |
| c.1813C>T: p.R605* | c.1813C>T: p.R605* | Parry et al2 Jalili4 | 3 | 1 | 2 | 5, 6, 10 | 2/60 to 6/60 | Fine | +2.0 to 4.0 | Normal macula, few RPE changes (1/3), optic disc unremarkable | b-wave impaired, NR at age 10, cone: impaired to NR | Gaza B |
| c.586T>C: p.S196P | c.586T>C: p.S196P | Parry et al2 | 2b | 2 | 5, 6 | Turkey | ||||||
| c.1-?_1403+?del | c.1-?_1403+?del | Parry et al2 | 4b | 3 | 1 | Iran | ||||||
| c.2149C>T: p.Q717* | c.62_145 del: L21Hfs*185 | Parry et al2 | 5b | 5 | Guatemala | |||||||
| c.971T>C: p.L324P | c.1690T>C: p.Q564* | Parry et al2 | 1b | 1 | Scotland | |||||||
| c.707G>A: p.R236Q | c.707G>A: p.R236Q | Polok et al3 | 3 | 2 | 1 | 2, 6, 12 | Low | Rapid | Scotopic: normal to reduced, photopic: severely attenuated to NR | Lebanon | ||
| c.971T>C: p.L324P | c.971T>C: p.L324P | Polok et al3 | 1 | 0 | 1 | 38 | LP (10/200 at age 6) | Slight myopia, astigmatism | Optic atrophy, macular atrophy, bone spiculae in midperiphery | NR | ||
| c.1555C>T: p.R519* | c.1555C>T: p.R519* | Doucette et al8 | 4 | 1 | 3 | 16–28 (f.u. 16–20) | 20/200 to HM, progression | Yes | Myopia | 1/4 Maculopathy, 1/4 bone spiculae, 2/4 ND | 1/4 Rod normal, cone absent, 1/4 rod borderline, cone absent, 2/4 ND | Northern Europe |
| c.1484C>T p.T495I | c.1484C>T p.T495I | Abu-Safieh et al9 | 1 | CRD (not sure if ERG was done) | ||||||||
| c.189del p.D63Efs*12 | c.189del p.D63Efs*12 | Coppieters et al10 | 3 | 2 | 1 | 1/3 Reported: maculopathy, outer retinal atrophy w/ pigmentation | Algeria |
Abbreviations: CRD, cone–rod dystrophy; Dpt, diopter; ERG, full-field electroretinography; F, female; f.u., follow-up; HM, hand motion detection; LP, light perception; M, male; ND, not done; NLP, no light perception; NR, non-recordable; RPE, retinal pigment epithelium; SE, spherical equivalent; VA, visual acuity; w/, with; yrs, years.
ERG description as written in publication.
Numbers are based on the pedigree shown in publication.
Figure 2.
Genetic testing results: Sanger DNA sequencing revealed a homozygous mutation in the two affected siblings (III:4 and III:5) as shown in the forward and reverse sequencing profiles from the DNA of the two patients. The mutation elongates the Oligo-C stretch in exon 1 of the CNNM4 gene, which consists of six cytosine residues in the reference sequence, by 1 nucleotide (c.1312dup; p.Leu438Profs*9). The DNA from the mother (II:4) is heterozygous for this mutation.
A similar dental phenotype with the characteristics of AI is described in all publications of patients with CNNM4 mutations. In his phenotype dissection, Jalili described anterior open bite (AOB) in 2/30 and posterior open bite in 1/30 ‘type A' patients, whereas AOB was present in all three patients of the ‘type B' phenotype. No open bite abnormality was seen on examination in our two patients and in one of the two patients reported by Luder et al.5
The intra-familial variability presented here is not consistent with a strict phenotype–genotype correlation, and may also argue against a rigid phenotype differentiation.4 As the patients with ‘type B' phenotype were examined at a younger age than the patient with ‘type A', it is possible that those patients with ‘type B' may have shown minimal macular signs as most of them had a visual impairment and signs of a cone–rod dystrophy.
The proposed strict differentiation between type A and B may not be applicable to all affected patients and families.
Acknowledgments
This study was funded in part by a grant from the Swiss National Science Foundation (grant number: 31003A 122359 to WB).
The authors declare no conflict of interest.
Footnotes
Data were presented partially at the 2013 ARVO Annual Meeting.
References
- Jalili IK, Smith NJ. A progressive cone-rod dystrophy and amelogenesis imperfecta: a new syndrome. J Med Genet. 1988;25:738–740. doi: 10.1136/jmg.25.11.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DA, Mighell AJ, El-Sayed W, Shore RC, Jalili IK, Dollfus H, et al. Mutations in CNNM4 cause Jalili syndrome, consisting of autosomal-recessive cone-rod dystrophy and amelogenesis imperfecta. Am J Hum Genet. 2009;84:266–273. doi: 10.1016/j.ajhg.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polok B, Escher P, Ambresin A, Chouery E, Bolay S, Meunier I, et al. Mutations in CNNM4 cause recessive cone-rod dystrophy with amelogenesis imperfecta. Am J Hum Genet. 2009;84:259–265. doi: 10.1016/j.ajhg.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili IK. Cone-rod dystrophy and amelogenesis imperfecta (Jalili syndrome): phenotypes and environs. Eye (Lond) 2010;24:1659–1668. doi: 10.1038/eye.2010.103. [DOI] [PubMed] [Google Scholar]
- Luder HU, Gerth-Kahlert C, Ostertag-Benzinger S, Schorderet DF. Dental phenotype in Jalili syndrome due to a c.1312 dupC homozygous mutation in the CNNM4 gene. PLoS One. 2013;8:e78529. doi: 10.1371/journal.pone.0078529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M, Bloch-Zupan A, Holder GE, Hunt DM, Moore AT. An autosomal recessive cone-rod dystrophy associated with amelogenesis imperfecta. J Med Genet. 2004;41 (6:468–473. doi: 10.1136/jmg.2003.015792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobor D, Kaufmann DH, Weckerle P, Sauer A, Wissinger B, Wilhelm H, et al. Cone-rod dystrophy associated with amelogenesis imperfecta in a child with neurofibromatosis type 1. Ophthalmic Genet. 2012;33 (1:34–38. doi: 10.3109/13816810.2011.592178. [DOI] [PubMed] [Google Scholar]
- Doucette L, Green J, Black C, Schwartzentruber J, Johnson GJ, Galutira D, et al. Molecular Genetics of Achromatopsia in Newfoundland Reveal Genetic Heterogeneity, Founder Effects and the First Cases of Jalili Syndrome in North America. Ophthalmic Genet. 2013;34 (3:119–129. doi: 10.3109/13816810.2013.763993. [DOI] [PubMed] [Google Scholar]
- Abu-Safieh L, Alrashed M, Anazi S, Alkuraya H, Khan AO, Al-Owain M, et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013;23 (2:236–247. doi: 10.1101/gr.144105.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters F, Van Schil K, Bauwens M, Verdin H, De Jaegher A, Syx D, et al. Identity-by-descent-guided mutation analysis and exome sequencing in consanguineous families reveals unusual clinical and molecular findings in retinal dystrophy. Genet Med. 2014;16 (9:671–680. doi: 10.1038/gim.2014.24. [DOI] [PubMed] [Google Scholar]