Abstract
Purpose of review
Early initiation of combination antiretroviral therapy (ART) in infants below 12 weeks of age reduces morbidity and mortality. A recent report of transient HIV remission in a child beginning ART from the second day of life has focused attention on very early therapy in the first days of life.
Recent findings
In the randomized children with HIV, early antiretroviral limited ART beginning at a median of 7.4 weeks of age lowered mortality and disease progression significantly compared with deferred ART beginning at a median of 21 weeks on study. In high-burden settings, infants initiating ART appear sicker than in children with HIV early antiretroviral and start at a later age. Many could be diagnosed on the first day of life. There are still programmatic obstacles to early diagnosis and initiation of ART in high-burden settings. There is growing but insufficient information on ART dosages in newborn infants.
Summary
There is now increased focus on initiating ART as postexposure prophylaxis in newborn infants at high risk of vertical transmission in the hope of limiting morbidity and dissemination of the virus.
Keywords: early, obstacles, postexposure prophylaxis
INTRODUCTION
With improvements in preventing mother-to-child transmission (PMTCT) of HIV, the number of infected infants is diminishing globally. Despite a 37% decline in vertical infections between 2009 and 2012, 210 000 children still acquired infection in 2012, the majority in sub-Saharan Africa [1]. Therefore, young infants will still require optimal diagnosis and treatment for many decades. Even in countries with excellent PMTCT programs, HIV infection in newborn infants still occurs. For example, in the USA, despite great success in PMTCT implementation, 167 infants acquired vertical HIV infection in 2010 [2]. Since 2008, early combination antiretroviral therapy (ART) in the first year of life became the standard of care after release of data from the randomized children with HIV early anti-retroviral (CHER) trial [3]. Subsequently, immediate ART recommendations were broadened to include children below 2 years of age and then below 5 years of age [4]. This review focuses mainly on the completed CHER trial and then on the changing emphasis to even earlier initiation of ART in the first days of life [5▪▪,6].
EARLY ANTIRETROVIRAL THERAPY
The rationale for broadening the age for immediate treatment was to circumvent barriers, such as poor access to viral loads and CD4 assays, which delay the treatment. However, there are other reasons. Modeling CD4 data from a large treatment strategy trial in Uganda and Zimbabwe showed that for below 5 years of age, CD4 recovery was possible, but CD4 counts in children initiating ART later would never fully recover [7]. Also, recent data from the Pediatric Randomized Early versus Deferred Initiation in Cambodia and Thailand trial showed improved quality of life in older children receiving early ART [8].
The CHER trial represents one of the largest infant cohorts initiating early ART and with adequate follow-up [9▪]. The trial hypothesis was that early limited ART in infancy would delay HIV disease progression and facilitate a prolonged period without the need for continuous ART according to CD4 and clinical criteria. The primary endpoint was time to failure of first-line ART (immunological, clinical, or virological) or death. Mothers from community PMTCT clinics were invited to bring their infants for screening, and those with CD4 of at least 25% and below 12 weeks of age were eligible for study entry. Postexposure prophylaxis consisted of single-dose nevirapine (NVP) to all infants and zidovudine for the first week in Cape Town, one of the two study centers. Infants were then randomized to either of three ART strategies: deferred ART (ART-Def) according to the WHO criteria of 2005 and 2006 or immediate ART until close to either the first or second birthday (ART-40W and ART-96W, respectively).
For ART-Def, therapy commenced for CD4 below 25% in the first year of life or for the Centers Disease Control and Prevention stage C or protocol-defined severe stage B conditions (HIV-related nephropathy, chronic lung disease, or cardiomyopathy). In ART-40W, ART was given from baseline until week 40 and in ART-96W, until week 96. Thereafter, ART was restarted for a CD4 below 20% or the clinical criteria listed above. With the realization that with absolute lymphopenia, the CD4 percent could be falsely elevated, absolute CD4 counts for restarting ART were added: 12–35 months below 750 cells/μl and 36–59 months above 500 cells/μl. Additional criteria for restarting ART in ART-40W and ART-96W were failure to thrive after excluding other causes, severe oral candidiasis, and recurrent bacterial pneumonia.
Median age at baseline was 7.4 weeks and median CD4 percent was 35% in the 377 infants enrolled. Although median HIV RNA was log 5.7 copies/ml, almost two-thirds (64%) had viral loads above 750 000 HIV RNA copies/ml, suggesting that viral replication was already well established. Levels above 750 000 copies were not quantified because of cost and insufficient plasma volume.
For the first 40 weeks on study, two-thirds of infants were randomized to early ART and a third to ART-Def. Therefore, the first part of the study was a comparison between early versus ART-Def. Time to ART initiation in ART-Def was 20 weeks (interquartile range [IQR 16–25]). After 2 years, the data safety monitoring board, noting that mortality in infants receiving early ART was 75% less than in ART-Def, recommended that recruitment into ART-Def be stopped [3].
The trial ended in September 2011 with a median follow-up of 4.8 (3.5–5.9) years [9▪]. Of 125 children in ART-Def, 48 (38%) reached the primary endpoint, 32 (25%) of 126 in ART-40W, and 26 (21%) of 126 in ART-96W. Relative to ART-Def, both early treatment arms had significantly better outcomes. The hazard ratio, relative to ART-Def, was 0.59 [95% confidence interval (CI) 0.38–0.93, P =0.02] for ART-40W and 0.47 (0.27–0.76, P =0.002) for ART-96W, confirming that early limited ART improved outcome compared with closely monitored deferred strategy.
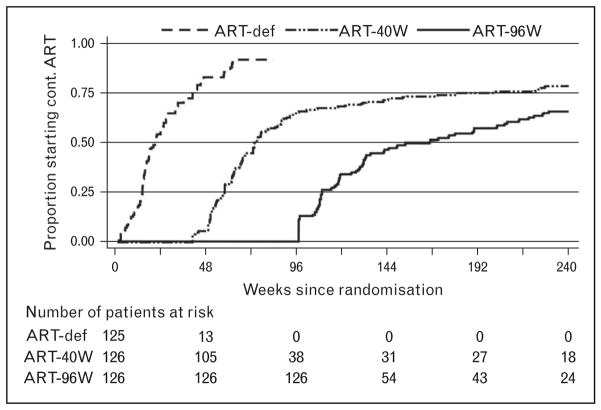
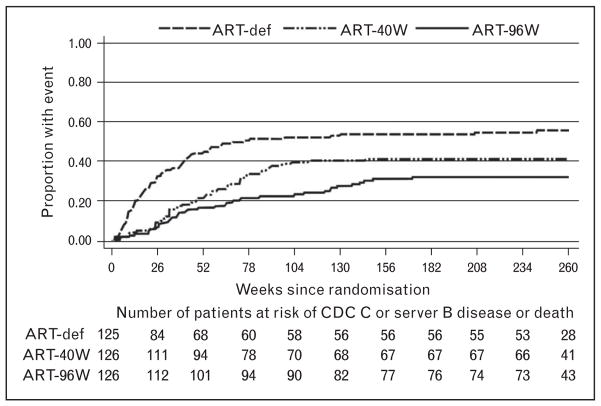
Comparisons between ART-40W and ART-96W showed a trend for better outcomes in ART-96W. Time to restarting ART was longer in ART-96W than in ART-40W. Median duration of time off ART was 33 weeks (26–45) in ART-40W and 70 weeks (35–109) in ART-96W (Fig. 1) [9▪]. In a secondary analysis, considering all site-reported death or the Centers Disease Control and Prevention severe stage B or stage C events, the benefits of early limited ART are well seen. The ART-Def arm showed continued increased mortality and disease progression until week 78, despite the ART being initiated at a median of 21 weeks on study. Relative to ART-40W, the hazard for events in ART-96W was 0.706 (0.462–1.081; P =0.1081), again with a trend to a better outcome after a longer period of primary therapy (Fig. 2) [9▪].
FIGURE 1.
Use of antiretroviral therapy over time in the CHER trial: time to start of continuous antiretroviral therapy. ART-Def, deferred antiretroviral therapy. Reproduced with permission from [9▪].
FIGURE 2.
Time to progression to the Centers Disease Control and Prevention stage C disease or severe stage B disease or death in 377 children with baseline CD4 of at least 25%. (All site reported events.) ART-Def, deferred antiretroviral therapy; CDC, Centers Disease Control and Prevention. Reproduced with permission from [9▪].
The highest event rate per 100 person-years was in ART-Def: 13.1 versus 7 and 5.3 in ART-40W and ART-96W, respectively. During ART interruption, event rates were 8.8 and 1.7, respectively, favoring a longer period of primary therapy. In contrast, in ART-Def, prior to ART, the event rate was 55.6 events per 100 patient-years. The high event rate preceding ART was not replicated after a period of primary limited ART, when the children were older.
At the end of the trial, 24 (19%) in ART-40W and 40 (31.7%) in ART-96W had not yet restarted ART. Three children in ART-Def, three in ART-40W, and one in ART-ART-96W switched to second-line ART. Of 331 children on ART, 280 (85%) had a viral load below 400 copies/ml, confirming the durability of a lopinavir/ritonavir-based regimen [9▪].
An important point in the CHER trial is that viral load had no role in either initiating or restarting ART. Instead, apart from a baseline level to confirm infection, the intention was to store plasma for post-hoc analysis, when funds became available. The rationale for this decision was that viral load assays were not readily available when the study was planned and were expensive. We reasoned that CD4 testing would be adequate and would assist programmatically should early limited ART become standard of care. However, during the trial, viral loads became standard of care in South Africa, and therefore were included every 3 months while on ART from October 2009, 4 years into the study. Also, a virological endpoint (plasma HIV above 10 000 copies/ml, two occasions within 4 weeks) was added to the composite endpoint of ART failure. However, during ART interruption, although specimens were stored every 3 months, viral loads were not measured and played no role in decisions to reinitiate ART. Studies are now in progress to evaluate viral rebound after limited ART in the ART-40W and ART-96W arms.
Of great importance was the lack of serious HIV-related disease in children receiving early limited ART compared with ART-Def, suggesting that young infants were far more vulnerable to the effects of HIV than in older children. A major limitation of the study, however, was the absence of an early continuous ART arm. Also, numbers in ART-40W and ART-96W were insufficient for adequate comparison. Nevertheless, the findings of the CHER trial support the concept of early limited primary therapy in infants. Indeed, the Mississippi child maintained virological suppression for almost 3 years after very early limited ART [5▪▪].
TIMING OF INFECTION AND PROGRESSION OF HIV
With improvements in PMTCT, most infant HIV infection occurs in utero and can potentially be detected immediately after birth. In well resourced settings, a diagnostic polymerase chain reaction (PCR) on the first day of life and immediate post-exposure prophylaxis with three antiretrovirals is standard practice when there is a high risk of vertical transmission [10]. Since 2001, increasing use of triple antiretroviral prophylaxis was documented in a review from Europe, mainly associated with a more recent calendar year, detectable maternal viral load close to delivery, lack of antenatal ART, and prematurity [11▪].
PROGRAMMATIC ISSUES AND RESOURCE LIMITATION
The CHER trial provided sufficient resources for screening, rapid turn-around of early diagnostic tests, and rapid initiation of ART in a high-burden setting, beginning just 2 years after the launch of the South African PMTCT program in 2003. The rapid pace of HIV disease progression in infants is evident in the screening data in the CHER study [9▪]. Of 585 HIV-infected infants screened for the study, 127 (21.7%) were excluded because of the advanced disease. In addition, two infants died over the brief period (usually 1–2 weeks) between screening and study entry.
A follow-up study conducted between June 2007 and September 2010 in the two CHER trial centers highlighted programmatic challenges in high-burden settings. The aim of the study was to evaluate the status of HIV-infected infants initiating ART below 12 weeks of age. Infants followed the same recruitment patterns as in the CHER trial. In the Cape Town Metropole, 88 infants were identified from community clinics. In Soweto, 315 infants were identified from a single wellness clinic linked to the perinatal HIV research unit. Median age at ART initiation was 8.4 weeks (IQR: 7.2–9.7). At ART initiation, 250 infants (62%) already had advanced HIV disease (CD4 percent <25% or absolute CD4 <1500 cells/μl or WHO clinical stage 3 or 4). Median age at ART initiation by site was 10.3 (IQR: 8.2–11.9) weeks in Cape Town and 8.6 (IQR: 7.7–10.0) weeks in Soweto infants (P <0.0001). In Cape Town, 73 infants (83%) had advanced HIV disease at ART initiation compared with 177 infants (56%) in Soweto (P <0.0001). This comparison showed that a single clinic functioned more efficiently than several smaller community clinics. Each month increase in age at ART initiation lowered the odds of initiating ART in an optimal state (Odds ratio: 0.56, CI: 0.36–0.94) and increased the odds of advanced HIV disease at ART initiation (Odds ratio: 1.69, CI: 1.05–2.71) [12▪]. In another study [13] from Johannesburg, in 864 infants initiating ART between 2004 and 2011, median age for ART initiation was 10 months of age, much older than in the CHER trial.
A study [14▪▪] from Johannesburg, South Africa, evaluating very early infant diagnosis, provided programmatic data supporting a first diagnostic test on first day of life. At the time, a routine diagnostic PCR was undertaken at 6 weeks of age. However, in the study, dried blood spots were collected at regular intervals from birth. Of 38 HIV infections, 29 (76%) were detectable on day 1 of life. The median age for ART initiation was 16 weeks, with only 14 (37%) still in care after a year, again highlighting programmatic difficulties in high burden settings.
VERY EARLY ANTIRETROVIRAL THERAPY AND THE MISSISSIPPI BABY
The report of the Mississippi baby changed the focus of early ART to the first days of life [5▪▪]. The mother’s rapid test for HIV antibodies was positive when presenting in active labor at 35-weeks gestation. No antenatal or intrapartum antiretrovirals were given, but zidovudine (ZDV) was commenced for postexposure prophylaxis, converted to full combination ART when NVP and lamivudine were added at 31 h of age. Of note, the lamivudine dosage was at twice the recommended dose for neonates, but appropriate for older infants and NVP was given twice daily, without the standard single dose lead-in strategy 14 days [15].
At that time, the plasma HIV RNA was 19 812 copies/ml and a qualitative HIV DNA PCR test was positive. NVP was replaced by lopinavir-ritonavir after a week. The CD4 percent for day 8 was 69%. Viral load became undetectable (<48 copies/ml) by day 29 of life. Poor adherence was suspected at 18 months of age when the red cell mean corpuscular volume, which had been above 101 fl before 15 months of age and indicative of ZDV exposure, decreased to 95 fl. Pharmacy records indicated that the prescription was last refilled at 15 months of age.
After several missed clinic visits, the infant was reassessed at 23 months of age. The viral load was, surprisingly, still undetectable by standard assay, antibodies to HIV were absent, and a repeat HIV DNA PCR was negative.
Extensive and highly sensitive tests were undertaken to look for evidence of HIV. At 24 months of age, plasma viral load was 1 HIV RNA copy/ml and HIV DNA was detected at 37.6 (lower limit of detection – 23.3) copies per 106 monocyte-derived adherent cells. No replication-competent virus was detected in 22 × 106 resting CD4+ T cells. HIV DNA was detected at 4.2 (lower limit of detection – 2.9) copies per 106 peripheral blood mononuclear cells at 26 months of age.
Regarding innate protection, neither infant nor mother had the Δ32 deletion in the chemokine receptor-5 gene. HIV entry into macrophages requires chemokine receptor-5 as coreceptor to the CD4 receptor, with the deletion being associated with slower progression. Also, human leukocyte antigen typing did not show a profile associated with long-term nonprogression.
At 41 months of age, traces of HIV DNA were still detectable at the lower limits of assay sensitivity but without evidence of HIV RNA or replication-competent virus [16▪]. However, 4 months later, during routine monitoring, a viral load of 16 750 copies/ml was detected, confirmed by a level of 10 564 copies 3 days later. The CD4 count had decreased marginally. The description of this outcome has correctly shifted focus from ‘cure’ to ‘remission’, in line with terminology for cancer. More relevant, however, is the duration of ‘remission’ that was almost 30 months, with undetectable viral load off ART. Usually viral rebound occurs quite quickly, within weeks. In adults, posttreatment remission has been identified after initiating ART within weeks of infection and then discontinuing ART after approximately 3 years of continuous therapy [17].
OTHER EXPERIENCE OF VERY EARLY ANTIRETROVIRAL THERAPY IN NEWBORN INFANTS
Several other cases of early recognition and treatment have been described. An infant born to a nonadherent mother in Long Beach, California, began ART at 4 h of age. HIV infection was confirmed by a viral load of 217 copies/ml and the HIV DNA PCR was positive at baseline. By day 11, plasma HIV RNA was below 20 copies/ml [16▪]. This infant remains on ART.
In a Canadian cohort, recruited over 10–15 years, four premature infants with in-utero infection and commencing ART within 72 h were identified [18▪▪]. The standard regimen comprised NVP at 150 mg/m2 (with daily dose lead-in for 14 days), lamivudine, and ZDV. Virological suppression was first documented between 66 and 189 days of life (median 99 days). For comparison, virological suppression in the Mississippi child was on day 29 and the Long Beach baby by day 11. Two of the Canadian infants maintained an undetectable viral load for 2–3 years, but had rapid virological rebound after poor ART adherence that led to treatment cessation [19].
MORBIDITY DESPITE EARLY ANTIRETROVIRAL THERAPY
In the Long Beach baby, 32 copies of HIV RNA/ml were detected in cerebrospinal fluid on day 6 of life when a spinal tap was required to exclude bacterial sepsis. This observation shows early penetration by HIV of the central nervous system, despite ART from birth [16▪]. In children from the CHER study, white matter signal abnormalities were detected on MRI in five infants at a mean age of 30 months. All had suspected HIV encephalopathy and were on continuous ART from 10 weeks of age. Therapy had not been interrupted as these children had already reached an endpoint in the trial. This observation also suggests early HIV presence in the brain [20].
ANTIRETROVIRALS FOR EARLY ANTIRETROVIRAL THERAPY
There are still limited formulations and information on appropriate dosage for young infants, especially in the first 2 weeks of life and also for low birth weight and premature infants [15]. NVP given at 150 mg/m2 daily for 14 days and then twice daily gave good exposure in the first 4 weeks of life as part of 3-drug postexposure prophylaxis [21▪]. Raltegravir has been approved from 4 weeks of age in an appropriate granule formulation [22].
CONCLUSION
Early ART, the norm after the CHER trial, limits morbidity associated with HIV. There are still major programmatic difficulties in the implementation of early ART in high-burden settings. It is likely that HIV dissemination to many organs, including the central nervous system, occurs at a very early stage, potentially limited by very early ART. An added bonus is the possibility of prolonged virological remission, thus obviating the need for continuous ART for a certain period, but the frequency of such remissions is unknown and requires study.
KEY POINTS.
Early diagnosis and initiation of ART in infants is now a standard of care.
The emphasis is now shifting from under 12 weeks of age to the first days of life.
Rapid suppression of viral replication may limit spread of virus and morbidity in areas, such as the central nervous system.
Initiating both infant diagnosis and ART consisting of three antiretrovirals for postexposure prophylaxis as soon as possible after birth is widely practiced when there is a risk for vertical transmission and could, in some circumstances, produce a period of virological remission.
Acknowledgments
None.
Financial support and sponsorship
Mark Cotton is funded on 2 UM1AI068632-08 for the International Maternal Pediatric Adolescent AIDS Clinical Trial (IMPAACT) group through the National Institute of Allergy and Infectious Diseases, USA. He also receives funding for trials from the Drugs for Neglected Diseases Initiative and Vakzine Projekt Management GmbH. Helena Rabie receives funding through IMPAACT 2 UM1AI068632-08 and DNDi.
Footnotes
Conflicts of interest
Mark Cotton was co-principal investigator for the CHER study (funded through the Comprehensive International Program of Research on AIDS (CIPRA) network grant U19 AI53217. He participates in trials through ViiV Healthcare, Bristol Myers Squibb, and Gilead. He is on a ViiV Advisory Board for Dolutegravir. Helena Rabie was an investigator on the CHER trial. She consults for Aid for AIDS Healthcare management.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.UNAIDS. 2013 progress report on global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. [Accessed 26 June 2014];HIV among pregnant women, infants and children in the US. 2012 http://www.cdc.gov/
- 3.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siberry G, Tindyebwa D. Commentary: when to start and what to use in children – recommendations and rationale. AIDS. 2014;28:S133–S135. doi: 10.1097/QAD.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. Landmark case report that established new research agenda and standard of care for infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.‘Mississippi Baby’ now has detectable HIV, researchers find. Vol. 10. National Institute of Allergy and Infectious Diseases; Jul 10, 2014. [Google Scholar]
- 7.Picat MQ, Lewis J, Musiime V, et al. Predicting patterns of long-term CD4 reconstitution in HIV-infected children starting antiretroviral therapy in sub-Saharan Africa: a cohort-based modelling study. PLoS Med. 2013;10:e1001542. doi: 10.1371/journal.pmed.1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunupuradah T, Kosalaraksa P, Vibol U, et al. PREDICT Study Group. Impact of antiretroviral therapy on quality of life in HIV-infected Southeast Asian children in the PREDICT study. AIDS Patient Care STDS. 2013;27:596–603. doi: 10.1089/apc.2013.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Cotton MF, Violari A, Otwombe K, et al. CHER Study Team. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382:1555–1563. doi: 10.1016/S0140-6736(13)61409-9. Largest randomized study of early ART in infants. This study established both early treatment and early infant diagnosis as critical for HIV-infected infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor GP, Clayden P, Dhar J, et al. British HIV Association guidelines for the management of HIV infection in pregnant women. HIV Med. 2012;13:87–157. doi: 10.1111/j.1468-1293.2012.01030_2.x. [DOI] [PubMed] [Google Scholar]
- 11▪.Chiappini E, Galli L, Giaquinto C, et al. Use of combination neonatal prophylaxis for the prevention of mother-to-child transmission of HIV infection in European high-risk infants. AIDS. 2013;27:991–1000. doi: 10.1097/QAD.0b013e32835cffb1. Good summary of neonatal postexposure prophylaxis. [DOI] [PubMed] [Google Scholar]
- 12▪.Innes S, Lazarus E, Otwombe K, et al. Early severe HIV disease precedes early antiretroviral therapy in infants: are we too late? J Int AIDS Soc. 2014;17:18914. doi: 10.7448/IAS.17.1.18914. First programmatic assessment of status of infants initiating ART below 12 weeks of age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Technau KG, Lazarus E, Kuhn L, et al. Poor early virologic performance and durability of abacavir-based first-line regimens for HIV-infected children. Pediatr Infect Dis J. 2013;32:851–855. doi: 10.1097/INF.0b013e31828c3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪▪.Lilian RR, Kalk E, Technau KG, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J. 2013;32:1080–1085. doi: 10.1097/INF.0b013e318290622e. The only study thus far to establish utility of HIV diagnosis on day 1 of life and the consequences of later identification in large antiretroviral programs in high-burden settings. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed 21 February 2014];Guidelines for the use of antiretroviral agents in pediatric HIV infection. 2014 http://aidsinfo.nih.gov/guidelines.
- 16▪.Persaud D, Deveikis A, Gay H, et al. Very early combination antiretroviral therapy in perinatal HIV Infection: two case studies. 21st Conference of Retroviruses and Opportunistic Infection; Boston, Massachussetts, USA. 2014. Excellent narrative of two pivotal case studies. [Google Scholar]
- 17.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. ANRS VISCONTI Study Group. Posttreatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪▪.Bitnun A, Samson L, Chun TW, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborn infants can achieve sustained virologic suppression with low frequency of CD4+ T-cells carrying HIV in peripheral blood. Clin Infect Dis. 2014;59:1012–1019. doi: 10.1093/cid/ciu432. The first description of three-drug ART as postexposure prophylaxis merging into treatment within 72 h of life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brophy J, Chun T-W, Samson L, et al. Impact of early initiation of combination antiretroviral therapy on measures of virus in peripheral blood of vertically HIV-1-infected children. 20th International AIDS Conference; Melbourne, Australia. 2014. p. TUAB0206LB. [Google Scholar]
- 20.Ackermann C, Andronikou S, Laughton B, et al. White matter signal abnormalities in children with suspected HIV-related neurologic disease on early combination antiretroviral therapy. Pediatr Infect Dis J. 2014;33:e207–e212. doi: 10.1097/INF.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Lau E, Brophy J, Samson L, et al. Nevirapine pharmacokinetics in HIV-exposed neonates receiving triple combination therapy as post exposure prophylaxis. 6th International workshop on HIV pediatrics. Melbourne: reviews in Antiviral Therapy and Infectious Diseases; Melbourne, Australia. 2014. Good summary of NVP pharmacokinetics in first 4 weeks of life. [Google Scholar]
- 22. [Accessed 4 August 2014];New Isentress (raltegravir) dosage form: oral suspension. 2013 http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/hivandaidsactivities/ucm379632.htm.