Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) infections are a leading cause of death among all fatalities caused by antibiotic-resistant bacteria. With the rise of increasing resistance to current antibiotics, new antimicrobials and treatment strategies are urgently needed. Thiazole compounds have been shown to possess potent antimicrobial activity. A lead thiazole 1 and a potent derivative 2 were synthesized and their activity in combination with glycopeptide antibiotics was determined against an array of MRSA and vancomycin-resistant Staphylococcus aureus (VRSA) clinical isolates. Additionally, the anti-biofilm activity of the novel thiazoles was investigated against Staphylococcus epidermidis. Compound 2 behaved synergistically with vancomycin against MRSA and was able to re-sensitize VRSA to vancomycin, reducing its minimum inhibitory concentration (MIC) by 512-fold in two strains. Additionally, both thiazole compounds were superior to vancomycin in significantly reducing S. epidermidis biofilm mass. Collectively the results obtained demonstrate compounds 1 and 2 possess potent antimicrobial activity alone or in combination with vancomycin against multidrug-resistant staphylococci and show potential for use in disrupting staphylococcal biofilm.
Keywords: Antimicrobials, Biofilm disruption, Combination therapy, Drug-resistance, Methicillin-resistant Staphylococcus aureus (MRSA), Re-sensitization, Thiazole compounds
Introduction
Antibiotic-resistant bacteria are a major global health concern resulting in 23,000 deaths each year in the United States alone1. Two species alone, methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus epidermidis, are responsible annually for the majority of skin and soft-tissue infections and infections caused by bacterial biofilms present on indwelling medical devices2,3. Biofilms are responsible for 80% of microbial infections which develop in the human body and bacterial biofilms on implanted biomedical devices and tissue surfaces (chronic wound) constitute an ever-increasing threat to human health and place a significant burden on healthcare systems4. Biofilms consist of a cluster of bacterial cells enclosed within an extracellular matrix which collectively attach to an animate or inanimate surface4. Cells present within a biofilm pose a key challenge as they demonstrate increased resistance to the effect of antimicrobials5.
Antibiotics have been key allies in the treatment of bacterial infections for more than 80 years. While several classes of antibiotics were once capable of treating staphylococci-induced infections, strains have emerged which are resistant to an array of antimicrobials once deemed effective including β-lactams6, macrolides2, and fluoroquinolones6,7. Further exacerbating the issue is the rise of strains (such as vancomycin-resistant Staphylococcus aureus (VRSA)), which are resistant to antibiotics deemed drugs of last resort for treatment of staphylococcal infections, including glycopeptide antibiotics like vancomycin8. Conventional antibiotics face an added challenge in the treatment of biofilm infections as bacteria present within a biofilm may be 1000-fold more resistant to antibiotics compared to their planktonic equivalents5. Thus there is a critical need for the discovery of novel antimicrobials and treatment strategies to circumvent this growing public health concern.
Several thiazole compounds have been shown to be effective anticonvulsant9, anticancer10,11, and antiviral agents12. However, limited studies have been performed to characterize their abilities as antimicrobial agents, particularly against MRSA. Darwish et al, synthesized a series of thiadiazole analogues incorporating a sulfonamide group and found they possessed activity against Streptococcus pneumonia and Bacillus subtilis13. Additionally, Desai et al, constructed a series of novel hybrid compounds which combined the thiazole and 1,3,4-oxadiazole pharmacophores but found they had limited activity against S. aureus (minimum inhibitory concentration (MIC) of six of 12 analogues constructed was 500 µg/mL or higher)14. Furthermore, a third study assessing a series of disubstituted 1,3-thiazole derivatives found the most potent analogue possessed modest activity against a single strain of S. aureus tested (MIC of 50 µg/mL)15. None of these studies assessed broader therapeutic applications of thiazole compounds beyond use as single agents to inhibit bacterial growth in vitro.
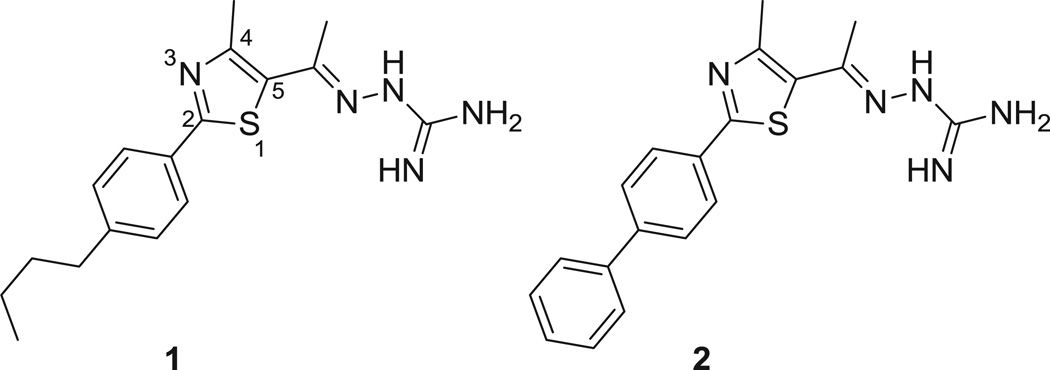
We recently discovered a novel lead thiazole compound 1 which exhibited potent antimicrobial activity against MRSA (Figure 1)16. The lead compound is composed of a thiazole nucleus connected to a cationic amino head at the C5-position and a lipophilic phenylalkyl tail at the C2-position. The aims of the present study are to identify if the lead compound 1 and the most potent synthesized derivative 2 have potential to be used in combination with glycopeptide antibiotics commonly used to treat MRSA infections, to analyze the ability of 1 and 2 to re-sensitize VRSA strains to glycopeptide antibiotics, and to assess if these compounds are capable of disrupting staphylococcal biofilms using an in vitro model of S. epidermidis. Results garnered from this study will provide valuable insight into potential therapeutic applications of thiazole compounds for use as antibacterial agents.
Figure 1.
Chemical structure of thiazole compounds 1 and 2.
Materials and methods
Bacterial Strains and Reagents
The bacterial strains of methicillin-resistant and vancomycin-intermediate Staphylococcus aureus utilized were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA). Staphylococcus epidermidis ATCC 35984 was obtained from the American Tissue Culture Collection. Antibiotics were purchased commercially from Gold Biotechnology (St. Louis, MO, USA) (vancomycin hydrochloride) and Biotang Inc. (Waltham, MA, USA) (teicoplanin). Both antibiotics were dissolved in dimethyl sulfoxide to obtain a stock 10 mM solution.
Synthesis of Thiazole Compounds 1 and 2
The detailed synthetic protocols and spectral data of final products 1 and 2 as well as all intermediates have been previously reported16,17.
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Against MRSA, VISA, VRSA, and S. epidermidis
The MICs of the thiazole compounds, vancomycin, and teicoplanin against MRSA, VISA, VRSA, and S. epidermidis were determined using the broth microdilution method, in accordance with the recommendations contained in the CLSI guidelines (with the exception that Mueller-Hinton broth (MHB) was used instead of cation-adjusted MHB)18. Bacteria were prepared in phosphate buffered saline (PBS) until a McFarland standard of 0.5 was achieved. The solution was diluted 1:300 in MHB to reach a starting inoculum of 1 × 105 colony-forming units (CFUml−1). Bacteria were transferred to a 96-well microtiter plate. Thiazole compounds and antibiotics were added (in triplicate) to wells in the first row of the microtitier plate and then serially diluted along the ordinate. The plate was incubated at 37°C for 20–24 h before the MIC was determined. The MIC was categorized as the concentration at which no visible growth of bacteria was observed in a particular well.
The MBC was determined by plating 5 µL from wells on the 96-well microtiter plate (where the MIC was determined) were no growth was observed onto Tryptic soy agar (TSA) plates. The TSA plates were then incubated at 37°C for 20–24 h before the MBC was determined. The MBC was categorized as the concentration where ≥99% reduction in bacterial cell count was observed.
Time-kill Analysis of Thiazole Compounds and Glycopeptide Antibiotics Against MRSA
MRSA NRS123 (USA400) cells in the logarithmic growth phase were diluted to ~1 × 108 colony-forming units (CFUml−1) and exposed to concentrations equivalent to 2, 4, and 8 × MIC (in triplicate) of thiazole compounds 1 and 2, teicoplanin, and vancomycin in Mueller-Hinton broth (MHB). 20 µL samples were collected after 0, 2, 4, 6, 8, 10, 12, and 24 h of incubation at 37°C and subsequently serially diluted in PBS. Bacteria were then transferred to TSA plates and incubated at 37°C for 18–20 h before viable CFUml−1 was determined. The test agent was deemed bactericidal if it successfully produced a 3-log10 reduction in the bacterial count within 24 hours, as reported elsewhere19.
Single-step Resistance Selection
The frequency of spontaneous single-step resistance of the thiazole compounds and glycopeptide antibiotics to five MRSA strains was determined as reported elsewhere20,21. Briefly, bacterial cultures (>1 × 109 CFUml−1) were spread onto Mueller-Hinton agar plates (10-mm diameter) containing each compound/antibiotic at 4 × MIC. Plates were incubated aerobically at 37°C for 48 h. The frequency of resistance was calculated as the number of resistant colonies per inoculum21.
Combination Therapy Analysis of Thiazole Compounds with Glycopeptide Antibiotics
The relationship between the thiazole compounds and glycopeptide antibiotics (vancomycin and teicoplanin) was assessed via a standard checkerboard assay22. Bacteria equivalent to a McFarland standard of 0.5 were prepared in PBS. The bacteria were then diluted in MHB to achieve a starting cell density of 1 × 105 CFUml−1. MHB was transferred to all wells of a 96-well microtiter plate. The thiazole compounds and glycopeptide antibiotics were diluted in MHB to achieve a starting concentration equivalent to 2 × or 4 × MIC, respectively. The glycopeptide antibiotic was serially diluted along the abscissa of the microtiter plate while the thiazole compound was serially diluted along the ordinate. The plate was incubated for 20–24 h at 37°C. The MIC of the test compound in combination with each glycopeptide antibiotic studied was determined as the lowest concentration of each compound/antibiotic where no visible growth of bacteria was observed. The fractional inhibitory concentration index (ΣFIC) was calculated for each combination as follows:
 |
A synergistic relationship was classified as an FIC index less than or equal to 0.5. FIC values above 0.5 but less than 4.0 were characterized as indifference while FIC values above 4.0 were classified as antagonistic.
Re-sensitization of VRSA Strains to Vancomycin Using Broth Microdilution Method
MHB was inoculated with VRSA (5×105 CFUml−1), as described elsewhere23. 5-ml aliquots of the bacterial suspension were divided into microcentrifuge tubes. Compound 1 or 2 (at ½ × MIC) was introduced into each tube. After sitting at room temperature for 30 min, 1 ml samples from each tube were transferred to a new centrifuge tube prior to addition of the antibiotic (either vancomycin or teicoplanin at a concentration equivalent to their MIC). Using a 96-well microtiter plate, rows 2–12 were filled with the remaining 4 ml bacterial suspension (containing either compound 1 or 2). 200-µl aliquots from tubes containing both the thiazole compound and glycopeptide antibiotic were transferred to row 1 of the 96-well plate. After aspirating contents in the first row 4–6 times, 100 µL was transferred from wells in row 1 to row 2. This process was repeated to dilute the remaining wells containing no antibiotic. Untreated bacteria served as a control. The plate was incubated at 37°C for 20 h before the MIC was recorded. The MIC was categorized as the concentration at which no visible growth of bacteria was observed in a particular well. A fold reduction was calculated by comparing the MIC of the antibiotic alone compared to the MIC of the antibiotic given in combination with 1 or 2.
Staphylococcus Biofilm Mass Reduction Determination
The microtiter dish biofilm formation assay24 was utilized to assess the ability of the thiazole compounds to disrupt an adherent staphylococcal biofilm, similar to what has been described elsewhere25. S. epidermidis ATCC 35984 was transferred to tryptic soy broth and incubated at 37°C for 24 h before being diluted 1:200 in tryptic soy broth + 1% glucose. This solution was transferred to each well of a 96-well microtiter plate and incubated at 37°C for 24 h to permit biofilm formation on the well surface. Bacteria were removed and wells were washed twice with PBS. Compounds 1, 2, or vancomycin were added (in triplicate) to wells and serially diluted. The microtiter plate was then incubated at 37°C for 24 h. The plate was washed twice by submerging in deionized water. 0.1% (w/v) crystal violet was added to each well and allowed to stain the biofilm for 20 min before addition of 95% ethanol to decolorize. Using a kinetic microplate reader (Molecular Devices, Sunnyvale, CA, USA), the optical density of each well at 595 nm was measured. Percent biofilm mass reduction was calculated for each treatment regimen as compared to the control (wells receiving no treatment).
Kinetic Solubility Determination of Compound 2
Serial dilutions of compound 2 were prepared in DMSO at 100× the final concentration. Compound 2 was then diluted 100-fold into PBS in a 96-well plate and mixed. The absorbance of the PBS-containing plate was measured prior to addition of the test agents to determine the background absorbance. After 2 h, the presence of precipitate was detected by turbidity (absorbance at 540 nm). An absorbance value of greater than (mean + 3× standard deviation of the blank), after subtracting the pre-experiment background, was indicative of turbidity. The solubility limit is reported as the highest experimental concentration for compound 2 with no evidence of turbidity.
Caco-2 Bidirectional Permeability Assessment of Compound 2
To assess the ability of compound 2 to passively permeate through epithelial tissue, a Caco-2 permeability assay was performed as described previously16. Caco-2 cells grown in tissue culture flasks were trypsinized, suspended in medium, and the suspensions were applied to wells of a Millipore 96 well Caco-2 plate. The cells were allowed to grow and differentiate for three weeks, feeding at 2-day intervals. For Apical to Basolateral (A→B) permeability, compound 2 was added to the apical (A) side and amount of permeation was determined on the basolateral (B) side; for Basolateral to Apical (B→A) permeability, compound 2 was added to the B side and the amount of permeation was determined on the A side. The A-side buffer contained 100 µM Lucifer yellow dye, in Transport Buffer (1.98 g/L glucose in 10 mM HEPES, 1.0 × Hank’s Balanced Salt Solution) at pH 6.5, and the B-side buffer contained Transport Buffer at pH 7.4. Caco-2 cells were incubated with these buffers for 2 h, and the receiver side buffer was removed for analysis by LC/MS/MS. To verify the Caco-2 cell monolayers were properly formed, aliquots of the cell buffers were analyzed by fluorescence to determine the transport of the impermeable dye Lucifer Yellow. Any deviations from control values are reported. Data are expressed as permeability where is the rate of permeation, C0 is the initial concentration of test agent, and A is the area of the monolayer. In bidirectional permeability studies, the efflux ratio (RE) is also calculated: . An RE > 2 indicates a potential substrate for P-glycoprotein or other active efflux transporters.
Statistical Analysis
All statistical analysis was performed using the two-tailed Student’s t-test (P < 0.05) utilizing Microsoft Excel software.
Results and Discussion
Determination of the Antimicrobial Activity of the Thiazole Compounds and Glycopeptide Antibiotics
We have designed and synthesized a series of thiazole derivatives containing modifications to the lipophilic alkyl side chain of 116. Antimicrobial susceptibility analysis of these derivatives, using the standard broth microdilution assay26, revealed compound 2 exhibited the most potent antibacterial activity against multidrug-resistant staphylococci. As Table 1 demonstrates, the minimum inhibitory concentration (MIC) for 1 was 1.38 µgml−1; compound 2 showed similar activity inhibiting growth of the same strains at a concentration of 1.40 µgml−1. At these concentrations, the compounds are not toxic to mammalian cells as confirmed in a previous study16.
Table 1.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of thiazole compounds 1 and 2, teicoplanin, and vancomycin against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate Staphylococcus aureus (VISA), and vancomycin-resistant Staphylococcus aureus (VRSA) strains.
| MIC and MBC (µgml−1) of thiazole compounds, teicoplanin, and vancomycin against S. aureus |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | 1 | 2 | Teicoplanin | Vancomycin | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| MRSA | NRS107 | 1.38 | 2.77 | 1.40 | 1.40 | 0.94 | 0.94 | 0.74 | 1.49 |
| NRS119 | 1.38 | 1.38 | 1.40 | 1.40 | 0.94 | 0.94 | 0.74 | 1.49 | |
| NRS123 (USA400) | 1.38 | 11.07 | 1.40 | 5.58 | 0.94 | 15.04 | 0.37 | 0.37 | |
| NRS194 | 1.38 | 5.54 | 1.40 | 11.17 | 0.94 | 15.04 | 0.74 | 0.74 | |
| NRS384 (USA300) | 1.38 | 1.38 | 1.40 | 2.80 | 0.94 | 15.04 | 0.74 | 0.74 | |
| NRS385 (USA500) | 1.38 | 1.38 | 1.40 | 1.40 | 0.94 | 3.76 | 0.74 | 0.74 | |
| ATCC 43300 | 1.38 | 2.77 | 1.40 | 2.80 | 0.94 | 3.76 | 0.74 | 0.74 | |
| VISA | NRS1 | 1.38 | 1.38 | 0.70 | 1.40 | 3.76 | 7.52 | 2.97 | 2.97 |
| NRS19 | 1.38 | 1.38 | 1.40 | 1.40 | 0.94 | 0.94 | 2.97 | 2.97 | |
| NRS37 | 1.38 | 1.38 | 0.70 | 1.40 | 7.52 | 7.52 | 2.97 | 2.97 | |
| VRSA | VRS1 | 2.77 | 2.77 | 1.40 | 1.40 | 120.30 | 240.60 | 760.68 | 760.68 |
| VRS4 | 2.77 | 2.77 | 0.70 | 2.80 | 60.15 | 60.15 | 760.68 | 760.68 | |
| VRS5 | 2.77 | 5.54 | 1.40 | 5.58 | 60.15 | 60.15 | 760.68 | 760.68 | |
| S. epidermidis | ATCC 35984 | 2.77 | N.D. | 0.70 | N.D. | N.D. | N.D. | 0.74 | N.D. |
Abbreviation: N.D. = Not Determined
The thiazole compounds exhibited activity against MRSA strains resistant to several different classes of antibiotics including macrolides (NRS384), fluoroquinolones (NRS385), aminoglycosides (NRS385), tetracyclines (NRS384), and oxazolidinones (NRS119). Additionally, both 1 (MIC from 1.38–2.77 µgml−1) and 2 (MIC from 0.70–1.40 µgml−1), unlike vancomycin (MIC from 2.97–760.68 µgml−1), retained their antimicrobial activity against strains of vancomycin-intermediate S. aureus (VISA) and VRSA strains. Furthermore, both thiazole compounds were more potent than teicoplanin against two VISA strains (MICTeicoplanin from 0.94–7.52 µgml−1) and all three VRSA strains tested (MICTeicoplanin from 60.51–120.30 µgml−1). Thus, 1 and 2 exhibit a selective advantage over vancomycin and teicoplanin in their antibacterial activity against both VISA and VRSA.
Antimicrobial agents that exhibit bactericidal activity are hypothesized to contribute to a more rapid recovery from infection and a better clinical outcome, compared to their bacteriostatic counterparts27. To ascertain whether the thiazole compounds were bacteriostatic or bactericidal, the minimum bactericidal concentration (MBC) was determined. The MBC was calculated as the lowest concentration of compound/drug that produced a ≥99.9% reduction in the bacterial cell count as compared to the initial inoculum28. As Table 1 demonstrates, both thiazole compounds are bactericidal. Against five MRSA strains (NRS107, NRS119, NRS123, NRS385, and ATCC 43300), all VISA strains, and two VRSA strains (VRS1 and VRS4), 1 and 2 possess MBC values equivalent to their MICs or one-fold higher than the MICs. This is similar to what is observed with vancomycin, a known bactericidal antibiotic, with MBC values equal to or one-fold higher than the MICs for all MRSA and VISA strains tested. Teicoplanin exhibits MBC values equivalent to its MIC against two MRSA strains, four-fold higher than its MIC against two additional MRSA strains, and MBC values 16-fold higher than the MIC values against three strains of MRSA (NRS194, USA300, and USA400).
Time-kill Analysis of Thiazole Compounds and Glycopeptide Antibiotics
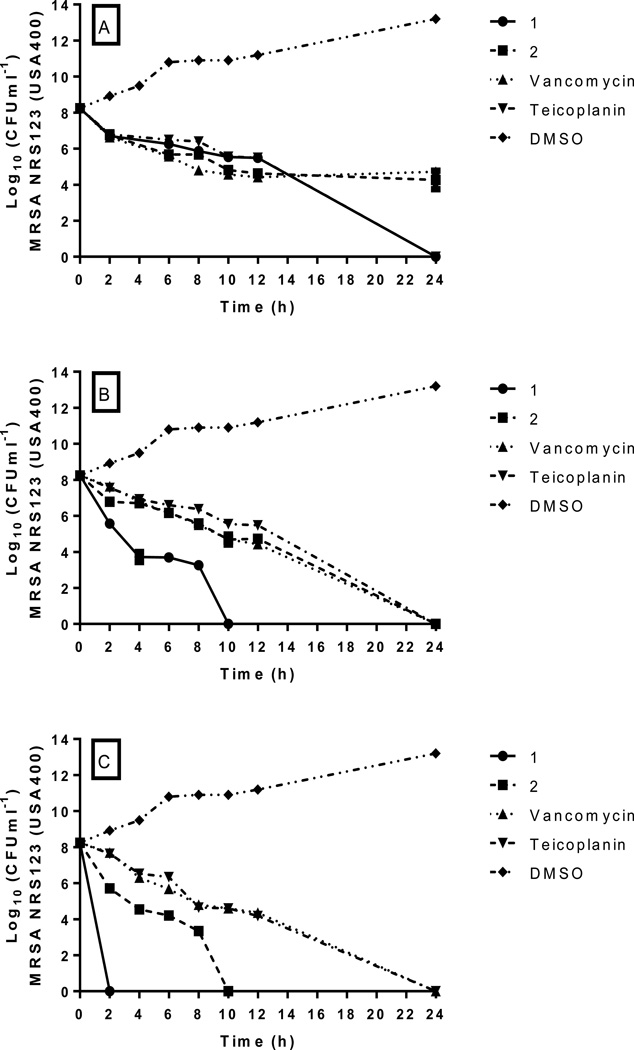
In order to confirm that 1 and 2 were bactericidal agents, we next examined how rapidly the thiazole compounds were able to kill a high inoculum of MRSA. Using a standard time-kill assay, MRSA USA400 (NRS123), a predominant strain linked to many community-acquired MRSA infections29, was exposed to 2, 4, and 8 × MIC of 1, 2, teicoplanin, or vancomycin. Samples were collected at specific time points and transferred to Tryptic soy agar (TSA) plates to determine the number of viable bacteria remaining post-treatment.
As depicted in Figure 2, both 1 and 2 exhibit bactericidal activity at all concentrations tested; however the time to achieve a 3-log10 reduction in CFUml−1 differs depending on the concentration of the test agent. For compound 1, MRSA is completely eliminated after 24 h at 2 × MIC, after 10 h at 4 × MIC, and after only two h at 8 × MIC. Analogue 2 produces a 3-log10 reduction in CFUml−1 after 10 h at 2 × MIC; however, it is not able to completely eradicate MRSA similar to the parent compound. At higher concentrations, 2 successfully eliminates MRSA completely after 24 h (at 4 × MIC); at the highest concentration tested (8 × MIC), analogue 2 proves superior to both antibiotics tested as it rapidly eliminates MRSA within 10 hours. Vancomycin required 24 h to completely eradicate MRSA at both 4 and 8 × MIC; at 2 × MIC, vancomycin produced a 3-log10 reduction in CFUml−1 within 24 h but was not able to eliminate all bacteria completely (similar to analogue 2). These results are similar to what has been previously published regarding vancomycin’s slow bactericidal activity30. Teicoplanin required 24 h to completely eliminate MRSA at all three concentrations tested. Thus, in addition to retaining antimicrobial activity against VISA and VRSA strains, 1 and 2 possess an additional advantage over vancomycin and teicoplanin in their ability to rapidly kill MRSA, particularly at higher concentrations. Rapid bactericidal activity is an important factor in reducing the emergence of bacterial resistance to an antimicrobial agent and is important clinically in preventing an infection from spreading27. Additionally, bactericidal agents have been shown both clinically and in in vivo studies to be superior to bacteriostatic agents for the treatment of certain invasive diseases such as endocarditis31. Furthermore, rapid bactericidal activity is an important quality for consideration in using a particular agent in combination with other antibiotics, such as vancomycin30. The results from the time-kill assay provided valuable insight into the possibility that the thiazole compounds could be potentially paired with other antibiotics against MRSA, given 1 and 2 possess rapid bactericidal activity.
Figure 2.
Time-kill analysis of the lead compound 1, derivative 2, teicoplanin, and vancomycin against methicillin-resistant Staphylococcus aureus (MRSA) strain NRS123 (USA400) at A) 2 × MIC, B) 4 × MIC, and C) 8 × MIC. Error bars represent standard deviation values.
Assessment of Single-step Resistance
After confirming compounds 1 and 2 possessed rapid bactericidal activity against MRSA, we next turned our attention to assessing the likelihood MRSA would develop resistance quickly to these thiazole compounds. A single-step resistance selection experiment was performed by subculturing a high inoculum of MRSA (>1 × 109 CFUml−1) onto TSA plates containing 1, 2, vancomycin, or teicoplanin at a concentration equivalent to 4 × MIC. The likelihood of bacterial resistance arising (via spontaneous mutations in the bacterial genome) to these compounds/antibiotics was examined using five MRSA strains. Table 2 presents the mutation frequencies generated against each tested agent: for 1, 1.19×10−8 to >1.73×10−10; for 2, >1.73×10−10 to >2.33×10−10; for teicoplanin, 2.73×10−7 to 3.03×10−9; and for vancomycin, 3.03×10−10 to >8.47×10−10. The values obtained for teicoplanin and vancomycin are similar to what has been reported elsewhere32.
Table 2.
Single-step frequency of resistance determination for compounds 1 and 2, teicoplanin, and vancomycin against methicillin-resistant Staphylococcus aureus (MRSA).
| MRSA Strain | Compound/Antibiotic Name | |||
|---|---|---|---|---|
| 1 | 2 | Teicoplanin | Vancomycin | |
| NRS107 | >1.73×10−10 | >1.73×10−10 | 1.91×10−8 | >1.73×10−10 |
| NRS119 | 1.99×10−8 | >8.47×10−10 | 2.73×10−7 | >8.47×10−10 |
| NRS123 (USA400) | 5.93×10−9 | >2.33×10−10 | 2.21×10−9 | >2.33×10−10 |
| NRS384 (USA300) | 1.35×10−8 | >3.03×10−10 | 3.03×10−9 | 3.03×10−10 |
| NRS385 (USA500) | 1.19×10−8 | >3.31×10−10 | 1.79×10−8 | >3.31×10−10 |
The thiazole compounds produce a similar mutation frequency as both teicoplanin and vancomycin. Interestingly, 2 demonstrates a mutation frequency similar to or better than vancomycin against the five MRSA strains tested. Even at lower (2 × MIC) concentrations, resistant mutants are difficult to isolate against this particular compound (data not published). It took 30 years to isolate a strain of S. aureus exhibiting resistance to vancomycin1. Thus the results presented here support the notion that MRSA is unlikely to develop rapid resistance to the thiazole compounds, in particular compound 2. The data obtained from both the time-kill and single-step resistance selection experiments demonstrate the thiazole compounds possess two important characteristics necessary for an ideal antibiotic for MRSA, rapid bactericidal activity and low potential for bacterial resistance development33.
Combination Testing of Thiazole Compounds with Glycopeptide Antibiotics
Glycopeptide antibiotics, chiefly vancomycin, have been a principal source of treatment of MRSA infections for many years33. However, extensive use of these antibiotics opens the door for the emergence of strains with reduced susceptibility to these antibiotics30. Combination therapy, pairing vancomycin with another antimicrobial, has been used in the healthcare setting both to reduce the likelihood of resistant strains to vancomycin from rapidly emerging and to improve the morbidity associated with MRSA infections. For example, vancomycin has been combined with subinhibitory concentrations of clindamycin and linezolid to reduce toxins generated by S. aureus during infection34,35. Identifying other antimicrobial partners capable of being paired with vancomycin can potentially prolong the clinical utility of this antibiotic.
To ascertain whether 1 and 2 have potential to be combined with vancomycin against MRSA, the checkerboard assay was utilized22. In this assay, one antibiotic/compound is serially diluted along the abscissa followed by diluting the second antibiotic/compound along the ordinate in a 96-well plate. The fractional inhibitory concentration (FIC) is then calculated as a ratio of the MIC of each antibiotic/compound when given in combination relative to the MIC of each antibiotic/compound given alone. The FIC index (ΣFIC) is a summation of the FICs for each antibiotic/compound tested in combination. ΣFIC ≤ 0.50 is indicative of synergism between the antibiotic and compound. Results from the checkerboard assay experiment are presented in Table 3. Both thiazole compounds were found to exhibit a synergistic relationship with vancomycin against six of the seven MRSA strains tested with ΣFIC values ranging from 0.07 to 0.50 for 1 and ΣFIC values ranging from 0.13 to 0.50 for 2. At ¼ × MIC for 2, a 16-fold reduction in the MIC for vancomycin (when combined with 2) was observed for all six MRSA strains where synergy was detected (data not presented). As vancomycin is known to be a nephrotoxic agent, using a lower concentration of this drug in MRSA infections is highly desirable as it has the potential benefit of reducing this side effect in patients33. When tested against VISA, 1 failed to exhibit synergy with vancomycin while 2 demonstrated a synergistic relationship with vancomycin against one strain (NRS19).
Table 3.
Fractional inhibitory concentration index (ΣFIC) range of thiazole compounds 1 and 2 in combination with teicoplanin and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus (MRSA and VISA).
| Strain | Vancomycin | Teicoplanin | ||||||
|---|---|---|---|---|---|---|---|---|
| ΣFIC1 (+1) |
Result | ΣFIC (+2) |
Result | ΣFIC (+1) |
Result | ΣFIC (+2) |
Result | |
| MRSA NRS107 | 0.50–0.56 | S/I | 0.31–0.50 | S | 0.53–1.00 | I | 0.53–0.53 | I |
| MRSA NRS119 | 0.07–0.31 | S | 0.28–0.31 | S | 0.50–0.53 | S/I | 0.53–0.75 | I |
| MRSA NRS123 | 0.19–0.50 | S | 0.19–0.31 | S | 0.53–1.00 | I | 0.53–1.00 | I |
| MRSA NRS194 | 0.13–0.50 | S | 0.13–0.56 | S/I | 0.28–1.00 | S/I | 0.28–1.00 | S/I |
| MRSA NRS384 | 0.16–0.50 | S | 0.13–0.31 | S | 0.53–1.00 | I | 0.53–0.75 | I |
| MRSA NRS385 | 0.13–0.50 | S | 0.13–0.31 | S | 0.53–1.00 | I | 0.53–1.00 | I |
| MRSA ATCC 43300 | 0.09–0.50 | S | 0.09–0.31 | S | 0.50–0.52 | I | 0.50–0.56 | S/I |
| VISA NRS1 | 0.26–0.56 | S/I | 0.63 | I | 0.16–1.00 | S/I | 0.19–2.00 | S/I |
| VISA NRS19 | 0.53–0.56 | I | 0.50 | S | 0.53–1.03 | I | 0.53–1.03 | I |
| VISA NRS37 | 0.53–0.56 | I | 0.75 | I | 0.09–1.01 | S/I | 0.13–2.00 | S/I |
Results for the FIC index are as follows: ≤ 0.5, synergistic (S); >0.5 to ≤4.0, indifference (I); >4, antagonistic (A). Results are reported from two independent experiments.
We were curious to explore if the synergistic relationship observed was limited just to vancomycin or could be observed with other glycopeptide antibiotics as well. Teicoplanin was used to further explore the partnership between thiazole compounds and glycopeptide antibiotics. Interestingly, the checkerboard assay revealed that neither 1 nor 2 exhibited a synergistic relationship with teicoplanin against MRSA. This suggests that combination therapy involving the thiazole compounds may be limited to only being paired with vancomycin though further studies with other glycopeptide antibiotics are needed to confirm this observation. Additionally, as vancomycin targets cell wall biosynthesis in S. aureus, it would be worthwhile to explore if a synergistic relationship would be observed between these thiazole compounds and other cell wall biosynthesis inhibitors (such as β-lactam antibiotics). Collectively, the results shed valuable insight into thiazole compounds serving as potential future partners with vancomycin against MRSA. This discovery can potentially prolong the usage of vancomycin as a therapeutic agent for MRSA infections by reducing the likelihood of strains developing resistance to vancomycin used in monotherapy.
Re-sensitization of VRSA to Glycopeptide Antibiotics
The emergence of S. aureus strains resistant to vancomycin presents an additional challenge to clinical care providers dealing with the growing epidemic of multidrug-resistant bacterial infections. Identifying clever strategies to prolong the use of current antibiotics against multidrug-resistant bacteria is necessary. One strategy that has been explored recently is suppressing antibiotic resistance by re-sensitizing resistant bacteria using a secondary compound23. As the thiazole compounds were found to possess a synergistic relationship with vancomycin against MRSA, we postulated that the thiazole compounds may be capable of re-sensitizing VRSA strains to vancomycin. Initially, the MIC of 1 and 2 was determined using the broth microdilution assay. Next, Mueller-Hinton broth was inoculated with either compound 1 or 2 (at ½ × MIC). Vancomycin was then serially diluted in both the inoculated media alone and media supplemented with the thiazole compounds. The MICs of vancomycin in the presence of the thiazole compounds was compared to vancomycin used alone. A fold-reduction was calculated by dividing the MIC of vancomycin alone by the MIC of vancomycin + the thiazole compound.
As Table 4 presents, both thiazole compounds were capable of re-sensitizing VRSA to vancomycin. Compound 1 was able to produce a four-fold reduction in the MIC of vancomycin when the two agents were combined against VRSA. Amazingly, compound 2 proved to be superior to 1 as it produced a 512-fold reduction in the MIC of vancomycin against two VRSA strains tested. Furthermore, compound 2 produced a 32-fold reduction in the MIC of teicoplanin against two VRSA strains (VRS4 and VRS5) and a 64-fold reduction against strain VRS1. Thus compound 2 was capable of re-sensitizing VRSA to both vancomycin and teicoplanin. Substitution of the alkane side chain (in 1) with a phenyl group (in 2) produced a dramatic improvement in the thiazole compounds’ ability to re-sensitize VRSA to the effect of glycopeptide antibiotics. Using the checkerboard assay, we found that compound 2 exhibited a synergistic relationship with both vancomycin and teicoplanin against two VRSA strains (VRS4 and VRS5) with ΣFIC = 0.50. Thus, compound 2 holds promise for future use to suppress vancomycin-resistance in VRSA strains, prolonging the utility of glycopeptide antibiotics against these strains.
Table 4.
Re-sensitization of vancomycin-resistant Staphylococcus aureus (VRSA) to vancomycin and teicoplanin using a subinhibitory concentration (½ × MIC) of compound 1 or 2.
| Strain | 1 + Teicoplanin | 2 + Vancomycin | 1 + Teicoplanin | 2 + Teicoplanin | ||||
|---|---|---|---|---|---|---|---|---|
| Re- sensitization |
ΣFIC1 | Re- sensitization |
ΣFIC | Re- sensitization |
ΣFIC | Re- sensitization |
ΣFIC | |
| VRS1 | <4-fold | >1.50 | <4-fold | 1.00 | 0-fold | >2.00 | 64-fold | 0.63 |
| VRS4 | <4-fold | 1.13 | 512-fold | 0.50 | 0-fold | 2.00 | 32-fold | 0.50 |
| VRS5 | 4-fold | 1.25 | 512-fold | 0.50 | 2-fold | 1.50 | 32-fold | 0.50 |
Results for the FIC index (ΣFIC) are as follows: ≤ 0.5, synergistic (S); >0.5 to ≤4.0, indifference (I); >4, antagonistic (A).
S. epidermidis Biofilm Mass Reduction
Bacterial biofilms which form on the surface of indwelling medical devices, such as intravascular catheters, are a major problem in hospitals. These biofilms can lead to life-threatening bloodstream infections associated with high mortality and treatment costs36. Staphylococci, primarily S. epidermidis and S. aureus, are responsible for many invasive infections which develop from bacterial biofilms that form on the surface of medical devices3,37. Further exacerbating this problem, traditional antibiotics are not effective at disrupting these biofilms as cells present within the biofilm exhibit increased resistance to antibiotics5. Identifying antimicrobials capable of disrupting these biofilms is necessary to combat this growing problem.
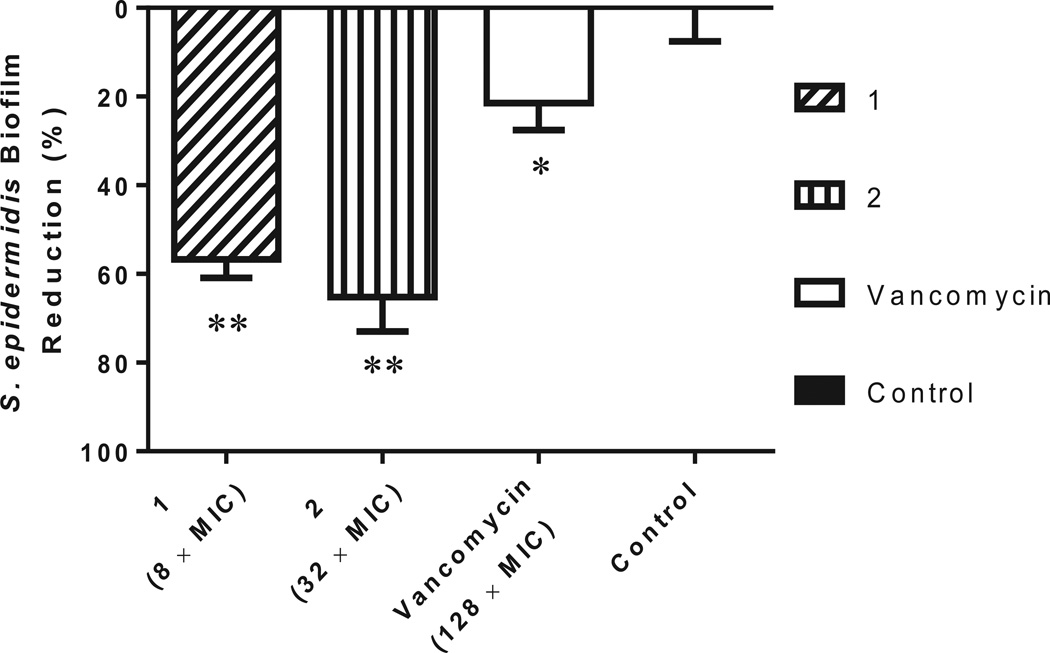
Recent studies have demonstrated that thiazole and thiazolidinone compounds possess the ability to disrupt bacterial biofilms38,39. To examine if the potential therapeutic application of 1 and 2 could be expanded beyond just inhibition of planktonic bacteria, the ability of both thiazole compounds to disrupt staphylococcal biofilm was analyzed. First, to confirm the thiazole compounds were capable of inhibiting planktonic bacteria, the MIC of each compound and vancomycin against a biofilm-forming clinical isolate of methicillin-resistant S. epidermidis was assessed using the broth microdilution technique. Compounds 1 and 2 were found to inhibit bacterial growth at 2.77 and 0.61 µgml−1, respectively (Table 1). Vancomycin inhibited growth of planktonic S. epidermidis at a concentration of 0.74 µgml−1. Next, to determine if compounds 1 and 2 had the potential to disrupt staphylococcal biofilm, the crystal violet reporter assay was used against a mature S. epidermidis biofilm24. As Figure 3 demonstrates, 1 (at 8 × MIC) and 2 (at 32 × MIC) significantly disrupted S. epidermidis biofilm, reducing the biofilm mass by 56.7% and 65.2% respectively. These compounds proved to be far superior to vancomycin; even at 128 × MIC, vancomycin was only able to reduce S. epidermidis biofilm mass by 21.5%. The thiazole compounds thus possess anti-biofilm activity and are capable of disrupting adherent staphylococci biofilm much better than a traditional antibiotic, vancomycin.
Figure 3.
Efficacy of thiazole compounds 1 and 2 and vancomycin (all at 64 µM) in disrupting an established methicillin-resistant S. epidermidis ATCC 35984 biofilm. Bacteria were incubated at 37°C in MHB medium supplemented with glucose for 24 h to allow biofilm formation. Wells were subsequently rinsed with PBS before MHB containing different concentrations of each test agent was added. Following incubation for 24 h, wells were washed again and left to dry. The adherent biofilm was stained with crystal violet and then the dye was extracted with ethanol before turbidity was measured at 595 nm. Data are presented as percentage of biofilm mass reduction compared to untreated wells (control). All experiments were done in triplicate. One asterisk (*) indicates data are statistically different when compared to the control (P < 0.05). Two asterisks (**) indicate the data are statistically different from the vancomycin-treated wells (P < 0.05).
Pharmacokinetic Analysis of Compound 2
Assessment of a compound’s drug-like properties is important early in drug development to identify and address potential issues, especially those associated with aqueous solubility and permeability. Previously we reported the lead thiazole compound 1 possessed moderate aqueous solubility (21.6 µgml−1) but poor permeability across a biological membrane (Caco-2 apparent permeability, Papp (A → B) = 0.0 × 10−6 cm/sec)16. We were interested to examine if compound 2, containing a phenyl ring substitution in place of the linear alkane side chain present in 1, would exhibit an improved pharmacokinetic profile. Initially, a turbidometric solubility screen was used to assess the maximum concentration compound 2 was able to dissolve in an aqueous buffer (phosphate-buffered saline). Table 5 demonstrates that the phenyl ring substitution resulted in a significant decrease in the aqueous solubility of compound 2 (2.70 µgml−1) relative to 1. After determining compound 2 exhibited poor aqueous solubility, this compound’s ability to passively diffuse across a biological membrane was assessed.
Table 5.
Evaluation of solubility of thiazole compound 2, reserpine, tamoxifen, and verapamil in phosphate-buffered saline (PBS).
| Compound Tested | Solubility Limit (µgml−1)1 |
|---|---|
| 2 | 2.70 |
| Reserpine | 19.05 |
| Tamoxifen | 5.80 |
| Verapamil | >227.30 |
Solubility limit corresponds to the highest concentration of test compound where no precipitate was detected.
The Caco-2 permeability assay was utilized to determine if compound 2 was more permeable than compound 1. 10 µM of 2 was added to the apical (A) surface of a Caco-2 bilayer and the rate of transfer of the compound to the basolateral (B) surface was measured. The process was then repeated in reverse to assess the rate of transfer from the B to A direction. As Table 6 demonstrates, the rate of transfer of compound 2 from the apical to basolateral surface was not measurable (Papp (A → B) = 0.0 × 10−6 cmsec−1). However, the rate of transfer from the B to A surface was measured to be 1.2 × 10−6 cm/sec. This is similar to what is observed with the poorly permeable drug control ranitidine (Papp (B → A) = 1.7 × 10−6 cmsec−1). Thus the results indicate compound 2 does not exhibit improved permeability relative to 1. The discrepancy between the rate of transfer of compound 2 across the basolateral and apical surfaces results in an efflux ratio >2; this suggests that 2 may be a substrate for an efflux transporter (such as P-glycoprotein). One method to overcome the effect of efflux transporters is to saturate the transporters, by using a higher concentration than 10 µM of compound 2 used for the assay, thus permitting passive transfer of the compound across the apical surface of the membrane.
Table 6.
Evaluation of physicochemical properties (apparent permeability) of thiazole compound 2, ranitidine, warfarin, and talinolol via the Caco-2 permeability assay.
| Compound Tested |
Mean A → B1 Papp (10−6 cmsec−1) |
Mean B → A2 Papp (10−6 cmsec−1) |
Efflux Ratio3 |
|---|---|---|---|
| 2 | 0.04 | 1.2 | >2 |
| Ranitidine | 0.2 | 1.7 | 8.5 |
| Warfarin | 27.6 | 11.1 | 0.4 |
| Talinolol | 0.1 | 8.3 | 83 |
Mean A → B Papp = mean apparent permeability of test compound from apical to basolateral surface
Mean B → A Papp = mean apparent permeability of test compound from basolateral to apical surface
Compound not detected in receiver compartment (peak below limit of detection); permeability may be underestimated
While limited solubility and permeability characteristics are not encouraging to consider biologically-active compounds as drug-candidates for subsequent clinical steps, recent formulation technology has been able to overcome such limitations to propel valuable compounds with similar kinetic profiles into the market. For instance, the orally administrated protease inhibitor telaprivir possesses an aqueous solubility profile similar to compound 2. By using a spray drying dispersion technique, telaprivir’s water solubility, permeability and the consequent bioavailability were dramatically improved40. Moreover, formulators have more techniques to handle poor water solubility such as using the solvent/antisolvent method41. By shedding light on the limited pharmacokinetic profile of compound 2, we are opening a gate for formulators to investigate their time and effort improving the pharmacokinetic profile of this very promising antimicrobial agent.
Conclusion
We have successfully developed an approach to synthesize phenylthiazole compounds with potent antibacterial activity against methicillin-resistant (MRSA), vancomycin-intermediate (VISA), and vancomycin-resistant Staphylococcus aureus (VRSA). The most potent derivative 2 exhibited MIC values ranging from 0.70 to 1.40 µgml−1 and MBC values ranging from 1.40 to 11.17 µgml−1 against MRSA, VISA, and VRSA. Both compounds 1 and 2 rapidly eliminated MRSA within 10 h, at 8 × MIC, while vancomycin required 24 h; additionally both thiazole compounds exhibited low resistance frequencies, similar to vancomycin. Lead 1 behaved synergistically when combined with vancomycin exhibiting ΣFIC ranging from 0.07 to 0.50 against six MRSA strains while derivative 2 behaved synergistically with vancomycin exhibiting ΣFIC ranging from 0.09 to 0.50 against six MRSA strains. Interestingly, compound 2 demonstrated the ability to re-sensitize two VRSA strains to vancomycin and teicoplanin reducing their MIC by 512-fold and 32-fold, respectively. Additionally, both compounds 1 and 2 exhibited strong anti-biofilm activity reducing adherent S. epidermidis biofilm by 56.7% and 65.2%, respectively. As compound 2 did not demonstrate good solubility or permeability properties, incorporating advanced formulation techniques are a must to improve its pharmacokinetic profile. In addition, further derivatives will be constructed with the aim of improving the thiazole compounds’ drug-like properties while maintaining their strong antibacterial properties. Collectively, the thiazole compounds prepared here have the versatility to potentially be used for multiple therapeutic applications including being used alone or in combination with vancomycin against multidrug-resistant staphylococci, to re-sensitize VRSA to vancomycin, or to disrupt mature staphylococcal biofilms.
Acknowledgements
The authors would like to thank the Network of Antimicrobial Resistance in Staphylococcus aureus (NARSA) program supported under NIAID/NIH Contract # HHSN272200700055C for providing MRSA, VISA, and VRSA strains used in this study.
References
- 1.Prevention, C.f.D.C.a. Antibiotic Resistance Threats in the United States, 2013. 2013:1–114. [Google Scholar]
- 2.Moran GJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. The New England journal of medicine. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 3.Wang R, et al. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. The Journal of clinical investigation. 2011;121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies D. Understanding biofilm resistance to antibacterial agents. Nature reviews. Drug discovery. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 5.Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends in microbiology. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 6.Chambers HF. Community-associated MRSA--resistance and virulence converge. The New England journal of medicine. 2005;352:1485–1487. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 7.Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerging infectious diseases. 2005;11:928–930. doi: 10.3201/eid1106.040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. The Lancet infectious diseases. 2001;1:147–155. doi: 10.1016/S1473-3099(01)00091-3. [DOI] [PubMed] [Google Scholar]
- 9.Hays SJ, et al. Substituted 2-Benzothiazolamine as Sodium Flux Inhibitors - Quantitative Structure-Activity-Relationships and Anticonvulsant Activity. J Pharm Sci. 1994;83:1425–1432. doi: 10.1002/jps.2600831013. [DOI] [PubMed] [Google Scholar]
- 10.Das J, et al. Discovery of 2-amino-heteroaryl-benzothiazole-6-anilides as potent p56(lck) inhibitors. Bioorganic & medicinal chemistry letters. 2003;13:2587–2590. doi: 10.1016/s0960-894x(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson I, Bradshaw TD, Matthews CS, Stevens MF, Westwell AD. Antitumour benzothiazoles. Part 20: 3'-cyano and 3'-alkynyl-substituted 2-(4'-aminophenyl)benzothiazoles as new potent and selective analogues. Bioorganic & medicinal chemistry letters. 2003;13:471–474. doi: 10.1016/s0960-894x(02)00930-7. [DOI] [PubMed] [Google Scholar]
- 12.Paget CJ, Kisner K, Stone RL, DeLong DC. Heterocyclic substituted ureas. II. Immunosuppressive and antiviral activity of benzothiazole- and benzoxazoleureas. Journal of medicinal chemistry. 1969;12:1016–1018. doi: 10.1021/jm00306a011. [DOI] [PubMed] [Google Scholar]
- 13.Darwish ES, Fattah AMA, Attaby FA, Al-Shayea ON. Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety. Int J Mol Sci. 2014;15:1237–1254. doi: 10.3390/ijms15011237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai NC, Bhatt N, Somani H, Trivedi A. Synthesis, antimicrobial and cytotoxic activities of some novel thiazole clubbed 1,3,4-oxadiazoles. European Journal of Medicinal Chemistry. 2013;67:54–59. doi: 10.1016/j.ejmech.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadek B, Al-Tabakha MM, Fahelelbom KMS. Antimicrobial Prospect of Newly Synthesized 1,3-Thiazole Derivatives. Molecules. 2011;16:9386–9396. doi: 10.3390/molecules16119386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammad H, et al. Discovery and Characterization of Potent Thiazoles versus Methicillin- and Vancomycin-Resistant Staphylococcus aureus. Journal of medicinal chemistry. 2014 doi: 10.1021/jm401905m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayhoub AS, et al. An investigation of phenylthiazole antiflaviviral agents. Bioorganic & medicinal chemistry. 2011;19:3845–3854. doi: 10.1016/j.bmc.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically - Seventh Edition: Approved Standard M7-A7. Wayne, PA: USA; 2011. Clinical and Laboratory Standards Institute. [Google Scholar]
- 19.Messick CR, Rodvold KA, Pendland SL. Modified time-kill assay against multidrug-resistant Enterococcus faecium with novel antimicrobial combinations. J Antimicrob Chemoth. 1999;44:831–834. doi: 10.1093/jac/44.6.831. [DOI] [PubMed] [Google Scholar]
- 20.Baldoni D, Haschke M, Rajacic Z, Zimmerli W, Trampuz A. Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection. Antimicrobial agents and chemotherapy. 2009;53:1142–1148. doi: 10.1128/AAC.00775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai K, et al. In vitro selection of resistance to clinafloxacin, ciprofloxacin, and trovafloxacin in Streptococcus pneumoniae. Antimicrobial agents and chemotherapy. 2000;44:2740–2746. doi: 10.1128/aac.44.10.2740-2746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orhan G, Bayram A, Zer Y, Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. Journal of clinical microbiology. 2005;43:140–143. doi: 10.1128/JCM.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furlani RE, Yeagley AA, Melander C. A flexible approach to 1,4-disubstituted 2-aminoimidazoles that inhibit and disperse biofilms and potentiate the effects of B-lactams against multi-drug resistant bacteria. European Journal of Medicinal Chemistry. 2013;62:59–70. doi: 10.1016/j.ejmech.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 24.O'Toole GA. Microtiter dish biofilm formation assay. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed MF, Hamed MI, Panitch A, Seleem MN. Targeting Methicillin-Resistant Staphylococcus aureus with Short Salt-Resistant Synthetic Peptides. Antimicrobial agents and chemotherapy. 2014;58:4113–4122. doi: 10.1128/AAC.02578-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS approved standard M7-A9. NCCLS, Wayne, Pa. 2012 [Google Scholar]
- 27.Alder J, Eisenstein B. The Advantage of Bactericidal Drugs in the Treatment of Infection. Current infectious disease reports. 2004;6:251–253. doi: 10.1007/s11908-004-0042-1. [DOI] [PubMed] [Google Scholar]
- 28.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 29.McDougal LK, et al. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. Journal of clinical microbiology. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49:1072–1079. doi: 10.1086/605572. [DOI] [PubMed] [Google Scholar]
- 31.Finberg RW, et al. The importance of bactericidal drugs: future directions in infectious disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39:1314–1320. doi: 10.1086/425009. [DOI] [PubMed] [Google Scholar]
- 32.Kosowska-Shick K, et al. Activity of telavancin against staphylococci and enterococci determined by MIC and resistance selection studies. Antimicrobial agents and chemotherapy. 2009;53:4217–4224. doi: 10.1128/AAC.00742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen HM, Graber CJ. Limitations of antibiotic options for invasive infections caused by methicillin-resistant Staphylococcus aureus: is combination therapy the answer? J Antimicrob Chemoth. 2010;65:24–36. doi: 10.1093/jac/dkp377. [DOI] [PubMed] [Google Scholar]
- 34.Dumitrescu O, et al. Effect of antibiotics, alone and in combination, on Panton-Valentine leukocidin production by a Staphylococcus aureus reference strain. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2008;14:384–388. doi: 10.1111/j.1469-0691.2007.01947.x. [DOI] [PubMed] [Google Scholar]
- 35.Stevens DL, et al. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. The Journal of infectious diseases. 2007;195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 36.Leonidou L, Gogos CA. Catheter-related bloodstream infections: catheter management according to pathogen. International journal of antimicrobial agents. 2010;36:S26–S32. doi: 10.1016/j.ijantimicag.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Vuong C, Otto M. Staphylococcus epidermidis infections. Microbes and infection / Institut Pasteur. 2002;4:481–489. doi: 10.1016/s1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 38.More PG, Karale NN, Lawand AS, Narang N, Patil RH. Synthesis and anti-biofilm activity of thiazole Schiff bases. Med Chem Res. 2014;23:790–799. [Google Scholar]
- 39.Rane RA, Sahu NU, Shah CP. Synthesis and antibiofilm activity of marine natural product-based 4-thiazolidinones derivatives. Bioorganic & medicinal chemistry letters. 2012;22:7131–7134. doi: 10.1016/j.bmcl.2012.09.073. [DOI] [PubMed] [Google Scholar]
- 40.Kwong AD, Kauffman RS, Hurter P, Mueller P. Discovery and development of telaprevir: an NS3-4A protease inhibitor for treating genotype 1 chronic hepatitis C virus. Nature biotechnology. 2011;29:993–1003. doi: 10.1038/nbt.2020. [DOI] [PubMed] [Google Scholar]
- 41.Allegrini PB. Enrico;. Process for the preparation of a viral protease inhibitor in amorphous form. In: SRL DF, editor. 2013. [Google Scholar]