Abstract
Background:
Hemorrhages, cerebrospinal fluid (CSF) fistula and infections are the most challenging postoperative complications in Neurosurgery. In this study, we report our preliminary results using a fully autologous fibrin sealant agent, the Vivostat® system, in achieving hemostasis and CSF leakage repair during cranio-cerebral procedures.
Methods:
From January 2012 to March 2014, 77 patients were studied prospectively and data were collected and analyzed. Autologous fibrin sealant, taken from patient's blood, was prepared with the Vivostat® system and applied on the resection bed or above the dura mater to achieve hemostasis and dural sealing. The surgical technique, time to bleeding control and associated complications were recorded.
Results:
A total of 79 neurosurgical procedures have been performed on 77 patients. In the majority of cases (98%) the same autologous fibrin glue provided rapid hemostasis and dural sealing. No patient developed allergic reactions or systemic complications in association with its application. There were no cases of cerebral hematoma, swelling, infection, or epileptic seizures after surgery whether in the immediate or in late period follow-up.
Conclusions:
In this preliminary study, the easy and direct application of autologous fibrin sealant agent helped in controlling cerebral bleeding and in providing prompt and efficient dural sealing with resolution of CSF leaks. Although the use of autologous fibrin glue seems to be safe, easy, and effective, further investigations are strongly recommended to quantify real advantages and potential limitations.
Keywords: Autologous fibrin glue, cerebral hemorrhage, cerebrospinal fistula, dura mater, skull base
INTRODUCTION
Hemorrhages with or without neurological signs, cerebrospinal fluid (CSF) fistula and infections are the most challenging postoperative complications in Neurosurgery. Fibrin sealant agents have been developed with the aim to provide efficient hemostasis and safe dural closure. In this study we report our preliminary results using the Vivostat® System (Vivostat A/S, Alleroed, Denmark) in achieving hemostasis and CSF leakage repair during cranio-cerebral procedures. We describe and show the unique features of this autologous fibrin sealant, pioneered with stunning success in many surgical procedures known to be at high risk of peri- and postoperative bleeding (i.e. nephrectomies, pulmonary lobectomies, ballistic injuries, arthroplasties, coronary bypass grafting, etc.), but still not exploited at its best in the field of Neurosurgery.[1,2,3,7,8,11,12,14,15,18,19,20,22,25,26,30]
MATERIALS AND METHODS
Patients’ population
Upon approval of the local Institutional Review Board, between January 2012 and March 2014 we performed 79 neurosurgical procedures in 77 patients testing the capability of the autologous fibrin sealant in achieving hemostasis and dural sealing in different kinds of cranial surgical scenario. Given the purposes of this study no inclusion criteria such as age, medical conditions, type and location of the pathology or symptomatology were considered, and patients were enrolled consecutively. Autologous fibrin sealant was prepared with the Vivostat® system and during all surgeries no other sealant or hemostatic agents were used in combination with the fibrin glue. Patients with major intracranial procedures, after surgery, were moved for 24 h intubated, under pharmacological coma, to the intensive care unit (ICU) in order to gain a continued monitoring of the hemodynamic parameters and to provide a controlled and graduated awakening. At the end of the surgical procedures, all patients underwent CT scan to verify potential hemorrhages in the surgical site. Within 3 days from the procedure, the majority of patients (70%) underwent brain magnetic resonance imaging (MRI) with and without the Gadolinium administration.
Cases description
The intracranial procedures included 60 supratentorial and 17 infratentorial lesions [Table 1]. A total of 57 craniotomies and of 20 craniectomies were performed. In detail, the Vivostat® was used in 52 tumors (21 glial tumors, 8 brain metastasis, 21 brain meningiomas, IV ventricle epidermoids), 19 vascular lesions (6 arterial aneurysms, trigeminal neuralgia, 7 intracerebral hemorrhages, 2 arteriovenous malformations), and in 6 miscellanea cases (4 primitive CSF fistula; 1 Arnold–Chiari I malformation, 1 frontal abscess, and 1 bone defect) [Table 1]. In 42.6% of cases (54), the autologous fibrin glue was used both as an hemostatic agent and a sealing agent (applied along the surgical site and above the artificial or proper dura); in 16.5% of cases (21) it was used only as sealing agent during the dural repair procedures and in 1.58% of cases (2), it was used only as hemostatic agent.
Table 1.
Patient data including sixty sovratentorial and seventeen infratentorial lesions
Vivostat® system administration
Vivostat® (Vivostat A/S, Alleroed, Denmark) is a system for on-site preparation of autologous fibrin sealant or platelet-rich fibrin (PRF®) The fully automated system prepares 5–6 ml of autologous fibrin sealant from 120 ml of the patients own blood in 23 min. In our study, the fibrin sealant was prepared directly from patient's own blood on the day scheduled for the surgical procedure. Whenever the autologous fibrin glue was used as hemostatic agent, it was sprayed uniformly in the resection bed, taking care to wait almost 30 s before washing out the blood clots formed afterwards around the surgical cavity. During this time, the autologous fibrin becomes a thin, dense, and white coat covering completely the cavity or the dura, avoiding the potential leakage of blood or CSF [Figure 1]. During the crucial phase of hemostasis, the application of autologous fibrin glue reduced the use of bipolar coagulation or diathermia, maintaining the integrity of the tissues. Whenever the Vivostat® system was used in the dural closure, the patient's dura was sutured (without always attempting a watertight fashion closure) and covered with an additional nonsuturable dural patch, then the autologous fibrin glue was uniformly sprayed above the dural repair and the bone flap was repositioned with the usual technique.
Figure 1.
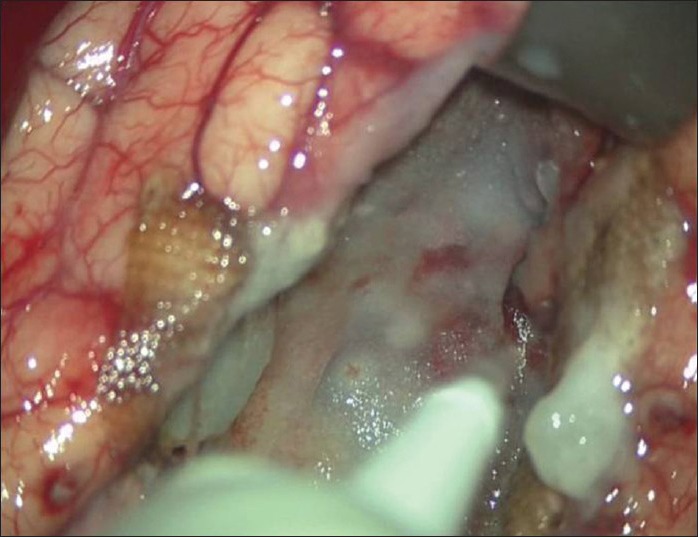
Intraoperative picture of the surgical cave covered by the Vivostat fibrin glue. In few seconds, it becomes a thin, dense, and white coat that covered completely the cavity, avoiding the potential leakage of blood or CSF
RESULTS
Technical considerations
All patients were monitored for a median follow-up of 18 months. Five patients died for reasons not related to the surgical procedures, whereas one patient died following surgical complications [Table 1].
The autologous fibrin glue was used mostly in oncological cases, specifically the most common procedure was the resection of fronto-temporal meningiomas, always with satisfactory results and, thanks to the elevated biocompatibility of this autologous fibrin glue and to its rapid degradation, no local parenchymal toxicity and no CSF obstruction have been documented [Figure 2] (case Nos. 6, 14, 18, 29, 41, 48, 49, 55, 70). This system proved to be effective even in cases of large vessels lacerations such as superior sagittal sinus (case Nos. 21, 47) where it favored a complete repair of the sinus laceration and preserved the venous drainage.
Figure 2.
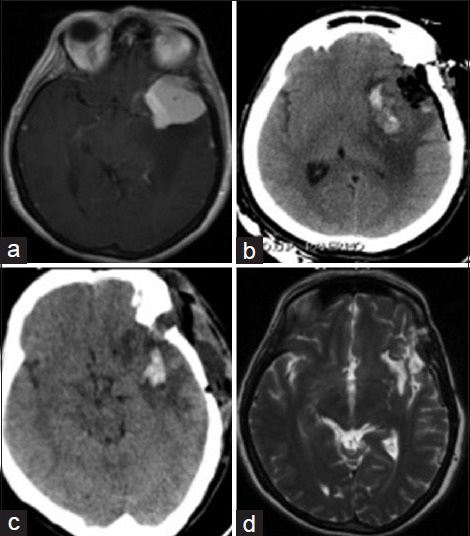
(a-d) Pre- and postoperative MRI images and postoperative CT scan. (a) Axial view, postcontrast: Left sphenoid wing meningioma surrounded by edema. (b) Immediate postoperative TC control: Hyperintense region corresponding to hemostatic materials applied. (c) One month postoperative CT control: A fluid extracranial subcutaneous collection is depicted. Thus, the surgical site was reopened; dural patch was applied and sealed with autologous fibrin glue. (d) Postoperative MRI control, axial view, T2: Eight months after the fistula repair, subcutaneous fluid was completely reabsorbed
In the entire series, we did not count any cases of local parenchymal toxicity, allergic reactions, infection, or systemic complications. The immediate hemostatic effect of the Vivostat® agent avoided symptomatic postoperative cerebral hemorrhages, moreover, even in all cases when the fibrin glue was applied along the surgical bed, the gentle and thin distribution of the product eliminated the potential risk of epileptic seizures. Unfortunately, in four cases where Vivostat® was applied with a hemostatic intent only, CSF fistulas occurred in the postoperative period. In two of those cases, we successfully used again the Vivostat® to achieve a surgical repair of the fistula during dural repair. In two other cases (case Nos. 14, 34) a bulge underneath the skin flap formed in the early postoperative days but it resolved with a conservative treatment, including compressive medications, syringes aspirations, and semi-sitting position. Definitely, considering that in two out four cases, the CSF collections resolved spontaneously, we had a 1.5% incidence of CSF fistula, which were managed with the same product.
In patients operated for skull base craniectomies, without application of artificial bone, the Vivostat® was able to achieve a durable tissues sealing without any difference with the other craniotomy cases. No differences were appreciated between the cases in whom artificial dura was placed above the proper dura previously closed in a watertight fashion, respect cases in whom artificial dura was instead just placed above the proper dura.
Economic advantages and limitations
The cost per kit needed for automated preparation of 6.5 ml of fibrin glue is around 700 USD. This price is slightly superior considering other industrial pharmaceutical nonautologous fibrin glue preparations. Autologous fibrin glue can also be produced in another way that is cheaper (10 dollars/10 ml of plasma): From pooled plasma (at least eight donors), precipitating fibrinogen by protamine sulfate. In the near future it will be interesting to compare the economic burden of these devices in relation to clinical outcome.
DISCUSSION
Postoperative hemorrhagic risk represents one of the most challenging complications during major intracranial procedures, and several studies have demonstrated the significant mortality and morbidity associated with this postoperative complication.[17,23,29] The definition of postoperative intracranial bleeding has been controversial. Indeed, it is common evidence finding a moderate amount of bleeding along the surgical site that is not always correlated to clinical neurological deterioration. Thus, a postoperative bleeding in the surgical site has to be considered as intracranial hemorrhage only when associated with potential neurological deterioration.[29] The overall risk of postoperative hemorrhage has been calculated to be around 0.8–1.1% (43–60% intraparenchymal hematoma, 28–33% epidural, 5–7% intrasellar, 8% mixed, 11% confined to the superficial wound).[27] The incidence of postoperative hemorrhage, however, changes in relation to the definition of hemorrhage; it lies between 0.8% and 6.9% if it relates to the occurrence of neurological alterations. Conversely, it is greatly superior, between 10.8% and 50% on the base of accidental neuroradiological findings.[27] Following neurosurgical procedures, another risky complication is represented by the occurrence of CSF fistulas that address the outcome and the survival of the patients.[4] Due to a loosing of the physical barrier, which may avoid the development of infection, the risk of morbidity or mortality, in the immediate postoperative time, is increased.[5] The incidence of cranial CSF fistula has been estimated around 2% or 3%. Nowadays, several fibrin sealant agents have been realized and they are largely used during several surgical procedures in order to limit potential complications.[3,5,10,21,28]
Vivostat® (Vivolution A/S, Alleroed, Denmark) is a system for on-site preparation of autologous fibrin sealant and PRF®.[3,6,31] It represents a valuable tool in many surgical procedures, and in our series it has demonstrated its efficacy not only in achieving dural closure, repair of dural defects, and hemostasis after tumor resections, but also to act as a safe and re-absorbable filler in “dead spaces”. Compared to conventional products, the Vivostat® presents unique features, and being patient derived, demonstrates excellent biocompatibility.[3,5,6,10,16,24,31,32] Indeed the autologous nature of Vivostat® efficiently eliminates the risks of bovine or human borne contaminants, protecting the patient against viral or prionic diseases. Vivostat® fibrin sealant can be applied at very close range allowing for pinpoint application, and the rapid polymerization ensures that the fibrin remains where it is applied.[16,32]
Nowadays, the Vivostat® system is widely used in several specialties with high rate of postoperative satisfaction.[8,11,12,13] To our knowledge, one case series has been published in the English literature on the use of Vivostat system in endoscopic trans nasal procedures for CSF leaks during ear–neck–throat (ENT) surgery with high success.[30] And recently a case describing the use of the Vivostat in a cranial procedure has been published.[9] Our study is the first prospective and observational analysis on the use of the Vivostat® system in Neurosurgery. Even though it is an observational study (a comparative study is ongoing in our Department), our preliminary results suggest the utility and convenience on the use of the Vivostat® system in terms of reduction of institutional costs and of postoperative better neurological outcome. In our clinical series, the Vivostat® was able to ensure immediate hemostasis of the surgical bed, reducing the use of bipolar coagulation and the application of other hemostatic agents; this aspect seems to us particularly important whenever the surgical site is close to eloquent areas or cortical tracts. Moreover, the safety demonstrated by Vivostat® in terms of epileptogenic activity suggests a possible niche of exploitation in all awake surgeries where the use of bipolar must be as limited as possible, but the risk of seizures is unfortunately high because of the intraoperative neurophysiological stimulation. Compared with the overall rate of CSF postoperative fistula, which it has been estimated of 2–3%, our study reported a reduction of CSF fistula incidence of 1.5%. In cases of profuse and unexpected bleeding or in the cases of continuous, slow bleeding, the fibrin sealant achieved a prompt support without the need to use other hemostatic agents. In the cases of skull base craniectomies, without application of artificial bone, Vivostat® was able to achieve a durable tissues sealing without noticeable differences from the craniotomy cases. No differences were also appreciated between the cases in whom the artificial dura was placed above dura closed by a continue stitches or those where the nonsuturable dural patch was applied above the patient's dura without attempting a watertight closure.
CONCLUSION
Vivostat® system is a fully autologous hemostatic and sealant agent. In our opinion, the advantages in the use of this product are based on four principle features:
The unique composition and mechanism of action which makes it able to adhere on the tissues forming a compact thin velum suddenly after its application;
The possibility to apply it on wet or dry tissues;
The capability to act independently of the hemostatic pattern of the patient;
The possibility to use it, in the same time, as hemostatic and as sealing agent.
Despite the limitations related to the design of observational studies such as the one conducted in our Department, the results achieved in our neurosurgical series are highly encouraging. Nonetheless, it is important to point out that even if this system presents unique features, which potentially may make it an efficacious substitute of other hemostatic and sealant agents, further studies are warranted to test its efficacy and convenience.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/1/77/156871
Disclaimer: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Contributor Information
Francesca Graziano, Email: franeurosurgery@libero.it.
Francesco Certo, Email: cicciocerto@yahoo.it.
Luigi Basile, Email: lbasile64@libero.it.
Rosario Maugeri, Email: rosario.maugeri77@gmail.com.
Giovanni Grasso, Email: giovanni.grasso@unipa.it.
Flavia Meccio, Email: flaviameccio@yahoo.it.
Mario Ganau, Email: marioganau@gmail.com.
Domenico G. Iacopino, Email: gerardo.iacopino@gmail.com.
REFERENCES
- 1.Antuña S, Barco R, Martínez Diez JM, Sánchez Márquez JM. Platelet-rich fibrin in arthroscopic repair of massive rotator cuff tears: A prospective randomized pilot clinical trial. Acta Orthop Belg. 2013;79:25–30. [PubMed] [Google Scholar]
- 2.Belboul A, Dernevik L, Aljassim O, Skrbic B, Radberg G, Roberts D. The effect of autologous fibrin sealant (Vivostat) on morbidity after pulmonary lobectomy: A prospective randomised, blinded study. Eur J Cardiothorac Surg. 2004;26:1187–91. doi: 10.1016/j.ejcts.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Belcher E, Dusmet M, Jordan S, Ladas G, Lim E, Goldstraw P. A prospective, randomized trial comparing BioGlue and Vivostat for the control of alveolar air leak. J Thorac Cardiovasc Surg. 2010;140:32–8. doi: 10.1016/j.jtcvs.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 4.Black P. Cerebrospinal fluid leaks following spinal or posterior fossa surgery: Use of fat grafts for prevention and repair. Neurosurg Focus. 2000;9:e4. doi: 10.3171/foc.2000.9.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Buchta C, Hedrich HC, Macher M, Hocker P, Redl H. Biochemical characterization of autologous fibrin sealants produced by CryoSeal and Vivostat in comparison to the homologous fibrin sealant product Tissucol/Tisseel. Biomaterials. 2005;26:6233–41. doi: 10.1016/j.biomaterials.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Dodd RA, Cornwell R, Holm NE, Garbarsch A, Hollingsbee DA. The Vivostat application system: A comparison with conventional fibrin sealant application systems. Technol Health Care. 2002;10:401–11. [PubMed] [Google Scholar]
- 7.Drake DB, Wong LG. Hemostatic effect of Vivostat patient-derived fibrin sealant on split-thickness skin graft donor sites. Ann Plast Surg. 2003;50:367–72. doi: 10.1097/01.SAP.0000041484.22953.6D. [DOI] [PubMed] [Google Scholar]
- 8.Gidaro S, Cindolo L, Lipsky K, Zigeuner R, Schips L. Efficacy and safety of the haemostasis achieved by Vivostat system during laparoscopic partial nephrectomy. Arch Ital Urol Androl. 2009;81:223–7. [PubMed] [Google Scholar]
- 9.Giugno A, Maugeri R, D’Arpa S, Visocchi M, Iacopino DG. Complex reconstructive surgery following removal of extra-intracranial meningiomas, including the use of autologous fibrin glue and a pedicled muscle flap. Interdiscipl Neurosurg. 2014;1:84–7. [Google Scholar]
- 10.Hanks JB, Kjaergard HK, Hollingsbee DA. A comparison of the haemostatic effect of Vivostat patient-derived fibrin sealant with oxidised cellulose (Surgicel) in multiple surgical procedures. Eur Surg Res. 2003;35:439–44. doi: 10.1159/000072229. [DOI] [PubMed] [Google Scholar]
- 11.Hevia M, Abascal-Junquera JM, Sacristán R, Suárez J, Lobo B, Méndez S, et al. Haemostasis control during laparoscopic partial nephrectomy without parenchymal renorrhaphy: The VIVOSTAT(®) experience. Actas Urol Esp. 2013;37:47–53. doi: 10.1016/j.acuro.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Holcomb J, MacPhee M, Hetz S, Harris R, Pusateri A, Hess J. Efficacy of a dry fibrin sealant dressing for hemorrhage control after ballistic injury. Arch Surg. 1998;133:32–5. doi: 10.1001/archsurg.133.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Kjaergard HK, Pedersen JH, Krasnik M, Weis-Fogh US, Fleron H, Griffin HE. Prevention of air leakage by spraying vivostat fibrin sealant after lung resection in pigs. Chest. 2000;117:1124–7. doi: 10.1378/chest.117.4.1124. [DOI] [PubMed] [Google Scholar]
- 14.Kjaergard HK, Trumbull HR. Bleeding from the sternal marrow can be stopped using vivostat patient-derived fibrin sealant. Ann Thorac Surg. 2000;69:1173–5. doi: 10.1016/s0003-4975(99)01560-x. [DOI] [PubMed] [Google Scholar]
- 15.Kjaergard HK, Trumbull HR. Vivostat system autologous fibrin sealant: Preliminary study in elective coronary bypass grafting. Ann Thorac Surg. 1998;66:482–6. doi: 10.1016/s0003-4975(98)00470-6. [DOI] [PubMed] [Google Scholar]
- 16.Kjaergard HK, Velada JL, Pedersen JH, Fleron H, Hollingsbee DA. Comparative kinetics of polymerisation of three fibrin sealants and influence on timing of tissue adhesion. Thromb Res. 2000;98:221–8. doi: 10.1016/s0049-3848(99)00234-0. [DOI] [PubMed] [Google Scholar]
- 17.Landriel Ibanez FA, Hem S, Ajler P, Vecchi E, Ciraolo C, Baccanelli M, et al. A new classification of complications in neurosurgery. World Neurosurg. 2011;75:709–15. doi: 10.1016/j.wneu.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Lassen MR, Solgaard S, Kjersgaard AG, Olsen C, Lind B, Mittet K, et al. A pilot study of the effects of Vivostat patient-derived fibrin sealant in reducing blood loss in primary hip arthroplasty. Clin Appl Thromb Hemost. 2006;12:352–7. doi: 10.1177/1076029606291406. [DOI] [PubMed] [Google Scholar]
- 19.Lardinois D, Jung FJ, Opitz I, Rentsch K, Latkoczy C, Vuong V, et al. Intrapleural topical application of cisplatin with the surgical carrier Vivostat increases the local drug concentration in an immune-competent rat model with malignant pleuromesothelioma. J Thorac Cardiovasc Surg. 2006;131:697–703. doi: 10.1016/j.jtcvs.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Larson MJ, Bowersox JC, Lim RC, Jr, Hess JR. Efficacy of a fibrin hemostatic bandage in controlling hemorrhage from experimental arterial injuries. Arch Surg. 1995;130:420–2. doi: 10.1001/archsurg.1995.01430040082018. [DOI] [PubMed] [Google Scholar]
- 21.Ochsner MG. Fibrin solutions to control hemorrhage in the trauma patient. J Long Term Eff Med Implants. 1998;8:161–73. [PubMed] [Google Scholar]
- 22.Rousou JA. Use of fibrin sealants in cardiovascular surgery: A systematic review. J Card Surg. 2013;28:238–47. doi: 10.1111/jocs.12099. [DOI] [PubMed] [Google Scholar]
- 23.Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–55. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 24.Schexneider KI. Fibrin sealants in surgical or traumatic hemorrhage. Curr Opin Hematol. 2004;11:323–6. doi: 10.1097/01.moh.0000142104.21058.df. [DOI] [PubMed] [Google Scholar]
- 25.Schips L, Dalpiaz O, Cestari A, Lipsky K, Gidaro S, Zigeuner R, et al. Autologous fibrin glue using the Vivostat system for hemostasis in laparoscopic partial nephrectomy. Eur Urol. 2006;50:801–5. doi: 10.1016/j.eururo.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt SC, Langrehr JM. Autologous fibrin sealant (Vivostat) for mesh fixation in laparoscopic transabdominal preperitoneal hernia repair. Endoscopy. 2006;38:841–4. doi: 10.1055/s-2006-944609. [DOI] [PubMed] [Google Scholar]
- 27.Seifman MA, Lewis PM, Rosenfeld JV, Hwang PY. Postoperative intracranial haemorrhage: A review. Neurosurg Rev. 2011;34:393–407. doi: 10.1007/s10143-010-0304-3. [DOI] [PubMed] [Google Scholar]
- 28.Shorter CD, Connor DE, Jr, Thakur JD, Gardner G, Nanda A, Guthikonda B. Repair of middle fossa cerebrospinal fluid leaks using a novel combination of materials: Technical note. Neurosurg Focus. 2012;32:E8. doi: 10.3171/2012.4.FOCUS1258. [DOI] [PubMed] [Google Scholar]
- 29.Theodosopoulos PV, Ringer AJ, McPherson CM, Warnick RE, Kuntz C, 4th, Zuccarello M, et al. Measuring surgical outcomes in neurosurgery: Implementation, analysis, and auditing a prospective series of more than 5000 procedures. J Neurosurg. 2012;117:947–54. doi: 10.3171/2012.7.JNS111622. [DOI] [PubMed] [Google Scholar]
- 30.Tomazic PV, Edlinger S, Gellner V, Koele W, Gerstenberger C, Braun H, et al. Vivostat: An autologous fibrin sealant as useful adjunct in endoscopic transnasal CSF-leak repair. Eur Arch Otorhinolaryngol. 2014 Aug 8; doi: 10.1007/s00405-014-3230-0. [In Press] [DOI] [PubMed] [Google Scholar]
- 31.Velada JL, Hollingsbee DA. Physical characteristics of Vivostat patient- derived sealant. Implications for clinical use. Eur Surg Res. 2001;33:399–404. doi: 10.1159/000049737. [DOI] [PubMed] [Google Scholar]
- 32.Velada JL, Hollingsbee DA, Menzies AR, Cornwell R, Dodd RA. Reproducibility of the mechanical properties of Vivostat system patient-derived fibrin sealant. Biomaterials. 2002;23:2249–54. doi: 10.1016/s0142-9612(01)00359-3. [DOI] [PubMed] [Google Scholar]


