Abstract
Background:
Osmotherapy is the frequently used for the treatment of intracranial pressure. The purpose of the study was to compare the effect of equiosmolar solution of 3% hypertonic saline and 20% mannitol on brain relaxation in supratentorial tumor surgery.
Methods:
After institutional review board approval and written informed consent, 50 patients aged >18, Glasgow Coma Scale (GCS) >13 with ASA physical status 1, 2, and 3 scheduled to undergo craniotomy for supratentorial tumors were enrolled in this prospective, randomized study. Patients received 5 ml/kg of either 3% hypertonic saline (n = 25) or 20% mannitol (n = 25). Hemodynamic variables (heart rate [HR], SBP, DBP, MBP, and central venous pressure [CVP]), serum electrolytes, serum osmolality, urine output, and fluid balance were measured. The surgeon assessed the brain condition on four point scale (1 = perfectly relaxed, 2 = satisfactorily relaxed, 3 = firm brain, and 4 = bulging brain), who was blinded to study drug.
Results:
Brain relaxation was comparable in two groups and there was no significant difference (P = 0.633). The number of brain conditions classified as perfectly relaxed, satisfactorily relaxed, firm brain, and bulging brain in the HS group was 8, 13, 3, and 1, respectively, whereas it was 5, 17, 3, and 0, respectively, in the M group. There was no significant difference in hemodynamic variables between the two groups except CVP at 30 min (P = 048). Compared with mannitol, hypertonic saline caused increase in the serum osmolality at 120 min (P = 0.008) and in serum sodium at 120 min (P = 0.001). Urine output was higher with mannitol than hypertonic saline (P = 0.001).
Conclusion:
3% hypertonic saline and 20% mannitol are equally effective for brain relaxation in elective supratentorial tumor surgery and compared with mannitol, hypertonic saline was associated with less diuretic effect.
Keywords: Brain relaxation, hypertonic saline, mannitol
INTRODUCTION
One of the main goals of anesthesia during any neurosurgical procedure is to provide a relaxed or ‘slack’ brain that will allow retraction of the brain and thereby reduce brain retractor ischemia. Various adjuncts are used to relax the brain. Although practices vary, they are all directed at the components of the intracranial vault, namely the brain tissue volume, cerebrospinal fluid (CSF) volume and blood volume. Osmotherapy is frequently used to reduce brain tissue volume and intracranial pressure (ICP) during neurosurgical operation. Mannitol (20%) and hypertonic saline (HS) are most commonly used hyperosmolar solutions in the treatment of elevated ICP.[14,23]
Mannitol is recommended as a first-choice hyperosmotic agent for the treatment of increased ICP.[23] The hyperosmolarity of mannitol reduces ICP by withdrawing water from the brain parenchyma to the intravascular tissue with intact blood–brain barrier (BBB). It reduces blood viscosity and transiently increases cerebral blood flow leading to reflex cerebral vasoconstriction. In addition mannitol facilitates decrease in ICP by decreasing CSF volume by virtue of decreasing the rate of formation of CSF. The use of mannitol is frequently associated with serious adverse effects, such as intravascular volume depletion, hyponatremia, rebound ICP elevation, and renal failure.[20]
Initially, HS solutions were used in small volumes for resuscitation of hypovolemic trauma patients. Now HS solutions are increasingly used as an alternative to mannitol for reduction of ICP.[21] Brain trauma foundation guidelines recommend HS, when mannitol fails to reduce ICP.[15]
HS is used in various concentrations and with varying osmolar loads. HS reduces ICP by dehydration of brain tissue by creation of the osmotic gradient, decreasing blood viscosity leading to cerebral vasoconstriction and increasing plasma tonicity, which favors rapid absorption of CSF. Compared with mannitol, HS have additional beneficial effects like enhancement of cardiac output, mean arterial pressure, and reduction of extravascular lung volume, leading to improved gas exchange and improved partial pressure of oxygen in the blood.[1,3,7,11,13]
Clinical trials that compared the effects of HS and mannitol on ICP have suggested that HS is at least as effective, if not better, than mannitol in the treatment of increased ICP.[2,5,6,14,15,18] Few studies have shown that HS is better than mannitol for brain relaxation[4,7,9,10,23,24,25] and others have revealed that they are equally effective.[19] Moreover, these studies differ in the amount and osmolarity of mannitol or HS used, thus making it difficult to interpret the results. Hence this study was designed to compare the equiosmolar concentrations of mannitol (20%) and HS (3%) for brain relaxation in patients undergoing elective craniotomies for supratentorial tumors.
MATERIALS AND METHODS
After obtaining institutional ethical committee approval on patients posted for elective supratentorial craniotomies, written informed consent was obtained from the patients or relatives of the patients. Fifty patients were enrolled in this prospective, randomized study and they were divided into two groups of 25 each assigned to receive 5 ml/kg of either 20% mannitol or 3% HS. The surgeon was blinded to the agents under study. Patients with age group 18–65 years, with Glasgow coma scale (GCS) >13, and ASA physical status 1–3 were included in the study. Patients with the presence of raised ICP, electrolyte imbalance, with severe cardiac, respiratory, or renal disease were excluded from the study. Patients who are already on mannitol or HS treatment were also excluded from the study.
Procedure
All patients satisfying the inclusion criteria were recruited to the study, after approval by ethics committee and written informed consent from the patients or their relatives. Patients were randomly allocated into two groups – mannitol (M group) and HS (HS group) by a computer-generated randomization chart. In the operation room, peripheral intravenous line was secured and 0.9% normal saline was given for fluid management. The standard monitors including electrocardiogram (ECG), noninvasive blood pressure (NIBP), and pulse oximeter were attached and baseline heart rate (HR), NIBP, and SPO2 readings were recorded. After preoxygenation with 100% O2 for 3 min, general anesthesia was induced with 2 mcg/kg fentanyl hydrochloride and 2 mg/kg propofol. Muscle relaxation was achieved using 0.1 mg/kg vecuronium bromide. Postinduction radial artery was cannulated and central venous catheter was placed depending on the need of surgery. Intraoperative anesthesia was maintained using oxygen–nitrous oxide mixture (40:60) with 0.7–1 minimal alveolar concentration (MAC) of isoflurane. Opioids for analgesia and vecuronium bromide for muscle relaxation were repeated as needed. PaCO2 was maintained between 30 and 34 mmHg. HR and invasive arterial blood pressure were kept within ± 20% of the baseline values. Normal saline (0.9%) was used as maintenance fluid at 2 ml/kg/h with additional replacement for urine output, third space loss, and blood loss. At the time of scalp incision patients received 5 ml/kg of either 20% mannitol (1 g/kg, osmolarity = 1098 mOsm/l; M group) or 3% HS (osmolarity = 1024 mOsm/l; HS group) over a period of 15 min through central line for intraoperative brain relaxation.
Brain relaxation was assessed by the operating neurosurgeon, who was blinded to the study drug upon opening the dura on four point scale:
Perfectly relaxed – 1
Satisfactorily relaxed – 2
Firm brain – 3
Bulging brain – 4.
If the surgeon was not satisfied with the degree of brain relaxation on dural opening, an additional bolus dose of 3 ml/kg of same study drug was administered and hyperventilation to a PaCO2 of 25 – 30 mmHg was initiated to provide relaxation for surgical access.
Parameters studied
Hemodynamic variables, including (HR), nonNIBP/IBP, central venous pressure (CVP) were recorded before infusion (T0) and after administration of study drugs at 15 min (T15), 30 min (T30), 60 min (T60), 90 min (T90), and 120 min (T120) and at the end of the surgery
Serum electrolytes at T0, T60, T120 min
Serum osmolality at T0 and T120 min
Urine output at T60, T120, T180, T240, and T300 and T360 min.
Details of fluid input, blood loss, blood transfused and duration of anesthesia were noted. At the end of surgery patients were either extubated or shifted to neurosurgical intensive care unit for elective mechanical ventilation.
Statistical analysis
For power analysis calculation, we considered a difference of 1 point in brain relaxation score between the groups to be clinically significant. A power analysis based on 95% confidence interval with 90% power, the sample size of 25 in each group was sufficient. The total sample size required was 50 for 90% statistical power and 5% level of significance assuming 1 point difference of brain relaxation between two groups.
Statistical analysis was carried out using SPSS 19.0. Results on continuous measurements are presented as Mean ± SD and median was used for non-Gaussian distribution. Categorical measurements were presented in number and percentages. Student t-test (two tailed, independent) was used to find the significance of study parameters on a continuous scale between two groups on metric parameters. Differences between the mannitol and HS groups were analyzed using Chi-square/Fisher exact test (demographic variables), Mann–Whitney U test (brain relaxation scores). To compare the effects of drugs between the groups over time, one-way repeated ANOVA was used (hemodynamic variables). All statistical analysis was carried out at 5% level of significance. P < 0.05 was considered significant.
RESULTS
Fifty neurosurgical patients admitted at our institution between October 2010 and march 2013 were enrolled in the study after satisfying the inclusion and exclusion criteria, and were randomized into group HS and group M to receive either 3% HS (n = 25) or 20% mannitol (n = 25).
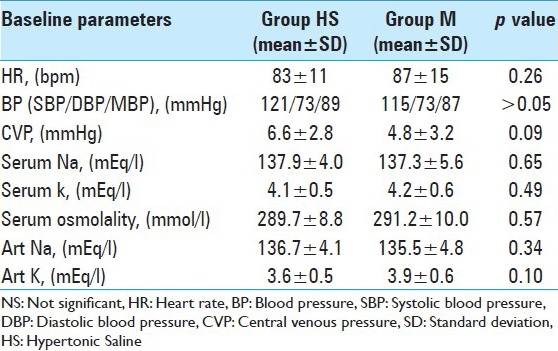
There was no significant difference between the groups in age, sex, weight, severity of illness, and brain pathology [Table 1] and the baseline hemodynamic and laboratory parameters were comparable in two groups [Table 2].
Table 1.
Patients demographic data
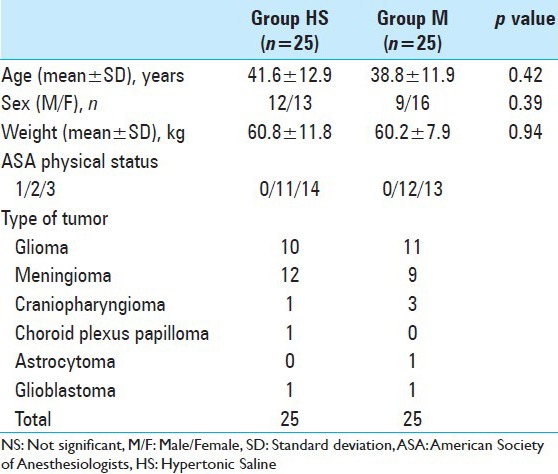
Table 2.
Baseline hemodynamic and laboratory parameters in two groups
INTRAOPERATIVE DATA
Hemodynamic parameters
There was no significant difference in the HR and mean blood pressure in two groups [Figures 1 and 2].
Figure 1.
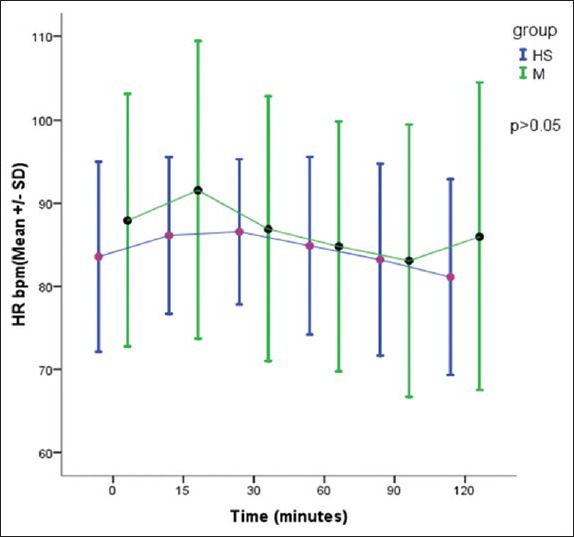
Comparison of heart rate in two groups, P > 0.05 – Not significant
Figure 2.
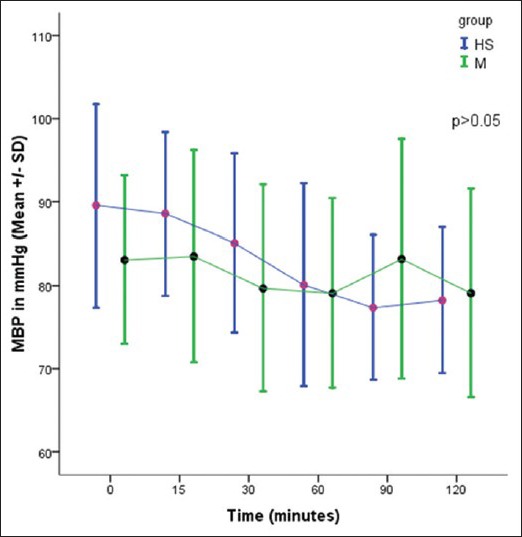
Comparison of mean blood pressure in two groups, P > 0.05 – Not significant
CVP was recorded in 19 patients in HS group and 18 patients in the mannitol group. There was a significant difference in CVP between two groups after 30 min of study drug infusion [Figure 3].
Figure 3.
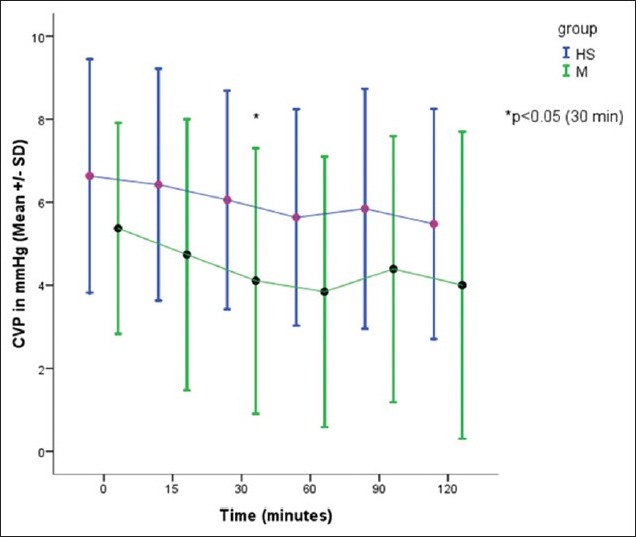
Comparison of CVP in two groups, *P< 0.05 – significant
Brain relaxation
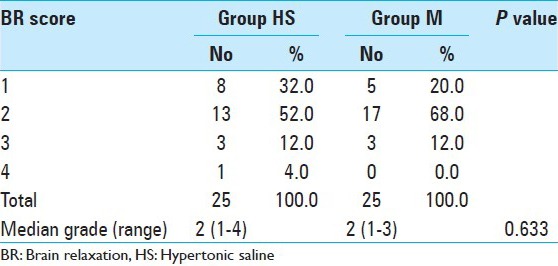
The number of conditions classified as perfectly relaxed, satisfactorily relaxed, firm brain, and bulging brain in the HS group was 8, 13, 3, and 1, respectively, whereas it was 5, 17, 3, and 0, respectively, in the M group. Brain relaxation was comparable in two groups and there was no significant difference (P = 0.633) [Table 3]. However, two patients in the HS group required the administration of additional study drug for brain relaxation (P = 0.49).
Table 3.
Comparison of brain relaxation score in two groups
Serum osmolality and electrolytes
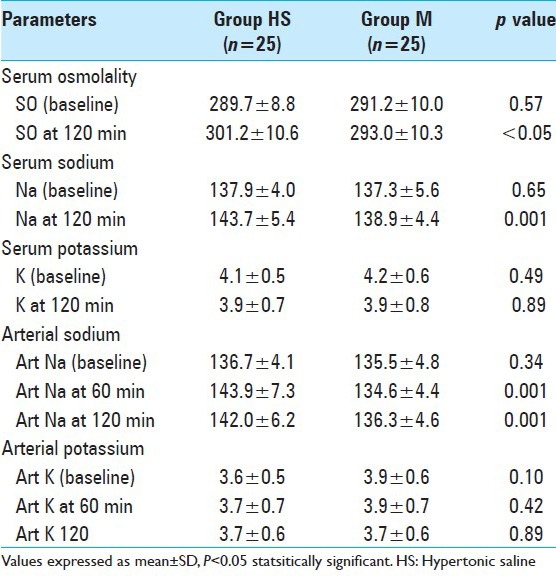
Compared with mannitol, HS caused increase in the serum osmolality at 120 min (P = 0.008) and in serum sodium at 120 min (P = 0.001) but there was no significant difference in serum potassium at 0 and 120 min in two groups. Compared with group M, there was significant increase in the arterial sodium at 60 and 120 min in the HS group but arterial potassium were comparable in two groups [Table 4].
Table 4.
Comparison of intraoperative laboratory parameters in two groups
Urine output
There was a significant increase in the urine output in the 1st, 2nd, 3rd, and 4th hour after the infusion in group M, when compared with the group HS. There was a significant increase in the total urine output in the mannitol group, when compared with HS group [Figure 4].
Figure 4.
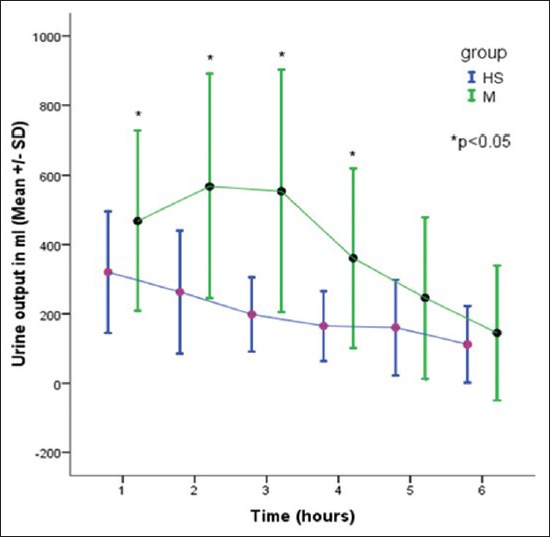
Comparison of urine output in two groups
Others
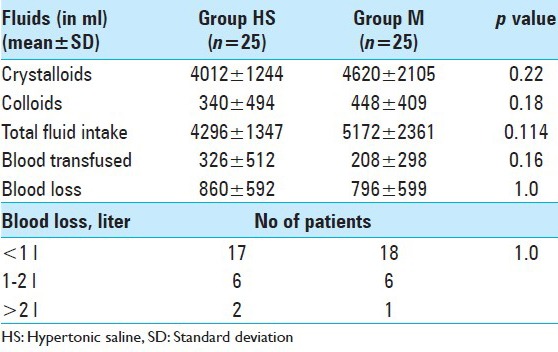
There was no significant difference in the fluid input, blood loss [Table 5]. The duration of surgery were comparable in both the groups (Group M 456 ± 126 min vs. Group HS 459 ± 151 min, P > 0.05).
Table 5.
Comparison of intraoperative fluids and blood loss in two groups
DISCUSSION
Our study compared the effects of equiosmolar boluses of 3% HS (group HS) and 20% mannitol (group M) on brain relaxation in patients with supratentorial tumor who were posted for elective craniotomies. Fifty patients (25 in each group) were randomly allocated to receive either mannitol or HS. We also compared the hemodynamic effect of these drugs in these patients.
At the end of infusion both HS and mannitol has created an osmolar gradient across the intact BBB, causing an increase in serum osmolality due to the impermeability of the BBB to both HS and mannitol, this leads to water absorption from the brain tissue parenchyma to intravascular tissue causing decreased water content and ICP, which has been shown in several animal[16,22] and human[12] studies in patients with traumatic brain injury and brain tumors, treated with either HS or mannitol. In our study, both mannitol and HS provided acceptable brain relaxation. HS provided perfect and satisfactory brain relaxation in 8 patients (32%) and 13 patients (52%), respectively, whereas mannitol in 5 patients (20%) and 17 patients (68%), respectively, (P > 0.05). The physical effects of HS and mannitol on the brain relaxation have been investigated previously by several researchers. In a similar study by Rozet et al.,[14] comparing equiosmolar solutions of HS and mannitol on intraoperative brain relaxation found no difference in the brain relaxation score in two groups. Gemma et al.,[6] and Di vivo et al.,[4] reported satisfactory brain relaxation in equal volumes of nonequiosmolar solutions of 7.5% HS and 20% mannitol. Wu et al.,[23] reported that HS provided better brain condition as compared with mannitol at equiosmolar doses (0.02). They have classified the brain relaxation on 3 point scale of 1 - soft, 2 – adequate, and 3 - tight as compared with our study, where we have used 4 point scale, which is more objective way of classifying brain relaxation.
All the patients in both the groups remained hemodynamically stable, with no significant changes in HR, systolic, diastolic, and mean blood pressure. This could be due to better anesthesia and fluid management to avoid fluctuations in blood pressure. We monitored CVP in 19 patients in HS group and 18 patients in the mannitol group. In the mannitol group, there was a gradual reduction in CVP with time as compared with HS group. At 30 min there was a significant reduction in CVP with mannitol as compared with HS (P < 0.05), coinciding with the onset of peak action of mannitol.
Being an osmotic diuretic, infusion of mannitol leads to significant diuresis. In our study, hourly urine output for the first 4 h was more in the mannitol group as compared with HS (P < 0.05), whereas during 5th and 6th hour of the surgery there was no difference in urine output between the groups, indicating fading action of mannitol. The total urine output was significantly more in the mannitol group as compared with HS group. These results are in concordance with earlier studies.[5,14,19,23]
We measured both serum and arterial sodium values. HS lead to increase in arterial sodium levels at 60 and 120 min as compared with mannitol (P < 0.05). At the end of 2 h, serum sodium was higher in HS group as compared with mannitol group.[4,5,8,14,17,23] The serum and arterial potassium were comparable at all time intervals. Rozet et al.,[14] found HS caused increased sodium levels but also a transient hypokalemia. Mannitol leads to a gradual increase in potassium levels. In our study we have measured the arterial and serum sodium at less frequent intervals as compared with Rozet et al.,[14] which might be responsible for missing arterial and serum potassium level changes.
CONCLUSIONS
Administration of equiosmolar concentrations of mannitol and HS provided acceptable brain relaxation. Mannitol, being an osmotic diuretic, resulted in a significant increase in urine output. HS resulted in a significant increase in serum sodium and osmolality compared with mannitol, without diuretic effect. HS can be routinely used in place of mannitol to achieve brain relaxation in elective supratentorial craniotomies.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/1/73/156771
Contributor Information
A. Raghava, Email: dr.raghava25@gmail.com.
Prasanna Udupi Bidkar, Email: drprasannabidkar@gmail.com.
M. V. S. Satya Prakash, Email: munuganuri1975@yahoo.co.in.
B. Hemavathy, Email: hemavathi.balachander@gmail.com.
REFERENCES
- 1.Bentsen G, Stubhaug A, Eide PK. Differential effects of osmotherapy on static and pulsatile intracranial pressure. Crit Care Med. 2008;36:2414–9. doi: 10.1097/CCM.0b013e318180fe04. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee N, Chaudhury A, Mukherjee S, Prusty GK, Chattopadhyay T, Ch SS. Efficacy of different hypertonic solutes in the treatment of refractory intracranial hypertension in severe head injury patients: A comparative study of 2ml/kg 7.5% hypertonic saline and 2ml/kg 20% mannitol. Indian J Neurotrauma. 2007;4:101–8. [Google Scholar]
- 3.Da Silva JC, de Lima F de MT, Valença MM, de Azevedo Filho HR. Hypertonic saline more efficacious than mannitol in lethal intracranial hypertension model. Neurol Res. 2010;32:139–43. doi: 10.1179/174313209X405119. [DOI] [PubMed] [Google Scholar]
- 4.De Vivo P, Del Gaudio A, Ciritella P, Puopolo M, Chiarotti F, Mastronardi E, et al. Hypertonic saline solution: A safe alternative to mannitol 18% in neurosurgery. Minerva Anestesiol. 2001;67:603–11. [PubMed] [Google Scholar]
- 5.Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36:795–800. doi: 10.1097/CCM.0B013E3181643B41. [DOI] [PubMed] [Google Scholar]
- 6.Gemma M, Cozzi S, Tommasino C, Mungo M, Calvi MR, Cipriani A, et al. 7.5% hypertonic saline versus 20% mannitol during elective neurosurgical supratentorial procedures. J Neurosurg Anesthesiol. 1997;9:329–34. doi: 10.1097/00008506-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients—a randomized clinical trial [ISRCTN62699180] Crit Care. 2005;9:R530–40. doi: 10.1186/cc3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SJ, Chang L, Han YY, Lee YC, Tu YK. Efficacy and safety of hypertonic saline solutions in the treatment of severe head injury. Surg Neurol. 2006;65:539–46. doi: 10.1016/j.surneu.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Kamel H, Navi BB, Nakagawa K, Hemphill JC, 3rd, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: A meta-analysis of randomized clinical trials. Crit Care Med. 2011;39:554–9. doi: 10.1097/CCM.0b013e318206b9be. [DOI] [PubMed] [Google Scholar]
- 10.Mirski AM, Denchev ID, Schnitzer SM, Hanley FD. Comparison between hypertonic saline and mannitol in the reduction of elevated intracranial pressure in a rodent model of acute cerebral injury. J Neurosurg Anesthesiol. 2000;12:334–44. doi: 10.1097/00008506-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Oddo M, Levine JM, Frangos S, Carrera E, Maloney-Wilensky E, Pascual JL, et al. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. J Neurol Neurosurg Psychiatr. 2009;80:916–20. doi: 10.1136/jnnp.2008.156596. [DOI] [PubMed] [Google Scholar]
- 12.Peterson B, Khanna S, Fisher B, Marshall L. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000;28:1136–43. doi: 10.1097/00003246-200004000-00037. [DOI] [PubMed] [Google Scholar]
- 13.Rockswold GL, Solid CA, Paredes-Andrade E, Rockswold SB, Jancik JT, Quickel RR. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery. 2009;65:1035–41. doi: 10.1227/01.NEU.0000359533.16214.04. [DOI] [PubMed] [Google Scholar]
- 14.Rozet I, Tontisirin N, Muangman S, Vavilala MS, Souter MJ, Lee LA, et al. Effect of equiosmolar solutions of mannitol versus hypertonic saline on intraoperative brain relaxation and electrolyte balance. Anesthesiology. 2007;107:697–704. doi: 10.1097/01.anes.0000286980.92759.94. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of hypertonic (10%) saline in patients with raised intracranial pressure after stroke. Stroke. 2002;33:136–40. doi: 10.1161/hs0102.100877. [DOI] [PubMed] [Google Scholar]
- 16.Shackford SR, Zhuang J, Schmoker J. Intravenous fluid tonicity: Effect on intracranial pressure, cerebral blood flow, and cerebral oxygen delivery in focal brain injury. J Neurosurg. 1992;76:91–8. doi: 10.3171/jns.1992.76.1.0091. [DOI] [PubMed] [Google Scholar]
- 17.Upadhyay P, Tripathi VN, Singh RP, Sachan D. Role of hypertonic saline and mannitol in the management of raised intracranial pressure in children: A randomized comparative study. J Pediatr Neurosci. 2010;5:18–21. doi: 10.4103/1817-1745.66673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vialet R, Albanèse J, Thomachot L, Antonini F, Bourgouin A, Alliez B, et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 2003;31:1683–7. doi: 10.1097/01.CCM.0000063268.91710.DF. [DOI] [PubMed] [Google Scholar]
- 19.Vilas Boas WW, Marques MB, Alves A. Hydroelectrolytic balance and cerebral relaxation with hypertonic isoncotic saline versus mannitol (20%) during elective neuroanesthesia. Rev Bras Anestesiol. 2011;61:456–68. doi: 10.1016/S0034-7094(11)70053-8. [DOI] [PubMed] [Google Scholar]
- 20.Wakai A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2007;1:CD001049. doi: 10.1002/14651858.CD001049.pub4. [DOI] [PubMed] [Google Scholar]
- 21.Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: A preliminary study. Neurosurgery. 2005;57:727–36. [PubMed] [Google Scholar]
- 22.Wisner DH, Schuster L, Quinn C. Hypertonic saline resuscitation of head injury: Effects on cerebral water content. J Trauma. 1990;30:75–8. doi: 10.1097/00005373-199001000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Wu CT, Chen LC, Kuo CP, Ju DT, Borel CO, Cherng CH, et al. A comparison of 3% hypertonic saline and mannitol for brain relaxation during elective supratentorial brain tumor surgery. Anesth Analg. 2010;110:903–7. doi: 10.1213/ANE.0b013e3181cb3f8b. [DOI] [PubMed] [Google Scholar]
- 24.Yildizdas D, Altunbasak S, Celik U, Herguner O. Hypertonic saline treatment in children with cerebral edema. Indian Pediatr. 2006;43:771–9. [PubMed] [Google Scholar]
- 25.Zeng HK, Wang QS, Deng YY, Jiang WQ, Fang M, Chen CB, et al. A comparative study on the efficacy of 10% hypertonic saline and equal volume of 20% mannitol in the treatment of experimentally induced cerebral edema in adult rats. BMC Neurosci. 2010;11:153. doi: 10.1186/1471-2202-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]


