Abstract
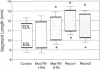
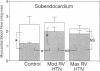
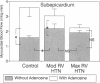
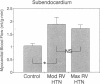
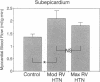
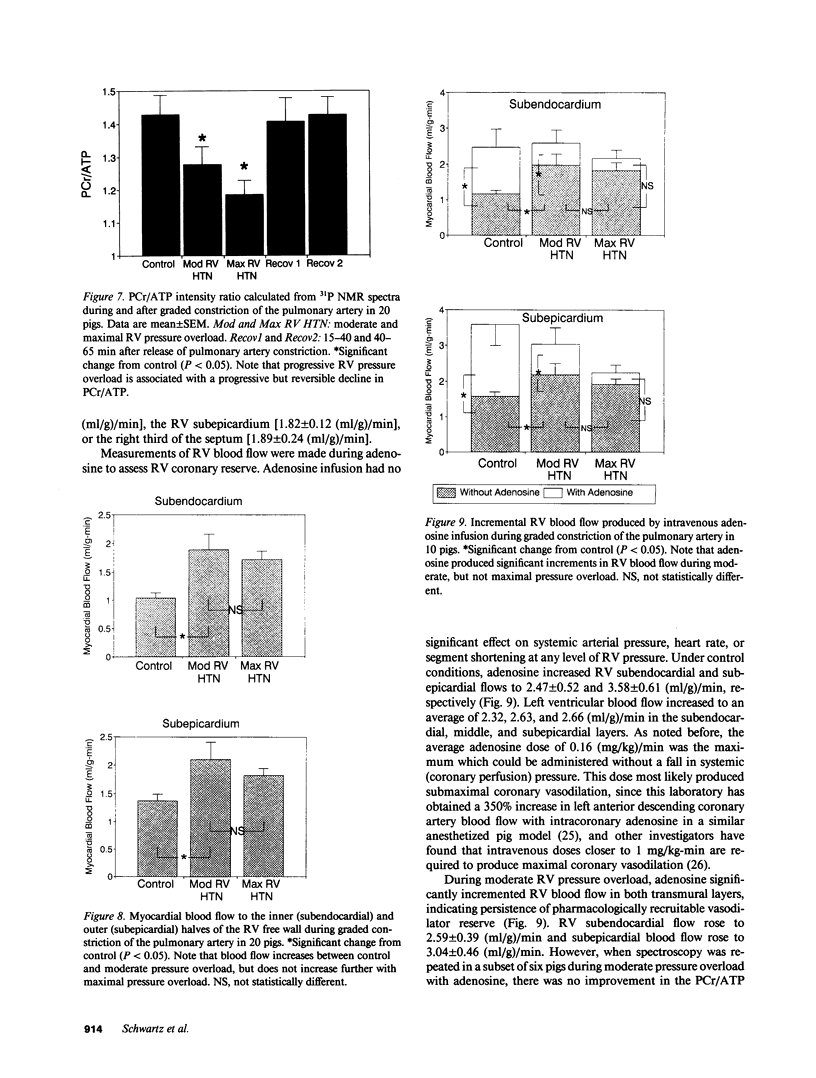
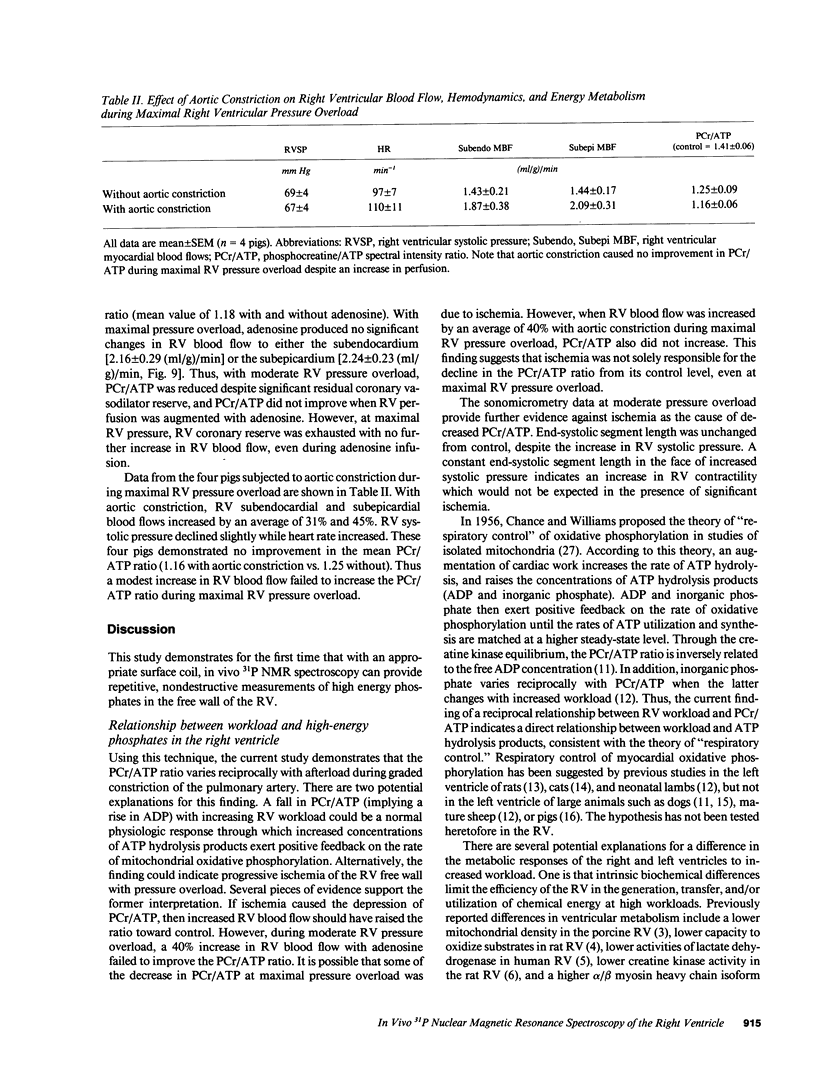
In vivo 31P nuclear magnetic resonance (NMR) spectroscopy of the right ventricular (RV) free wall was employed to determine (a) whether phosphorus energy metabolites vary reciprocally with workload in the RV and (b) the mechanisms that limit RV contractile function in acute pressure overload. In 20 open-chest pigs, phosphocreatine (PCr)/ATP ratio (an index of energy metabolism inversely related to free ADP concentration), myocardial blood flow (microspheres), and segment shortening (sonomicrometry, n = 14) were measured at control (RV systolic pressure 31 +/- 1 mm Hg), and with pulmonary artery constriction to produce moderate pressure overload (RV systolic pressure 45 +/- 1 mm Hg), and maximal pressure overload before overt RV failure and systemic hypotension (RV systolic pressure 60 +/- 1 mm Hg). With moderate pressure overload, PCr/ATP declined to 89% of control (P = 0.01), while contractile function increased. Adenosine (n = 10, mean dose 0.16 mg/kg-min) increased RV blood flow by an additional 41% without increasing PCr/ATP, indicating that coronary reserve was not depleted and that the decrease in PCr/ATP from control was not due to ischemia. With maximal pressure overload and incipient RV failure, PCr/ATP fell further to 81% of control and RV blood flow did not increase further, even with adenosine. Thus: (a) The decline in PCr/ATP with moderate RV pressure overload, without evident ischemia or contractile dysfunction, supports the positive regulation of oxidative phosphorylation by ATP hydrolysis products. (b) Depletion of RV coronary flow reserve accompanies the onset of RV failure at maximal pressure overload.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Austin R. E., Jr, Aldea G. S., Coggins D. L., Flynn A. E., Hoffman J. I. Profound spatial heterogeneity of coronary reserve. Discordance between patterns of resting and maximal myocardial blood flow. Circ Res. 1990 Aug;67(2):319–331. doi: 10.1161/01.res.67.2.319. [DOI] [PubMed] [Google Scholar]
- Baer R. W., Payne B. D., Verrier E. D., Vlahakes G. J., Molodowitch D., Uhlig P. N., Hoffman J. I. Increased number of myocardial blood flow measurements with radionuclide-labeled microspheres. Am J Physiol. 1984 Mar;246(3 Pt 2):H418–H434. doi: 10.1152/ajpheart.1984.246.3.H418. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Kantor H. L., Katz L. A., Briggs R. W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986 May 30;232(4754):1121–1123. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- Bittl J. A., Balschi J. A., Ingwall J. S. Effects of norepinephrine infusion on myocardial high-energy phosphate content and turnover in the living rat. J Clin Invest. 1987 Jun;79(6):1852–1859. doi: 10.1172/JCI113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvagnet P., Mairhofer H., Leger J. O., Puech P., Leger J. J. Distribution pattern of alpha and beta myosin in normal and diseased human ventricular myocardium. Basic Res Cardiol. 1989 Jan-Feb;84(1):91–102. doi: 10.1007/BF01907006. [DOI] [PubMed] [Google Scholar]
- Brooks W. W., Bing O. H., Blaustein A. S., Allen P. D. Comparison of contractile state and myosin isozymes of rat right and left ventricular myocardium. J Mol Cell Cardiol. 1987 May;19(5):433–440. doi: 10.1016/s0022-2828(87)80395-4. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Clark B. J., Maris J., Kent J., Nioka S., Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold F. L., Bache R. J. Transmural right ventricular blood flow during acute pulmonary artery hypertension in the sedated dog. Evidence for subendocardial ischemia despite residual vasodilator reserve. Circ Res. 1982 Aug;51(2):196–204. doi: 10.1161/01.res.51.2.196. [DOI] [PubMed] [Google Scholar]
- Gold F. L., Horwitz L. D., Bache R. J. Adrenergic coronary vasoconstriction in acute right ventricular hypertension. Cardiovasc Res. 1984 Jul;18(7):447–454. doi: 10.1093/cvr/18.7.447. [DOI] [PubMed] [Google Scholar]
- Kainulainen H., Komulainen J. Effects of training on regional substrate oxidation in the hearts of ageing rats. Gerontology. 1989;35(5-6):289–296. doi: 10.1159/000213039. [DOI] [PubMed] [Google Scholar]
- Kantor H. L., Briggs R. W., Balaban R. S. In vivo 31P nuclear magnetic resonance measurements in canine heart using a catheter-coil. Circ Res. 1984 Aug;55(2):261–266. doi: 10.1161/01.res.55.2.261. [DOI] [PubMed] [Google Scholar]
- Kusachi S., Nishiyama O., Yasuhara K., Saito D., Haraoka S., Nagashima H. Right and left ventricular oxygen metabolism in open-chest dogs. Am J Physiol. 1982 Nov;243(5):H761–H766. doi: 10.1152/ajpheart.1982.243.5.H761. [DOI] [PubMed] [Google Scholar]
- Ligeti L., Osbakken M. D., Clark B. J., Schnall M., Bolinger L., Subramanian H., Leigh J. S., Chance B. Cardiac transfer function relating energy metabolism to workload in different species as studied with 31P NMR. Magn Reson Med. 1987 Feb;4(2):112–119. doi: 10.1002/mrm.1910040203. [DOI] [PubMed] [Google Scholar]
- Lin L., Sylvén C., Sotonyi P., Somogyi E., Kaijser L., Jansson E. Lactate dehydrogenase and its isoenzyme activities in different parts of the normal human heart. Cardiovasc Res. 1989 Jul;23(7):601–606. doi: 10.1093/cvr/23.7.601. [DOI] [PubMed] [Google Scholar]
- Martin J. F., Guth B. D., Griffey R. H., Hoekenga D. E. Myocardial creatine kinase exchange rates and 31P NMR relaxation rates in intact pigs. Magn Reson Med. 1989 Jul;11(1):64–72. doi: 10.1002/mrm.1910110106. [DOI] [PubMed] [Google Scholar]
- Meier G. D., Bove A. A., Santamore W. P., Lynch P. R. Contractile function in canine right ventricle. Am J Physiol. 1980 Dec;239(6):H794–H804. doi: 10.1152/ajpheart.1980.239.6.H794. [DOI] [PubMed] [Google Scholar]
- Portman M. A., Heineman F. W., Balaban R. S. Developmental changes in the relation between phosphate metabolites and oxygen consumption in the sheep heart in vivo. J Clin Invest. 1989 Feb;83(2):456–464. doi: 10.1172/JCI113904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembert J. C., Boyd L. M., Watkinson W. P., Greenfield J. C., Jr Effect of adenosine on transmural myocardial blood flow distribution in the awake dog. Am J Physiol. 1980 Jul;239(1):H7–13. doi: 10.1152/ajpheart.1980.239.1.H7. [DOI] [PubMed] [Google Scholar]
- SARNOFF S. J., MITCHELL J. H. The regulation of the performance of the heart. Am J Med. 1961 May;30:747–771. doi: 10.1016/0002-9343(61)90211-x. [DOI] [PubMed] [Google Scholar]
- Schwartz G. G., Schaefer S., Meyerhoff D. J., Gober J., Fochler P., Massie B., Weiner M. W. Dynamic relation between myocardial contractility and energy metabolism during and following brief coronary occlusion in the pig. Circ Res. 1990 Aug;67(2):490–500. doi: 10.1161/01.res.67.2.490. [DOI] [PubMed] [Google Scholar]
- Singh S., White F. C., Bloor C. M. Myocardial morphometric characteristics in swine. Circ Res. 1981 Aug;49(2):434–441. doi: 10.1161/01.res.49.2.434. [DOI] [PubMed] [Google Scholar]
- Smith S. H., Kramer M. F., Reis I., Bishop S. P., Ingwall J. S. Regional changes in creatine kinase and myocyte size in hypertensive and nonhypertensive cardiac hypertrophy. Circ Res. 1990 Dec;67(6):1334–1344. doi: 10.1161/01.res.67.6.1334. [DOI] [PubMed] [Google Scholar]
- Theroux P., Franklin D., Ross J., Jr, Kemper W. S. Regional myocardial function during acute coronary artery occlusion and its modification by pharmacologic agents in the dog. Circ Res. 1974 Dec;35(6):896–908. doi: 10.1161/01.res.35.6.896. [DOI] [PubMed] [Google Scholar]
- Vlahakes G. J., Turley K., Hoffman J. I. The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation. 1981 Jan;63(1):87–95. doi: 10.1161/01.cir.63.1.87. [DOI] [PubMed] [Google Scholar]
- Vøllestad N. K., Sejersted O. M. Biochemical correlates of fatigue. A brief review. Eur J Appl Physiol Occup Physiol. 1988;57(3):336–347. doi: 10.1007/BF00635993. [DOI] [PubMed] [Google Scholar]