Abstract
Background and Aim:
The obstructive sleep apnea–hypopnea syndrome (OSAHS) is a common chronic respiratory disease, characterized by repetitive complete or partial collapse of the upper airway during sleep. The clinical spectrum extends between stoppage of breathing, snoring, daytime somnolence, and fatigue, to serious cardiovascular disease, stroke, metabolic syndrome, increased morbidity, and mortality. We aim to evaluate the short-term use of nasal continuous positive airway pressure (nCPAP) therapy for the clinical profile and exercise capacity of patients with OSAHS.
Patient Selection:
Twenty patients diagnosed with moderate-to-severe OSAHS were enrolled in the study (study group — 15; clinically and PSG-matched control group — 5).
Materials and Methods:
Each patient was clinically evaluated for sleep-related symptoms, and also assessed with spirometry, the six-minute walk test (6MWT), and a symptom-limited incremental cardiopulmonary exercise test (CPET). The study group patients were administered nCPAP therapy for eight hours each night for four weeks, while the control group patients were just observed. They were re-assessed after four weeks and the data were statistically analyzed between the two groups.
Results:
The study group patients showed a significant (P- < 0.05) improvement in the OSAHS symptoms—the Epworth sleepiness score, six-minute walk distance; duration of exercise, power output, peak oxygen uptake, anaerobic threshold, diastolic blood pressure, dyspnea, and fatigue—in comparison with the control group patients. The improvement in exercise capacity following nCPAP therapy was attributed to the relief of disabling the OSAHS symptoms and improved cardiovascular, ventilator, and musculoskeletal functions.
Conclusion:
All OSAHS patients must be treated with nCPAP.
KEY WORDS: CPAP, CPET, OSAHS, polysomnography, 6-MWT
INTRODUCTION
The obstructive sleep apnea-hypopnea syndrome (OSAHS) is a common chronic respiratory disease — next only to asthma and chronic obstructive pulmonary disease (COPD). Epidemiological surveys estimate that this syndrome affects 3 – 7% males and 2 – 5% females in the 30- to 60-year age group in western countries and Asia, including India.[1,2,3,4]
The hallmark of OSAHS is an anatomically small upper airway, which may result from craniofacial anomalies and/or a physiologically more collapsible pharyngeal airway. There occurs a repetitive, complete or partial collapse of the pharyngeal airway during sleep. Even as complete obstruction produces apnea, partial occlusion may produce only snoring and hypopnea. The event often terminates in the arousal of the patient with sudden awakening accompanied by an intense respiratory activity to re-establish ventilation through the obstructed airway and sympathetic nervous system overactivity.[5,6]
Repetitive apnea-hypopnea produces intermittent hypoxia and re-oxygenation, which promotes oxidative stress in the presence of increased production of reactive oxygen species (ROS). The latter promotes systemic inflammation and atherosclerosis, which may have significant adverse cardiovascular effects.[7,8]
The patient complains of loud snoring, stoppage of breathing, daytime somnolence, and excessive chronic fatigue. As the disease progresses the patient may develop a range of morbidities. The latter manifests as daytime hypoxemia, systemic hypertension,[9] myocardial ischemia,[10,11] stroke,[12] diabetes, metabolic syndrome,[13] increased cardiovascular morbidity, and mortality,[14] and higher risk of automobile and workplace accidents.[15]
Obstructive sleep apnea-hypopnea syndrome patients often suffer from pulmonary disability due to poor exercise capacity of multifactorial origin. These include cardiovascular abnormalities including pulmonary hypertension and cardiac failure; nocturnal/daytime hypoxemia and tissue hypoxia; fragmented sleep; excessive daytime sleepiness (EDS) causing muscle fatigue; obesity requiring high energy cost of breathing and a sensation of dyspnea; smoking-related COPD overlap; diabetic myopathy, and de-conditioning of the musculoskeletal system.
Treatment with nasal continuous positive airway pressure (n-CPAP) and BIPAP is known to be effective in treating OSAHS. It relieves sleep-related symptoms, improves cardiopulmonary indices and exercise endurance, and is also reported to reduce mortality.[16,17] Most workers acknowledge that four or more hours of CPAP use per night significantly improves the symptoms.[18] Data on the exercise characteristics of OSAHS patients is fairly limited in the published literature. Assessment of the cardiopulmonary responses to exercise may be relevant to predicting mortality from cardiovascular disease and understanding the physiological adaptation that occurs in response to hypoxia-re-oxygenation injury during sleep apnea.[19] To the best of our knowledge, a study on cardiopulmonary responses to exercise in OSAHS has not been reported from India.
The present study was undertaken in a group of patients diagnosed with moderate-to-severe OSAHS, to evaluate short-term nocturnal nCPAP therapy for clinical effects and cardiopulmonary responses to exercise.
MATERIALS AND METHODS
An outpatient-based prospective study in patients diagnosed with moderate and severe OSAHS was conducted between April 2010 and January 2012, in the Department of Respiratory, Sleep, Allergy, and Critical Care Medicine of the Metro Group of Hospitals, NOIDA. The study was approved by the Institutional Ethics Committee. The purpose and protocol of the study were discussed with each patient and his/her consent was also obtained in writing.
Patient selection and study protocol
During the period of study, a total of 96 adult patients, referred to our Sleep Laboratory for symptoms of loud snoring, daytime somnolence, and fatigue were clinically evaluated for OSAHS. Patients with thyroid disease, abnormal chest radiograph or with clinical evidence of neurological, hepatic, renal or peripheral vascular disease were excluded. Those with a history of alcohol or substance abuse, current smokers, and those already on CPAP treatment were also excluded.
Patients with an Epworth sleepiness score (ESS) of ≥10[20] were subjected to attend overnight polysomnography (PSG). Twenty consecutive patients, who met the criteria of moderate to severe OSAHS with an apnea-hypopnea index (AHI) of ≥15 per hour, were inducted into the present study. A verbal assurance of full cooperation for at least five weeks or till conclusion of the study was obtained from each of the twenty patients. All patients were advised nCPAP therapy for eight hours daily, without interruption, for four weeks. Fifteen patients who readily agreed to undergo nCPAP therapy constituted the ‘study group’. Five patients, who declined nCPAP therapy, but agreed to remain under observation for the next five weeks, were assigned to the ‘control group’.
Investigations
Overnight polysomnography
The patient slept overnight in the Sleep Laboratory to get familiarized with the environment of the actual sleep study to be performed the next night. Attended-PSG was performed with a 32-channel polysomnograph (Medcare Embla S7000 with Video) and the sleep data were scored as per international standards.[21,22] The video attachment helped in visualizing the patients at arousal. Electrodes for electroencephalogram (EEG-C4/A1, C3/A2), electro-oculogram (EOG), and sub-mental electromyography (EMG) were placed as per the international 10-20 system for sleep staging. Respiratory movements and nasal/oral airflow were recorded by inductance plethysmography and a thermocouple, respectively. Arterial oxygen saturation and pulse rate were also continuously recorded. The bilateral tibial EMG and electrocardiogram (EKG) were monitored from surface electrodes. The following measurements were made or derived from the overnight sleep data as per international practice.[22]
Sleep variables: Apnea-hypopnea index (AHI), respiratory disturbance index (RDI), oxygen desaturation events (ODE), arousal index (AI), and sleep efficacy
Sleep stages
Movement time, body movements, and movement arousals
Sleep stage data summary: Time on bed (TOB), total sleep time, wake-after-sleep-time, sleep onset latency, rapid eye movement (REM), sleep latency; and sleep efficacy index
Arousals
-
American Academy of Sleep Medicine (AASM) - Chicago criteria for scoring sleep related respiratory events:
- Apnea - defined as cessation of breathing for ten seconds or longer; types: (i) obstructive, (ii) central or (iii) mixed
- Hypopnea - defined as a clear decrease of >50% from baseline in the amplitude of a valid measure of breathing from an oronasal airflow, nasal pressure or thoracic-abdominal motion; or an associated >3% reduction of arterial oxygen saturation in the absence of a clear reduction of a valid measure of breathing.
Overnight fixed nasal continuous positive airway pressure titration
All study group patients underwent manual CPAP titration in the laboratory, using the Resmed machine and a comfortably fitting nasal mask, ensuring ≥90% arterial oxygen saturation, minimal leak, and AHI below five per hour. The median, highest, and optimal pressures were recorded.
Spirometry
A baseline spirometry test was performed on all patients with Quark PFT-4, as per the American Thoracic Society (ATS) guidelines.[23] The flow-volume loops were analyzed for FVC and FEV1 - before and after inhalation of 500 μg salbutamol aerosol.
Cardiac evaluation
A 12-lead EKG and 2-D echocardiography were performed on each patient. The echocardiograph was analyzed for wall-motion abnormality, left ventricular ejection fraction, systolic pulmonary hypertension, intracardiac shunts, and left ventricular (LV) systolic and diastolic dysfunction. Cardiac failure was diagnosed as per the European Society of Cardiologists criteria.[24]
Cadiopulmonary exercise test
Symptom-limited incremental CPET was performed according to the American Thoracic Society protocol,[25] on an elecronically braked bicycle ergometer (ERGOSELECT 200P/200K), with the seat adjusted, to avoid maximal extension of the knee joint during biking. The various exercise parameters (see RESULTS) were either measured directly or derived from the raw data.
The six-minute walk test was performed as per the ATS guidelines.[26]
Nocturnal continuous positive airway pressure therapy
All study group patients were administered nocturnal nCPAP therapy for at least eight hours each night for an uninterrupted period of four weeks. Treatment for concomitant health problems, if any, was continued as before without altering the dosing or frequency. Each patient was supplied with an nCPAP machine for home use after fully training him/her in its use, routine care, and maintenance. If faced with any technical problem the patient would contact the hospital maintenance staff for prompt redressal or rectification. Compliance to CPAP use was checked on the downloaded data from the machine. Patients in the control group, who were not treated with nCPAP, were simply kept under ‘routine observation’ for four weeks. At the end of four weeks all patients were again evaluated clinically and assessed with spirometry, 6MWT, ECG, echocardiography, and CPET.
Statistical analysis of data
Clinical and other investigational data were analyzed using SPSS 16, mainly to find the level of significance and the difference in various parameters before and after intervention in the study group and the initial and after four-week ‘just-observation’ in the control group patients. Similarly, between-group comparisons were also made.
RESULTS
The results of comparative statistical analysis of clinical, sleep, 6MWT, and CPET data between the study group and control group patients are presented below.
Anthropometric, clinical, spirometry, and sleep data
The findings of between-group analysis of demographic data - study group versus control group were: Gender - M/F (11/4 vs. 5/0); age (56.5 ± 9.1 vs. 57.8 ± 9.7 years - P = 0.783); height (161.8 ± 7.4 vs. 166.8 ± 2.8 cm - P = 0.162); weight (79.5 ± 9.0 vs. 91.6 ± 22.8 kg - P = 0.096); and body mass index (30.7 ± 5.2, range = 22.8 to 39.5 vs. 32.7 ± 7.4, range = 23.4 to 44.3 kg/m2, - P = 0.51). Fifty-three percent of the patients in the study group were obese (BMI >30) against 80% in the control group. Eight of the fifteen patients in the study group and three of the five patients in the control group were smokers.
Comorbidities
Seven (47%) of the study group and two (40%) of the control group patients had COPD; three (20%) of the study group and one (20%) of the control group patients suffered from mild hypertension; and seven (47%) patients of the study group and two (40%) patients of the control group had diabetes mellitus. They were all under satisfactory clinical control with treatment for their respective ailments.
Table 1 shows that the baseline Epworth sleepiness score (ESS), polysomnography data, and pre- and post-bronchodilator spirometry test values did not show any statistically significant difference in the two groups of patients.
Table 1.
Epworth sleepiness score (ESS), polysomnography, and spirometry test data: Study group compared with control group patients
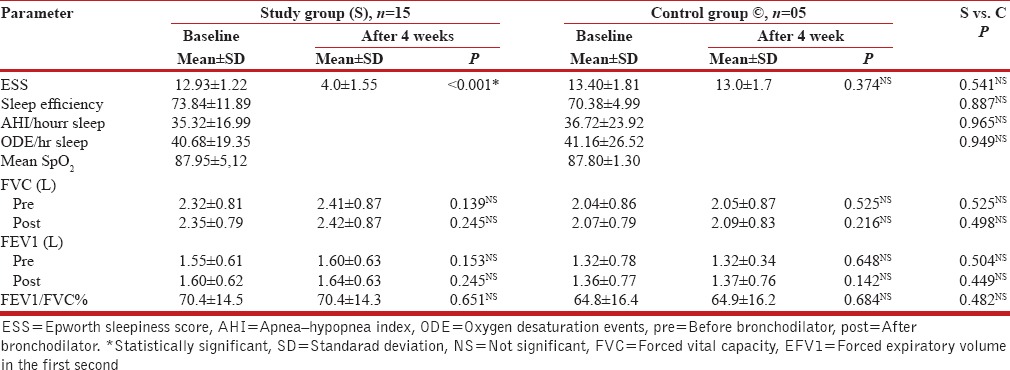
It can be concluded, therefore, that the two groups of patients were indeed matched and comparable for their clinical profile, associated comorbidities, sleep, PSG, and spirometry test values - the small number of patients in the control group notwithstanding.
Evaluation of nasal continuous positive airway pressure therapy
The Epworth sleepiness score showed statistically significant improvement after four-weeks of nCPAP therapy in the study group; the same was unchanged after four weeks in the control group patients, who were not treated with nCPAP [Table 1].
It can be seen from Table 2 that a four-week nasal CPAP therapy had produced a statistically significant relief of OSAHS symptoms in the study group, but not in the control group patients.
Table 2.
Effect of nCPAP on the severity of symptoms of OSAHS: Study group compared with control group patients
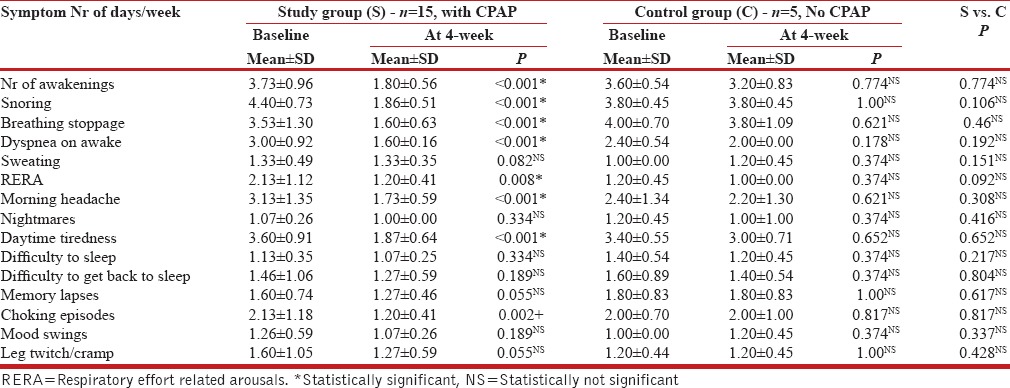
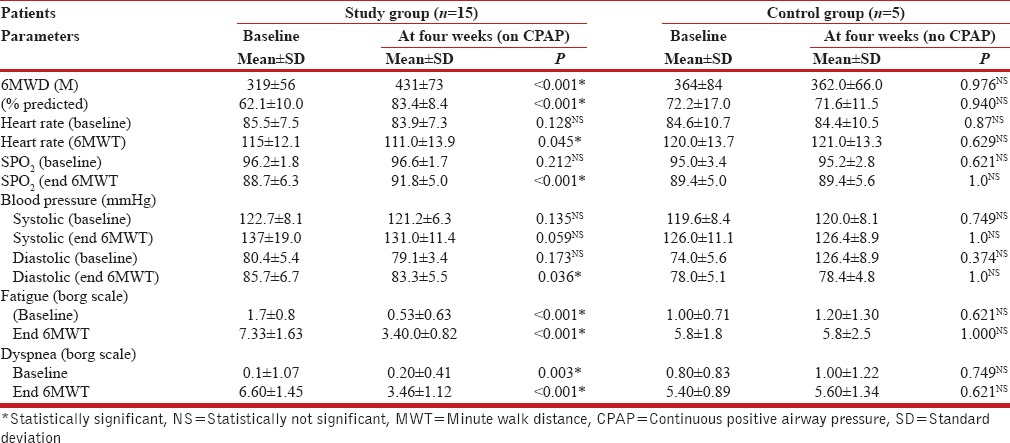
Table 3 shows that the nCPAP therapy produced statistically significant improvement (35%) in the 6MWD, as well as dyspnea and fatigue in the study group patients. No such beneficial effects were seen after four weeks of ‘just observation’ in the control group.
Table 3.
Six minute walk test (6MWT) parameters: Study group compared with control group patients
Cardiopulmonary exercise test
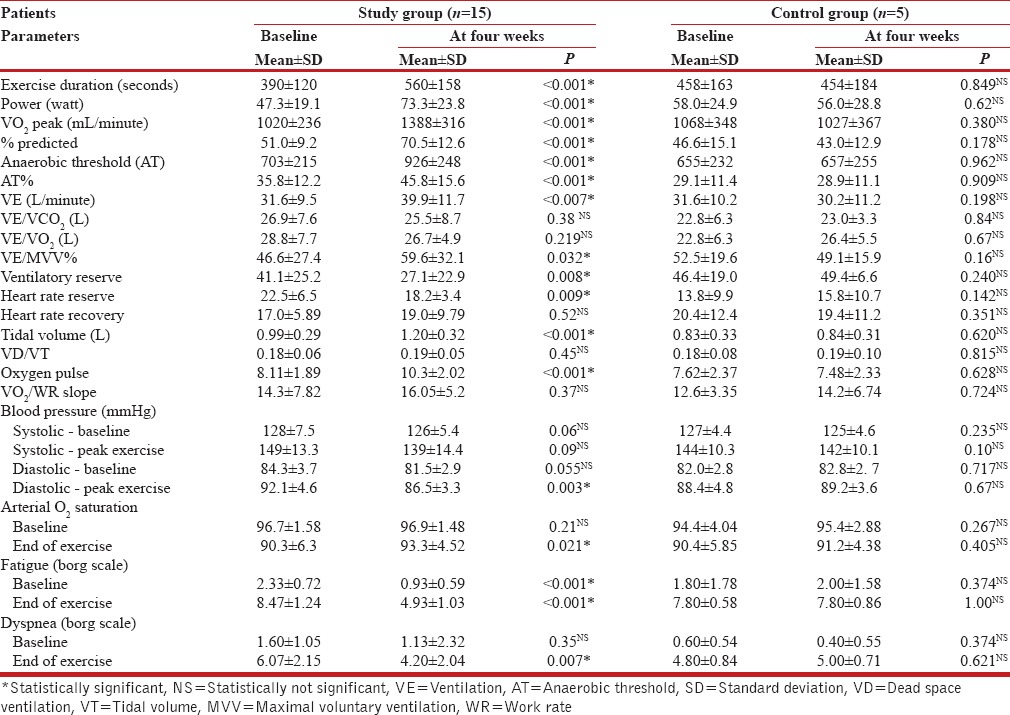
All patients in the study and control groups successfully completed the symptom-limited incremental exercise test at the baseline as well as after four weeks, without any suggestive evidence of cardiac ischemia, exaggerated by hypertensive response or dysrrhythmia. The CPET data presented in Table 4 shows that a four-week treatment with nCPAP produced statistically significant improvement in the exercise capacity of the study group patients. The duration of exercise and power output — before the limiting symptoms appeared — increased by approximately 44 and 36%, respectively. The peak oxygen uptake increased by 36% (from 51 to 71% predicted). Furthermore, the anaerobic threshold value increased; the symptoms of dyspnea and fatigue were relieved; and at the peak of exercise, the SpO2 and oxygen pulse showed significant improvement. A small, but statistically significant decrease in diastolic blood pressure was observed at the peak of exercise. The findings were suggestive of a significant improvement in the cardiovascular function with better muscle blood flow, pulmonary gas exchange, and energy production. No such beneficial effects were discernible in the control group patients.
Table 4.
Cardiopulmonary exercise test (CPET) parameters-baseline and after four weeks: Study group compared with control group patients
Mechanisms underlying the limitation of exercise capacity
The limitation of exercise capacity in OSAHS can be adequately explained by: (i) Limitation of ventilation (VEMAX /MVV X 100 >85% or a respiratory frequency that is >60 per minute); (ii) cardiovascular impairment (heart rate reserve <15 beats per minute or an oxygen pulse <80%); and deconditioning of the musculoskeletal system (AT is low and there is a shift in the HR/VO2 relationship to the left).
Table 5 and Figure 1 depict the role of impaired ventilatory, cardiovascular, and musculoskeletal systems, underlying the limitation of exercise in the study group compared to the control group patients. The mechanisms in the study group patients were assessed to be ventilatory in three; cardiovascular in thirteen; and deconditioning of the musculoskeletal system in three patients. Following nCPAP therapy, the corresponding numbers of patients were: Six, six, and three, respectively. The findings strongly suggested that post nCPAP there was a significant improvement in the function of the ventilatory, cardiovascular, and musculoskeletal systems. The corresponding figures in the control group patients at baseline/four-week were: Ventilatory (0/0); cardiovascular (5/5); and deconditioning of the musculoskeletal system (4/3) - indicating no change in the pattern of mechanisms underlying limitation of exercise.
Table 5.
Mechanisms underlying exercise limitation: Study group compared with control group patients
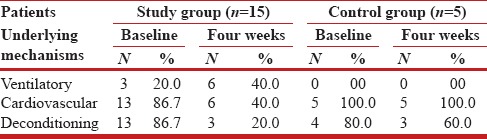
Figure 1.
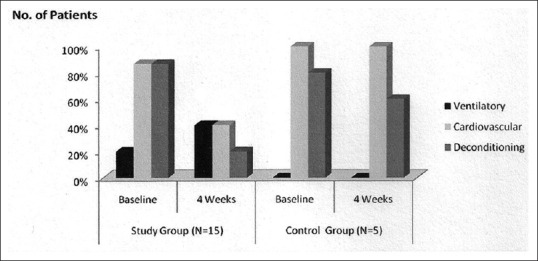
Mechanisms underlying exercise limitation: Study group compared with control group patients
DISCUSSION
The present study was apparently limited by the following factors: (i) It was not a double-blind, placebo-controlled study and allotment to each arm was not randomized. This could not have been done for ethical considerations. (ii) The sample size was inadequate, as the control group had only five patients compared to fifteen in the study group. There was a practical difficulty to enroll patients in the control group, as very few patients actually refused nCPAP therapy. (iii) Comorbidities such as COPD, hypertension, and diabetes were seen in significant numbers in both groups. It was difficult to get patients with pure OSAHS in a tertiary care hospital. A statistical analysis of the baseline data of patients in the two groups, however, provided convincing evidence that the two groups, for the purpose of the present study, were comparable, and the results of the nCPAP therapy were conclusive. For example, the control group patients were matched with the study group patients with no statistically significant difference in their physical characteristics, smoking habits, clinical profile, including comorbidities, baseline sleep, and PSG variables, and the indices of a 6MWT and CPET. Furthermore, the results of the nCPAP therapy in study group patients were statistically significantly superior to those of the control group patients.
Several factors may singly or in combination contribute to the limitation of exercise in OSAHS patients. These include: (i) Lack of physical fitness resulting from disturbed sleep, daytime somnolence, obesity, chronic tiredness, and body deconditioning on account of a reduced level of daily activity; (ii) obesity-related increased resistance to breathing and respiratory muscle dysfunction adding to dyspnea and daytime hypoxemia; (iii) there was also direct and indirect evidence to suggest that impaired cardiovascular function played a significant role in the impairment of exercise capacity.[9,10,11,14]
In a large Polish study of OSAHS (111 participants; age = 50.2 ± 10 years; BMI = 31.4 ± 4.6 kg/m2 ; AHI = 47.2 ± 23.1/hour), the impairment of exercise tolerance was directly correlated to the severity of OSA and exaggerated hemodynamic response to exercise (BP >220 mmHg in 20% patients) and slow post-exercise blood pressure recovery of blood pressure.[27] In the present study (15 participants, age = 56.5 ± 9.1 years; BMI 30.1 ± 4.6 kg/m2 ; AHI = 35.32 ± 16.99/hr), comparable hemodynamic responses to exercise, although smaller in magnitude, were observed. The quantitative difference in the responses may be explained by the fact that our patients had comparatively less severe OSAHS than that of the Polish study and they were also of a different ethnicity.
The results of the present study clearly demonstrated that even short-term use of the nCPAP therapy significantly relieved the disabling symptoms of OSAHS and improved the exercise capacity, when compared to the control group patients, who were not treated with it. The improvement of exercise capacity is possibly multifactorial in nature. Earlier studies reported that CPAP produced a significant reduction in the Epworth sleepiness score (ESS) in OSAHS patients after CPAP therapy, more so if the baseline ESS was >11.0,[28] The findings of this study are supportive of this observation, in that, ESS decreased from a baseline value of 12.93 ± 1.22 to 4.0 ± 1.55 after nCPAP therapy, while it remained high and unaffected in the control group patients.
Compliance to CPAP is the single most important determinant of the clinical outcome, but can be problematic. ‘What is good compliance’ is highly debatable, but most workers agree that four or more hours of nCPAP per night is adequate to relieve the symptoms.[18] In our study, the CPAP machine had an in-built mechanism to record the time duration for which the machine was actually used. Relief of OSAHS symptoms would directly improve the general well-being and also elevate the mood of the patient, which helped in increasing exercise tolerance.
The improvement in exercise capacity may be attributed to a decrease in the production of ROS (reactive oxygen species), IL-8, TNF-alpha, and IL-6, and the consequent improvement in the function of the cardiovascular, ventilatory, and musculoskeletal systems.[7,8,29] A small, but statistically significant decrease in diastolic ABP at the peak of 6MWT in our patients strongly suggested a decrease in peripheral vascular resistance and improved muscle blood flow during exercise. However, this finding needs to be confirmed in a larger study sample.
Going by the analogy of COPD patients in whom >70 m improvement in 6MWD after intervention is considered significant,[30] a mean increase of 112 m in 6MWD after nCPAP in our study definitely indicates a marked improvement in the exercise capacity.
The sensations of fatigue and perceived dyspnea (Borg scale) both at baseline and at the peak of 6MWT were significantly relieved after nCPAP therapy. It may be noted that echocardiography showed evidence of a diastolic dysfunction in 60% of the cases. After the nCPAP therapy, a decrease in heart rate and diastolic blood pressure, with improved muscle blood flow as a result of improved cardiac function, suggest a cardiac etiology for fatigue and perceived dyspnea.
At baseline, 80% of the patients in the study group and 40% of the patients in the control group showed a significant decrease (>4%) in arterial oxygen saturation, along with a fall in the oxygen pulse value, post exercise. After the CPAP therapy, a significant improvement was seen in both these parameters. This could be attributed to an improvement in the cardiac output and possibly improved gas exchange in a proportion of patients who had COPD.[31] At baseline, the cardiovascular function was limited in 87% of the patients, as reflected by the decreased heart rate reserve and low oxygen pulse, while after CPAP therapy only 40% of the patients showed residual cardiovascular limitation.
Significant improvement in the anaerobic threshold meant an increase in the aerobic capacity, and hence, exercise duration and mean maximum load (work done/power) sustained by the study group patients. Obstructive sleep apnea produced reduction of ventilatory and heart rate reserves. After CPAP therapy, there was a significant improvement in exercise ventilation, suggesting that the patients could achieve exercise VE close to the predicted maximal ventilation, with decreased dyspnea and fatigue.
It may be noted that most patients stopped exercise primarily due to leg fatigue or a subjective sensation of dyspnea, but with no ventilatory limitation such as significant ventilatory reserve was seen at the termination of the exercise. After CPAP therapy, many more patients could perform the test to the maximal ventilation capacity - secondary to improvement in the degree of fatigue after CPAP therapy. Prior to CPAP therapy 87% of the patients had deconditioning of the musculoskeletal system, as reflected by low AT and shift of the AT and HR/VO2 relationship to the left, while after CPAP therapy only 20% of patients showed deconditioning of the musculoskeletal system. The findings are supported by a significant improvement in AT and normalization of the HR/VO2 relationship. Additionally, there has been significant improvement in fatigue. These beneficial effects could either result from decreased peripheral vascular resistance and improved muscle blood flow or decreased sympathetic activity improving the glycogen synthetase activity, and thus, the intramyocytic glycogen store.
Another possible explanation of improvement in exercise capacity could be the nocturnal CPAP acting as a noninvasive ventilator support to patients with an overlap of COPD with OSAHS.
CONCLUSION
To conclude, short-term use of nCPAP therapy for four weeks in OSAHS executing the requisite compliance in our study has undoubtedly produced very encouraging results, although the long-term benefits still remain to be demonstrated. Overall, considerable improvement in the general well-being and exercise capacity of patients is likely to have a far-reaching impact, as improved daytime functioning with enhanced quality of life perceived by patients may not only be reflected in improved compliance in CPAP use, but may also lead to the long-term beneficial effects on the cardiometabolic profile of these patients. Hence, more rigorous, multicentered clinical trials should be planned to further elaborate on this aspect of OSAHS.
It may be of some interest to readers; to know that since the completion of this study; the definitions of apnea and hypopnea have been revised by the American Academy of Sleep Medicine.[32] “Apnea is scored when there is a drop in the peak signal excursion by more than or equal to 90% of the pre-event baseline using an oronasal thermal sensor for more than or equal to 10 seconds.”
“Hypopnea is scored when peak signal excursions drop by more than or equal to 10 seconds in association with either more than or equal to 3% arterial oxygen desaturation or an arousal”. The criteria of defining apnea/hypopnea used in the present study are perhaps slightly more stringent.[21] However, the results of the present study, in light of the revised definitions, remain unaffected.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lindberg E. Epidemiology of OSA. Eur Respir Mon. 2010;50:51–68. [Google Scholar]
- 2.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169:168–73. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 3.Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest. 2006;130:149–56. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 4.Reddy EV, Kadhiravan T, Mishra HK, Sreenivas V, Handa KK, Sinha S, et al. Prevalence and risk factors of obstructive sleep apnea among middle aged urban Indians: A community-based study. Sleep Med. 2009;10:913–8. doi: 10.1016/j.sleep.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Gastaut H, Tassinari C, Duran B. Etude polygraphique des manifestations episodiques (hypniques et respirtoires) diurnes et nocturnes du syndrome de Pickwick. Rev Neurol (Paris) 1965;112:568–79. [PubMed] [Google Scholar]
- 6.Bradley TD, Brown IG, Grossman RF, Zamel N, Martinez D, Phillipson EA, et al. Pharyngeal size in snorers, non-snorers, and patients with obstructive sleep apnea. N Eng J Med. 1986;315:1327–31. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 7.Lavie L. Obstructive sleep apnea syndrome: An oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 8.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 9.Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnea and sex: A population-based case-controlled study. Eur Respir J. 2006;27:564–70. doi: 10.1183/09031936.06.00042105. [DOI] [PubMed] [Google Scholar]
- 10.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: A 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 11.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnea: A long term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 12.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol (1985) 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 14.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnea-hypopnea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 15.Rodenstein D. Sleep apnea: Traffic and occupational accidents-individual risks, socioeconomic and legal implications. Respiration. 2009;78:241–8. doi: 10.1159/000222811. [DOI] [PubMed] [Google Scholar]
- 16.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157:858–65. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 17.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1998;94:9–14. [PubMed] [Google Scholar]
- 18.Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnea/hypopnea syndrome. Thorax. 1994;49:263–6. doi: 10.1136/thx.49.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savonen KP, Lakka TA, Laukkenen JA, Halonen PM, Rauramaa TH, Salonen JT, et al. Heart rate response during exercise test and cardiovascular mortality in middle-aged men. Eur Heart J. 2006;27:582–8. doi: 10.1093/eurheartj/ehi708. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Bibbs MB, Hirshkowitz M. Event scoring in polysomnography: Scoring arousals, respiratory events, and leg movements. Respir Care Clin N Am. 2005;11:709–30, ix. doi: 10.1016/j.rcc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardization of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Remme WJ, Swedberg K. Task Force for the Diagnosis and Treatment of Chronic Heart Failure, European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–60. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 25.American Thoracic Society; American College of Chest Physicians Statement. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 26.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 27.Przybyłowski T, Bielicki P, Kumor M, Hildebrand K, Maskey-Warzechowska M, Korczyñski P, et al. Exercise capacity in patients with obstructive sleep apnea syndrome. J Physiol Pharmacol. 2007;58(Suppl 5):563–74. [PubMed] [Google Scholar]
- 28.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airway pressure for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006:CD001106. doi: 10.1002/14651858.CD001106.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am j Respir Crit Care Med. 2000;162:566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 30.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: The six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–82. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 31.American Association for Respiratory Care: AARC clinical practice guidelines: Exercise testing for evaluation of hypoxemia and/or desaturation. 2001 revision and update. Respir Care. 2001;46:514–22. [Google Scholar]
- 32.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]


