Abstract
A 20-month-old boy presented with 1-year history of persistent fever, cough, and progressive abdominal distention. Abdominal ultrasonography showed hepatomegaly and multiple calcifications in the liver and spleen. Thoracic computed tomography showed multiple mediastinal lymph nodes and consolidation in both lungs. Additionally, there was a 2-cm thick retroperitoneal soft tissue mass destroying the T7-8 and L1-L2 vertebral bodies. The patient was preliminarily diagnosed with miliary tuberculosis (TB) and Pott's disease, and began administering anti-TB treatment consisting of isoniazid, rifampin, ethambutol, and pyrazinamide. Acid-resistant bacilli analysis and mycobacterial culture of the biopsy specimen of Pott's abscess were positive. Mycobacterial culture and PCR of gastric aspirate were also positive. The patient's condition progressively improved with anti-TB treatment and he received 12 months of antiTB therapy. At the end of the treatment all of the patient's symptoms were relieved and he was well except for kyphosis. Miliary TB complicated by Pott's abscess is a very rare presentation of childhood TB. The presented case shows that when Pott's abscess is diagnosed and surgically corrected without delay, patients can recover without squeal.
KEY WORDS: Infant, miliary disease, Pott's abscess, tuberculosis
INTRODUCTION
The World Health Organization (WHO) Global Tuberculosis (TB) Report published in 2012 showed that worldwide there were an estimated 8.7 million new cases of active tuberculosis (TB) and 1.4 million TB-related deaths in 2011. In developing countries 15% of the total burden of TB cases is children under the 15 years of age. It is reported that there were a total of 16.551 TB cases in Turkey in 2010, 5.5% of which were aged 0-14 years. Worldwide, there are 1.3 billion childhood TB cases and every year 400,000 children die due to TB.[1,2] Low socioeconomic level, poor nutrition, and overcrowded living conditions lead to the transmission and spread of disease. Although treatment of TB is very effective and inexpensive, difficulty accessing healthcare services, human immunodeficiency virus (HIV) co-infection, and diagnostic difficulty lead to delays in the diagnosis of TB and cause dissemination of TB. Young children higher risk for the severe form of TB than older children. Miliary TB is associated with high disability and mortality rates, particularly in cases of delayed diagnosis.[2,3] Miliary TB disease, which constitutes 1-3% of all TB cases, results from hematogeneous spread of Mycobacterium tuberculosis bacilli. Herein we present an older infant with miliary TB disease complicated by Pott's abscess.
CASE REPORT
A 20-month-old male presented with a 1-year history of persistent fever, cough, and progressive abdominal distention. His medical history showed that he had been diagnosed with pneumonia several times by a local physician, and was hospitalized five times and treated with intravenous antibiotics. Following each hospitalization and treatment his fever resolved, but cough persisted. His family history was not significant. He had a negative history of contact with active tuberculous and had not received the Bacillus Calmette-Guérin (BCG) vaccine.
The patient presented to our hospital upon referral for further evaluation of the underlying cause of recurrent pneumonia. Physical examination showed he was malnourished and pale; his weight was 8.2 kg (<5th percentile), height was 73 cm (<5th percentile), body temperature was 37°C, blood pressure was 100/60 mm Hg, and heart rate was 112 beats per min. There was no BCG scar. He was tachypneic at 56 breaths per minute and oxygen saturation was 89% on room air. His respiratory sounds were rough and anteroposterior diameter of the chest was increased. There was abdominal distention and mild hepatomegaly, without splenic enlargement. Musculoskeletal examination revealed moderate thoracic kyphosis. Initial laboratory findings were as follows: White blood cell count 22.000/μL with 26% lymphocytes and 74% polymorphonuclear leukocytes; hemoglobin 9.8 g/dL; platelet count 809.000/μL; the erythrocyte sedimentation rate 65 mm/h (normal: <20 mm/h), C-reactive protein 31.5 mg/L (normal: <2.9 mg/L). Serum electrolytes, renal and hepatic function test results were normal. Chest X-ray showed atelectasis and consolidation in the upper lobe of the right lung, and perihilar and pericardial infiltration in the left lung.
He was hospitalized and empirical intravenous cefepime was commenced for the non-specific treatment of pneumonia. Immunologic examinations were performed to determine the underlying cause of recurrent pneumonia. Serum quantitative immunoglobulin levels, the absolute neutrophil and lymphocyte counts, and the CH50 level were in normal range. The nitroblue tetrazolium (NBT) test result was 100%. Investigation of bone marrow smear showed no abnormalities. Thoracic computed tomography (CT) showed multiple paratracheal, precarinal, and right hilar lymph nodes, and consolidation in the middle and inferior lobes of the right lung and the inferior lobe of the left lung. Additionally, there was a 2-cm thick retroperitoneal soft tissue mass destroying the T7-8 and L1-L2 vertebral bodies, and infiltrating the spinal cord, pushing the aorta anteriorly. The mass extended from the level of bifurcation of the aorta to the bottom of the diaphragm and contained punctuate calcification. In addition to these findings, spinal MRI showed signal intensity changes in compatible with spinal cord edema, and an abscess extending to the medial fibers of the right and left psoas muscle at the L1-L3 level. Abdominal ultrasonography (USG) showed hepatomegaly and several calcifications in the liver, and widespread splenic calcifications. Tuberculin skin test (TST) was negative. Early morning gastric aspirate and urine were negative for acid-fast bacilli (AFB). We preliminarily diagnosed the patient with miliary TB and Pott's disease, and began administering antituberculous treatment consisting of isoniazid, rifampin, ethambutol, and pyrazinamide on day 11 of hospitalization.
Tru-Cut biopsy for Pott's abscess was performed on day 14 of hospitalization, and AFB analysis and mycobacterial culture of the biopsy specimen was positive, whereas PCRs of biopsy specimen were negative. Histopathological examination showed severe degeneration and necrosis of muscle fibers. Mycobacterial culture and PCR of gastric aspirate were also positive, and as such definitive diagnoses of miliary TB, tuberculous spondylitis, and tuberculous iliopsoas abscess were established. Screening of household contacts did not identify any cases of active TB. The patient's condition progressively improved; respiratory symptoms and findings resolved, and he began to gain weight during the following weeks. The patient was discharged after 28 day of anti-TB treatment. After 2 months of treatment, the anti-TB medication regimen was reduced to rifampin and isoniazid. After the 11th month of anti-TB treatment, spinal MRI findings were normal, except for angulation at the T9 level. Furthermore, thoracic CT was completely normal, but abdominal USG showed that hepatosplenic calcifications persisted. The patient completed 12 months of anti-TB therapy without any adverse effect. At the end of the anti-TB therapy all of the patient's symptoms were relieved and he was well except for kyphosis. The patient's family declined surgery to correct his kyphosis. Six years after completion of treatment, he had thoracic kyphosis, his height was under the 3 percentile. His throracic kyphosis deformity at 9 years old is demonstrated in Figure 1. Control MRI in sagittal T2-weighted images revealed kyphosis at the level of T 7-8 vertebra due to the collapsed vertebral body. The signal intensity is normal as it is in chronic stage. The L1-3 vertebrae are also collapsed. No soft tissue mass consistent with abscess is observed. The signal intensity of the spinal cord is normal [Figure 2].
Figure 1.
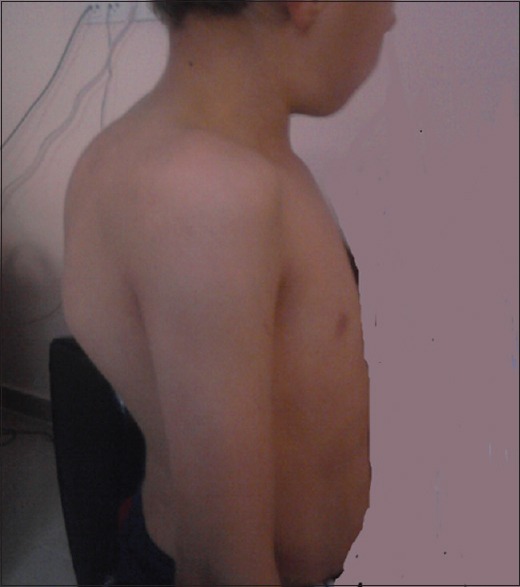
His thoracic kyphosis deformity at 9 years old
Figure 2.
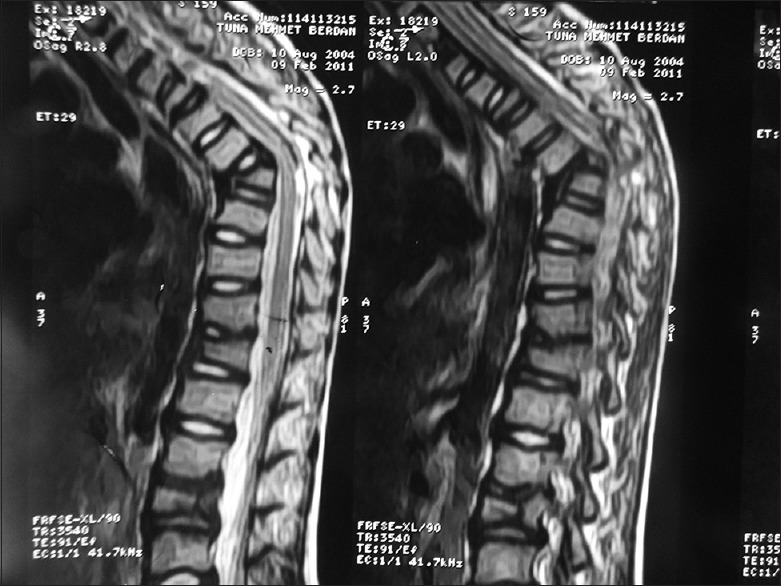
Control MRI of the patient showed kyphosis at the level of T 7-8 vertebra due to the collapsed vertebral body. The signal intensity is normal as it is in chronic stage. The L1-3 vertebrae are also collapsed
DISCUSSION
Miliary TB is a potentially fatal form of TB caused by widespread dissemination of M. tuberculosis bacilli. In total, 9-10% of pediatric TB cases are complicated by dissemination.[4] Miliary TB can be either a late manifestation of chronic disease or represent rapid spread during primary infection. Miliary TB usually complicates the primary infection, occurring within 2-6 months of initial infection. Although miliary TB is the most commonly seen in infants and young children, it also occurs in adolescents and older adults due to the breakdown of a previously healed primary pulmonary lesion. Because miliary TB occurs most commonly in infants, and malnourished or immunosuppressed patients, host immune incompetence most likely also plays a role in its pathogenesis.[5,6] Our patient had malnutrition and young age as risk factors of miliary disease. Miliary TB occurs when massive numbers of tubercle bacilli are released into the bloodstream, causing disease in two or more organs. It was reported that in the case of disseminated TB the lungs, liver, and spleen are the most commonly involved organs (80-100%), followed by the kidneys (60%) and bone morrow (25-75%). Bones and joints were reported in another study to be the second most frequently involved organ, following the lungs.[7] Any organ can be involved in disseminated TB; the presented patient had lung, liver, spleen, and vertebral involvement (the kidneys and bone morrow were spared).
A study performed in the USA analyzed the TST results for 64.238 persons with culture-confirmed TB from the National Tuberculosis Surveillance System of the Centers for Disease Control and Prevention (CDC) between 1 January 1993 and 31 December 2010. The analysis included anatomical site of disease, HIV status, and birthplace (US- or foreign-born). Years, male, non-Hispanic white, born in the USA, infected with HIV, and had an AFB-positive sputum smear at baseline. Persons with miliary disease, and combined pulmonary and extrapulmonary disease had high rates of TST negativity-36.7% and 22.9%, respectively. The researchers reported that persons with a TST ≥15 mm were less likely to have miliary, or combined pulmonary and extrapulmonary disease, but were more likely to have cavitary pulmonary disease than non-cavitary pulmonary disease. They suggested that the absence of BCG immunization might be associated with both disseminated disease and TST negativity.[8] Similarly, the presented patient hadn't received BCG vaccination, although BCG vaccine is a routine as a part of national immunization schedule in Turkey, and his TST result was negative.
Mycobacteria can cause osteoarticular infection via three routes: By direct inoculation following a traumatic injury or during surgical procedures, hematogeneous dissemination, spread from a contiguous focus. Spinal spondylitis is the most common manifestation of osteoarticular TB and 1-3% of patients with TB have skeletal involvement. The clinical symptoms of spinal TB in children are often insidious and include back pain, fever, paraparesis, sensory disturbance, and bowel and bladder dysfunction. TB vertebral osteomyelitis most commonly affects the thoracic or thoracolumbar segment, followed by the lumbar and, rarely, the cervical segment. Because thoracolumbar TB was a component of miliary TB in the presented case, we thought spinal TB was caused by hematogenous dissemination of mycobacteria.[5,9]
Computerized tomography of spine provides bony detail, whereas spinal MRI is used to evaluate involvement of soft tissue and abscess formation. MRI should be performed when spinal infection is suspected. A major advantage of MRI over CT is early detection of inflammatory bone morrow changes. In later stages MRI findings of TB spondylitis include posterior element involvement, spinal canal stenosis, spinal cord or nerve root compression, intervertebral disc enhancement, vertebral collapse, and kyphosis.[5,10] Although there are no findings specific to spinal TB, a decrease in signal intensity of the involved bone and soft tissues on T2-weighted images, and an increase in intensity of a uniform thin rim enhancement is a pathogenomic finding suggesting caseation necrosis or a cold abscess in TB.[11] In the presented case spinal cord edema and the boundaries of abscess were discerned more clearly using MRI in addition to CT.
The vertebral cortex is disrupted, and infection may spread to adjacent intervertebral discs and extend into the surrounding soft tissue, resulting in paravertebral, epidural, and ilio-psoas abscess formation. In long-standing cases there may be multiple levels of vertebral body collapse, resulting in Gibbus deformity. Compression of the spinal cord, the cauda equina, or other nerve roots can result in neurological deficits. TB spondylitis without spinal cord compression or neurological deficits can be treated with antituberculous chemotherapy; however, surgery is recommended for patients with more than 50% of vertebral body destruction, spinal cord compression or neurological compromise, persistent severe axial pain, more than 5° of kyphosis, unresponsiveness to medical treatment.[5,12] If surgical treatment of Pott's disease is delayed, severe kyphosis leading to respiratory system dysfunction, painful costopelvic compression, and paraplegia can occur.[11] About 3% of children with spinal TB develop severe kyphosis >60°. The risk factors for development of severe kyphotic deformity are as follows: Age <10 years, involvement of ≥3 vertebral bodies, and thoracic spine localization. Children with these risk factors should undergo surgical correction.[11,13] Although the presented patient had all three risk factors, his family declined surgical treatment and the patient eventually healed, but with kyphosis sequelae. Any surgical approach for TB spondylitis should consider the skeletal immaturity of young children. Iatrogenic short trunk, skeletal deformity, and pulmonary hypoplasia due to restricted growth of the rib cage could occur as a complication of surgery due to a large number of fused segments, especially in the thoracic spine.[13]
Although untreated disseminated TB was once uniformly fatal, with treatment the mortality rate is 29-64%, depending on the frequency of meningitis in reported series. For children with suspected disseminated TB recent WHO guidelines recommend a standard four-drug regimen of isoniazid (10 mg/kg; maximum dose: 300 mg/d), rifampicin (15 mg/kg; maximum dose: 600 mg/d), pyrazinamide (35 mg/kg), and ethambutol (20 mg/kg) during the first 2 months of therapy, followed by a two-drug regimen with isoniazid and rifampin. Additionally 10 months duration of continuation phase is recommended.[14] The presented patient recovered completely with 12-month treatment.
CONCLUSION
In conclusion, disseminated TB complicated by Pott's abscess is a very rare presentation of childhood TB. Pott's abscess is a leading cause of kyphosis in TB-endemic countries. The presented case shows that early diagnosis and surgical correction of Pott's abscess are important for avoiding sequelas.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.WHO Global Tuberculosis Report 2012. [Last accessed on 2014 October 20]. Available from: http://www.who.int/tb/publications/global_report/gtbr12_main.pdf .
- 2. [Last accessed on 2014 October 20]. Available from: http://tuberkuloz.thsk.saglik.gov.tr/Dosya/Dokumanlar/raporlar/turkiyede_verem_savasi_2012_raporu.pdf .
- 3.Starke JR, Jacobs RF. Mycobacterium tuberculosis. In: Long SS, Pickering LP, Prober CG, editors. Principles and Practice of Pediatric Infectious Diseases. Pennsylvania: Churchill Livingstone; 2008. pp. 771–88. [Google Scholar]
- 4.Pekcan S, Tana Aslan A, Kiper N, Uysal G, Gürkan F, Patıroğlu T, et al. Multicentric analysis of childhood tuberculosis in Turkey. Turk J Pediatr. 2013;55:121–9. [PubMed] [Google Scholar]
- 5.Dahab R, Barrett C, Dunn A, De Matas M, Pillay R. Unusual presentation of tuberculosis with multiple spinal deposits. Br J Neurosurg. 2012;26:925–6. doi: 10.3109/02688697.2012.697222. [DOI] [PubMed] [Google Scholar]
- 6.Wen LS, Noble JA. A case of a young girl with fever and seizure. Acad Emerg Med. 2011;18:e86–92. doi: 10.1111/j.1553-2712.2011.01193.x. [DOI] [PubMed] [Google Scholar]
- 7.Gunal S, Yang Z, Agarwal M, Koroglu M, Arıcı ZK, Durmaz R. Demographic and microbial characteristics of extrapulmonary tuberculosis cases diagnosed in Malatya, Turkey, 2001-2007. BMC Public Health. 2011;11:154. doi: 10.1186/1471-2458-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auld SC, Click ES, Heilig CM, Miramontes R, Cain KP, Bisson GP, et al. Association between tuberculin skin test result and clinical presentation of tuberculosis disease. BMC Infect Dis. 2013;13:460. doi: 10.1186/1471-2334-13-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pigrau-Serrallach C, Rodríguez-Pardo D. Bone and join tuberculosis. Eur Spine J. 2013;22(Suppl 4):556–66. doi: 10.1007/s00586-012-2331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vuyst D, Vanhoenacker F, Gielen J, Bernaerts A, De Schepper AM. Imaging features of musculoskeletal tuberculosis. Eur Radiol. 2003;13:1809–19. doi: 10.1007/s00330-002-1609-6. [DOI] [PubMed] [Google Scholar]
- 11.Rasouli MR, Mirkoohi M, Vaccaro AR, Yarandi KK, Rahimi-Movaghar V. Spinal tuberculosis: Diagnosis and management. Asian Spine J. 2012;6:294–308. doi: 10.4184/asj.2012.6.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wardle N, Ashwood N, Pearse M. Orthopaedic manifestations of tuberculosis. Hosp Med. 2004;65:228–33. doi: 10.12968/hosp.2004.65.4.12737. [DOI] [PubMed] [Google Scholar]
- 13.Vadivelu S, Effendi S, Starke JR, Luerssen TG, Jea A. A review of the neurological and neurosurgical ımplications of tuberculosis in children. Clin Pediatr (Phila) 2013;52:1135–43. doi: 10.1177/0009922813493833. [DOI] [PubMed] [Google Scholar]
- 14.Guidance for national tuberculosis programmes on the management of tuberculosis in children. [Last accessed on 2014 October 20]. Available from: http://whqlibdoc.who.int/hq/2006/WHO_HTM_TB_2006.371_eng.pdf . [PubMed]


