Abstract
Paragonimiasis is a food-borne parasitic zoonosis caused by the genus Paragonimus. Fresh water snails, crabs, and crayfish are the first and second intermediate hosts, respectively. Humans acquire this infection by ingesting uncooked/undercooked crustaceans. Laboratory diagnosis of Paragonimiasis is done by demonstration of ova in the sputum/feces/pleural fluid or by serology. A case of pulmonary Paragonimiasis is presented herewith; the patient having been diagnosed with pulmonary tuberculosis earlier. The aim of this presentation is to highlight this entity so that it is considered in the differential diagnosis in a case of hemoptysis.
KEY WORDS: Endemic hemoptysis, pulmonary paragonimiasis, zoonosis
INTRODUCTION
Paragonimiasis is one of the most important food-borne parasitic zoonosis caused by one or more of the trematode species of the genus Paragonimus. It is also known as endemic hemoptysis, oriental lung fluke infection, pulmonary distomiasis, parasitical hemoptysis, parasitare haemopte, Gregarinosis pulmonum, and so on. It is endemic to many parts of Asia, Africa, and South America.[1]
Fresh water snails, crabs, as well as crayfish act as first and second intermediate hosts, respectively. Humans acquire infection mostly by ingestion of uncooked or undercooked crustaceans containing metacercariae, the larval stage of the parasite, and rarely by ingestion of infected, uncooked or undercooked meat of pig and wild boar. The latter are paratenic hosts.[2,3]
The parasites primarily infect the lung, but extrapulmonary infections are also encountered. The laboratory diagnosis of Paragonimiasis is by microscopic demonstration of Paragonimus ova in the sputum and other specimens, such as, feces and pleural fluid or by serology.[4]
CASE REPORT
A 26-year-old male patient, presented with the history of blackish red, streaky hemoptysis daily. He quantified it as up to 15 to 20 ml per day. There was no history of fever, chest pain, syncope, orthopnea, paroxysmal nocturnal dyspnea (PND), pedal edema, pain abdomen, abdominal distension, jaundice, altered bowel habits, oliguria, facial puffiness, nocturia, generalized swelling, joint pains, rashes or sore throat. The patient also did not give any history of anorexia or weight loss. There was past history of the patient having been given anti-tubercular treatment for one year, for tubercular pleural effusion and eosinophilic polyserositis (bilateral pleural effusion and ascites) at a peripheral hospital, as a therapeutic trial. There was no history of hypertension or diabetes mellitus. The patient was an occasional nonvegetarian, a nonsmoker, and consumed alcohol occasionally. He gave a history of eating raw and pickled crabs on being asked leading questions. His bowel and bladder habits were normal. There was no history of high risk behavior. On examination, the patient was 165 cm tall, weighed 50 kg, and had a body mass index of 18.38 kg/m2. His general physical examination was within normal limits with a respiratory rate of 20/minute. On systemic examination, the chest was bilaterally symmetrical and moving equally bilaterally. There were reduced breath sounds in the right infrascapular region. No cackles or rhonchi were present. Other systems were essentially within normal limits on examination. The patient did not give any history of tuberculosis in the family. A family history of bronchial asthma was present. Clinical differential diagnoses of tuberculosis, bronchiectasis, fungal infection, and malignancy were considered.
Routine investigations revealed hemoglobin to be 15.2 g%, a mildly raised total leukocyte count of 18,200/cumm, eosinophilia of 12%, and a normal platelet count. The absolute eosinophil count was 2000/cu mm and the coagulation profile was unremarkable. Sputum examination for acid fast bacilli (AFB) done for three consecutive days was negative. Mantoux test was negative. Sputum was negative for eosinophils. The routine urine and microscopic examinations were within normal limits. The stool examination showed that the antinuclear antibodies (ANA), antinuclear cytoplasmic antibodies (p ANCA), hepatitis B surface antigen (HBS Ag), anti-Hepatitis C virus (HCV), and human immunodeficiency virus (HIV) were within normal limits. Spirometry showed mild restriction. An x-ray of the chest and contrast-enhanced computed tomography (CECT) revealed fibrosis with traction bronchiectasis in the left upper lobe (LUL) [Figure 1], with few discrete nodules in both upper lobes [Figure 2], mediastinal lymphadenopathy (maximum 14 mm), pleural effusion (right side), and mild left pleural thickening. Post fibreoptic bronchoscopy, the sputum showed operculated eggs of P. westermani [Figure 3]. The patient was put on tablet Praziquantel 600 mg − two tablets, thrice a day, for three days. On follow-up, the patient's hemoptysis had abated, his repeat sputum analysis was normal, and the hemogram showed that the eosinophilia had subsided.
Figure 1.
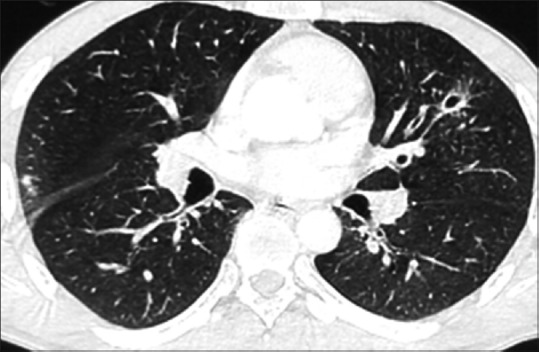
CECT of chest showing bronchiectasis
Figure 2.
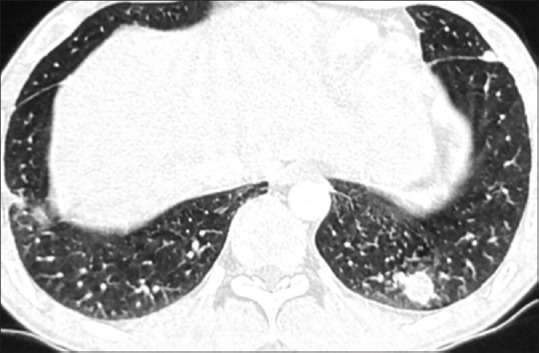
CECT of chest showing pulmonary nodules
Figure 3.
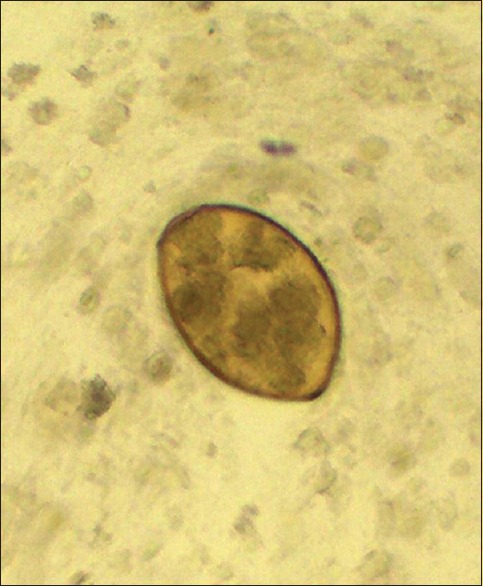
Operculated egg of Paragonimus westermani; post flexible fibreoptic bronchoscopy (FOB) sputum, direct examination, iodine mount, 1000x
DISCUSSION
Paragonimiasis is an important food-borne parasitic zoonosis caused by one or more of the trematode species of the genus Paragonimus. It is endemic in some pockets of Northeastern India.[4] Two Paragonimus species were first described from India − P. compactus from an Indian mongoose (Herpestes edwardsi), in 1859, and P. westermani from two Bengal tigers, in 1878.[5,6]
P. westermani enters a human host after ingestion of metacercariae, its infective stage. They excyst in the small intestine, and the larvae then penetrate the intestinal wall to enter the peritoneum. The diaphragm and pleura are penetrated thereafter, and they enter the lung in three to eight weeks, where they mature to adult flukes.[7]
Clinically, Paragonimiasis may be classified into pulmonary, extrapulmonary, and pleuropulmonary forms. Alternatively, it can be categorized into acute and chronic forms and ectopic paragonimiasis.[1]
Pulmonary paragonimiasis presenting with hemoptysis is commonly misdiagnosed as pulmonary tuberculosis, as had happened in the present case.
The incubation period may vary from one to two months or even longer. The patients may present with signs and symptoms of mild pleural effusion, pneumonitis, bronchiectasis or bronchopneumonia. The common presenting symptoms are pain or tightness in the chest, difficulty in breathing, rusty brown or blood-stained sputum or recurrent hemoptysis. Chronic infection presents with fever, anemia, weakness, and weight loss.[4] Our patient presented with hemoptysis only. He had a past history of pleural effusion, ascites, and eosinophilic polyserositis.
Definitive diagnosis of Paragonimiasis can be made by demonstrating characteristic golden brown, ellipsoidal operculated Paragonimus ova in the sputum, body fluids or feces. Occasionally, an adult worm may be found in the biopsy specimens/autopsy/pleural fluid or sputum.[8,9]
Paragonimus ova and Charcot-Leyden crystals are demonstrated in early morning sputum specimens of patients with pleuropulmonary Paragonimiasis.[4] Literature has shown Paragonimus ova in 55.6 to 72% of the sputum specimens of pulmonary paragonimiasis.[10,11]
On CT, air-space consolidation was noted in 82%, nodules in 41%, linear opacities extending from the pleura to the lung in 41%, and bronchiectasis in 35%, by Im et al., in a study of the radiological findings in 71 cases of pleuropulmonary paragonimiasis, with CT scans being available in 17 patients. Subpleural linear opacities or a tubular structure communicating with a cyst were found to be suggestive of worm migration tracks. On chest x-rays, 83% of the patients were found to have pulmonary lesions (consolidation, cysts, nodules, and linear opacities) and 61% had pleural lesions (pleural effusion, hydropneumothorax, and pleural thickening).[12] Adjacent bronchectasis has been found in 55% of the cases and pleural effusion in 26% of the cases studied by Kim et al.[13] The patient in the present case had a few discrete nodules in both upper lobes, fibrosis with traction bronchiectasis in the LUL, mediastinal lymphadenopathy, right pleural effusion, and left pleural thickening.
CONCLUSION
Paragonimus westermani infection of the lung is a perplexing diagnostic entity. The present case is reported here to discuss the approach to a case of hemoptysis and to highlight the fact that every case of hemoptysis is not and must not be treated as pulmonary tuberculosis. An underdiagnosed entity of ‘endemic hemoptysis’ is highlighted here.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Procop GW. North American paragonimiasis (caused by paragonimus kellicotti) in the context of global praragonimiasis. Clin Microbiol Rev. 2009;22:415–46. doi: 10.1128/CMR.00005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong HL, He LY, Xu ZB, Cao WJ. Recent progress in studies of paragonimus and paragonimiasis control in China. Chin Med J (Engl) 1981;94:483–94. [PubMed] [Google Scholar]
- 3.Miyazaki I, Hirose H. Immature lung flukes first found in the muscles of the wild boar in Japan. J Parasitol. 1976;62:836–7. [PubMed] [Google Scholar]
- 4.Singh TS, Sugiyama H, Rangsiruji A. Paragonimus and paragonimiasis in India. Indian J Med Res. 2012;136:192–204. [PMC free article] [PubMed] [Google Scholar]
- 5.Cobbold TS. On some new forms of Entozoa. Trans Linn Soc London. 1859;22:363–6. [Google Scholar]
- 6.Kerbert C. Knowledge to Trematodes. Zool Anz. 1878;1:271–3. [Google Scholar]
- 7.Yokogawa M. Paragonimus and paragonimiasis. Adv Parasitol. 1965;3:99–158. doi: 10.1016/s0065-308x(08)60364-4. [DOI] [PubMed] [Google Scholar]
- 8.Vidamaly S, Choumlivong K, Keolouangkhot V, Vannavong N, Kanpittaya J, Strobel M. Paragonimiasis: A common cause of persistent pleural effusion in Lao PDR. Trans R Soc Trop Med Hyg. 2009;103:1019–23. doi: 10.1016/j.trstmh.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Vanijanonta S, Radomyos P, Bunnag D, Harinasuta T. Pulmonary paragonimiasis with expectoration of worms: A case report. Southeast Asian J Trop Med Public Health. 1981;12:104–6. [PubMed] [Google Scholar]
- 10.Singh TS, Mutum SS, Razaque MA. Pulmonary paragonimiasis: Clinical features, diagnosis and treatment of 39 cases in Manipur. Trans R Soc Trop Med Hyg. 1986;80:967–71. doi: 10.1016/0035-9203(86)90275-0. [DOI] [PubMed] [Google Scholar]
- 11.Komiya Y, Yokogawa M. The recovering of paragonimus eggs from stools of paragonimiasis patients by AMS III centrifuging technic. Jpn J Med Sci Biol. 1953;6:207–11. doi: 10.7883/yoken1952.6.207. [DOI] [PubMed] [Google Scholar]
- 12.Im JG, Whang HY, Kim WS, Han MC, Shim YS, Cho SY. Pleuropulmonary paragonimiasis: Radiologic findings in 71 patients. AJR Am J Roentgenol. 1992;159:39–43. doi: 10.2214/ajr.159.1.1609718. [DOI] [PubMed] [Google Scholar]
- 13.Kim TS, Han J, Shim SS, Jeon K, Koh WJ, Lee I, et al. Pleuropulmonary paragonimiasis: CT findings in 31 patients. AJR Am J Roentgenol. 2005;185:616–21. doi: 10.2214/ajr.185.3.01850616. [DOI] [PubMed] [Google Scholar]


